Open Access Article
Open Access ArticleSynthetic cation channel: reconstructing the ion permeation pathway of TRPA1 in an artificial system†
Pengyang Xin *,
Wenke Ren,
Qiuhui Zhu,
Jie Wang,
Yonghui Sun*,
Junbiao Chang and
Gongming Zhu*
*,
Wenke Ren,
Qiuhui Zhu,
Jie Wang,
Yonghui Sun*,
Junbiao Chang and
Gongming Zhu*
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, NMPA Key Laboratory for Research and Evaluation of Innovative Drug, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453007, China. E-mail: pyxin27@163.com; syonghui1994@163.com; zhugm1201@163.com
First published on 27th August 2024
Abstract
A novel artificial cation channel was developed by rebuilding the ion permeation pathway of the natural channel protein (TRPA1) in a synthetic system. This tubular molecule can effectively embed into lipid bilayers and form transmembrane channels, thereby mediating cation transport. Furthermore, due to its carboxyl-modified ion permeation pathway, the transport activity of this artificial channel can be modulated by the pH of the buffer solution.
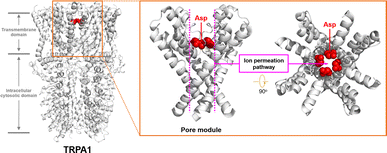
The cellular ion homeostasis is of vital importance for many physiological functions, and the regulation and maintenance of this ion gradient are implemented by natural proteins on the cell membrane with transmembrane transport capabilities.1 For example, the transient receptor potential A1 (TRPA1) channel protein is proposed to function as a sensor of the somatosensory nervous system.2 It can be activated by environmental stimuli, and facilitate the influx of extracellular cations, thereby initiating neural signal transmission. Compelling studies have demonstrated the presence of an ion transport pathway formed by pore-helices in the transmembrane domain of TRPA1 channel proteins, wherein the Asp 915 residue, positioned centrally in this pathway, plays a crucial role as an integral component of its selectivity ion filter (Fig. 1).3 The carboxyl group of Asp 915 can facilitate the dehydration process of hydrated cations and stabilize the cations through electrostatic interactions, thereby promoting the transmembrane transport of cations.4
The engaging details and diverse functionalities of native ion channels have sparked the curiosity of chemists, prompting extensive research in recent years to mimic natural channel proteins within synthetic systems.5,6 These researches strive to deepen our understanding of the underlying transport mechanisms of natural ion channels and uncover novel therapeutic options for channel-related diseases. However, the reconstruction of a stable ion permeation pathway resembling natural channel proteins within an artificial system and the incorporation of key functional groups such as Asp 915 found in TRPA1 to facilitate transmembrane ion transport pose significant challenges.
Previously, we found that the hybrid molecules comprising α-cyclodextrin (α-CD) and pillar-[5]arene possess the remarkable ability to construct single-molecule tubular architectures, which can create stable ion permeation pathways within membranes.7 The use of click reactions between scaffold molecules to construct this tubular molecules provide a simplified approach for synthesizing single-molecular artificial ion channels. Furthermore, the α-CD modules within these tubular molecules possess multiple modifiable sites, offering the potential for the incorporation of key functional groups, thus enabling the development of functionalized artificial transmembrane transport systems. Herein, we have introduced multiple carboxyl groups into the α-CD modules of the hybrid molecule. The hybrid molecule 7 was prepared by the click reaction of the corresponding ester-functionalized azido-α-cyclodextrin 5 and bialkynyl-pillar[5]arene 6. Then, the ethyl ester group deprotection of 7 afforded tubular molecule 1 (Fig. 2), and the structure of 1 were characterized by nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (ESI, Section S2†).
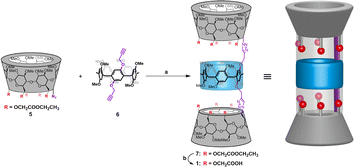 | ||
| Fig. 2 Synthesis and structural features of channel 1: (a) CuSO4·5H2O, sodium ascorbate, DMSO, r.t.; (b) LiOH·H2O, MeOH/H2O, r.t. | ||
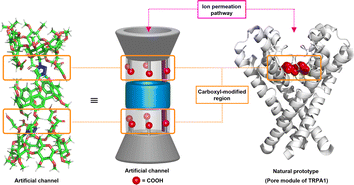
Computational studies reveal that this molecule is capable of adopting a stable tubular conformation and possesses a carboxyl-modified ion permeation pathway (ESI, Section S3†), which shares structural features resembling the ion transport pathway observed in TRPA channel proteins, potentially serving as synthetic analogues of TRPA channel proteins (Fig. 3).
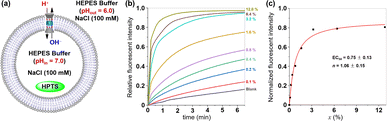
The ionophoric capability of compound 1 was initially evaluated using assays with 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS).8 In this experiment, the pH-sensitive dye HPTS was encapsulated within egg yolk phosphatidylcholine (EYPC) liposomes (pH = 7.0), and subsequently exposed to a pH gradient by introducing these liposomes into a buffer solution with a pH of 6.0 (Fig. 4a). Upon adding varying concentrations of 1 to the liposome suspensions, a significant increase in fluorescence intensity was observed for HPTS as the concentration of compound 1 increased (Fig. 4b), indicating that this tubular molecule could be inserted into the lipid bilayer and mediated the transmembrane transport of ions. From the Hill analysis of the dose–response curves, the EC50 (effective concentrations required for 50% activity) value was determined to be 0.75 ± 0.13 (Fig. 4c).9 The calculated Hill coefficients (n) of 1 indicated a value of 1.06 ± 0.15, suggesting that this tubular molecule mediates ion transport in a single-molecular manner.
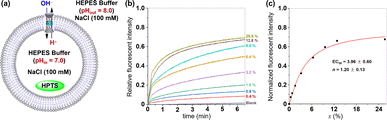
Because the ion permeation pathway of tubular molecule 1 bears multiple carboxyls, we envisioned that the pH value of the buffer solution may influence the membrane-incorporation ability and ion transport activity of this molecule. To investigate this possibility, the ionophoric capabilities of 1 were tested by using HPTS-assays under different pH values. We introduced EYPC liposome suspension (pH = 7.0) containing HPTS into buffer solutions with pH values of 4.0, 5.0, 6.0, 8.0, 9.0, and 10.0 respectively. After addition of 1, the fluorescence intensity of HPTS was continuously monitored for 6.5 minutes. As shown in Fig. S22,† upon exposure of liposomes to an acidic external buffer solution (pH 4.0, 5.0, or 6.0), a significant increase in the fluorescence intensity of HPTS is observed, suggesting that tubular molecule 1 can efficiently insert into the membrane and facilitate ion transport in an acidic milieu. Interestingly, under alkaline conditions (pH 8.0, 9.0, or 10.0), there is only a slight increase in the fluorescence intensity of HPTS, indicative of a substantial decrease in the ionophoric capacity of this molecule in a basic environment. To further quantitatively assess the ionophoric abilities of the tubular molecule in acidic and alkaline environments, we also conducted a transport experiment of 1 over a concentration ramp under the condition of alkaline external buffer solution (pH = 8.0) (Fig. 5). From the Hill analysis of the dose–response curves, the EC50 value was determined to be 3.96 ± 0.60, which is 5.3 times higher than the corresponding EC50 value under acidic condition (pH = 6.0), indicating that the ionophoric abilities of such molecule under acidic conditions is significantly higher than that under alkaline conditions. This observation may be attributed to the deprotonation of multiple carboxyl groups within the ion transport pathway of the tubular molecule in alkaline environments, which changes the amphiphilicity of this molecule, thereby reducing its membrane integration capability and ionophoric ability.
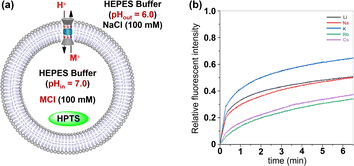
Then, the transmembrane transport activities of 1 towards alkali metal ions were investigated by the HPTS assay.10 Briefly, EYPC liposomes were filled with HPTS and 100 mM MCl (M = Li+, Na+, K+, Rb+, Cs+) in a HEPES buffer (10 mm, pH 7.0). The LUVs were suspended in an external HEPES buffer (10 mm, pH 6.0) containing 100 mM NaCl. After addition of 1 (x = 0.8%), the fluorescence intensity of HPTS was continuously monitored for 6.5 minutes. As seen in Fig. 6b, tubular molecule 1 demonstrates effective transmembrane transport of tested cations, and the transport activities of this molecule towards alkali metal ions are in the order of K+ > Li+ ≈ Na+ > Rb+ ≈ Cs+. The above observation not only provides clear evidence for the role of alkali metal ions as transport species in transmembrane transport processes, but also excludes the possibility of H+/Cl− symport and Cl−/OH− antiport serving as primary charge-balancing mechanisms during such processes, since they inherently preclude the participation of alkali metal cations.11
To gain deeper insights into the transport mechanisms of this molecule, we employed two commercial agents: FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenyl hydrazone) as an H+ transporter and VA (valinomycin) as a K+ carrier, in the HPTS assays, respectively.10a,12 The efflux of K+ facilitated by this channel needs to be accompanied by either the influx of H+ or the efflux of OH− in order to maintain electrical neutrality. If the transport rates of H+ or OH− are slower than that of K+, the presence of FCCP will result in an increase in fluorescence intensity observed from the HPTS dye. In the FCCP-HPTS assay, compound 1 (x = 0.8%) exhibited a transport efficiency of 44.0%, while FCCP (0.5 μM) showed a transport efficiency of 10.4% (Fig. 7). However, when both 1 and FCCP were simultaneously introduced into the LUVs suspension, there was a significant increase in the fluorescence intensity of HPTS to 77.0%. These findings suggest that co-injected FCCP enhances the rate of H+ transportation, implying that the transport rate of either H+ or OH− across the membrane is significantly slower compared to the compound 1-mediated transport of K+ ions. These observations have also been verified in the VA-HPTS assay (see Fig. S24 in the ESI†).
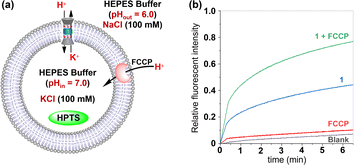 | ||
| Fig. 7 (a) Schematic representation for the FCCP-HPTS assay. (b) Changes in the fluorescence intensity of HPTS after the addition of 1 and FCCP. | ||
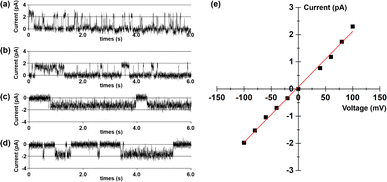
To further elucidate the transport mechanism and membrane behaviour of tubular molecule 1, its planar bilayer conductance was measured (ESI, Section S9†).13 Two chambers, each containing a 1.0 M KCl solution, were separated by a planar lipid bilayer composed of diphytanoylphosphatidylcholine (diPhyPC). Then, add a DMSO solution containing 1 into the cis chamber to achieve a final concentration of 0.25 μM. In the presence of this compound, regular square-like single channel currents were observed upon the application of multiple voltage levels (+100, +80, −80 and −100 mV) across the membrane (Fig. 8). These findings provide compelling evidence that this tubular molecule can be incorporated into lipid bilayers, thus forming transmembrane channels.14 The current–voltage (I–V) curves for artificial channel was derived from bilayer lipid membrane (BLM) electrophysiology measurements conducted at varying voltages, exhibiting a linear relationship in the range of −100 to +100 mV (Fig. 8e). Utilizing this I–V curve, the conductance (γ) of 1 was calculated to be 20.4 ± 0.5 pS. Additionally, the K+/Cl− selectivity of this artificial channel was evaluated by measuring the I–V curve in asymmetrical KCl solutions (see Fig. S26 in the ESI†).15 The permeability ratio (P) for K+ and Cl− was calculated using the Goldman–Hodgkin–Katz equation, resulting in a PK+/PCl− value of 2.8. The above results indicate that this tubular molecule can serve as an artificial cation channel.
In conclusion, inspired by the ion permeation pathway modulated by carboxyl groups in the natural cation channel protein TRPA1, we have developed a novel artificial transmembrane channel by rebuilding this pathway in a synthetic system. The carboxyl-modified ion permeation pathway in this artificial channel can be easily established via click reactions among the modular components. The vesicle-based transmembrane transport assays and BLM electrophysiology measurements have confirmed that this artificial channel can effectively embed into lipid bilayer membranes and form transmembrane channels, thereby mediating the transmembrane transport of cations. Additionally, due to the carboxyl-modified ion permeation pathway of such channels, their transmembrane transport activity can be modulated by the pH of the external buffer solution. These findings could enhance our understanding of the structure–function relationship in natural channel proteins and potentially have applications in molecular devices and channel-related drug discovery.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding from the National Natural Science Foundation of China (22271079, 82130103), Research Project from Pingyuan Laboratory (2023PY-ZZ-0201), Special Project for Fundamental Research in the University of Henan Province (21ZX005), Central Plains Scholars and Scientists Studio Fund (2018002) and Henan Normal University Research Foundation for the Doctoral Program (QD2023058). We also acknowledge financial support from the Henan Key Laboratory of Organic Functional Molecules and Drug Innovation.Notes and references
- B. Hille, Ionic Channels of Excitable Membranes, Sinauer Associates, Sunderland, MA, 3rd edn, 2001 Search PubMed.
- (a) G. M. Story, A. M. Peier, A. J. Reeve, S. R. Eid, J. Mosbacher, T. R. Hricik, T. J. Earley, A. C. Hergarden, D. A. Andersson, S. W. Hwang, P. McIntyre, T. Jegla, S. Bevan and A. Patapoutian, Cell, 2003, 112, 819 CrossRef PubMed; (b) S. E. Jordt, D. M. Bautista, H. Chuang, D. D. McKemy, P. M. Zygmunt, E. D. Högestätt, I. D. Meng and D. Juliuset, Nature, 2004, 427, 260 CrossRef PubMed; (c) D. Julius, Annu. Rev. Cell Dev. Biol., 2013, 29, 355 CrossRef PubMed.
- (a) J. Payandeh, T. Scheuer, N. Zheng and W. A. Catterall, Nature, 2011, 475, 353 CrossRef PubMed; (b) X. Wang, Y. Li, H. Wei, Z. Yang, R. Luo, Y. Gao, W. Zhang, X. Liu and L. Sun, Cell Discovery, 2023, 9, 36 CrossRef CAS PubMed.
- C. E. Paulsen, J. P. Armache, Y. Gao, Y. Cheng and D. Julius, Nature, 2015, 520, 511 CrossRef CAS.
- (a) K. S. Akerfeldt, J. D. Lear, Z. R. Wasserman, L. A. Chung and W. F. DeGrado, Acc. Chem. Res., 1993, 26, 191 CrossRef CAS; (b) X. Li, Y.-D. Wu and D. Yang, Acc. Chem. Res., 2008, 41, 1428 CrossRef CAS PubMed; (c) A. V. Jentzsch, A. Hennig, J. Mareda and S. Matile, Acc. Chem. Res., 2013, 46, 2791 CrossRef; (d) M. Barboiu and A. Gilles, Acc. Chem. Res., 2013, 46, 2814 CrossRef PubMed; (e) G. W. Gokel and S. Negin, Acc. Chem. Res., 2013, 46, 2824 CrossRef; (f) P. Reis and U. Koert, Acc. Chem. Res., 2013, 46, 2773 CrossRef PubMed; (g) M. Mayer and J. Yang, Acc. Chem. Res., 2013, 46, 2998 CrossRef CAS; (h) T. M. Fyles, Acc. Chem. Res., 2013, 46, 2847 CrossRef CAS; (i) B. Gong and Z. Shao, Acc. Chem. Res., 2013, 46, 2856 CrossRef CAS PubMed; (j) S. J. Webb, Acc. Chem. Res., 2013, 46, 2878 CrossRef CAS; (k) F. Otis, M. Auger and N. Voyer, Acc. Chem. Res., 2013, 46, 2934 CrossRef CAS PubMed; (l) J. Montenegro, M. R. Ghadiri and J. R. Granja, Acc. Chem. Res., 2013, 46, 2955 CrossRef CAS; (m) Y. Zhao, H. Cho, L. Widanapathirana and S. Zhang, Acc. Chem. Res., 2013, 46, 2763 CrossRef PubMed; (n) W. Si, P. Xin, Z.-T. Li and J.-L. Hou, Acc. Chem. Res., 2015, 48, 1612 CrossRef; (o) S. Howorka, Nat. Nanotechnol., 2017, 12, 619 CrossRef PubMed; (p) R. D. Mukhopadhyay, Y. Kim, J. Koo and K. Kim, Acc. Chem. Res., 2018, 51, 2730 CrossRef PubMed.
- (a) T. Muraoka, T. Shima, T. Hamada, M. Morita, M. Takagi, K. V. Tabata, H. Noji and K. Kinbara, J. Am. Chem. Soc., 2012, 134, 19788 CrossRef CAS PubMed; (b) M. D. Vincenzo, A. Tiraferri, V.-E. Musteata, S. Chisca, R. Sougrat, L.-B. Huang, S. P. Nunes and M. Barboiu, Nat. Nanotechnol., 2021, 16, 190 CrossRef PubMed; (c) J. Shen, C. Ren and H. Zeng, Acc. Chem. Res., 2022, 55, 1148 CrossRef CAS PubMed; (d) I. M. Andrei, A. Chaix, B. T. Benkhaled, R. Dupuis, C. Gomri, E. Petit, M. Polentarutti, A. van der Lee, M. Semsarilar and M. Barboiu, J. Am. Chem. Soc., 2023, 145, 21213 CrossRef CAS; (e) A. Mondal, S. N. Save, S. Sarkar, D. Mondal, J. Mondal, S. Sharma and P. Talukdar, J. Am. Chem. Soc., 2023, 145, 9737 CrossRef PubMed; (f) R. Cao, R. B. Rossdeutcher, Y. Zhong, Y. Shen, D. P. Miller, T. A. Sobiech, X. Wu, L. S. Buitrago, K. Ramcharan, M. I. Gutay, M. F. Figueira, P. Luthra, E. Zurek, T. Szyperski, B. Button, Z. Shao and B. Gong, Nat. Chem., 2023, 15, 1559 CrossRef PubMed; (g) A. Docker, T. G. Johnson, H. Kuhn, Z. Zhang and M. J. Langton, J. Am. Chem. Soc., 2023, 145, 2661 CrossRef PubMed; (h) W.-L. Huang, X.-D. Wang, Y.-F. Ao, Q.-Q. Wang and D.-X. Wang, Angew. Chem., Int. Ed., 2023, 62, e202302198 CrossRef PubMed; (i) P. Xin, L. Xu, W. Dong, L. Mao, J. Guo, J. Bi, S. Zhang, Y. Pei and C.-P. Chen, Angew. Chem., Int. Ed., 2023, 62, e202217859 CrossRef CAS; (j) W.-L. Huang, X.-D. Wang, Y.-F. Ao, Q.-Q. Wang and D.-X. Wang, Chem. Commun., 2023, 59, 14689 RSC; (k) C. Li, Y. Wu, Y. Zhu, J. Yan, S. Liu, J. Xu, S. Fa, T. Yan, D. Zhu, Y. Yan and J. Liu, Adv. Mater., 2024, 36, 2312352 CrossRef CAS PubMed; (l) Z. Chen, X. Xie, C. Jia, Q. Zhong, Q. Zhang, D. Luo, Y. Cao, Y. Mu and C. Ren, Angew. Chem., Int. Ed., 2024, 63, e202318811 CrossRef CAS; (m) L. Zhang, J. Tian, Z. Lin and Z. Dong, J. Am. Chem. Soc., 2024, 146, 8500 CrossRef CAS; (n) L. Shi, W. Zhao, Z. Jiu, J. Guo, Q. Zhu, Y. Sun, B. Zhu, J. Chang and P. Xin, Angew. Chem., Int. Ed., 2024, 63, e202403667 CrossRef CAS; (o) Y.-H. Fu, Y.-F. Hu, T. Lin, G.-W. Zhuang, Y.-L. Wang, W.-X. Chen, Z.-T. Li and J.-L. Hou, Nat. Chem., 2024 DOI:10.1038/s41557-024-01519-8.
- P. Xin, H. Kong, Y. Sun, L. Zhao, H. Fang, H. Zhu, T. Jiang, J. Guo, Q. Zhang, W. Dong and C.-P. Chen, Angew. Chem., Int. Ed., 2019, 58, 2779 CrossRef PubMed.
- Y. J. Jeon, H. Kim, S. Jon, N. Selvapalam, D. H. Oh, I. Seo, C.-S. Park, S. R. Jung, D.-S. Koh and K. Kim, J. Am. Chem. Soc., 2004, 126, 15944 CrossRef PubMed.
- N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernandez, R. Perez-Tomas and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136 CrossRef PubMed.
- (a) R. Ye, C. Ren, J. Shen, N. Li, F. Chen, A. Roy and H. Zeng, J. Am. Chem. Soc., 2019, 141, 9788 CrossRef PubMed; (b) C. Lang, X. Deng, F. Yang, B. Yang, W. Wang, S. Qi, X. Zhang, C. Zhang, Z. Dong and J. Liu, Angew. Chem., Int. Ed., 2017, 56, 12668 CrossRef CAS PubMed.
- C. Ren, F. Zeng, J. Shen, F. Chen, A. Roy, S. Zhou, H. Ren and H. Zeng, J. Am. Chem. Soc., 2018, 140, 8817 CrossRef CAS PubMed.
- S. V. Shinde and P. Talukdar, Angew. Chem., Int. Ed., 2017, 56, 4238 CrossRef CAS PubMed.
- R. H. Ashley, Ion Channels: A Practical Approach, Oxford University Press, Oxford, UK, 1995 Search PubMed.
- J. K. W. Chui and T. M. Fyles, Chem. Soc. Rev., 2012, 41, 148 RSC.
- T. M. Fyles, D. Loock, W. F. van Straaten-Nijenhuis and X. Zhou, J. Org. Chem., 1996, 61, 8866 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05676k |
| This journal is © The Royal Society of Chemistry 2024 |