Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fatty aldehyde bisulfite adducts as a purification handle in ionizable lipid synthesis†
Graham Atwood,
Sona Purbiya,
Cassandra Reid,
Brandon Smith,
Kuljit Kaur,
Drew Wicks,
Peter Gaudet,
K. Cory MacLeod and
Jean-Francois Vincent-Rocan
and
Jean-Francois Vincent-Rocan *
*
BioVectra Inc., Charlottetown, Prince Edward Island C1E 0A1, Canada. E-mail: jvincent-rocan@biovectra.com
First published on 19th August 2024
Abstract
Rapid access to ALC-0315, a crucial component of the formulated Pfizer Covid vaccine, was obtained by employing solid adduct formation and filtration after an oxidation step in place of the standard chromatographic separation, allowing for a more scalable synthesis. Impurities were removed by formation of this fatty aldehyde bisulfite adduct at the penultimate step and by performing the final reductive amination directly with the fatty aldehyde bisulfite adduct. This eliminates chromatographic separations for all prepared aldehyde containing intermediates. Along with ALC-0315, FTT5 and SM-102 ionizable lipids were prepared utilizing this strategy. This work paves the way for more sustainable access to these critical ionizable lipids that would de-risk the world supply of important vaccines and medicines in the future.
Introduction
Lipid nanoparticles (LNPs) play a crucial role in the formulation of nucleic acid derived medicines. Ionizable lipids are the major component of these LNPs, and their chemical and physical properties are responsible for the entrapment and delivery of the payload.1–5 These lipids are required in significantly larger quantities than the nucleic acids they carry. However, their synthesis includes major drawbacks from a process chemistry perspective such as excessive use of chlorinated solvents and multiple column chromatography operations. ALC-0315 1 is the ionizable lipid in the Pfizer-BioNTech Covid-19 vaccine, Comirnaty, (0.43 mg per dose) and an estimated 4.6 billion doses were shipped to 181 countries.6 Therefore, since 2020 more than 2 MT of ALC-0315 was produced. Herein, we present our effort to make ionizable lipid production more sustainable by developing a route that relies on bisulfite adduct formation and filtration rather than chromatographic purification of intermediates to obtain analytically pure lipid.There are select few syntheses of ALC-0315 1 that are reported (Scheme 1).7–10 However, they all have significant drawbacks from a process chemistry perspective, using skin sensitizing reagents, chlorinated solvents and having a low control on impurity management requires the use of column chromatography purification at multiple steps. To overcome these challenges and allow for a more sustainable and scalable synthesis, it was envisioned that the desired lipid could be obtained via a fatty aldehyde bisulfite adduct intermediate 4 of the analogous aldehyde 3 which would itself act as a purification handle in the synthesis. Additionally, the bisulfite adduct can be used directly in the next reductive amination step to produce 1.
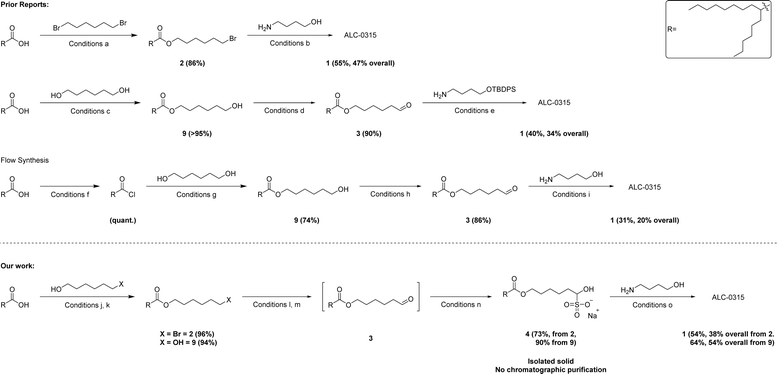 | ||
Scheme 1 Comparison of prior reports and our work in the synthesis of ALC-0315 1. Overall yields are with respect to hexyldecanoic acid (acid). (a) Acid (1 equiv.), 1,6-dibromohexane (4 equiv.), K2CO3 (1.5 equiv.), DMF, 72 h.7 (b) 2 (2.2 equiv.), 4-aminobutanol (1 equiv.), K2CO3 (2.2 equiv.), 72 h, DMF.7 (c) Acid (1 equiv.), DCC (1.1 equiv.), DMAP (1.2 equiv.), CH2Cl2, 72 h.8 (d) 9 (1 equiv.), TEMPO (0.2 equiv.), bleach, NaHCO3 aq. (sat.), CH2Cl2, 2 h. (e) 3 (2.3 equiv.), Na(CH3CH2CO2)3BH (3 equiv.), cat. acetic acid, 4-aminobutanol (1 equiv.), CH2Cl2, 0 °C to RT, 3 h, followed by: HF-pyridine (4 equiv.), THF, 0 °C, overnight.8 (f) Acid (1 equiv.), SOCl2 (1.1 equiv.), DMF (5 mol%), 2-MeTHF, 80 °C, 20 min.9 (g) Acyl chloride (1 equiv.), 1,6-hexanediol (3 equiv.), 2-MeTHF, 100 °C, 4 min.9 (h) 9 (1 equiv.), TEMPO (7 mol%), NaClO (2.3 equiv.), KBr (0.35 equiv.), 2-MeTHF, 2.3 min.9 (i) 3 (3 equiv.), 4-aminobutanol (1 equiv.), (CH3)4N(CH3CO2)3BH (3 equiv.), methanol, 2-MeTHF and NMP, 16 min.9 (j) X = Br, acid (1 equiv.), 6-bromo-1-hexanol (0.98 equiv.), TsOH (10 mol%), toluene, reflux, 16 h. (k) X = OH, acid (1 equiv.), 1,6-hexanediol (1 equiv.), EDC HCl (1.7 equiv.), DMAP (1.2 equiv.) CH2Cl2, 16 h. (l) 2 (1 equiv.), pyridine N-oxide (6 equiv.), NaOAc (2 equiv.), n-propyl acetate, reflux, 16 h. (m) 9 (1 equiv.), PIDA (1.1 equiv.), TEMPO (10 mol%), 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane 1 heptane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2, 3–16 h. (n) Na2S2O5 (0.6 equiv.). (o) 4 (2.3 equiv.), 4-amino-1-butanol (1 equiv.), NEt3 (2.4 equiv.), NaBH(OAc)3 (4.3 equiv.), 2-MeTHF, 16 h. CH2Cl2, 3–16 h. (n) Na2S2O5 (0.6 equiv.). (o) 4 (2.3 equiv.), 4-amino-1-butanol (1 equiv.), NEt3 (2.4 equiv.), NaBH(OAc)3 (4.3 equiv.), 2-MeTHF, 16 h. | ||
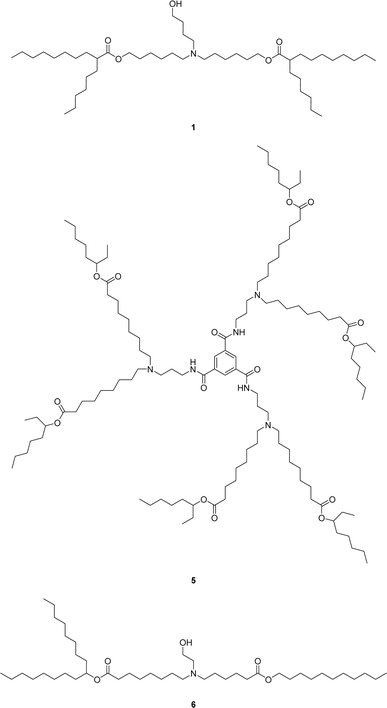
To generate the required aldehyde 3, an acid-catalyzed esterification using the non-symmetrical 6-bromo-1-hexanol could be employed to avoid bis-ester formation. Whereas, prior reports relied on non-selective esterification of symmetrical diols, which typically requires the use of chromatographic separation of the undesired bis-ester byproduct or the use of a large molar excess of diol starting material relative to the carboxylic acid in order to disfavor bis-ester formation. The obtained bromo-ester 2 could then undergo a Ganem type oxidation to furnish the aldehyde 3.11,12 This would allow the aldehyde 3 to be purified via bisulfite adduct formation. Alternatively, the direct alkylation of the amine with the bromo-ester 2 to form ALC-0315 1 is known in the literature but produces the quaternary ammonium salt as a side-product which is not easily removed or controlled (Scheme 1, conditions b).7 Finally, a modification of the known reductive amination would provide ALC-0315 1 by direct reaction of the fatty aldehyde bisulfite adduct 4 in 2-MeTHF as an alternative to the commonly used CH2Cl2, increasing the selectivity without needing to add solubilizing groups such as silyl ethers (Scheme 1, conditions e)8 which can increase step count as well as unit operations dramatically. These modifications greatly improve the yield of the reductive amination to those reported in the literature.7–10 The strategy of purification via solid fatty aldehyde bisulfite adduct followed by reductive amination directly with the bisulfite adduct lends itself well to a large range of ionizable lipids due to structural similarities (Chart 1) and, therefore, retrosynthetic approaches. Select examples of other ionizable lipids (FTT5 5,13 a lipid-like compound which has found in vivo success, and SM-102 6 from Moderna's mRNA-1273 COVID-19 vaccine) were synthesized to further demonstrate the utility of this synthetic strategy.
To the best of our knowledge, solid fatty aldehyde bisulfite adduct intermediates have not been previously reported in the field of ionizable lipid synthesis nor used in subsequent reactions directly.
Results
The initial step in the synthesis of ALC-0315 1 being an esterification allows for a variety of potential reaction conditions to be utilized. While carbodiimides are commonly used to couple an alcohol to a carboxylic acid for this step, an acid-catalyzed esterification can be envisioned to avoid skin sensitizers.14–17 Using standard Dean–Stark azeotropic conditions the bromo-ester 2 is generated in good yield (96%) and purity (>95% by HPLC-CAD).With the bromo-ester 2 in hand, subsequent oxidation to the aldehyde was developed, which was adapted from an analogous oxidation (see ESI† for more details).12 Optimization of this reaction (Table S3†) culminates in bromide 2 being dosed into a refluxing solution of pyridine N-oxide, NaOAc, and n-propyl acetate over 4 hours to generate aldehyde 3 in good yield (88% NMR yield).
With general conditions for the alkyl bromide oxidation to an aldehyde, purification of the crude aldehyde 3 via bisulfite adduct is seen as a means of not only overcoming impurities18 generated in the developed oxidation but as a potentially more appropriate starting material for the final reductive amination to generate 1.19–21 This would also act as a more suitable hold point when compared to typical aldehyde stability.22
Initial attempts to purify the crude aldehyde 3 involved dissolution of the crude oil in EtOH. This solution is then subjected to an aqueous solution of sodium metabisulfite dropwise. The bisulfite adduct 4 forms within 30 minutes (monitored by 1H NMR) but gives a sticky, gummy solid material which is not suitable for filtration. Aqueous extraction of the adduct is not feasible due to limited solubility.
Removal of the water that is introduced during bisulfite adduct formation via azeotropic distillation with toluene gives a more workable solid after addition of EtOH, but unfortunately is still unsuitable for filtration due to clogging.
Utilizing acetone as a bisulfite adduct anti-solvent has been reported previously23 and gives a well filtering mixture of 4, however a significant portion of the free aldehyde product is lost to the filtrates resulting in a decreased yield of the isolated solid adduct. It is proposed that a small equilibrium of the bisulfite adduct to the free aldehyde and bisulfite anion can cause the degradation of the adduct over time as acetone can also form a bisulfite adduct with the in situ generated bisulfite anion. Therefore, alternative anti-solvents were evaluated to avoid loss of the bisulfite adduct and to further improve the purification.
The results of a stability test of the adduct 4 indicate complete degradation of adduct and loss of product in acetone at extended contact times. Changing from acetone to ethyl formate improves the stability of the bisulfite adduct, as determined by 1H NMR (Fig. S1†), while still enabling filtration. This is likely due to the similar size and polarity of ethyl formate, while not reacting with the in situ generated bisulfite anions as acetone does. Additionally, spiking experiments also indicate that water does not have an impact on bisulfite stability (mechanistic detail Scheme S3†).
Upon addition of ethyl formate to the solid bisulfite material, a brown solution is obtained along with an easily filterable light-tan colored solid giving 23.86 g (73% yield) of 4 as a light-brown to tan solid from the starting bromo-ester 2. This tan solid is noted to be hygroscopic as before, and can be stored either under nitrogen, or in a desiccator. A significant amount of semi-solids are found to have collected on the underside of the filter paper, slowing filtration. This is likely due to the relatively low boiling point of ethyl formate and the low pressure experienced after passing through the filter. A simple change to DMC negates this issue and allows for a more rapidly filtering mixture.
The isolated adduct 4 is readily converted to the free aldehyde via washing with 10% sodium carbonate solution and extracting into EtOAc. The free aldehyde product is obtained with good purity (85–88%, HPLC-CAD) and can be used in the final reductive amination step. With the free aldehyde in hand from 4, the reductive amination to generate ALC-0315 1 is preformed via a dosed addition of the free aldehyde with portion-wise addition of the reducing agent, NaBH(OAc)3. Portion-wise addition of the reducing agent is needed to overcome process limitations.8 These conditions give purity of ALC-0315 1 in the isolated crude oil of 68% as analyzed by HPLC-CAD. Alternatively, the reductive amination can be performed directly from the isolated solid bisulfite adduct 4 (Scheme 1, conditions o) to give an improved crude purity (86% HPLC-CAD), while also allowing 2-MeTHF to replace CH2Cl2 as the solvent. Column chromatography gives purified ALC-0315 1 as a clear slightly yellow oil (53% isolated yield, 94.9% HPLC-CAD). This represents a 37% overall yield from the starting hexyldecanoic acid.
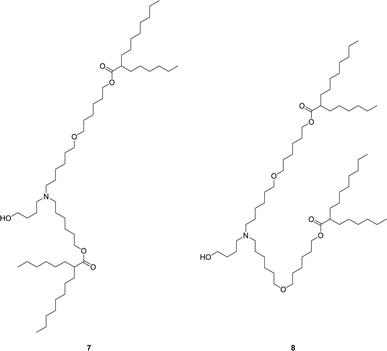
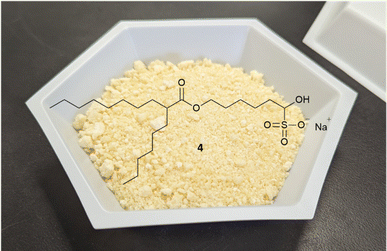
The main impurities in the final isolated ALC-0315 1 are two structurally related ether impurities, 7 and 8 (Chart 2), which stems from an ether impurity present in the 6-bromo-1-hexanol starting material. This ether impurity in the esterification starting material culminates in two structurally similar compounds to ALC-0315 which are not easily separated via column chromatography. Therefore, an alternative synthetic route was evaluated, avoiding 6-bromo-1-hexanol which contains the impurity.
A straightforward change of the first two steps eliminated the dependence on the problematic starting material and avoids the structurally similar impurities, 7 and 8, albeit with the introduction of a coupling reagent. An initial EDC coupling of hexyldecanoic acid and 1,6-hexandiol gives the mono-esterified diol 9 in high yield and good purity (94%, 88.0% HPLC-CAD), with the main impurity being the bis-ester byproduct (9.5%), which can be easily removed in a later step during purification of the aldehyde bisulfite-adduct. Alcohol-ester 9 is then oxidized to aldehyde 3 with PIDA in the presence of cat. TEMPO.24–26 The crude aldehyde 3 is then subjected to the general bisulfite adduct purification conditions. This gives crystalline off-white solid which filters rapidly to give the purified product as the sodium bisulfite adduct 4 (Fig. 1) in a 90% yield (83% purity by HPLC-CAD) from 9, and overall yield of 85% from hexyldecanoic acid. This solid isolate eliminates the need for a chromatographic purification prior to its use in the following step, significantly reducing waste that is typically generated in such chromatographic operations (Table S4†). The adduct 4, as before, is subjected to the improved reductive amination conditions and column purified to give ALC-0315 1 in a 64% yield for the final reductive amination step and 54% overall yield of ALC-0315 1 from the starting material, hexyldecanoic acid. This yield is an improvement when compared to other literature preparations of ALC-0315 1 (20–47%, Scheme 1)7–9 as well as patented procedures (50%).10 The culmination of the process yields ALC-0315 1 with a purity of 97.3% as analyzed by HPLC-CAD with no structurally similar impurities, 7 or 8, present. This material was subsequently used in a formulation preparation, encapsulating mRNA producing LNPs which match the physiochemical properties of formulation batches that utilize commercially available ALC-0315 (Table S6†).
With the ability to synthesize ALC-0315 1 in this manner, FTT5 5, a proven lipid in vivo,13 and SM-102 6 were the next targets for evaluating the route's feasibility across multiple lipids.
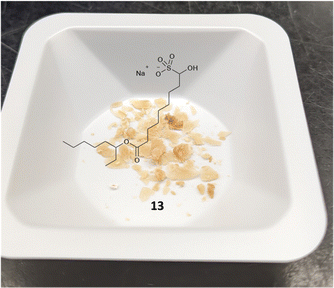
Preparation of the bisulfite adduct intermediate 13 of FTT5 5 follows analogously to 4 (Schemes 2 and S1† for full route) and gives a well behaving flaky tan solid (Fig. 2). This, again, eliminates the need for a chromatographic separation of the aldehyde intermediate. Direct reaction of amine 10 with bisulfite adduct 13 gives FTT5 5 (26%), closely matching the literature yield (27%).13 The inclusion of IPA in the reaction is required to solubilize the amine 10, otherwise a very slow reaction is observed.
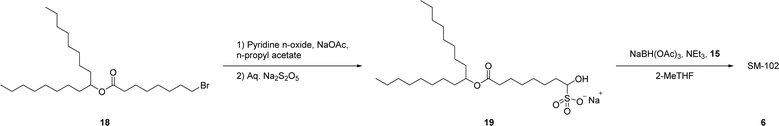
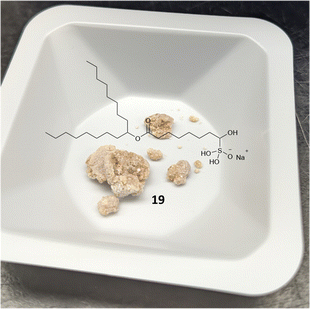
The synthetic route to SM-102 6 proceeds through bisulfite adduct 19, after oxidation of bromo-ester 18 (Schemes 3 and S2† for full route). Isolation of the bisulfite adduct 19 gives a well behaving off-white to tan solid (Fig. 3). After direct reaction of bisulfite adduct 19 with amine 15 and column purification, SM-102 6 is obtained in good yield and purity (67% yield, 96.0% purity by HPLC-CAD). As with ALC-0315 1 and FTT5 5, SM-102 6 is readily prepared via a solid aldehyde bisulfite adduct intermediate, enabling the elimination of chromatographic purification of the aldehyde intermediate, as well as the use of the bisulfite adduct 19 directly in the final step to produce the lipid.
Experimental
Synthetic procedures
Conclusions
A general route has been developed for multiple commonly used ionizable lipids which have been prepared through solid fatty aldehyde bisulfite adduct intermediates, eliminating the need for column purification of intermediates in these multi-step syntheses. Both ALC-0315 1 and SM-102 6, two ionizable lipids being used in COVID-19 vaccine drug products, as well as FTT5 5, a relatively recent lipid-like compound which has seen in vivo success, have been prepared by employing fatty aldehyde bisulfite adducts, which allows for easy isolation of the resulting solid intermediates. Additionally, the bisulfite adducts can be used directly in subsequent reductive amination reactions, in which they performed equally or better than the free aldehyde while also eliminating the need for CH2Cl2 in the reaction. We believe this to be the first reported isolation of solid fatty aldehyde bisulfite adduct intermediates and their use in the production of ionizable lipids.The production of ALC-0315 1 via a solid fatty aldehyde bisulfite adduct intermediate enabled an improved purification process to the typical column chromatography, improved yield when compared to other syntheses, and was found to perform equally when formulated with mRNA as other commercial sources of ALC-0315. Isolation of solid fatty aldehyde intermediates via bisulfite adduct formation and filtration is viewed as a general approach to improve synthesis of other lipids and the isolation and purification of other fatty alkyl aldehyde intermediates.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors have no acknowledgments.Notes and references
- X. Hou, T. Zaks, R. Langer and Y. Dong, Nat. Rev. Mater., 2021, 6, 1078–1094 CrossRef CAS PubMed.
- L. Xu, X. Wang, Y. Liu, G. Yang, R. J. Falconer and C.-X. Zhao, Adv. NanoBiomed Res., 2022, 2, 2100109 CrossRef CAS.
- R. H. Müller, K. Mäder and S. Gohla, Eur. J. Pharm. Biopharm., 2000, 50, 161–177 CrossRef.
- R. Paliwal, S. R. Paliwal, R. Kenwat, B. D. Kurmi and M. K. Sahu, Expert Opin. Ther. Pat., 2020, 30, 179–194 CrossRef CAS.
- M. Jeong, Y. Lee, J. Park, H. Jung and H. Lee, Adv. Drug Delivery Rev., 2023, 200, 114990 CrossRef CAS PubMed.
- L. Schoenmaker, D. Witzigmann, J. A. Kulkarni, R. Verbeke, G. Kersten, W. Jiskoot and D. J. A. Crommelin, Int. J. Pharm., 2021, 601, 120586 CrossRef CAS.
- I. A. Boldyrev, V. P. Shendrikov, A. G. Vostrova and E. L. Vodovozova, Russ. J. Bioorg. Chem., 2023, 49, 412–415 CrossRef CAS.
- F. Saadati, S. Cammarone and M. A. Ciufolini, Chem.–Eur. J., 2022, 28, e202200906 CrossRef CAS.
- J. B. Wolf, J. Weon Lee, M. B. Plutschack, D. Cambié, A. Seidel-Morgenstern and P. H. Seeberger, React. Chem. Eng., 2024, 9, 959–966 RSC.
- N. M. Do, S. A. Eisenbeis and O. A. Salman, Methods for Producing of Lipids, WO2022215002A1, World Intellectual Property Organization, 2022.
- A. G. Godfrey and B. Ganem, Tetrahedron Lett., 1990, 31, 4825–4826 CrossRef CAS.
- Z. Chen, N. Salehi Marzijarani, S. Quirie, G. F. Pirrone, S. M. Dalby, T. Wang, J. Kim, F. Peng and A. J. Fine, Org. Process Res. Dev., 2022, 26, 525–532 CrossRef CAS.
- X. Zhang, W. Zhao, G. N. Nguyen, C. Zhang, C. Zeng, J. Yan, S. Du, X. Hou, W. Li, J. Jiang, B. Deng, D. W. McComb, R. Dorkin, A. Shah, L. Barrera, F. Gregoire, M. Singh, D. Chen, D. E. Sabatino and Y. Dong, Sci. Adv., 2020, 6, eabc2315 CrossRef CAS.
- K. J. McKnelly, W. Sokol and J. S. Nowick, J. Org. Chem., 2020, 85, 1764–1768 CrossRef CAS.
- E. Fischer and A. Speier, Ber. Dtsch. Chem. Ges., 1895, 28, 3252–3258 CrossRef CAS.
- Z. Khan, F. Javed, Z. Shamair, A. Hafeez, T. Fazal, A. Aslam, W. B. Zimmerman and F. Rehman, J. Ind. Eng. Chem., 2021, 103, 80–101 CrossRef CAS.
- J. C. Graham, A. Trejo-Martin, M. L. Chilton, J. Kostal, J. Bercu, G. L. Beutner, U. S. Bruen, D. G. Dolan, S. Gomez, J. Hillegass, J. Nicolette and M. Schmitz, Chem. Res. Toxicol., 2022, 35, 1011–1022 Search PubMed.
- T. M. Bass, D. Zell, S. M. Kelly, T. C. Malig, J. G. Napolitano, L. E. Sirois, C. Han and F. Gosselin, Org. Process Res. Dev., 2024, 28, 2635–2645 CrossRef CAS.
- C. R. Pandit and N. S. Mani, Synthesis, 2009, 2009, 4032–4036 CrossRef.
- M. Barniol-Xicota, A. L. Turcu, S. Codony, C. Escolano and S. Vázquez, Tetrahedron Lett., 2014, 55, 2548–2550 CrossRef CAS.
- X. Li, K. S. Iyer, R. R. Thakore, D. K. Leahy, J. D. Bailey and B. H. Lipshutz, Org. Lett., 2021, 23, 7205–7208 CrossRef CAS.
- M. G. Kissane, S. A. Frank, G. A. Rener, C. P. Ley, C. A. Alt, P. A. Stroud, R. K. Vaid, S. K. Boini, L. A. McKee, J. T. Vicenzi and G. A. Stephenson, Tetrahedron Lett., 2013, 54, 6587–6591 CrossRef CAS.
- Y. Itoh, J. Horiuchi and K. Takahashi, Colloids Surf., A, 2007, 308, 118–122 CrossRef CAS.
- L. De Luca, G. Giacomelli and A. Porcheddu, Org. Lett., 2001, 3, 3041–3043 CrossRef CAS PubMed.
- R. Ciriminna and M. Pagliaro, Org. Process Res. Dev., 2010, 14, 245–251 CrossRef CAS.
- C. Guérin, V. Bellosta, G. Guillamot and J. Cossy, Org. Lett., 2011, 13, 3534–3537 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05189k |
| This journal is © The Royal Society of Chemistry 2024 |