Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transformation of 5-acylated N-fluoroalkyl-1,2,3-triazoles to trifluoromethylated ring-fused isoquinolines, 1,3-oxazines, and 1,3-oxazin-6-ones via ketenimines†
Lukáš Janecký ab,
Blanka Klepetářováa and
Petr Beier
ab,
Blanka Klepetářováa and
Petr Beier *a
*a
aThe Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Flemingovo nam. 2, 16610 Prague 6, Czech Republic. E-mail: beier@uochb.cas.cz
bDepartment of Organic Chemistry, Faculty of Science, Charles University, Hlavova 2030/8, 128 43 Prague 2, Czech Republic
First published on 27th August 2024
Abstract
A one-pot multistep methodology leading to trifluoromethylated cyclopenta[c]isoquinolines, indeno[1,2-c]isoquinolines, 6,6-difluoro-1,3-oxazines, or 1,3-oxazin-6-ones, based on the reaction of 5-acylated N-pentafluoroethyl-substituted 1,2,3-triazoles is presented. A thermal ring opening of the starting triazoles, followed by a 1,2-acyl shift formed reactive ketenimines which cyclized after a rearrangement in a substrate-specific manner to provide new trifluoromethylated heterocyclic products.
Introduction
Isoquinolines with fused 5-membered rings, 6H-1,3-oxazines, or oxazin-6-ones constitute important classes of biologically active compounds known as anti-tubercular, anti-inflammatory, sedative agents, or enzyme inhibitors (Fig. 1).1–5 Despite the few known synthetic strategies to indeno[1,2-c]isoquinolines1,6–8 or cyclopenta[c]isoquinolines,9 the preparation of 2-trifluoromethyl-5-membered ring-fused isoquinolines was described for only one specific example.10 Similarly, non-fluorinated fully substituted 6H-1,3-oxazin-6-ones can be synthesized from β-lactams,11 isoxazolones,12–14 cyclopropenones15 or ynamides.16 However, 2-trifluoromethyl-substituted 1,3-oxazin-6-ones or 1,3-oxazines remain unexplored (Fig. 1). Since trifluoromethylated heteroaromatics of novel structures are highly valued chemicals, which find use in medicinal chemistry17–20 and agrochemistry21–23 research programmes, we set out to investigate the synthetic approaches towards the proposed novel trifluoromethylated heteroarenes shown in Fig. 1.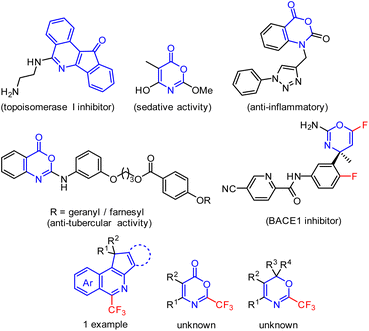 | ||
| Fig. 1 Selected examples of bioactive 5-membered ring fused isoquinolines or 1,3-oxazine and 1,3-oxazin-6-ones and compounds of interest – their trifluoromethylated derivatives. | ||
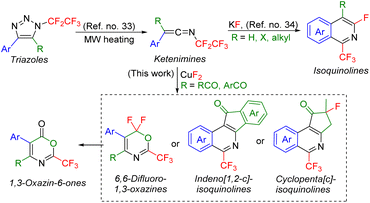
We recently reported a denitrogenation strategy for multisubstituted N-fluoroalkylated 1,2,3-triazoles24–31 with Brønsted or Lewis acids proceeding via vinyl cation intermediates and leading to various N-alkenyl compounds.10,24,30–32 We also showed that N-fluoroalkyl 1,2,3-triazoles in microwave reaction conditions undergo a rearrangement to form ketenimines,33 which can further cyclize to isoquinolines (Scheme 1).34
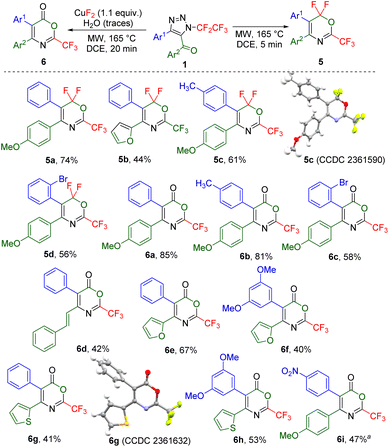
Herein, we propose a new synthetic methodology to prepare trifluoromethylated 5-membered ring fused isoquinolines, 6,6-difluoro-1,3-oxazines or 1,3-oxazin-6-ones from 5-acyl-N-pentafluoroethyl-1,2,3-triazoles involving ketenimine intermediates (Scheme 1).
Results and discussion
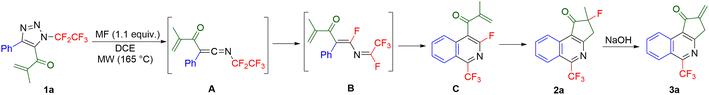
Denitrogenation of N-fluoroalkylated 1,2,3-triazoles to ketenimines by microwave heating33 was extended to 5-acylated triazoles.24 Thus, microwave heating of 5-methacryloyl-substituted triazole 1a resulted in the formation of a mixture of ring-fused 1-trifluoromethylisoquinolines 2a and 3a, presumably via ketenimine A, imidoyl fluoride B and isoquinoline C intermediates (Table 1, entry 1). The addition of fluoride salts can enhance the 1,3-fluorine shift of A to B, therefore an optimization study was conducted to improve the selectivity of the reaction. Copper(II) fluoride was identified as the most effective fluoride additive (entry 8). A combination of potassium fluoride and sodium hydroxide was used to obtain dehydrofluorinated 1-trifluoromethyl-isoquinoline 3a (entry 9).| Entry | Time (min) | Additive | Ratio 2a/3aa | 2a Yieldb (%) | 3a Yieldb (%) |
|---|---|---|---|---|---|
| a 19F NMR ratio.b Isolated yield. n.d. not determined.c With added NaOH (3 equiv.). | |||||
| 1 | 120 | — | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 52 |
n.d. | n.d. |
| 2 | 60 | KF | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
12 | 23 |
| 3 | 60 | AlF3 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
n.d. | n.d. |
| 4 | 60 | CsF | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 58 |
n.d. | n.d. |
| 5 | 60 | AgF | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
42 | 10 |
| 6 | 60 | NaF | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
n.d. | n.d. |
| 7 | 60 | FeF3 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
n.d. | n.d. |
| 8 | 30 | CuF2 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
41 | 12 |
| 9 | 30 | KFc | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 87 |
Traces | 31 |
A small library of 4-aryl-5-methacryloyl triazoles, obtained from the intercepted click reaction of aromatic copper(I) acetylides, azidopentafluoroethane and methacrylic chloride in the presence of DIPEA (see ESI† for details), was subjected to the reaction providing ring-fused isoquinolines 2 in moderate to good yields (Scheme 2). The structure of derivative 2c was confirmed by crystallography. Substrate with electron-acceptor group (nitro) on the aryl ring did not form the product (2e). Additionally, two examples of dehydrofluorinated isoquinolines 3 were prepared albeit in moderate to low yields.
When electron-rich 5-(3,5-dimethoxybenzoyl)-substituted 1,2,3-triazoles were used, ring-fused 1-trifluoromethyl-isoquinolines 4 bearing various substituents on the isoquinoline ring formed in good yields (Scheme 2).
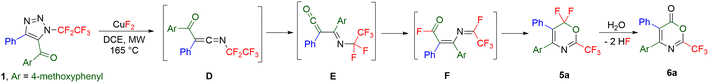
All other investigated 5-acylated 1,2,3-triazoles except strongly electron-rich 5-(3,5-dimethoxybenzoyl)- or 5-methacryloyl-substituted ones afforded different products under the thermal denitrogenation conditions. Thus, 5-(4-methoxyphenyl)-substituted triazole underwent a unique transformation presumably via ketenimine D, followed by 1,3-aryl group transfer to ketene E,35 1,5-fluorine shift to intermediate F, and cyclization involving another 1,5-fluorine shift to 6,6-difluoro-2-trifluoromethyl-1,3-oxazine 5a or a product of its hydrolysis 1,3-oxazin-6-one 6a (Table 2). Short reaction time (5 min) and no additive favoured the formation of product 5a, while a longer reaction time (20 min) and the use of CuF2 favoured the product of hydrolysis 6a. Four examples of 1,3-oxazines 5 were prepared in moderate to good yields, including the crystal structure of 5c and nine examples of 1,3-oxazinones 6 were synthesized in moderate to high yields including the crystal structure of 6g (Scheme 3). While the presence of an alkenyl group led to oxazinone 6d with this substitution in position 4, the products with alkyl groups in position 4 or 5 or an alkenyl group in position 5 did not form. Also, products 6 with the difluoromethyl or ethoxycarbonyl groups in position 2 did not form.
Conclusions
In conclusion, thermal denitrogenation of N-pentafluoroethylated 4-substituted-5-acyl-1,2,3-triazoles in the presence of copper(II) fluoride affords depending on the nature of 5-acyl substitution 1-trifluoromethylcyclopenta[c]-isoquinolines, indeno[1,2-c]-isoquinolines, 2-trifluoromethyl-6,6-difluoro-1,3-oxazines, or products of their hydrolysis 2-trifluoromethyl-1,3-oxazin-6-ones. All these compounds result from the formation of ketenimine intermediates which undergo either 1,3-fluorine shift, SEAr and SNAr sequence, or 1,3-aryl shift, 1,5-fluorine shift, cyclization and another 1,5-fluorine shift sequence. The presented methodology showcases advanced cyclization of ketenimine intermediates generated from triazoles and their application in the C–C bond formation for the synthesis of new heterocyclic structures.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
PB supervised the project. LJ contributed to experiments and product characterization. BK solved the crystal structures. LJ and PB jointly conceived the project, prepared the manuscript, and contributed to discussions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Czech Academy of Sciences (Research Plan RVO: 61388963) and by the Czech Science Foundation (Project 23-04659S).References
- D. B. Khadka, Q. M. Le, S. H. Yang, H. T. M. Van, T. N. Le, S. H. Cho, Y. Kwon, K. T. Lee, E. S. Lee and W. J. Cho, Design, Synthesis and Docking Study of 5-Amino Substituted Indeno[1,2-c]Isoquinolines as Novel Topoisomerase i Inhibitors, Biochem. Mol. Med., 2011, 19, 1924–1929 Search PubMed.
- P. Song, P. Yu, J. S. Lin, Y. Li, N. Y. Yang and X. Y. Liu, Transition-Metal-Free β-C-H Bond Carbonylation of Enamides or Amides with a Trifluoromethyl Group as CO Surrogate for the Synthesis of 1,3-Oxazin-6-Ones, Org. Lett., 2017, 19, 1330–1333 CrossRef CAS PubMed.
- B. Y. Lalaev, O. A. Petina, N. N. Kuz’mich, I. P. Yakovlev, G. M. Alekseeva and V. E. Zakhs, Potentiometric Study on Acid Properties of Some 4-Hydroxy-6H-1,3-Oxazin-6- Ones. Structure-Biological Activity Relationship, Russ. J. Gen. Chem., 2006, 76, 645–648 CrossRef CAS.
- W. Yang, Z. Zhou, Y. Zhao, D. Luo, X. Luo, H. Luo, L. Cui and L. Li, Copper Catalyzed Inverse Electron Demand [4+2] Cycloaddition Fot the Synthesis of Oxazines, Catalysts, 2022, 12, 526 CrossRef.
- N. Gupta, V. Saini, S. M. Basavarajaiah, M. O. Dar, R. Das and R. S. Dahiya, 1,3-Oxazine as a Promising Scaffold for the Development of Biologically Active Lead Molecules, ChemistrySelect, 2023, 8, 1–18 Search PubMed.
- M. A. Campo and R. C. Larock, Synthesis of Fluoren-9-Ones via of o-Halobiaryls, Org. Lett., 2000, 3, 1999–2001 Search PubMed.
- J. Dusemund and E. Kröger, Ein 1,3-Indandion-Derivat Aus Phthalaldehyd, Arch. Pharm., 1987, 320, 617–620 CrossRef.
- P. Halder, A. Iqubal, K. Mondal, N. Mukhopadhyay and P. Das, Carbonylative Transformations Using a DMAP-Based Pd-Catalyst through Ex Situ CO Generation, J. Org. Chem., 2023, 88, 15218–15236 CrossRef CAS.
- T. T. Pham, X. Chen, T. Söhnel, N. Yan and J. Sperry, Haber-Independent, Diversity-Oriented Synthesis of Nitrogen Compounds from Biorenewable Chitin, Green Chem., 2020, 22, 1978–1984 RSC.
- L. Janecký, A. Markos, B. Klepetářová and P. Beier, Lewis-Acid-Mediated Intramolecular Cyclization of 4-Aryl-5-Allyl-1,2,3-Triazoles to Substituted Cyclopentene Derivatives, J. Org. Chem., 2023, 88, 1155–1167 CrossRef PubMed.
- G. Cainelli, D. Giacomini, M. Gazzano, P. Galletti and A. Quintavalla, N-Acylation of 4-Alkylidene-b-Lactams: Unexpected Results, Tetrahedron Lett., 2003, 44, 6269–6272 CrossRef CAS.
- Y. M. Zhu, W. Zhang, H. Li, X. P. Xu and S. J. Ji, Palladium Catalyzed Ring Expansion Reaction of Isoxazolones with Isocyanides: Synthesis of 1,3-Oxazin-6-One Derivatives, Adv. Synth. Catal., 2021, 363, 808–818 CrossRef.
- B. F. Risitano, G. Grassi, F. Foti, F. Caruso, G. L. Vecchio and V. T. Cannizzaro, Ring-Enlargemen of Isoxazol-5-Ones to 1,3-Oxazin-6-Ones, J. Chem. Soc., Perkin Trans., 1979, 24, 1522–1524 RSC.
- E. M. Beccalli, T. Benincori and A. Marchesini, 1,3-Oxazin-6-Ones from 5(2H)-Isoxazolones, Synthesis, 1988, 8, 630–631 CrossRef.
- T. Matsuda, K. Yamanaka, Y. Tabata and T. Shiomi, Synthesis of Trisubstituted 1,3-Oxazin-6-Ones via Base-Catalyzed Ring-Opening Annulation of Cyclopropenones with N-(Pivaloyloxy)Amides, Tetrahedron Lett., 2018, 59, 1458–1460 CrossRef.
- O. A. Petina, I. P. Yakovlev and D. Geffken, Preparation of Arylpropynamides and Their Reaction with Malonyl Acid Derivatives, Synthesis, 2013, 45, 803–809 CrossRef CAS.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011), Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, Applications of Fluorine in Medicinal Chemistry, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed.
- K. Müller, C. Faeh and F. Diederich, Fluorine in pharmaceuticals: looking beyond intuition, Science, 2007, 317, 1881–1886 CrossRef.
- Fluorine in Pharmaceutical and Medicinal Chemistry, ed. V. Gouverneur and K. Müller, Imperial College Press, Ondon, 2012 Search PubMed.
- Y. Ogawa, E. Tokunaga, O. Kobayashi, K. Hirai and N. Shibata, Current contributions of Organofluorine Compounds to the Agrochemical Industry, iScience, 2020, 23, 101467 CrossRef CAS.
- P. Jeschke, Recent developments in fluorine-containing pesticides, Pest Manage. Sci., 2024, 80, 3065–3087 CrossRef CAS.
- Q. Wang, H. Song and Q. Wang, Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation, Chin. Chem. Lett., 2022, 33, 626–642 CrossRef.
- L. Janecký and P. Beier, Lewis Acid-Mediated Transformations of 5-Acyl-N-Fluoroalkyl-1,2,3-Triazoles to Cyclopentenones, Indenones, or Oxazoles, RSC Adv., 2024, 14, 13640–13645 RSC.
- Z. E. Blastik, S. Voltrová, V. Matoušek, B. Jurásek, D. W. Manley, B. Klepetářová and P. Beier, Azidoperfluoroalkanes: Synthesis and Application in Copper(I)-Catalyzed Azide–Alkyne Cycloaddition, Angew. Chem., Int. Ed., 2017, 56, 346–349 CrossRef PubMed.
- O. Bakhanovich and P. Beier, Synthesis, Stability and Reactivity of α-Fluorinated Azidoalkanes, Chem.–Eur. J., 2020, 26, 773–782 CrossRef.
- A. Markos, V. Matoušek and P. Beier, Fluoroalkyl Azides and Triazoles: Unlocking a Novel Chemical Space, Aldrichim Acta, 2022, 55, 37–44 CAS.
- S. Voltrová, M. Muselli, J. Filgas, V. Matoušek, B. Klepetářová and P. Beier, Synthesis of Tetrafluoroethylene-and Tetrafluoroethyl-Containing Azides and Their 1,3-Dipolar Cycloaddition as Synthetic Application, Org. Biomol. Chem., 2017, 15, 4962–4965 RSC.
- E. Shaitanova, V. Matoušek, T. Herentin, M. Adamec, R. Matyáš, B. Klepetářová and P. Beier, Synthesis and Cycloaddition Reactions of 1-Azido-1,1,2,2-Tetrafluoroethane, J. Org. Chem., 2023, 88, 14969–14977 CrossRef CAS PubMed.
- A. Markos, L. Janecký, T. Chvojka, T. Martinek, H. Martinez-Seara, B. Klepetářová and P. Beier, Haloalkenyl Imidoyl Halides as Multifacial Substrates in the Stereoselective Synthesis of N-Alkenyl Compounds, Adv. Synth. Catal., 2021, 363, 3258–3266 CrossRef CAS.
- A. Markos, S. Voltrová, V. Motornov, D. Tichý, B. Klepetářová and P. Beier, Stereoselective Synthesis of (Z)-β-Enamido Triflates and Fluorosulfonates from N-Fluoroalkylated Triazoles, Chem.–Eur. J., 2019, 25, 7640–7644 CrossRef CAS.
- A. Markos, L. Janecký, B. Klepetářová, R. Pohl and P. Beier, Stereoselective Synthesis of (Z)-β-Enamido Fluorides from N-Fluoroalkyl- And N-Sulfonyl-1,2,3-Triazoles, Org. Lett., 2021, 23, 4224–4227 CrossRef CAS.
- A. Kubíčková, A. Markos, S. Voltrová, A. Marková, J. Filgas, B. Klepetářová, P. Slavíček and P. Beier, Aza-Wolff Rearrangement of N-Fluoroalkyl Triazoles to Ketenimines, Org. Chem. Front., 2023, 10, 3201–3206 RSC.
- A. Kubíčková, S. Voltrová, A. Kleman, B. Klepetářová and P. Beier, One-Pot Multistep Synthesis of 1-Fluoroalkylisoquinolines and Fused Fluoroalkylpyridines from N-Fluoroalkyl-1,2,3-Triazoles, Org. Chem. Front., 2024, 11, 4442–4448 RSC.
- L. George, K. P. Netsch, G. Penn, G. Kollenz and C. Wentrup, Oxoketene-Oxoketene, Imidoylketene-Imidoylketene and Oxoketenimine- Imidoylketene Rearrangements. 1,3-Shifts of Phenyl Groups, Org. Biomol. Chem., 2006, 4, 558–564 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2361631 2361632 and 2361590. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra04794j |
| This journal is © The Royal Society of Chemistry 2024 |