Open Access Article
Open Access ArticlePrevalence and health risk evaluations of mycotoxins in drinking water sources in Nigeria†
Daniel T. Koko ab,
Moses O. Alfred
ab,
Moses O. Alfred ab,
Nathaniel B. Bolujokoab,
Damilare Olorunnisolaab,
Oluwaferanmi B. Otitojuab,
Peter Alabi
ab,
Nathaniel B. Bolujokoab,
Damilare Olorunnisolaab,
Oluwaferanmi B. Otitojuab,
Peter Alabi a,
Olumuyiwa O. Ogunlaja
a,
Olumuyiwa O. Ogunlaja ac,
Friday Okonofuadef,
Akhere A. Omonkhua
ac,
Friday Okonofuadef,
Akhere A. Omonkhua eg,
Titus A. M. Msagati
eg,
Titus A. M. Msagati h,
Martins O. Omorogie
h,
Martins O. Omorogie *ab,
Aemere Ogunlajaai,
Olumide D. Olukanniaj and
Emmanuel I. Unuabonah
*ab,
Aemere Ogunlajaai,
Olumide D. Olukanniaj and
Emmanuel I. Unuabonah *ab
*ab
aAfrican Centre of Excellence for Water and Environmental Research (ACEWATER), Redeemer's University, P.M.B 230, Ede, 232101, Osun State, Nigeria. E-mail: unuabonahe@run.edu.ng; omorogiem@run.edu.ng
bDepartment of Chemical Sciences, Faculty of Natural Sciences, Redeemer's University, P.M.B 230, Ede, Osun State, Nigeria
cDepartment of Chemical Sciences, Faculty of Natural and Applied Sciences, Lead City University, Ibadan, Nigeria
dWomen's Health and Action Research Centre (WHARC), Km 11 Benin-Lagos Expressway, Igue-Iheya, Benin City, Edo State, Nigeria
eCentre of Excellence in Reproductive Health Innovation, University of Benin, Benin City, Nigeria
fDepartment of Obstetrics and Gynaecology, University of Benin and University of Benin Teaching Hospital, Benin City, Nigeria
gDepartment of Medical Biochemistry, School of Basic Medical Sciences, University of Benin, Benin City, Nigeria
hInstitute for Nanotechnology and Water Sustainability, College of Science, Engineering and Technology, University of South Africa, South Africa, The Science Campus, 1709, Roodepoort, Johannesburg, South Africa
iDepartment of Biological Sciences, Faculty of Natural Sciences, Redeemer's University, P.M.B 230, Ede, Osun State, Nigeria
jDepartment of Biochemistry, Faculty of Basic Medical Sciences, Redeemer's University, Ede, Nigeria
First published on 29th October 2024
Abstract
Mycotoxins in drinking water are neglected pollutants that have serious health implications when ingested. Common mycotoxins with health concerns include deoxynivalenol (DON), ochratoxin A (OTA), and zearalenone (ZEN). This study considers the distribution and apparent health risks of these mycotoxins in drinking water sources (groundwater, surface water, bottled water, sachet water) in three Southwest Nigeria States: Osun, Oyo, and Lagos States, using the UHPLC-ESI-QTOF. ZEN and DON were found in all 95 water samples across all three states. ZEN in sachet water samples has the highest mean concentrations, with those from Osun, Oyo, and Lagos States having concentrations of 14.96 ± 4.46, 8.59 ± 3.86, and 10.56 ± 2.84 μg L−1, respectively. In contrast, the mean concentrations of all three mycotoxins (± Standard Error of Mean) in bottled water samples are the lowest across the three States. The mean concentrations of OTA in sachet water samples (2.93 ± 0.79, 1.24 ± 0.40, and 3.01 ± 1.50 μg L−1) are slightly higher than those in bottled water (1.47 ± 0.28, 1.53 ± 0.31, and 0.75 ± 0.31 μg L−1) for Osun, Oyo, and Lagos States, respectively. Groundwater samples across the three States had the lowest average pH values, below the WHO's lowest limit of 6.5. Principal Component Analysis studies indicate that all three mycotoxins in water samples are closely associated by source. Possible human exposure values for ZEN suggest some health concerns, especially with the use of sachet water type based on the estrogenicity of this mycotoxin. However, OTA values for all water samples analyzed, are much lower than the tolerable daily intake (TDI) of 4.73 μg kg−1 bw per day provided by European Food Safety Auhtority (EFSA). The levels of DON in all water sample types were of no serious health concern. However, human exposure levels to all three mycotoxins in bottled water fall within safe limits of health-based guidance values (HBGV) indicating that this drinking water type is better treated, unlike sachet water. Nevertheless, stakeholders need to re-examine water quality with respect to these mycotoxins and adopt stringent guidelines and new water treatment strategies to provide consumers with safe drinking water in line with the UN SDG #6.
1 Introduction
The vulnerability of drinking water sources to diverse pollutants from natural and anthropogenic activities is a global challenge, especially in developing nations where sanitation is very poor and most of the populace depends on rivers, streams, hand-dug wells, bore-holes, and lakes for potable water. Over the years scientists have focused on understanding the distribution, concentration, and risk associated with the presence of a large variety of chemical pollutants in drinking water including inorganic contaminants such as heavy metals,1,2 inorganic anions,3,4 radionuclides,5 organic pollutants,6–8 and inorganic–organic pollutants.9,10 Moreover, there is a large repository that reports the presence of micro-organisms in drinking water.11–14 However, there is scarce information on the presence of fungi metabolites (mycotoxins) in drinking water especially from Africa, except for reports that considered them in food.15,16A chronology of the study of fungi in water is provided by Hageskal, et al.17 The presence of filamentous fungi in water leads to the production of mycotoxins that are toxic to invertebrates, plants, micro-organisms18,19 and humans. Fungi of the genera Fusarium, Aspergillus, and Penicillium are known to produce toxins including aflatoxins, ochratoxins, deoxynivalenol, nivalenol, fumonisins, trichothecenes, fusaproliferin, moniliformin, beauvericin, enniatins, NX-2 toxin, and zearalenone.20,21 Among these toxins, aflatoxins, ochratoxins, deoxynivalenol, zearalenone, and fumonisins are some of the few mycotoxins dangerous to both humans and animals.22 For example, zearalenone (ZEN) mycotoxin which is produced by fungi of the Fusarium sp has a structure similar to estrogens such as estradiol, estrone, estriol, 7-β-estradiol, and 17-β-estradiol23 which imbues it with strong estrogenic and anabolic characteristics that result in fertility disorders (infertility or reduced fertility), vaginal prolapse, vulvar swelling and breast enlargement in females, testicular atrophy, and enlargement of the mammary glands in males in various animal species.24,25 Meanwhile, ochratoxin A (OTA) is a fungal secondary metabolite produced by several species of the genera Aspergillus (occurring in tropical climates) and Penicillium.26,27 This toxin (OTA) is the most toxic of all mycotoxins aside from aflatoxins and is known to be nephrotoxic, hepatotoxic, teratogenic, and immunotoxic to several species of animals and can cause kidney and liver tumours.28 OTA is classified as a possible human carcinogen (group 2B)29 and it causes liver tumours.30
On the other hand, deoxynivalenol (DON) is reported to cause sperm damage, oxidative stress, testicular apoptosis as well as diarrhoea, decreased weight, anorexia, decreased nutritional efficiency, and altered immune function31,32 and reduced quality of spermatozoa.33 DON is a naturally occurring mycotoxin among the many toxins from the genus Fusarium known to be an important pathogen of concern for small cereals. It is thermally and chemically stable in several matrices including water.34,35 For these reasons, these mycotoxins are now classified as emerging contaminants of interest.36
Water-borne diseases related to the presence of fungi and their toxins in water are on the increase, with immunocompromised patients who undergo chemotherapy, organs and bone marrow transplants being the most impacted.37 Changing climatic conditions (including rising temperatures and precipitation patterns) are now considered to support the growth of fungi in water systems, leading to more frequent mycotoxin release into water that affects the supply of clean water.38 In addition, mycotoxin contamination of drinking water sources is also possible through poor water storage, transportation, and wastewater from food supply chains,39 and even from grey water.40 The aquatic environment (including surface water and groundwater) is thus, a large reservoir for these mycotoxins.
For example, aflatoxins B1 and B2, fumonisin B3, and OTA were detected at concentrations up to 35 ng L−1 in surface water from Portugal.41 Aflatoxin B2 was found in bottled water from Spain at 0.48 ng L−1,42 DON and ZEN in the Swiss river43 and aflatoxin B1 in surface water from Spain.39 In another study by Huang and his colleagues, they monitored the presence of 23 mycotoxins and their metabolites in the Yangze River Delta in China and evaluated the cumulative health risk in 228 adults. They observed that DON, fumonisin B1 (FB1), and ZEN were the most prevalent mycotoxins, even though aflatoxin B1 and OTA risk study indicated adverse impacts on humans.44 In a separate study, Schenzel and co-investigators also investigated the presence of mycotoxins in Swiss midland river and detected 4 [3-acetyl-deoxynivalenol, deoxynivalenol (DON), nivalenol (NIV), and beauvericin (BEA)] out of the 33 mycotoxins analyzed for. DON was observed to have the maximum concentration (73.4 ng L−1) and occurred more in summer, whereas BEA had the least concentration (1.3 ng L−1), but prevailed during the winter.45
To the best of our knowledge, there is only one report of mycotoxins in water from Africa, South Africa46 with no report or data from Nigeria, even though published reports are abundant of their presence in food.47–49 For African nations to deliver on the United Nations Sustainability Goal #6 (clean water and sanitation for all), it is imperative to have a first-hand understanding of the presence of these contaminants of emerging concerns (mycotoxins) in our water systems before developing effective mitigation strategies.
The aim of this study is therefore to investigate the magnitude and health risks of the presence of some mycotoxins: deoxynivalenol (DON), ochratoxin A (OTA), and zearalenone (ZEN)in drinking water samples from three States in Southwest Nigeria: Osun, Oyo, and Lagos States. The targeted drinking water sources are groundwater (hand-dug wells, boreholes), surface water (rivers), and treated water (packaged water in bottles and sachets) collected across different sites in the three selected States. The major sources of drinking water for people living in Southwestern Nigeria are groundwater,50–52 bottled water and sachet water.50,53 In West Africa, bottled water and sachet water (treated water) quality are expected to meet national or international standards. However, it is observed that they are most times, poorly treated.54,55 Thus, it is important to assess these drinking water sources for the levels of these mycotoxins. The choice of toxins analyzed in this study encompasses mycotoxins from field-based fungi of the genius Fusarium (DON and ZEN) and from storage-based fungi of the genius A. ochraceous or P. viridicatum (OTA). These mycotoxins have been established to be prevalent in cereals, and animal feed and forages under various environmental conditions in Nigeria.56,57 The presence of these mycotoxins in drinking water sources will indicate whether untreated drinking water is an additional source of these mycotoxins in humans aside food. The analysis of total dissolved solid (TDS), electrical conductivity (EC), and pH is to ascertain the apparent quality of the water samples while the Principal Component analysis (PCA) was done on physicochemical data to establish if there were associations among mycotoxins and between mycotoxins, pH, EC, and TDS. Data from this study is expected to provide baseline information on the presence and levels of these mycotoxins in drinking water systems from Nigeria. It is also projected that results from this study will drive policy development that will evolve practices that prevent these mycotoxins from entering drinking water sources in Nigeria. Results from this study represent the very first effort to provide baseline data on the presence and levels of these mycotoxins in drinking water systems from West Africa.
2 Materials and methods
2.1 Standards and reagents
Analytical standards of deoxynivalenol (DON), ochratoxin A (OTA), and zearalenone (ZEN) were purchased from Sigma Aldrich (St. Louis, MO, USA). Acetonitrile and methanol of HPLC grade and Oasis HLB SPE cartridges (500 mg, 12 mL) were also purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was obtained with the Milli-Q Direct 8/16 System. Standard stock solutions of each targeted mycotoxin (1000 μg L−1) were prepared individually in methanol and preserved at 4 °C in a refrigerator. Working solutions (50 μg L−1) of each of the targeted mycotoxins were prepared by dilution of stock solution with ultrapure water fresh before use. Some physicochemical properties of DON, OTA, and ZEN are shown in Table S1.†2.2 Description of the sampling sites
The Southwestern region of Nigeria is made up of six (6) States which include Osun, Ondo, Ekiti, Oyo, Ogun, and Lagos. Samples were collected from three States: Osun, Oyo, and Lagos States in the region. These States are located in the tropical rainforest biome in Southwestern Nigeria, and they lie between Lat. 06° 30′ N and Long. 04° 30′ E, Lat. 7° 51′ N and Long. 3° 55′ E and Lat. 6° 27′ N and 3° 24′ 23′ E, encompassing areas of approximately 14,875, 28,454 and 3,577 sq. km respectively. Lagos State serves as one of Nigeria's commercial hubs, while Oyo State is a densely populated inland State in the Southwest of Nigeria regarded as one of the top ten agriculture-intensive states.58 Osun State is known for its agricultural activities. Surface water sites are open to everyone in the communities around the surface water and groundwater sites are mainly hand-dug wells serving households and in some instances several households. Coordinates of sampling sites used to develop the map are shown in Fig. S1–S3 (ESI document†).2.3 Sample collection
Groundwater samples (GW) from hand-dug wells and boreholes were collected from different locations across the three States. For hand-dug wells, a bailer was lowered into the deep end of the well to collect water samples into sample bottles, while borehole water samples were collected directly into the sample bottles from the tap connected to them. Likewise, water samples from surface water (SW) were collected at random from different locations along major rivers: Osun River (Osun State), Epe River (Lagos State), and Osun River (running through Oyo State); and from streams across the three States. We collected 34 groundwater samples, with 31 surface water samples respectively. For treated water, 29 packaged water samples comprising three popular commercial brands of bottled water (BTW) and sachet water (STW) were collected representing different brands sold across the three States. Samples were collected in triplicates throughout May 2021. Table S2† shows the sample collection distribution. For all water samples, onsite analysis was done to determine pH, electrical conductivity (EC), and total dissolved solids (TDS) using a HANNA pH/EC/TDS/Temperature (HI 9811-5) portable meter instrument.2.4 Sample preparations
The water samples were filtered using a cellulose ester filter membrane (0.22 μm) and 200 mL of each sample was spiked with 1 mL of known concentration (50 μg L−1) of the analytes. Solid Phase Extraction (SPE) cartridges (Oasis HLB, 500 mg, 12 mL) were conditioned with 3 mL of HPLC-grade methanol and equilibrated with 3 mL of Milli-Q water. An aliquot of 200 mL of each sample was passed through the cartridge at a flow rate range of 5–8 mL min−1. The SPE cartridges were washed by passing 3 mL of Milli-Q water through them. The cartridges were dried in a vacuum oven for 5 min and elution was done with 3 mL of HPLC-grade methanol, followed by 3 mL of acetonitrile. The eluate was evaporated to dryness in the vacuum oven and reconstituted with 0.5 mL of HPLC-grade methanol for analysis. Aliquot of samples were not buffered before extraction or analysis.2.5 Instrumental analysis
A Dionex Ultimate 3000 UHPLC system (Dionex Softron GmbH, Dornierstr. 4, Bayern, Germany), equipped with a high-resolution quadrupole-time-of-flight mass spectrometer (Impact II) system (Bruker Daltonics GmbH Fahrenheitstr. 4, Bremen, Germany), was used for the separation and the detection of analytes. Quality assurance and quality control for the method of analysis were done and details are provided in Section S1.† Similarly, the recovery and precision parameters were calculated via the method described in Section S2† while data are provided in Table 1. Details of instrument settings and accessories used are provided in Section S3.0 (ESI† document). The chromatograms for mycotoxins and their mass spectra are shown in Fig. S4.†| Mycotoxins | Retention time (min) | R2 | Linear range (μg L−1) | LOD (μg L−1) | LOQ (μg L−1) | Spiked conc. (μg L−1) | % Recovery ± SD | Instrument precision (RSD) |
|---|---|---|---|---|---|---|---|---|
| DON | 4.9 | 0.9999 | 2–500 | 0.62 | 1.84 | 50 | 105.2 ± 3.3 | 3.09 |
| 500 | 94.4 ± 2.0 | 2.11 | ||||||
| 1000 | 133.8 ± 11.5 | 8.57 | ||||||
| OTA | 6.8 | 0.9986 | 1–1000 | 0.16 | 0.48 | 50 | 81.6 ± 4.1 | 6.77 |
| 500 | 86.0 ± 4.4 | 5.17 | ||||||
| 1000 | 118.0 ± 21.7 | 18.41 | ||||||
| ZEN | 5.9 | 0.9999 | 1–1000 | 0.23 | 0.71 | 50 | 84.5 ± 3.9 | 4.66 |
| 500 | 90.9 ± 1.4 | 1.57 | ||||||
| 1000 | 89.3 ± 13.0 | 7.26 |
2.6 Risk assessment
The ecological risk (for groundwater and surface water), human exposure, and hazard risk assessments were evaluated using data obtained and relevant equations as described in Section S4 in the ESI document.†2.7 Statistical analysis
Statistical analyses were done using the IBM Statistical Package for the Social Sciences (SPSS) Statistics v21, Graph Pad Prism 8.4.2(679), Origin (Origin Lab 9.1), and Principal Component Analysis (PCA) software. Section S5† provides details of these analyses.3 Results and discussion
3.1 Physicochemical characterization
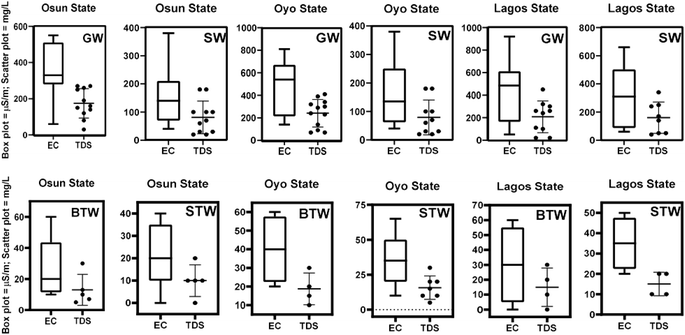
The physicochemical parameters considered for water samples in this study were pH, electrical conductivity (EC) and total dissolved solids (TDS). Fig. 1 shows data variability across the different sampling sites in the three States. The EC data for surface water and groundwater samples all fall within the WHO EC standard for freshwater (500 μS m−1)59 with the mean EC values being 329, 139, and 538 μS m−1 (groundwater samples) and 135, 486, and 308 μS m−1 (surface water samples) for Osun, Oyo, and Lagos States, respectively. Only the mean EC value for groundwater from Oyo State (538 μS m−1) exceeds the WHO EC standard limit.Similarly, mean TDS values for groundwater and surface water are 173 and 79; 239 and 78; 205 and 155 mg L−1 for Osun State, Oyo State and Lagos State, respectively, and these fall well within the WHO set limit of <1000 mg L−1.59 Typically, groundwater should have higher EC and TDS than surface water because it contains more dissolved ions and particulate matter picked up as it moves across the rocks and soils beneath the earth. The average pH of the water samples as shown in Fig. 2 suggests that surface water samples from the three States have the highest mean pH values which are above the WHO lower pH limit (Fig. 2). In contrast, groundwater samples have pH values that fall below the WHO lowest limit of 6.5. Weak acidic groundwater may be the result of the dissociation of H2CO3, the release of the absorbed H3O+ in clay layers, and the infiltration of acid rainwater.60 This may well explain the slightly acidic nature of groundwater samples across the States studied.
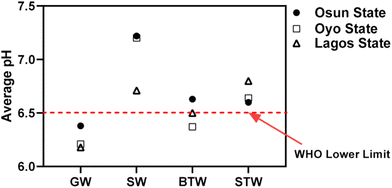 | ||
| Fig. 2 Average pH values for different drinking water sources in Osun, Oyo, and Lagos States, Nigeria (GW = Groundwater; SW = Surface water; BTW = Bottled water; STW = Sachet water). | ||
On the other hand, package water samples (BTW and STW) had lower mean EC and TDS values compared with those for groundwater and surface water, which were all below WHO standard values with Oyo state BTW samples (EC = 40 ± 9.1 μS m−1, TDS = 18.8 ± 4.3 mg L−1) and STW samples (EC = 35.7 ± 6.9 μS m−1, TDS = 15.7 ± 3.2 mg L−1) giving the highest values as shown in Fig. 1. This is because they were treated in one form or the other before they were packed. Sachet water samples appears to have higher EC values than bottled water with the exception of samples collected from Oyo State.
Generally speaking, bottled water and sachet water samples have average pH values that fall between the WHO lower and upper limits of 6.5 and 8.5 respectively (Fig. 2). It is reported that there is no significant difference in the percentage extraction of these mycotoxins between the pH range of 3.0–7.0 with percentage OTA extraction increasing as pH of solution increases up till 7.0.61 This implies that the extraction of mycotoxins in this study should be maximum at pH range of water samples collected as shown in Fig. 2 (pH 6.2–7.3). This is more so that fungi are known to often prefer acidic pH for their growth.46 The weakly acidic pH generally reported for water samples in this study may enhance fungal growth in packaged water (bottled water and sachet water), which could release these mycotoxins into these drinking water types.
3.2 Mycotoxin distribution and concentration
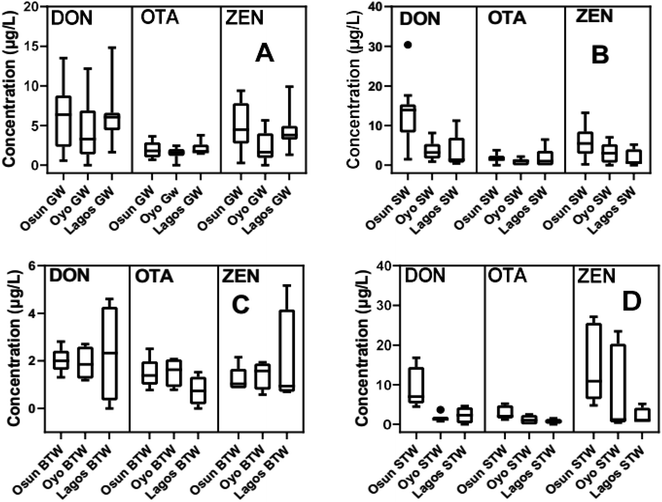
The percentage distribution of these mycotoxins shows that ZEN is the most prevalent mycotoxin with a 100% detection frequency in all water samples across the three States, except in GW (Oyo State) and SW (Oyo and Lagos States), as shown in Fig. S5.† Detection frequency is least for OTA (a storage mycotoxin) especially for water samples from Oyo and Lagos States (Fig. S5†). In Osun State, all three mycotoxins had 100% detection frequencies except for OTA in SW samples. The mean concentrations of these mycotoxins in natural water samples (GW and SW) with their standard error of mean values are shown in Fig. 3A–D and Table S3† respectively. In Osun State, the range of mean concentrations is between 1.21 μg L−1 to 14.96 μg L−1 with DON (9.30 ± 2.30 μg L−1), OTA (2.93 ± 0.79 μg L−1), and ZEN (14.96 ± 4.46 μg L−1) in sachet water samples being the highest (Fig. 3D). A similar trend is also found for water samples from Lagos State with sachet water samples having relatively, the highest mean concentrations of these mycotoxins with the mean concentrations for DON, OTA, and ZEN being 4.59 ± 0.59 μg L−1, 3.01 ± 1.50 μg L−1, and 10.56 ± 2.84 μg L−1 respectively (Fig. 3D). For surface water samples, mean concentrations of mycotoxins are in the range of 0.97 to 12.96 μg L−1, with SW samples from Osun State having the highest mean concentrations for all three mycotoxins (DON is 12.96 ± 2.23 μg L−1; OTA is 1.73 ± 0.30 μg L−1; ZEN is 5.84 ± 1.11 μg L−1) than SW samples from other two States (Fig. 3B). Between GW and SW samples, the mean concentrations of DON appear to be higher than those of ZEN largely because the former is more hydrophilic than the latter as shown in their log Kow and log Koc in Table S1.† We observe that GW has higher mean concentrations of both mycotoxins than SW except for Osun State. Similarly, OTA is higher in GW than in SW samples. The mean concentrations of all mycotoxins in the different drinking water types are presented in Table S3.†Generally, the mean concentrations of these mycotoxins in bottled water are in the range of 0.75 μg L−1 to 2.32 μg L−1 which are much lower than those found in sachet water (1.24 μg L−1 to 14.96 μg L−1), groundwater (1.49–6.06 μg L−1), or even surface water (0.97–12.96 μg L−1) across the three States (Table S3†). While DON shows some of the highest concentrations in bottled water across the three States with Osun, Oyo, and Lagos States having mean concentrations of 2.02 ± 0.24 μg L−1, 1.90 ± 0.35 μg L−1, and 2.32 ± 1.02 μg L−1 respectively, ZEN is highest in sachet water with Osun, Oyo and Lagos States having mean concentrations of 14.96 ± 4.46 μg L−1, 8.59 ± 3.86 μg L−1, and 10.56 ± 2.84 μg L−1 respectively. Similarly, the mean concentrations of OTA in sachet water samples (2.93 ± 0.79 μg L−1, 1.24 ± 0.40 μg L−1, and 3.01 ± 1.50 μg L−1 for Osun, Oyo and Lagos States respectively) are slightly higher than those in bottled water (1.47 ± 0.28 μg L−1, 1.53 ± 0.31 μg L−1, and 0.75 ± 0.31 μg L−1 for Osun, Oyo and Lagos States respectively) as shown in Fig. 3C and D. The mean concentrations of OTA in this study are far higher (1000 fold) than what was reported for bottled water (0.26 ± 0.02 ng L−1)42 and untreated surface water (8.5 ± 0.3 ng L−1)41 from Portugal. It is worrisome to note that bottled water and sachet water samples which are acclaimed to be treated before sale to the public, have relatively high mean concentrations of these mycotoxins (Fig. 3C and D). Even though the mean concentrations of these mycotoxins are lower in bottled water samples than in sachet water samples across the three States, the results, however, indicate that current treatment procedures by most drinking water vendors in Nigeria (most of whom use filtration and/or adsorption techniques) are insufficient to remove these mycotoxins and their associated fungi from water, completely. The ‘treated’ water sealed in bottles and sachets, after the filtration process, may still contain some fungi species62 that tend to grow and release more of these mycotoxins into the drinking water especially if the treated water still contains high enough nutrients to support the growth of these fungi species. Some fungi species have been reported to even resist chlorination methods during water treatment.63
Overall, among the various water types, bottled water samples show the lowest mean concentrations of mycotoxins (<3.0 μg L−1) especially for OTA (Fig. 3C). Indeed, the mean concentrations of OTA in all water sample types were generally lower than those DON or ZEN irrespective of the State and were ≤3.01 μg L−1 (Table S3†). The mean concentration of DON in surface water from Osun State is 12.96 ± 2.23 μg L−1 which is higher than that for groundwater (6.06 ± 1.02 μg L−1) for the same State. Between groundwater and surface water samples, the mean concentrations of ZEN were lower than those of DON (Table S3†). Furthermore, concentrations of DON recorded in this study are several hundred folds higher than those reported by Mhlongo et al.46 for water supply networks in South Africa. Furthermore, we observe that the mean concentrations of the mycotoxins follow the order DON > ZEN > OTA for all categories of water samples except sachet water which is ZEN > DON > OTA. This trend (DON > ZEN > OTA) is supported by the hydrophobicity of ZEN and OTA (as expressed by its octanol–water partitioning coefficient, Kow) which are higher than that for DON. Only the mean EC value for groundwater from Oyo State (538 μS m−1) exceeds the WHO EC standard limit (Fig. 1).
The main source of these mycotoxins in drinking water sources is still unclear64 but there is a high chance that a combination of poor storage of cereals and grains, wash-off from agricultural fields and emptying of grey water into open drainage systems,65 poor treatment of wastewater especially from the food industry,66 building infrasture with moisture problems,67 and weak regulatory oversight of drinking water quality could be responsible for the introduction of these mycotoxins into the drinking water sources that have been reported in this study. It is reported that mycotoxins are easily dispersed via aqueous carriers such as rainfall which wash them off from infected plant tissues and cereals in the field into water systems68 as with DON and ZEN which are field mycotoxins.
Indeed, it has been shown that DON and ZEN toxins can be leached off cereals in the field into the aquatic environment via runoff from Fusarium graminearum-infested agricultural fields43,69 especially DON which is more hydrophilic. Additionally, the practice of open grazing and using animal dung manure for crop production in Nigeria could be a diffuse mycotoxin source, as suggested by Kolpin et al.66 These mycotoxins (DON, OTA, and ZEN) have been reported to co-exist in contaminated food,70 polluted water matrices,14,71 and even from human body fluids.72 There are strong indications that the combined presence of DON and ZEN in surface water may result in oxidative stress, hepatotoxicity, apoptosis, and inflammation in zebrafish embryos and swine.73,74
3.3 Multivariate statistical analysis vs. principal component analysis
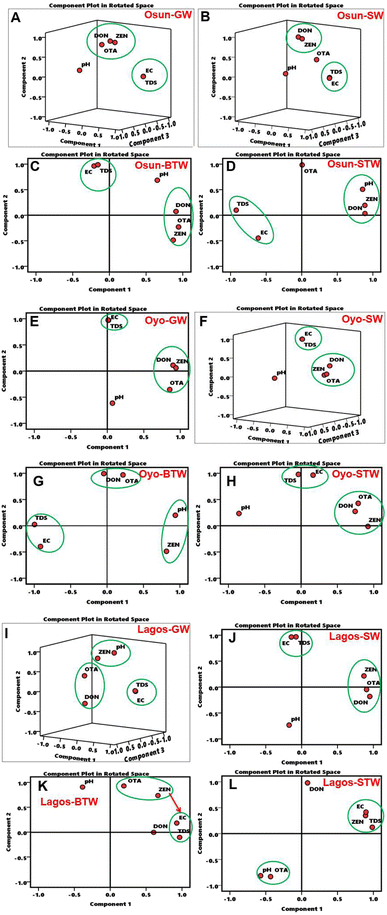
In groundwater samples from Osun State, three components dominated the PCA. PC1 was dominated by EC and TDS, with high loadings of 0.95 each (Table 2) which is corroborated by a short distance between TDS and EC in the 3-D PCA loading plot (Fig. 4). This suggests that similar factors may have contributed to the values of both EC and TDS. However, the loading for pH in PC1 was low (−0.24), suggesting that the pH of the groundwater samples from this state may be influenced by other factors than the presence of mycotoxins, EC and TDS.| Osun | Oyo | Lagos | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groundwater | Surface water | Groundwater | Surface water | Groundwater | Surface water | |||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | |
| DON | −0.29 | 0.76 | −0.50 | 0.0 | 0.94 | −0.1 | 0.90 | 0.11 | 0.88 | 0.32 | −0.13 | 0.11 | −0.2 | 0.93 | 0.95 | −0.18 |
| OTA | 0.00 | 0.81 | 0.28 | 0.067 | 0.29 | −0.7 | 0.86 | −0.35 | 0.87 | 0.10 | 0.08 | −0.04 | 0.45 | 0.74 | 0.91 | −0.05 |
| ZEN | 0.33 | 0.88 | 0.18 | 0.12 | 0.93 | 0.02 | 0.94 | 0.06 | 0.95 | 0.14 | 0.09 | 0.00 | 0.82 | 0.23 | 0.87 | 0.22 |
| pH | −0.24 | 0.23 | 0.89 | 0.18 | 0.17 | 0.78 | 0.07 | −0.61 | 0.03 | 0.07 | 0.99 | 0.18 | 0.91 | −0.2 | −0.18 | −0.73 |
| EC (μS m−1) | 0.98 | 0.05 | −0.11 | 0.99 | 0.05 | 0.06 | 0.02 | 0.97 | 0.20 | 0.98 | 0.05 | 0.99 | 0.08 | 0.05 | −0.14 | 0.97 |
| TDS (mg L−1) | 0.99 | 0.05 | −0.10 | 0.99 | 0.033 | 0.07 | 0.01 | 0.97 | 0.19 | 0.98 | 0.05 | 0.99 | 0.06 | 0.03 | −0.08 | 0.97 |
| Eigenvalues | 2.19 | 2.06 | 1.18 | 2.03 | 1.87 | 1.12 | 2.43 | 2.41 | 2.50 | 2.05 | 1.03 | 2.03 | 1.77 | 1.51 | 2.53 | 2.49 |
| % Total variance | 36.5 | 34.3 | 19.6 | 33.8 | 31.1 | 18.7 | 40.5 | 40.2 | 41.6 | 34.2 | 17.1 | 33.8 | 29.4 | 25.1 | 42.2 | 41.5 |
| Cumulative % | 36.5 | 70.7 | 90.3 | 33.8 | 64.9 | 83.6 | 40.5 | 80.7 | 41.6 | 75.8 | 92.9 | 33.8 | 63.2 | 88.3 | 42.2 | 84.7 |
PC2 dominated by DON, OTA and ZEN have high loadings of 0.76, 0.81 and 0.88, respectively (Table 2), and this is supported by the short distances between the mycotoxins in the 3-D PCA plot (Fig. 4A), indicating that these mycotoxins may have a common anthropogenic source. These mycotoxins (DON, OTA, and ZEN) have been reported to co-exist in contaminated food,75 polluted water matrices71,76 and even from human body fluids.72 Meanwhile, pH dominated PC3 with a high loading of 0.89 and other variables (EC, TDS, mycotoxins) show very low and negative loadings that are supported by the far distance of pH from the other variables (Fig. 4A). Pearson correlation correlates this (Fig. S6A–D†), implying that pH has no relationship with mycotoxins in these water samples.
As with groundwater, the PCA data for surface water samples from Osun State were dominated by three components. PC1 is dominated by EC and TDS, with a high loading value of 0.99 each while pH had a low loading of 0.18, indicating that the resulting surface water pH is influenced by other factors different from those of EC and TDS. The Pearson correlation analysis also supports this result (Fig. S6B). However, PC2 was dominated by high loadings of 0.94 and 0.93 for DON and ZEN, respectively (Table 2), as supported by high correlation coefficients (≥0.70) of both mycotoxins (Fig. S6B). This indicates that both mycotoxins (DON and ZEN) are likely to originate from the same source, corroborated by shorter distance from each other (Fig. 4B†). However, OTA has a very low loading of 0.29 in PC2 (Table 3), as confirmed by its distance from other mycotoxins in Fig. 4B.
| Osun | Oyo | Lagos | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bottled water | Sachet water | Bottled water | Sachet water | Bottled water | Sachet water | |||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| a Var. = variance. | ||||||||||||
| DON | 0.91 | 0.07 | 0.89 | 0.03 | −0.04 | 0.99 | 0.74 | 0.27 | 0.61 | −0.005 | 0.08 | 0.98 |
| OTA | 0.94 | −0.23 | 0.01 | 0.98 | 0.22 | 0.97 | 0.79 | 0.43 | 0.19 | 0.93 | −0.43 | −0.83 |
| ZEN | 0.87 | −0.49 | 0.89 | 0.19 | 0.82 | −0.49 | 0.92 | −0.01 | 0.67 | 0.74 | 0.89 | 0.35 |
| pH | 0.65 | 0.68 | 0.86 | 0.51 | 0.94 | 0.20 | −0.86 | 0.23 | −0.39 | 0.91 | −0.57 | −0.81 |
| EC (μS m−1) | −0.21 | 0.96 | −0.61 | −0.45 | 0.91 | −0.40 | 0.17 | 0.97 | 0.93 | 0.18 | 0.90 | 0.41 |
| TDS (mg L−1) | −0.16 | 0.99 | −0.92 | 0.10 | 0.99 | 0.02 | −0.04 | 0.98 | 0.98 | −0.11 | 0.98 | 0.12 |
| Eigenvalues | 2.96 | 2.66 | 3.53 | 1.47 | 3.42 | 2.36 | 2.78 | 2.20 | 2.79 | 2.27 | 3.09 | 2.61 |
| % Total var. | 49.3 | 44.4 | 58.9 | 24.6 | 57.0 | 39.4 | 46.3 | 36.7 | 46.5 | 37.8 | 51.5 | 43.6 |
| Cumulative % | 49.3 | 93.7 | 58.9 | 83.5 | 57.0 | 96.4 | 46.3 | 83.0 | 46.5 | 84.3 | 51.5 | 95.1 |
The PCA for Oyo State groundwater samples is dominated by two components. While PC1 showed high loadings of 0.90, 0.86 and 0.94 for DON, OTA and ZEN, respectively, a very low loading of 0.07 was observed for pH in PC1 which could suggest that the pH of groundwater samples from this state, as with Osun State, is influenced by different factors compared to those for the mycotoxins, EC and TDS (Fig. 4). These are again validated by the strong correlation coefficients of these mycotoxins (Fig. S6E†).
For surface water samples from Oyo State, PC1 show high loadings for DON (0.88), OTA (0.87) and ZEN (0.95), and PC2 show high loadings for EC and TDS (0.98 each), which are all supported with strong correlations coefficients of all three mycotoxins, EC and TDS (Fig. S4F†). A similar trend is observed for EC and TDS (PC1 groundwater and PC2 surface water) for Lagos State samples (Table 2). However, while high loadings of ZEN (0.82) and pH (0.91) dominate PC2 and high loadings of DON (0.93) and OTA (0.91) dominate PC3 for groundwater from Lagos State, PC1 for surface water samples from this same state is dominated by high loadings of DON (0.95), OTA (0.91), and ZEN (0.87), as shown in Table 2. The loadings for surface water samples from this state are supported by high correlation coefficients (Fig. S6J†), while those for groundwater show only moderate coefficients (Fig. S6I†). In any case, these results suggest a strong association between all three mycotoxins (i.e. similar anthropogenic inputs) and between EC and TDS in water samples from Lagos State. There appears to be a moderate correlation between pH and ZEN in groundwater samples from Lagos State as can be seen also from the 3-D PCA plot (Fig. 4I). This is one rare case found in all the groundwater and surface water samples examined in this study suggesting that pH may have an influence on ZEN in groundwater samples. The influence of pH in releasing ZEN bound to the vein in Maize has been recently reported.77
Considering the PCA for packaged water samples (bottled and sachet water samples) as shown in Table 3, we see that the presence of these mycotoxins and the physicochemical characteristics of the water samples are described by two principal components with PC1 for bottled water from Osun State, showing high loadings of DON (0.91), OTA (0.94), and ZEN (0.87); from Oyo State high loading for ZEN (0.82); and from Lagos State moderate loadings for DON (0.61) and ZEN (0.67). With PC2, there are high loadings for DON (0.99) and OTA (0.97) and high loadings for OTA (0.93) and ZEN (0.74) for bottled water samples from Oyo and Lagos States, respectively (Table 3). Similarly, sachet water samples show high PC loadings for these mycotoxins either in PC1 or in PC2 as shown in Table 3. Additionally, there are high loadings of PC1 and PC2 for EC and TDS in both bottled and sachet water samples, which is similar to what was observed with groundwater and surface water in this study. The pH of water samples appears to show moderate to strong correlations with mycotoxins in the packaged water samples (Fig. S6C, D, G, H, K and L), and this suggests that pH may have some influence on the mycotoxin in the packaged water samples.78 This is unlike what was observed with groundwater and surface water samples earlier discussed.
Overall, from the PCA results, all three mycotoxins (DON, OTA and ZEN) in all water sample types from each of the three states, appear to be closely associated possibly through agricultural run-offs from the fields and from storage points. On the one hand, aside from the isolated case of pH having a strong correlation with ZEN in groundwater from Lagos State, pH does not have any relationship with the mycotoxins in groundwater or surface water nor on either EC or TDS from water samples across the three states. This is unlike packaged water samples (bottled and sachet water). On the other hand, EC and TDS in all water samples across the three states are influenced by the same factors (i.e. ionic composition of the water and the concentration of dissolved species) due to their high correlation values but not by the pH of the water samples(Tables 2 and 3). The strong association between EC and TDS for all water samples across the three states is in line with previous reports from other studies.7,79 However, there is generally a negative correlation between pH and EC since pH measures the concentration of H+ in solution only, whereas electric conductivity measures the concentrations of all active ions present in the solution. As such, pH on its own, cannot specify the conductivity of the solution.
3.4 Ecological and human risk assessment
The risk posed by the levels of these mycotoxins in water on aquatic organisms could be serious with severe implications for the health of an ecosystem. Therefore, the ecological risk of target mycotoxins (DON, OTA and ZEN) was evaluated with the embryonic stage of a zebrafish (Zebrafish Embryo Toxicity, ZET) using the risk quotient index. Fish is used as the index organism because it is a vertebrate like man. Fig. 5 shows the plots of the risk quotients (RQs) for the different mycotoxins in water samples collected from the three states.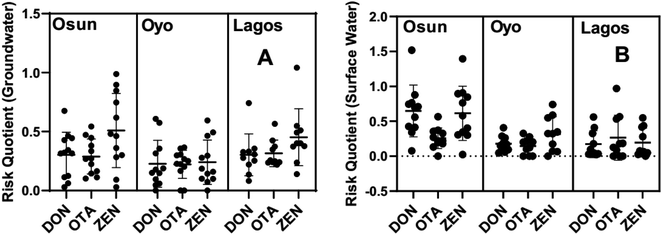 | ||
| Fig. 5 Nested scatter plots (mean with standard deviation) of risk quotients of mycotoxins in (A) groundwater and (B) surface water samples from the various states. | ||
From Fig. 5, it is observed that the RQs for the mean concentrations of ZEN in surface water and groundwater are relatively higher than those of DON and OTA with the latter being the least in most cases. For groundwater samples across the three states, the mean RQs for ZEN are 0.51 (Osun State), 0.24 (Oyo State), and 0.45 (Lagos State) (Fig. 5A). However, Fig. 5B show that the concentration of ZEN in surface water samples across the Osun and Oyo States have relatively higher mean RQs of 0.62 and 0.32 respectively while that for Lagos is lower, 0.19. The mean RQs for OTA mycotoxin in groundwater samples are 0.29, 0.22, and 0.32 and in surface water samples are 0.26, 0.14, and 0.27 for Osun, Oyo and Lagos States, respectively (Fig. 5A and B). The mean RQs for DON in groundwater and surface water are not significantly different from those of OTA (Fig. 5A and B).
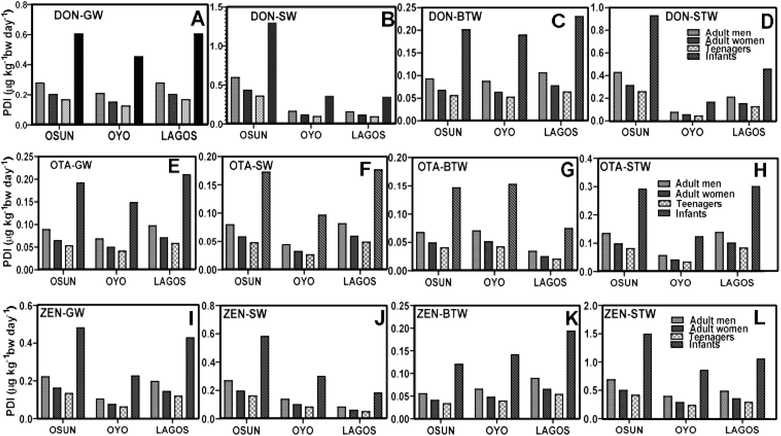
From the probable daily intake (PDI) values (Fig. 6A–6D) for humans, infants from Osun State face non-estrogenic health risks from the levels of DON surface and sachet water because the PDI values for this population group are either higher than or close to the JECFA health-based guidance value (HBGV) of 1 μg kg−1 bw per day.80 At the moment, it is, thus, not advisable to use either sachet water or surface water from Osun State to prepare formula food for infants. However, the PDI values for OTA in water samples across the three states (Fig. 6E–H) are less than the recently revised HBGV for benchmark dose lower confidence limit (BMDL10) of 14.5 μg kg−1 bw per day for neoplastic effect set by EFSA.81 In addition, these PDI values for OTA in all water sample types are ≈20-fold lower than the Tolerable Daily Intake (TDI) of 4.73 μg kg−1 bw per day for non-neoplastic effects (calculated from kidney lesions observed in pigs) as suggested by European Food Safety Authority (EFSA).82,83 In general, the levels of OTA in these water samples are still of concern. Consistent ingestion of these water could lead to liver tumours and other neoplastic effects in humans. Various studies have linked OTA exposure with chronic interstitial nephropathy (CIN) and human diseases such as Balkan endemic nephropathy (BEN) when there is a synergistic or additive effect between mycotoxins, long-term exposure to the same mycotoxins, and other renal diseases.84,85 It is only a matter of time, if the conditions remain the same and nothing is done to improve the quality of these drinking water sources, ingesting them will lead to a higher risk of nephrotoxicity and possible cancer among the populace living in these states.
There are serious estrogenic health concerns about the PDI values of ZEN groundwater, surface water, and sachet water in all three states (Fig. 6I–L) as they are either slightly below or above EFSA HBGV value for their Tolerable Daily Intake (TDI) of 0.25 μg kg−1 bw per day (ref. 86), especially for sachet water (Fig. 6L). A general observation is that the levels of these mycotoxins in bottled water (Fig. 6C, G and K) are all below their HBGVs which suggests that popular brands of bottled water in Southwest Nigeria use better treatment procedures that remove significant amounts of mycotoxins from the drinking water, unlike sachet water.
Overall, these results imply that urgent steps need to be taken to address the presence of these mycotoxins in water (especially from OTA and ZEN), otherwise, the human risk levels will increase with time, especially for OTA with the lowest excretion rate of the three mycotoxins.87 ‘Treated’ water from these states (bottled and sachet water) needs to be carefully re-examined and new treatment strategies and regulatory policies should be adopted to provide safe drinking water to consumers.
4 Conclusion
The presence of mycotoxins was investigated in four drinking water sources (groundwater, surface water, bottled water and sachet water) in Southwest Nigeria. To the best of our knowledge, this is the first study that reports the presence of mycotoxins in drinking water sources from West Africa. From our study, we observed that ZEN is the most detected with a 100% detection frequency in most of the drinking water types collected across the three States (Osun, Oyo, and Lagos). For groundwater, surface water and bottled water, DON showed the highest mean concentrations over ZEN and OTA while in sachet water it was ZEN. The mean concentrations of these mycotoxins in the various drinking water samples are several folds higher than those previously reported from across the globe. The trend for the average concentrations of these mycotoxins in water samples is DON > ZEN > OTA. Principal Component Analysis of data suggests that all three mycotoxins in water types are from the same source. This is strongly supported by data from Pearson Correlation analysis. The mean ecological risk quotients analysis suggests that the concentrations of these mycotoxins in water samples do not pose high risks to vertebrates like fish. The possible daily intake (PDI) evaluation showed levels of OTA in water samples that are ≈20-fold lower than the Tolerable Daily Intake (TDI) of 4.73 μg kg−1 bw per day for non-neoplastic effects. The PDI values for mycotoxin intake from bottled water are all below the HBGV values set for them. This suggests that this type of drinking water is the safest in the Southwest of Nigeria. However, the presence of these mycotoxins in drinking water sources in Nigeria strikes an urgent call for a sustainable and efficient way to tackle the presence of this dangerous mycotoxin in drinking water sources in Nigeria and across Africa. There is a need for the inclusion of mycotoxin testing in conventional water quality parameters. Although, we have not considered seasonal and settlement type variations on the presence of mycotoxins in drinking water, yet these will form part of future studies.Data availability
The authors hereby declare that the data of the submitted manuscript is available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding from The World Academy of Sciences-Islamic Development Bank (TWAS-IsDB) to the African Centre of Excellence for Water and Environmental Research (ACEWATER), Redeemer's University, Ede, Nigeria (Grant number: 4500420197) for sampling campaigns. The authors also appreciate the Centre of Excellence in Reproductive Health Innovation, University of Benin, Benin City, Nigeria for financial support through the Tertiary Education Trust Fund (TETFund/DR&D/CE/NRF/STI/44/VOL1) to purchase mycotoxin standards.References
- P. N. Obasi and B. B. Akudinobi, Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria, Appl. Water Sci., 2020, 10(7), 1–23 CrossRef.
- K. Rehman, et al., Prevalence of exposure of heavy metals and their impact on health consequences, J. Cell. Biochem., 2018, 119(1), 157–184 CrossRef CAS PubMed.
- H. Kwon, W. Jiang and E. T. Kool, Pattern-based detection of anion pollutants in water with DNA polyfluorophores, Chem. Sci., 2015, 6(4), 2575–2583 RSC.
- M. Chen, et al., Inorganic anion removal using micellar enhanced ultrafiltration (MEUF), modeling anion distribution and suggested improvements of MEUF: A review, Chem. Eng. J., 2020, 398, 125413 CrossRef CAS.
- A. Altıkulaç, Ş. Turhan and H. Gümüş, The natural and artificial radionuclides in drinking water samples and consequent population doses, J. Radiat. Res. Appl. Sci., 2015, 8(4), 578–582 Search PubMed.
- N. B. Bolujoko, et al., Occurrence and human exposure assessment of parabens in water sources in Osun State, Nigeria, Sci. Total Environ., 2022, 814, 152448 CrossRef CAS PubMed.
- O. B. Otitoju, et al., Pollution and risk assessment of phenolic compounds in drinking water sources from South-Western Nigeria, Environ. Sci. Pollut. Res., 2023, 30, 76798–76817 CrossRef CAS PubMed.
- T. Ruan, et al., Identification and Prioritization of Environmental Organic Pollutants: From an Analytical and Toxicological Perspective, Chem. Rev., 2023, 123(17), 10584–10640 CrossRef CAS PubMed.
- M. R. Gbadamosi, et al., Concentrations of organophosphate esters in drinking water from the United Kingdom: Implications for human exposure, Emerging Contam., 2023, 9(1), 100203 CrossRef CAS.
- R. Gutiérrez, et al., Presence of organochlorine contaminants in bottled drinking water from Mexico City, Water Sci. Technol.: Water Supply, 2012, 12(4), 470–479 Search PubMed.
- X. Wen, et al., Microbial indicators and their use for monitoring drinking water quality—A review, Sustainability, 2020, 12(6), 2249 Search PubMed.
- E. Gbotche, et al., National Survey of Stream Water Quality Revealing Threats to Antibio-Resistant Bacteria, Antibiotic Residues and Heavy Metals in Benin, Pollution, 2023, 9(2), 678–692 Search PubMed.
- V. Pereira, et al., Occurrence of filamentous fungi and yeasts in three different drinking water sources, Water Res., 2009, 43(15), 3813–3819 Search PubMed.
- N. T. Mhlongo, M. Tekere and T. Sibanda, Prevalence and public health implications of mycotoxigenic fungi in treated drinking water systems, J. Water Health, 2019, 17(4), 517–531 CrossRef PubMed.
- A. K. Pandey, et al., Fungal mycotoxins in food commodities: present status and future concerns, Front. sustain. food syst., 2023, 7, 1162595 CrossRef.
- I. Reinholds, et al., Mycotoxins in herbal teas marketed in Latvia and dietary exposure assessment, Food Addit. Contam.: Part B, 2019, 12(3), 199–208 CrossRef CAS PubMed.
- G. Hageskal, N. Lima and I. Skaar, The study of fungi in drinking water, Mycol. Res., 2009, 113(2), 165–172 CrossRef PubMed.
- B. R. Oliveira, et al., Production of mycotoxins by filamentous fungi in untreated surface water, Environ. Sci. Pollut. Res., 2018, 25, 17519–17528 CrossRef CAS.
- R. Russell and M. Paterson, Zearalenone production and growth in drinking water inoculated with Fusarium graminearum, Mycol. Prog., 2007, 6(2), 109–113 CrossRef.
- M. Picardo, et al., Recent advances in the detection of natural toxins in freshwater environments, TrAC, Trends Anal. Chem., 2019, 112, 75–86 CrossRef CAS.
- M. Picardo, O. Núñez and M. Farré, Suspect and target screening of natural toxins in the Ter River catchment area in NE Spain and prioritisation by their toxicity, Toxins, 2020, 12(12), 752 CrossRef CAS PubMed.
- D. Khodaei, F. Javanmardi and A. M. Khaneghah, The global overview of the occurrence of mycotoxins in cereals: A three-year survey, Curr. Opin. Food Sci., 2021, 39, 36–42 CrossRef CAS.
- C. Frizzell, et al., Endocrine disrupting effects of zearalenone, alpha-and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis, Toxicol. Lett., 2011, 206(2), 210–217 CrossRef CAS PubMed.
- M. Peraica, et al., Toxic effects of mycotoxins in humans, Bull. W. H. O., 1999, 77(9), 754 CAS.
- K. Ropejko and M. Twarużek, Zearalenone and its metabolites—General overview, occurrence, and toxicity, Toxins, 2021, 13(1), 35 CrossRef CAS PubMed.
- K. Cheong, et al., Effect of different light wavelengths on the growth and ochratoxin A production in Aspergillus carbonarius and Aspergillus westerdijkiae, Fungal Biol., 2016, 120, 745–751 CrossRef CAS PubMed.
- A. El Khoury, A. Atoui and A. Ochratoxin, General overview and actual molecular status, Toxins, 2010, 2(4), 461–493 CrossRef CAS.
- Y. Tao and A. Ochratoxin, et al., Toxicity, oxidative stress and metabolism, Food Chem. Toxicol., 2018, 112, 320–331 CrossRef CAS.
- IARC, Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins, in IARC Monographs on the Evaluation of Carcinogenic Risk to Humans, International Agency for Research on Cancer, Lyon, France, 1993 Search PubMed.
- S. D. Stoev, New evidences about the carcinogenic effects of ochratoxin A and possible prevention by target feed additives, Toxins, 2022, 14(6), 380 CrossRef CAS PubMed.
- J. Pestka, Deoxynivalenol: Toxicity, mechanisms and animal health risks, Anim. Feed Sci. Technol., 2007, 137(3–4), 283–298 CrossRef CAS.
- J.-h. Yang, et al., Toxic effects and possible mechanisms of deoxynivalenol exposure on sperm and testicular damage in BALB/c mice, J. Agric. Food Chem., 2019, 67(8), 2289–2295 CrossRef CAS PubMed.
- F. E. Okonofua, et al., Association of Urinary Mycotoxins with Sperm Quality: A Case-Control Study in Southern Nigeria, Toxins, 2024, 16(3), 119 CrossRef CAS.
- H. Fang, et al., Effect of Reactive Chemical Species on the Degradation of Deoxynivalenol, 3-Acetyldeoxynivalenol, and 15-Acetyldeoxynivalenol in Low-Temperature Plasmas, ACS Food Sci. Technol., 2022, 2(3), 558–567 CrossRef CAS.
- Y. Li, et al., Deoxynivalenol in food and feed: Recent advances in decontamination strategies, Front. Microbiol., 2023, 14, 1141378 CrossRef.
- A. Székács, Mycotoxins as emerging contaminants. Introduction to the special issue “Rapid Detection of Mycotoxin Contamination, Toxins, 2021, 13(7), 475 CrossRef PubMed.
- E. Fosso-Kankeu and A. K. Mishra, Photocatalytic degradation and adsorption techniques involving nanomaterials for biotoxins removal from drinking water, in Water Purification, Elsevier, 2017, pp. 323–354 Search PubMed.
- Q. Wan, et al., Occurrence and control of fungi in water: New challenges in biological risk and safety assurance, Sci. Total Environ., 2023, 860, 160536 CrossRef CAS PubMed.
- M. Picardo, et al., Suspect screening of natural toxins in surface and drinking water by high performance liquid chromatography and high-resolution mass spectrometry, Chemosphere, 2020, 261, 127888 CrossRef CAS PubMed.
- W. Li, et al., Fungi characteristics of biofilms from sewage and greywater in small diameter gravity sewers, Environ. Sci.: Water Res. Technol., 2020, 6(3), 532–539 RSC.
- B. R. Oliveira, et al., Production of mycotoxins by filamentous fungi in untreated surface water, Environ. Sci. Pollut. Res., 2018, 25, 17519–17528 CrossRef CAS.
- A. Mata, et al., Bottled water: Analysis of mycotoxins by LC–MS/MS, Food Chem., 2015, 176, 455–464 CrossRef CAS PubMed.
- T. D. Bucheli, et al., Fusarium mycotoxins: overlooked aquatic micropollutants?, J. Agric. Food Chem., 2008, 56(3), 1029–1034 CrossRef CAS PubMed.
- Q. Huang, et al., Exposure assessment of multiple mycotoxins and cumulative health risk assessment: a biomonitoring-based study in the Yangtze River Delta, China, Toxins, 2021, 13(2), 103 CrossRef CAS PubMed.
- J. Schenzel, K. Hungerbühler and T. D. Bucheli, Mycotoxins in the environment: II. Occurrence and origin in Swiss river waters, Environ. Sci. Technol., 2012, 46(24), 13076–13084 CrossRef CAS PubMed.
- T. N. Mhlongo, et al., Occurrence and diversity of waterborne fungi and associated mycotoxins in treated drinking water distribution system in South Africa: Implications on water quality and public health, Environ. Monit. Assess., 2020, 192, 1–16 CrossRef.
- C. C. Onyeke, Review of mycotoxins in foods in Nigeria, Food Control, 2020, 118, 107376 CrossRef CAS.
- A. Aasa, et al., A review of toxigenic fungi and mycotoxins in feeds and food commodities in West Africa, World Mycotoxin J., 2023, 16(1), 33–47 CAS.
- M. A. Egbuta, Occurrence of Mycotoxins in Nigerian Food Commodities and Health Risk Assessment, MTech thesis, Faculty of Health Sciences, University of Johannesburg, South Africa, 2011, p. 162.
- J. Oyelakin, et al., Water quality assessment of groundwater in selected potable water sources for household use in Ibadan, Southwest, Nigeria, Int. J. Energy Water Resour., 2021, 5, 125–132 CrossRef.
- A. Oyerinde and H. Jacobs, The complex nature of household water supply: An evidence-based assessment of urban water access in Southwest Nigeria, J. Water, Sanit. Hyg. Dev., 2022, 12(3), 237–247 CrossRef.
- D. Lapworth, et al., Drinking water quality from rural handpump-boreholes in Africa, Environ. Res. Lett., 2020, 15(6), 064020 CrossRef CAS.
- A. I. Airaodion, et al., Assessment of Sachet and Bottled Water Quality in Ibadan, Nigeria, J. Glob. J. Nutri. Food Sci., 2019, 518(1), 4 Search PubMed.
- J. Stoler, From curiosity to commodity: a review of the evolution of sachet drinking water in West Africa, Wiley Interdiscip. Rev.: Water, 2017, 4(3), e1206 CrossRef.
- P. N. Angnunavuri, et al., Evaluation of plastic packaged water quality using health risk indices: a case study of sachet and bottled water in Accra, Ghana, Sci. Total Environ., 2022, 832, 155073 CrossRef CAS PubMed.
- B. K. Olopade, et al., Occurrences of Deoxynivalenol, Zearalenone and some of their masked forms in selected cereals from Southwest Nigeria, NFS J., 2021, 23, 24–29 CrossRef CAS.
- A. Pfohl-Leszkowicz and R. A. Manderville, An update on direct genotoxicity as a molecular mechanism of ochratoxin a carcinogenicity, Chem. Res. Toxicol., 2012, 25(2), 252–262 Search PubMed.
- Top 10 Highest Agricultural Producing States in Nigeria, 2023, in https://www.onlineworld.ng/2023/02/agricultural-producing-states-nigeria.html Search PubMed.
- WHO, Guidelines for Drinking-Water Quality, World Health Organization, Geneva, 2011 Search PubMed.
- X. Zhou, et al., Hydrochemistry of the natural low pH groundwater in the coastal aquifers near Beihai, China, J. Ocean Univ. China, 2015, 14, 475–483 Search PubMed.
- V. Mohos, et al., Testing the extraction of 12 mycotoxins from aqueous solutions by insoluble beta-cyclodextrin bead polymer, Environ. Sci. Pollut. Res., 2022, 29, 210–221 CrossRef CAS.
- F. Ameen, et al., Diversity of Fungi in Bottled Water in Jeddah, Saudi Arabia, Water Sci. Technol.: Water Supply, 2018, 18(5), 1664–1673 CAS.
- V. Pereira, et al., Free chlorine inactivation of fungi in drinking water sources, Water Res., 2013, 47(2), 517–523 CrossRef CAS.
- S.-Y. Wang, et al., Occurrence of aflatoxins in water and decontamination strategies: A review, Water Res., 2023, 232, 119703 CrossRef CAS.
- J. Schenzel, et al., Mycotoxins in the environment: I. Production and emission from an agricultural test field, Environ. Sci. Technol., 2012, 46(24), 13067–13075 CrossRef CAS.
- D. W. Kolpin, et al., Mycotoxins: diffuse and point source contributions of natural contaminants of emerging concern to streams, Sci. Total Environ., 2014, 470, 669–676 CrossRef PubMed.
- T. Tuomi, et al., Mycotoxins in crude building materials from water-damaged buildings, Appl. Environ. Microbiol., 2000, 66(5), 1899–1904 CrossRef CAS PubMed.
- L. M. Juraschek, A. Kappenberg and W. Amelung, Mycotoxins in soil and environment, Sci. Total Environ., 2022, 814, 152425 CrossRef CAS PubMed.
- Z. Wang, et al., Deoxynivalenol: Signaling pathways and human exposure risk assessment—An update, Arch. Toxicol., 2014, 88, 1915–1928 CrossRef CAS PubMed.
- H. Guo, et al., Co-contamination and interaction of fungal toxins and other environmental toxins, Trends Food Sci., 2020, 103, 162–178 CrossRef CAS.
- F. E. Wettstein and T. D. Bucheli, Poor elimination rates in waste water treatment plants lead to continuous emission of deoxynivalenol into the aquatic environment, J. Water Res., 2010, 44(14), 4137–4142 CrossRef CAS.
- B. Arce-López, et al., Assessment of exposure to mycotoxins in Spanish children through the analysis of their levels in plasma samples, J. Toxins, 2021, 13(2), 150 CrossRef.
- X. Rong, et al., Combined effects of zearalenone and deoxynivalenol on oxidative stress, hepatotoxicity, apoptosis, and inflammation in zebrafish embryos, Sci. Total Environ., 2023, 859, 160233 CrossRef CAS PubMed.
- Z. Ren, et al., Combined effects of deoxynivalenol and zearalenone on oxidative injury and apoptosis in porcine splenic lymphocytes in vitro, Exp. Toxicol. Pathol., 2017, 69(8), 612–617 CrossRef CAS PubMed.
- H. Guo, et al., Co-contamination and interaction of fungal toxins and other environmental toxins, Trends Food Sci. Technol., 2020, 103, 162–178 CrossRef CAS.
- N. T. Mhlongo, et al., Prevalence and public health implications of mycotoxigenic fungi in treated drinking water systems, J. Water Health, 2019, 17(4), 517–531 CrossRef PubMed.
- H. Tan, et al., Effect of temperature and pH on the conversion between free and hidden zearalenone in zein, Food Chem., 2021, 360, 130001 CrossRef CAS.
- F. R. F. Passamani, et al., Effect of temperature, water activity, and pH on growth and production of ochratoxin A by Aspergillus niger and Aspergillus carbonarius from Brazilian grapes, J. Food Prot., 2014, 77(11), 1947–1952 CrossRef.
- N. H. Bakhtiar Jemily, et al., Relationship between electrical conductivity and total dissolved solids as water quality parameter in Teluk Lipat by using regression analysis, Progress in Engineering Technology. Advanced Structured Materials, ed. M. Abu Bakar, M. Mohamad Sidik and A. Öchsner, Springer, Cham, 2019, vol. 119, pp. 169–173 Search PubMed.
- JECFA, Evaluation of certain food additives and contaminants, in 72nd Report of the Joint FAO/WHO Expert Committee on Food Additive, WHO Technical Report Series, 2010 Search PubMed.
- D. Schrenk, et al., Risk assessment of ochratoxin A in food, EFSA J., 2020, e06113 Search PubMed.
- EFSA, et al., Risk assessment of ochratoxin A in food, EFSA J., 2020, 18(5), e06113 Search PubMed.
- B. De Santis, et al., Overall Exposure of European adult population to mycotoxins by statistically modelled biomonitoring data, Toxins, 2021, 13(10), 695 CrossRef CAS PubMed.
- T. R. Bui-Klimke and F. Wu, Ochratoxin A and human health risk: A review of the evidence, Crit. Rev. Food Sci. Nutr., 2015, 55(13), 1860–1869 CrossRef CAS PubMed.
- S. D. Stoev, Balkan Endemic Nephropathy–Still continuing enigma, risk assessment and underestimated hazard of joint mycotoxin exposure of animals or humans, Chem.-Biol. Interact., 2017, 261, 63–79 CrossRef CAS PubMed.
- EFSA, Scientific opinion on the risks for public health related to the presence of zearalenone in food, in EFSA Panel on Contaminants in the Food Chain): EFSA Journal, 2011, DOI:10.2903/j.efsa.2011.2197.
- B. De Santis, et al., Overall Exposure of European adult population to mycotoxins by statistically modelled biomonitoring data, Toxins, 2021, 13(10), 695 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04866k |
| This journal is © The Royal Society of Chemistry 2024 |