Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Adsorption of drugs on B12N12 and Al12N12 nanocages†
Remya Geetha Sadasivan Nair *,
Arun Kumar Narayanan Nair
*,
Arun Kumar Narayanan Nair * and
Shuyu Sun*
* and
Shuyu Sun*
Physical Science and Engineering Division (PSE), Computational Transport Phenomena Laboratory, King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia. E-mail: remya.nair@kaust.edu.sa; arun.narayanannair@kaust.edu.sa; shuyu.sun@kaust.edu.sa
First published on 8th October 2024
Abstract
The adsorption behavior of twelve drug molecules (5-fluorouracil, nitrosourea, pyrazinamide, sulfanilamide, ethionamide, 6-thioguanine, ciclopirox, 6-mercaptopurine, isoniazid, metformin, 4-aminopyridine, and cathinone) on B12N12 and Al12N12 nanocages was studied using density functional theory. In general, the drug molecules prefer to bind with the boron atom of the B12N12 nanocage and the aluminium atoms of the Al12N12 nanocage. However, a hydrogen atom is transferred from each of 5-fluorouracil, nitrosourea, 6-thioguanine, ciclopirox, and 6-mercaptopurine to the nitrogen atom of the Al12N12 nanocage. All the drug molecules are found to be chemisorbed on the B12N12 and Al12N12 nanocages. The adsorption energies of the drug/B12N12 system are linearly correlated with the molecular electrostatic potential minimum values of the drug molecules. The transfer of the hydrogen atom from the drug molecules to the nitrogen atom of the Al12N12 nanocage leads to relatively high adsorption energies. We observed significant changes in the reactivity parameters (e.g. electronic chemical potential) of the nanocages due to the chemisorption process. Overall, the QTAIM analysis indicates that the interactions between drug molecules and nanocages have a partial covalent character. Among the studied systems, the adsorption process was more spontaneous for the ciclopirox/Al12N12 system in water.
1 Introduction
Many drugs have been developed to treat various diseases and improve human health.1–10 For example, 5-fluorouracil, a pyrimidine containing drug, is widely used in the management of different types of cancers such as colon cancer and head and neck cancer.1 Nitrosoureas have long been of interest in the treatment of brain tumors and Hodgkin's disease.2 Pyrazinamide, ethionamide, and isoniazid play key roles in the treatment of tuberculosis.3,4 Sulfanilamide could be used in the treatment of vaginal infections.5 6-Thioguanine and 6-mercaptopurine are important in the treatment of lymphoblastic leukemia.6 Ciclopirox is an ideal candidate for the treatment of superficial dermatophyte and yeast infections.7 Metformin can be used to lower blood glucose in non-insulin-dependent diabetic patients.8 4-Aminopyridine is reported to be useful for managing the symptoms of multiple sclerosis.9 There has been interest in the therapeutic potential of cathinone as an antidepressant.10There has been growing interest in developing materials for drug delivery and sensing applications.11–34 Density functional theory (DFT) was used to study the adsorption of drug molecules on nanosheets and nanotubes.15–23 DFT investigations revealed that the adsorption energy of isoniazid on B-doped carbon nanotubes was higher than that on the pristine carbon nanotubes.15 The adsorption of metformin on carbon nanotube was physisorption in nature, while that on Al- and Si-doped carbon nanotubes was chemisorption in nature.16 5-Fluorouracil was physically adsorbed on the graphene oxide nanosheet.17 The adsorption process of 5-fluorouracil, 6-thioguanine, and 6-mercaptopurine on the boron nitride nanosheet was exothermic and occurred spontaneously.18 Nitrosourea was found to be physically adsorbed on the boron nitride nanosheet.19 The adsorption energy of 5-fluorouracil on Al-doped boron nitride nanotube was higher than that on the pristine boron nitride nanotube.20 The binding stability on a graphene flake decreased in the sequence 6-thioguanine > 6-mercaptopurine (thiol form) > 5-fluorouracil.21 5-Fluorouracil was physically adsorbed to the wall of the carbon nanotube, while a chemisorption occurred between 5-fluorouracil and doped carbon nanotube.22 The adsorption of 5-fluorouracil on AlN-nanotube was physisorption in nature.23
There have also been studies on the adsorption of drug molecules on fullerene-like nanocages such as B12N12 and Al12N12 using DFT.24–34 The nanocage clusters of B12N12 were synthesized by Oku et al. in 2004.35 The AlN nanostructures have also been successfully synthesized.36,37 The Al12N12 nanocage was predicted to be the most stable among the AlnNn (n = 2–41) nanocages.38 DFT investigations revealed that the B12N12 nanocage is a better sensor for 4-aminopyridine than the Al12N12 nanocage.27 6-Mercaptopurine binds via the unsubstituted nitrogen atom of the imidazole ring to the B12N12 nanocage.28 The B12N12 nanocage could be used as a potential sensor for the detection of metformin.29 The adsorption of 6-thioguanine onto the B12N12 nanocage was a strong chemisorption in the gas phase as well as in water.30 Sulfanilamide preferred to bind with the boron atom of the B12N12 nanocage and the aluminium atom of the Al12N12 nanocage.31 The oxygen atom of the carbonyl group of ciclopirox bound to the boron atom of the B12N12 nanocage.32 The B12N12 nanocage could be a potential candidate as a drug carrier for isoniazid.33 A hydrogen atom was transferred from ciclopirox to the nitrogen atom of the Al12N12 nanocage.34 However, the adsorption properties of drug molecules like ethionamide on the B12N12 and Al12N12 nanocages have yet to be investigated.
In this work, the adsorption behavior of twelve drug molecules on the B12N12 and Al12N12 nanocages was studied using DFT. Typically, the molecular electrostatic potential (MESP) minimum (Vmin) points appear along the electron-rich regions (e.g., π- and lone-pair regions).39–42 An interesting observation is that the adsorption energies of the drug/Bl12N12 system are linearly correlated with the MESP Vmin values of the drug molecules. The present study may be helpful for the exploration of nanocages in drug delivery and sensing applications.
2 Computational details
The adsorption of twelve drug molecules, namely, 5-fluorouracil, nitrosourea, pyrazinamide, sulfanilamide, ethionamide, 6-thioguanine, ciclopirox, 6-mercaptopurine, isoniazid, metformin, 4-aminopyridine, and cathinone onto the B12N12 and Al12N12 nanocages was studied using DFT. The chemical structures of the drug molecules examined in the present study are given in Fig. 1a. DFT computations were conducted using the Gaussian 16 program.43 All structures are optimized at the M062X/6-311G(d,p) level and confirmed as energy minima by frequency calculations.44 The MESP, V(r), is given as39,40,42,45
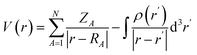 | (1) |
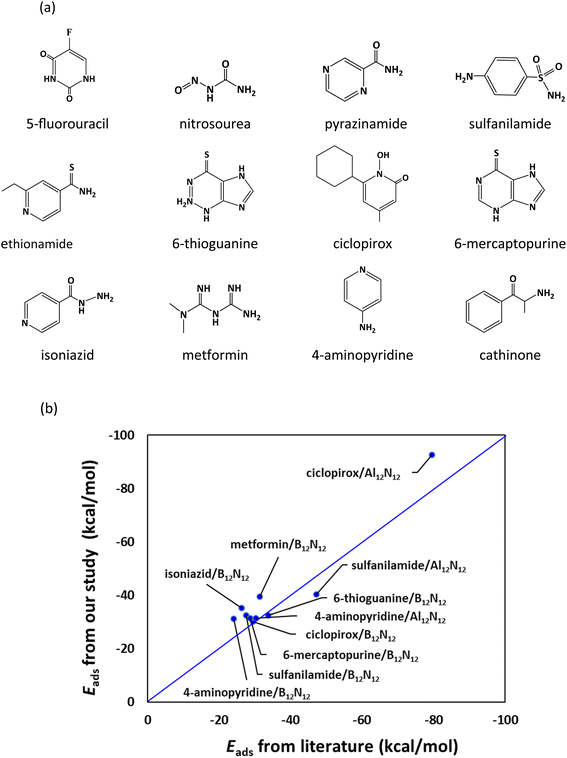 | ||
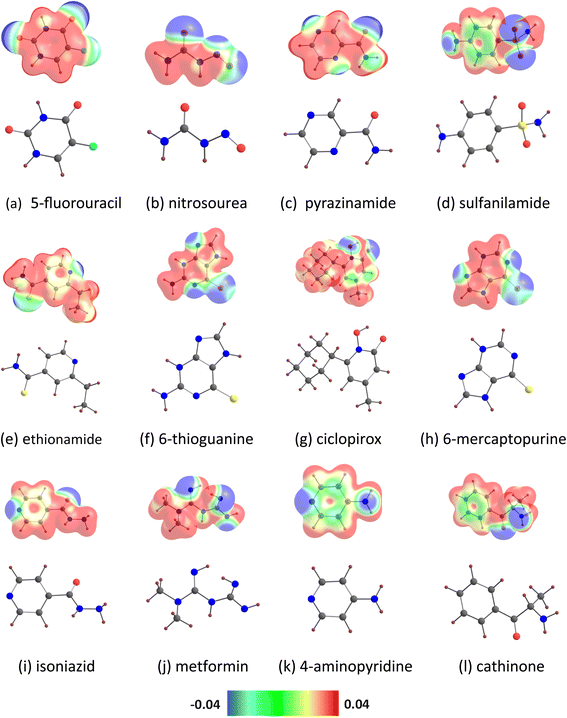
| Fig. 1 (a) Drug molecules examined in the present study. (b) Comparison of our results for adsorption energies with literature values.27–34 | ||
The adsorption energy of the drug molecule on the nanocage (Eads) is computed as follows:
| Eads = Edrug/nanocage − (Enanocage + Edrug) + EBSSE | (2) |
The Gibbs free energy change (ΔG), the enthalpy change (ΔH), and the entropy change (ΔS) were estimated by the following equations:
| ΔG = Gdrug/nanocage − (Gnanocage + Gdrug) | (3) |
| ΔH = Hdrug/nanocage − (Hnanocage + Hdrug) | (4) |
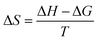 | (5) |
The DFT reactivity indices were estimated by the following equations:48
 | (6) |
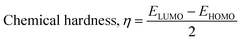 | (7) |
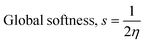 | (8) |
 | (9) |
Bader's quantum theory of atoms in molecules (QTAIM) analyses49 were conducted at the M062X/6-31G(d,p) level using the Multiwfn software.50 The values of ρb and its Laplacian (∇2ρb) and the total electron energy density (Hb) and its components (the kinetic electron energy density (Gb) and the potential electron energy density (Vb)) at the bond critical point can provide insights into the nature of the atomic interactions.51 For example, ∇2ρb < 0 generally indicates covalent interactions. ∇2ρb > 0 and Hb > 0 indicate noncovalent interactions such as van der Waals and electrostatic interactions, while ∇2ρb > 0 and Hb < 0 indicate partially covalent interactions. In addition, −Gb/Vb < 0.5, 0.5 < −Gb/Vb < 1, and −Gb/Vb > 1 indicate covalent, partially covalent and noncovalent interactions, respectively.39,42,51
3 Results and discussion
3.1 MESP
The MESP isosurfaces of the drug molecules are given in Fig. 2. The visual inspection of the MESP surfaces indicates that electron-rich regions (e.g., blue regions) are present in the drug molecules. For example, the blue regions in the MESP maps of 5-fluorouracil are mainly located near the oxygen atoms and of cathinone are located near the nitrogen and oxygen atoms. The locations of the MESP Vmin of the drug molecules are given in Fig. S1.† The MESP Vmin of 5-fluorouracil resides near the oxygen atom (in the ortho position relative to the fluorine). The MESP Vmin of nitrosourea, pyrazinamide, and ciclopirox is observed near the oxygen atom of the carbonyl group. The MESP Vmin points of sulfanilamide are found near the two oxygen atoms. The MESP Vmin of ethionamide and 4-aminopyridine is observed near the pyridinic nitrogen atom. The MESP Vmin of 6-thioguanine and 6-mercaptopurine is found near the unsubstituted nitrogen atom of the pyrimidine ring. The MESP Vmin of isoniazid is observed near the nitrogen atom of the terminal amino group. The MESP Vmin points of metformin are found near the nitrogen atoms of the two imine groups. The MESP Vmin of cathinone resides near its nitrogen atom. Furthermore, the MESP Vmin values of the drug molecules (represented as Vmin-X) are given in Table 1. Here the Vmin-X values are in the range of −48.19 (5-fluorouracil) to −71.35 kcal mol−1 (cathinone). A higher negative MESP Vmin value indicates a more electron rich character of the drug molecule. The MESP Vmin values of benzene-containing drug molecules follow the order: sulfanilamide < cathinone. The MESP Vmin values of pyridine-containing drug molecules follow the order: ethionamide < ciclopirox < isoniazid < 4-aminopyridine. The MESP Vmin value of 5-fluorouracil is lower than that of pyrazinamide (−53.53 kcal mol−1). The MESP Vmin values of purine-containing drug molecules follow the order: 6-thioguanine < 6-mercaptopurine. The MESP Vmin value of nitrosourea (−52.71 kcal mol−1) is lower than that of metformin (−68.02 kcal mol−1).| Drug | Vmin-X |
|---|---|
| 5-Fluorouracil | −48.19 |
| Nitrosourea | −52.71 |
| Pyrazinamide | −53.53 |
| Sulfanilamide | −54.47 |
| Ethionamide | −58.30 |
| 6-Thioguanine | −59.80 |
| Ciclopirox | −60.12 |
| 6-Mercaptopurine | −61.12 |
| Isoniazid | −65.01 |
| Metformin | −68.02 |
| 4-Aminopyridine | −70.28 |
| Cathinone | −71.35 |
The B12N12 nanocage consists of six tetragonal and eight hexagonal rings39,52 (Fig. S2†). This nanocage has two distinct B–N bonds (two hexagonal rings shared the shorter B–N bond (1.44 Å), and a tetragonal ring and a hexagonal ring shared the longer B–N bond (1.48 Å)). A similar structure was found for the Al12N12 nanocage (see Fig. S2†). Here, the shorter Al–N bond length is 1.78 Å, and the longer one is 1.85 Å. The visual inspection of the MESP surfaces indicates that electron-rich regions (e.g., blue regions) are situated close to the nitrogen atoms of the B12N12 and Al12N12 nanocages (see Fig. S2†). The values of the MESP Vmin of the B12N12 and Al12N12 nanocages (denoted as Vmin-C) were calculated to be −20.77 and −49.07 kcal mol−1, respectively.39
3.2 Adsorption of drug molecules on the B12N12 nanocage
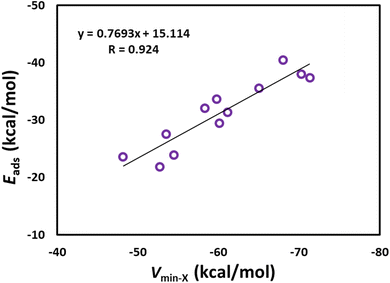
Fig. 3 shows the optimized structures of the drug molecules adsorbed on the B12N12 nanocage. We see that all drug molecules prefer to bind with the boron atom of the B12N12 nanocage. 5-Fluorouracil binds via the oxygen atom (at the para position relative to the fluorine) to the B12N12 nanocage. Nitrosourea and ciclopirox bind via the oxygen atom of the carbonyl group. Pyrazinamide binds via the nitrogen atom (far from the amide group) of the pyrazine ring. Sulfanilamide binds via the nitrogen atom of the amino group attached to the benzene ring. Ethionamide and 4-aminopyridine bind via the pyridinic nitrogen atom. 6-Thioguanine and 6-mercaptopurine bind via the unsubstituted nitrogen atom of the imidazole ring. Isoniazid binds via the nitrogen atom of the terminal amino group. Metformin binds via the nitrogen atom of the imine group. Cathinone binds via its nitrogen atom to the B12N12 nanocage. Furthermore, all the drug molecules are found to be chemisorbed on the B12N12 nanocage. For example, the adsorption distances are in the range of 1.50 (ciclopirox) to 1.65 Å (sulfanilamide). This observation is also supported by the Eads data (see Table 2) and other adsorption-induced structural changes (Table S2†). All these Eads values are negative, and they are in the range of −21.85 (nitrosourea) to −40.50 kcal mol−1 (metformin). A higher negative value of Eads generally indicates a stronger interaction between the drug molecule and the B12N12 nanocage. The Eads values of benzene-containing drug molecules follow the order: sulfanilamide < cathinone. The Eads values of pyridine-containing drug molecules follow the order: ciclopirox < ethionamide < isoniazid < 4-aminopyridine. The Eads value of 5-fluorouracil (−23.59 kcal mol−1) is lower than that of pyrazinamide (−27.53 kcal mol−1). The Eads values of purine-containing drug molecules follow the order: 6-mercaptopurine < 6-thioguanine. The Eads value of nitrosourea is about two times lower than that of metformin. A key finding is that these Eads values are well correlated with the MESP Vmin values of the drug molecules, with a correlation coefficient of 0.924 (Fig. 4). This result reflects the stronger interactions between the drug molecules and the B12N12 nanocage as the MESP Vmin values of the drug molecules become more negative. The angles of the hexagonal rings of the pristine B12N12 nanocage are about 125°. These angles at the adsorption sites decrease by about 9° due to the adsorption of drugs in all cases (see Table S2†).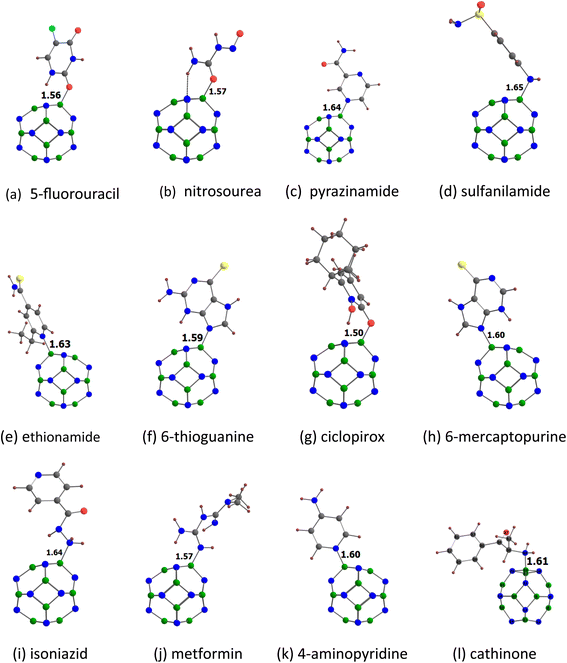 | ||
| Fig. 3 Optimized structures of drugs (a) 5-fluorouracil, (b) nitrosourea, (c) pyrazinamide, (d) sulfanilamide, (e) ethionamide, (f) 6-thioguanine, (g) ciclopirox, (h) 6-mercaptopurine, (i) isoniazid, (j) metformin, (k) 4-aminopyridine and (l) cathinone adsorbed on B12N12. The adsorption distances are given in Å. The color code is the same as in Fig. 2. In addition, B atom is denoted by green color. | ||
| Drug | Eads | ΔH | ΔS | ΔG | Vmin-C′ | ΔVmin-C |
|---|---|---|---|---|---|---|
| 5-Fluorouracil | −23.59 | −25.78 | −0.04 | −14.33 | −34.39 | −13.62 |
| Nitrosourea | −21.85 | −24.19 | −0.04 | −12.93 | −39.66 | −18.89 |
| Pyrazinamide | −27.53 | −28.65 | −0.04 | −17.61 | −41.29 | −20.52 |
| Sulfanilamide | −23.90 | −25.19 | −0.03 | −14.84 | −41.35 | −20.58 |
| Ethionamide | −32.09 | −33.08 | −0.04 | −21.27 | −36.58 | −15.81 |
| 6-Thioguanine | −33.64 | −35.35 | −0.04 | −23.85 | −47.44 | −26.67 |
| Ciclopirox | −29.44 | −33.61 | −0.04 | −21.58 | −45.87 | −25.10 |
| 6-Mercaptopurine | −31.35 | −32.90 | −0.04 | −21.41 | −48.19 | −27.42 |
| Isoniazid | −35.52 | −36.98 | −0.04 | −25.61 | −48.88 | −28.11 |
| Metformin | −40.50 | −42.77 | −0.04 | −31.47 | −49.39 | −28.62 |
| 4-Aminopyridine | −38.01 | −39.05 | −0.03 | −29.13 | −40.79 | −20.02 |
| Cathinone | −37.33 | −39.25 | −0.04 | −27.44 | −36.40 | −15.63 |
The enthalpy change (ΔH), the entropy change (ΔS), and the Gibbs free energy change (ΔG) (see eqn (3)–(5)) for the drug/B12N12 system are provided in Table 2. The negative values of ΔH in all systems indicate that the adsorption processes are exothermic in nature. The values of ΔH are in the range of −24.19 (nitrosourea/B12N12 system) to −42.77 kcal mol−1 (metformin/B12N12 system). The values of ΔS are negative (about −0.04 kcal mol−1 K−1 in all cases), indicating a decrease in entropy during the adsorption process. In all cases, the spontaneous nature of the adsorption processes may be deduced from the fact that the estimated values of ΔG are negative. The values of ΔG are in the range of −12.93 (nitrosourea/B12N12 system) to −31.47 kcal mol−1 (metformin/B12N12 system). Here the values of ΔG are less negative than those of ΔH due to the entropic effect.
The MESP isosurfaces of the drug-adsorbed B12N12 nanocage are shown in Fig. S3.† The visual inspection indicates major alterations in the MESP features of the isolated molecules due to the chemisorption process (see also Fig. 2 and S2†). For example, the blue region near the nitrogen atom of cathinone turns red in the presence of B12N12. The values of ΔVmin-C = Vmin-C′ − Vmin-C (Vmin-C′ is the MESP Vmin of the drug-adsorbed nanocage) are provided in Table 2. In all cases, the ΔVmin-C values are negative, implying that the B12N12 nanocage becomes electron-rich upon adsorption of the drug molecules. Here the values of ΔVmin-C are in the range of −13.62 (5-fluorouracil/B12N12 system) to −28.62 kcal mol−1 (metformin/B12N12 system).
The adsorption of the drug molecules onto the nanocage may have an impact on the DFT reactivity indices μ, η, s, and ω (see eqn (6)–(9)). The electrophilicity index ω incorporates the tendency of a system to accept additional electronic charge (described by μ2) and the resistance of a system to change its electronic configuration (described by η). Thus, a good electrophile can be identified by a high μ value and a low η value. The values of μ, η, s, and ω for the pristine B12N12 nanocage were −4.73 eV, 4.72 eV, 0.11 eV−1 and 2.37 eV, respectively.39 The change in the DFT reactivity indices, for instance, Δμ was estimated by taking the difference between the μ of the drug-adsorbed nanocage and the μ of the pristine nanocage. The values of Δμ, Δη, Δs, and Δω are given in Table 3. In all cases, we observe significant changes in μ, η, s, and ω due to the chemisorption process. For instance, the values of Δμ and Δη for the 5-fluorouracil/B12N12 system are 7.71 and −23.64% respectively.
| Drug | μ | Δμ | η | Δη | s | Δs | ω | Δω |
|---|---|---|---|---|---|---|---|---|
| 5-Fluorouracil | −5.09 | 7.71 | 3.60 | −23.64 | 0.14 | 26.12 | 3.60 | 51.93 |
| Nitrosourea | −5.21 | 10.15 | 3.31 | −29.90 | 0.15 | 37.37 | 4.10 | 73.08 |
| Pyrazinamide | −5.53 | 16.97 | 3.02 | −35.98 | 0.17 | 50.42 | 5.06 | 113.69 |
| Sulfanilamide | −4.84 | 2.30 | 3.85 | −18.40 | 0.13 | 18.02 | 3.04 | 28.26 |
| Ethionamide | −5.28 | 11.53 | 2.98 | −36.93 | 0.17 | 52.68 | 4.67 | 97.22 |
| 6-Thioguanine | −4.66 | −1.46 | 3.07 | −35.03 | 0.16 | 48.21 | 3.54 | 49.43 |
| Ciclopirox | −4.81 | 1.64 | 3.50 | −25.92 | 0.14 | 30.00 | 3.31 | 39.46 |
| 6-Mercaptopurine | −5.01 | 6.01 | 3.00 | −36.35 | 0.17 | 51.30 | 4.18 | 76.57 |
| Isoniazid | −5.22 | 10.35 | 3.66 | −22.49 | 0.14 | 24.25 | 3.72 | 57.12 |
| Metformin | −4.18 | −11.56 | 4.07 | −13.67 | 0.12 | 11.56 | 2.15 | −9.39 |
| 4-Aminopyridine | −4.48 | −5.20 | 3.68 | −21.94 | 0.14 | 23.38 | 2.73 | 15.13 |
| Cathinone | −4.90 | 3.68 | 3.60 | −23.70 | 0.14 | 26.22 | 3.34 | 40.89 |
The results from the QTAIM analyses of the drug-adsorbed B12N12 nanocage are given in Fig. S4† and Table 4. For all systems, the values of ρb are in the range of 0.108 (nitrosourea/B12N12 system) to 0.135 au (metformin/B12N12 system) and the values of ∇2ρb are positive (see Table 4). It can be seen that all the values of Hb are negative and 0.5 < −Gb/Vb < 1. These results imply the presence of partial covalent interactions between the drug molecules and the B12N12 nanocage.
| Drug | ρb | ∇2ρb | Hb | Gb | Vb | −Gb/Vb |
|---|---|---|---|---|---|---|
| 5-Fluorouracil | 0.111 | 0.496 | −0.060 | 0.185 | −0.245 | 0.753 |
| Nitrosourea | 0.108 | 0.489 | −0.059 | 0.181 | −0.240 | 0.755 |
| Pyrazinamide | 0.115 | 0.306 | −0.079 | 0.156 | −0.235 | 0.663 |
| Sulfanilamide | 0.114 | 0.277 | −0.080 | 0.149 | −0.229 | 0.651 |
| Ethionamide | 0.119 | 0.289 | −0.085 | 0.157 | −0.242 | 0.649 |
| 6-Thioguanine | 0.125 | 0.364 | −0.088 | 0.179 | −0.268 | 0.670 |
| Ciclopirox | 0.133 | 0.579 | −0.082 | 0.227 | −0.309 | 0.734 |
| 6-Mercaptopurine | 0.123 | 0.364 | −0.086 | 0.177 | −0.262 | 0.674 |
| Isoniazid | 0.116 | 0.301 | −0.081 | 0.156 | −0.237 | 0.659 |
| Metformin | 0.135 | 0.353 | −0.100 | 0.188 | −0.288 | 0.654 |
| 4-Aminopyridine | 0.127 | 0.317 | −0.092 | 0.171 | −0.264 | 0.650 |
| Cathinone | 0.126 | 0.307 | −0.092 | 0.169 | −0.260 | 0.647 |
3.3 Adsorption of drug molecules on the Al12N12 nanocage
Fig. 5 shows the optimized structures of the drug molecules adsorbed on the Al12N12 nanocage. In general, the drug molecules prefer to bind with the aluminium atoms of the Al12N12 nanocage. 5-Fluorouracil binds, for example, via the oxygen atom (at the para position relative to the fluorine) to the Al12N12 nanocage. Nitrosourea and ciclopirox bind, for example, via the oxygen atom of the carbonyl group. Pyrazinamide and sulfanilamide bind via the oxygen atom. Ethionamide and 4-aminopyridine bind via the pyridinic nitrogen atom. 6-Thioguanine and 6-mercaptopurine bind, for example, via the nitrogen atom of the imidazole ring. Isoniazid binds via the nitrogen atom of the terminal amino group. Metformin binds via the nitrogen atom of the imine group. Cathinone binds via its nitrogen atom to the Al12N12 nanocage. However, a hydrogen atom is transferred from each of 5-fluorouracil, nitrosourea, 6-thioguanine, ciclopirox, and 6-mercaptopurine to the nitrogen atom of the Al12N12 nanocage. Similar transfer of the hydrogen atom from the drug molecules to the nitrogen atom of the nanocage has been reported previously.34,53–61 Furthermore, all the drug molecules are found to be chemisorbed on the A12N12 nanocage. For example, the adsorption distances are in the range of 1.85 (ciclopirox) to 2.03 Å (isoniazid). This observation is also supported by the Eads data (see Table 5) and other adsorption-induced structural changes (Table S3†). All these Eads values are negative, and they are in the range of −39.31 (ethionamide) to −84.81 kcal mol−1 (ciclopirox). A higher negative value of Eads generally indicates a stronger interaction between the drug molecule and the Al12N12 nanocage. The transfer of the hydrogen atom from 5-fluorouracil, nitrosourea, 6-thioguanine, ciclopirox, and 6-mercaptopurine to the nitrogen atom of the A12N12 nanocage leads to relatively high Eads values. The Eads values of benzene-containing drug molecules follow the order: sulfanilamide < cathinone. The Eads values of pyridine-containing drug molecules follow the order: ethionamide < 4-aminopyridine < isoniazid < ciclopirox. The Eads value of 5-fluorouracil (−69.62 kcal mol−1) is higher than that of pyrazinamide (−42.27 kcal mol−1). The Eads values of purine-containing drug molecules 6-mercaptopurine and 6-thioguanine are close to each other. The Eads value of nitrosourea (−73.16 kcal mol−1) is higher than that of metformin (−50.71 kcal mol−1). These Eads values are not correlated with the MESP Vmin values of the drug molecules (Fig. S5†). The angles of the hexagonal rings of the pristine Al12N12 nanocage are about 125°. These angles at the adsorption sites decrease by at least 4° due to the adsorption of drugs (see Table S3†).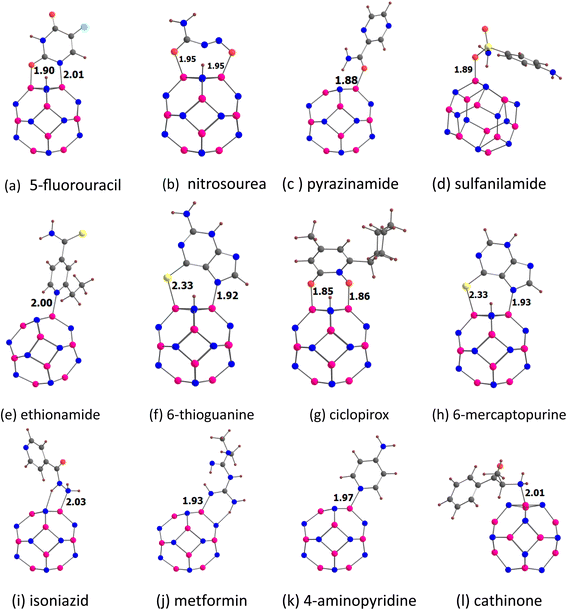 | ||
| Fig. 5 Optimized structures of drugs (a) 5-fluorouracil, (b) nitrosourea, (c) pyrazinamide, (d) sulfanilamide, (e) ethionamide, (f) 6-thioguanine, (g) ciclopirox, (h) 6-mercaptopurine, (i) isoniazid, (j) metformin, (k) 4-aminopyridine and (l) cathinone adsorbed on Al12N12. The adsorption distances are given in Å. The color code is the same as in Fig. 2. In addition, Al atom is denoted by purple color. | ||
| Drug | Eads | ΔH | ΔG | ΔS | Vmin-C′ | ΔVmin-C |
|---|---|---|---|---|---|---|
| 5-Fluorouracil | −69.62 | −75.76 | −63.18 | −0.04 | −58.23 | −9.16 |
| Nitrosourea | −73.16 | −81.63 | −68.62 | −0.04 | −64.51 | −15.44 |
| Pyrazinamide | −42.27 | −45.63 | −34.38 | −0.04 | −66.39 | −17.32 |
| Sulfanilamide | −40.38 | −46.48 | −33.20 | −0.04 | −66.20 | −17.13 |
| Ethionamide | −39.31 | −40.79 | −29.64 | −0.04 | −62.00 | −12.93 |
| 6-Thioguanine | −82.46 | −88.02 | −75.78 | −0.04 | −66.83 | −17.76 |
| Ciclopirox | −84.81 | −91.39 | −78.76 | −0.04 | −64.19 | −15.12 |
| 6-Mercaptopurine | −82.31 | −87.77 | −75.45 | −0.04 | −63.19 | −14.12 |
| Isoniazid | −44.08 | −46.41 | −35.95 | −0.04 | −57.04 | −7.97 |
| Metformin | −50.71 | −54.12 | −43.22 | −0.04 | −67.83 | −18.76 |
| 4-Aminopyridine | −42.69 | −44.03 | −34.48 | −0.03 | −69.09 | −20.02 |
| Cathinone | −42.98 | −46.46 | −34.72 | −0.04 | −64.95 | −15.88 |
The ΔH, ΔS, and ΔG (see eqn (3)–(5)) for the drug/Al12N12 system are provided in Table 5. The negative values of ΔH in all systems indicate that the adsorption processes are exothermic in nature. The values of ΔH are in the range of −40.79 (ethionamide/Al12N12 system) to −91.39 kcal mol−1 (ciclopirox/Al12N12 system). The values of ΔS are negative (about −0.04 kcal mol−1 K−1 in all cases), indicating a decrease in entropy during the adsorption process. In all cases, the spontaneous nature of the adsorption processes may be deduced from the fact that the estimated values of ΔG are negative. The values of ΔG are in the range of −29.64 (ethionamide/Al12N12 system) to −78.76 kcal mol−1 (ciclopirox/Al12N12 system). Here also the values of ΔG are less negative than those of ΔH due to the entropic effect.
The MESP isosurfaces of the drug-adsorbed Al12N12 nanocage are shown in Fig. S6.† The visual inspection indicates major alterations in the MESP features of the isolated molecules due to the chemisorption process (see also Fig. 2 and S2†). For example, the blue region near the nitrogen atom of the Al12N12 nanocage turns red due to the transfer of the hydrogen atom from 5-fluorouracil. In all cases, the ΔVmin-C values are negative, implying that the Al12N12 nanocage becomes electron-rich upon adsorption of the drug molecules (see Table 5). The values of ΔVmin-C are in the range of −7.97 (isoniazid/Al12N12 system) to −20.02 kcal mol−1 (4-aminopyridine/Al12N12 system).
The values of μ, η, s, and ω for the pristine Al12N12 nanocage were −4.86 eV, 3.16 eV, 0.16 eV−1 and 3.74 eV, respectively.39 The values of Δμ, Δη, Δs, and Δω of the drug/Al12N12 nanocage system are given in Table 6. In all cases, we observe significant changes in μ, η, s, and ω due to the chemisorption process. For instance, the values of Δμ and Δη for the 5-fluorouracil/Al12N12 system are −4.63 and −3.16% respectively. Overall, the changes in all these reactivity indices of the drug/Al12N12 system are lower when compared to the drug/B12N12 system (see Table 3).
| Drug | μ | Δμ (%) | η | Δη (%) | s | Δs (%) | ω | Δω (%) |
|---|---|---|---|---|---|---|---|---|
| 5-Fluorouracil | −4.63 | −4.63 | 3.06 | −3.16 | 0.16 | 2.12 | 3.51 | −6.15 |
| Nitrosourea | −4.49 | −7.71 | 2.94 | −7.04 | 0.17 | 6.38 | 3.42 | −8.44 |
| Pyrazinamide | −4.67 | −3.85 | 2.77 | −12.37 | 0.18 | 12.86 | 3.94 | 5.43 |
| Sulfanilamide | −4.47 | −8.02 | 3.05 | −3.64 | 0.16 | 2.63 | 3.28 | −12.26 |
| Ethionamide | −4.78 | −1.55 | 2.71 | −14.35 | 0.18 | 15.47 | 4.23 | 13.08 |
| 6-Thioguanine | −4.34 | −10.70 | 2.99 | −5.53 | 0.17 | 4.68 | 3.15 | −15.66 |
| Ciclopirox | −4.26 | −12.27 | 3.05 | −3.47 | 0.16 | 2.44 | 2.98 | −20.33 |
| 6-Mercaptopurine | −4.58 | −5.72 | 2.86 | −9.49 | 0.17 | 9.26 | 3.67 | −1.87 |
| Isoniazid | −4.68 | −3.76 | 3.15 | −0.28 | 0.16 | −0.83 | 3.47 | −7.19 |
| Metformin | −4.22 | −13.17 | 3.05 | −3.38 | 0.16 | 2.35 | 2.92 | −22.02 |
| 4-Aminopyridine | −4.16 | −14.48 | 3.10 | −1.87 | 0.16 | 0.78 | 2.79 | −25.53 |
| Cathinone | −4.38 | −9.98 | 3.05 | −3.64 | 0.16 | 2.63 | 3.14 | −15.96 |
The results from the QTAIM analyses of the drug-adsorbed Al12N12 nanocage are given in Fig. S7† and Table 7. For all systems, the values of ρb are in the range of 0.052 (6-mercaptopurine/Al12N12 system) to 0.068 au (6-thioguanine/Al12N12 system) and the values of ∇2ρb are positive (see Table 7). Overall, the values of Hb are negative and 0.5 < −Gb/Vb < 1, suggesting the presence of partial covalent interactions between the drug molecules and the Al12N12 nanocage. However, the values of Hb are positive and −Gb/Vb > 1 for the 5-fluorouracil/Al12N12 (Al–O bond), nitrosourea/Al12N12, pyrazinamide/Al12N12, sulfanilamide/Al12N12, and ciclopirox/Al12N12 systems. These results imply the presence of noncovalent interactions between these drug molecules and the Al12N12 nanocage.
| Drug | ρb | ∇2ρb | Hb | Gb | Vb | −Gb/Vb |
|---|---|---|---|---|---|---|
| 5-Fluorouracil (Al–O) | 0.061 | 0.419 | 0.004 | 0.101 | −0.097 | 1.042 |
| 5-Fluorouracil (Al–N) | 0.057 | 0.310 | −0.002 | 0.080 | −0.082 | 0.970 |
| Nitrosourea (Al–OC) | 0.054 | 0.347 | 0.003 | 0.083 | −0.080 | 1.044 |
| Nitrosourea (Al–ON) | 0.056 | 0.338 | 0.001 | 0.083 | −0.082 | 1.016 |
| Pyrazinamide | 0.062 | 0.457 | 0.007 | 0.108 | −0.101 | 1.067 |
| Sulfanilamide | 0.058 | 0.424 | 0.007 | 0.099 | −0.093 | 1.071 |
| Ethionamide | 0.057 | 0.321 | −0.001 | 0.082 | −0.083 | 0.985 |
| 6-Thioguanine (Al–S) | 0.053 | 0.159 | −0.013 | 0.053 | −0.066 | 0.801 |
| 6-Thioguanine (Al–N) | 0.068 | 0.411 | −0.002 | 0.105 | −0.107 | 0.980 |
| Ciclopirox (Al–ON) | 0.067 | 0.483 | 0.004 | 0.117 | −0.113 | 1.037 |
| Ciclopirox (Al–OC) | 0.066 | 0.490 | 0.005 | 0.117 | −0.112 | 1.048 |
| 6-Mercaptopurine (Al–S) | 0.052 | 0.157 | −0.013 | 0.052 | −0.065 | 0.802 |
| 6-Mercaptopurine (Al–N) | 0.067 | 0.405 | −0.002 | 0.103 | −0.105 | 0.982 |
| Isoniazid | 0.055 | 0.303 | −0.001 | 0.077 | −0.078 | 0.987 |
| Metformin | 0.066 | 0.400 | −0.002 | 0.102 | −0.103 | 0.984 |
| 4-Aminopyridine | 0.061 | 0.361 | −0.001 | 0.091 | −0.093 | 0.986 |
| Cathinone | 0.057 | 0.318 | −0.001 | 0.081 | −0.082 | 0.983 |
3.4 Recovery time
A shorter recovery time (τ) is often required for the reusability of a sensor material (e.g. B12N12).62,63 The recovery time was calculated using the following equation:
τ = ϑ−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Eads/(kBT)) exp(−Eads/(kBT))
| (10) |
3.5 Solvent effects
We investigated the effect of water, the most important biological solvent, on the interaction between the drug molecules and the nanocages. The M062X/6-311G(d,p) level energetics was corrected for solvation effects using the self-consistent reaction field method SMD.64 The solvent-corrected Gibbs free energy (ΔGW) was calculated by adding the solvent-phase single-point energy with the gas-phase Gibbs free energy correction. The ΔGW values for the drug/B12N12 complex are provided in Table 8. The values of ΔGW are in the range of −23.80 (5-fluorouracil/B12N12 complex) to −43.84 kcal mol−1 (metformin/B12N12 complex). A more negative ΔGW indicates that the adsorption is more exergonic for the metformin/B12N12 complex in water.| Drug | ΔGW | |
|---|---|---|
| Drug/B12N12 | Drug/Al12N12 | |
| 5-Fluorouracil | −23.80 | −55.20 |
| Nitrosourea | −24.64 | −62.98 |
| Pyrazinamide | −29.34 | −30.11 |
| Sulfanilamide | −28.75 | −25.77 |
| Ethionamide | −31.93 | −26.56 |
| 6-Thioguanine | −31.03 | −67.86 |
| Ciclopirox | −33.54 | −73.93 |
| 6-Mercaptopurine | −29.14 | −66.94 |
| Isoniazid | −33.77 | −28.76 |
| Metformin | −43.84 | −40.72 |
| 4-Aminopyridine | −41.28 | −33.10 |
| Cathinone | −39.91 | −33.51 |
The ΔGW for the drug/Al12N12 complex is also provided in Table 8. Here the values of ΔGW are in the range of −25.77 (sulfanilamide/Al12N12 complex) to −73.93 kcal mol−1 (ciclopirox/Al12N12 complex). A more negative ΔGW indicates that the adsorption is more spontaneous for the ciclopirox/Al12N12 complex in water. This is possibly due to the presence of the –OH group in ciclopirox.
The MESP is a real physical property which can be obtained by computational method or experimentally by X-ray diffraction technique.41 The MESP Vmin value would qualify as a good parameter for quantifying the strength of, for example, a lone pair.41 Typically, the electron-rich lone-pair regions of the drug molecules interact with the electron-deficient boron or aluminium atoms of the B12N12 and Al12N12 nanocages. We observed a linear correlation between the Eads values of the drug-adsorbed B12N12 nanocage and the MESP Vmin values of the drugs. This enables one to predict the adsorption energy once the MESP features of the drug molecules are known. Similar correlations were found for the lone pair-π interactions.41 However, the Eads values of the drug-adsorbed Al12N12 nanocage were not correlated with the MESP Vmin values of the drug molecules. Also, a hydrogen atom was transferred from each of 5-fluorouracil, nitrosourea, 6-thioguanine, ciclopirox, and 6-mercaptopurine to the nitrogen atom of the Al12N12 nanocage. These results may be attributed to the fact that the Al12N12 nanocage is more electron-rich compared to the B12N12 nanocage (MESP Vmin of B12N12 and Al12N12 nanocages are −20.77 and −49.07 kcal mol−1, respectively). More negative electrostatic potentials at nuclei (EPN) values indicate greater electron densities in a molecular region.65 For the B12N12 nanocage, we estimated the EPN values at B and N to be −11.37 and −18.39 au, respectively. For the Al12N12 nanocage, the EPN values at Al and N are −44.55 and −18.45 au, respectively. Also, the Al12N12 nanocage displayed a relatively high surface area.42 The B–N bond lengths in the B12N12 nanocage were in the range of 1.44 to 1.48 Å and the Al–N bond lengths in the Al12N12 nanocage were in the range of 1.78 to 1.85 Å.42
All the drug molecules investigated in this study were found to be chemisorbed on the B12N12 and Al12N12 nanocages. In contrast, for example, the adsorption of 5-fluorouracil on AlN-nanotube23 and nitrosourea on BN-nanosheet19 were physisorption in nature. Structural defects in the nanocages or changes in the surface chemical environment66,67 may also affect the adsorption properties of drugs. We will study these effects in a future publication.
4 Conclusions
DFT studies were conducted to understand the adsorption mechanism of twelve drug molecules (5-fluorouracil, nitrosourea, pyrazinamide, sulfanilamide, ethionamide, 6-thioguanine, ciclopirox, 6-mercaptopurine, isoniazid, metformin, 4-aminopyridine, and cathinone) on the B12N12 and Al12N12 nanocages. In general, the drug molecules prefer to bind with the boron atom of the B12N12 nanocage and the aluminium atoms of the Al12N12 nanocage. However, a hydrogen atom is transferred from each of 5-fluorouracil, nitrosourea, 6-thioguanine, ciclopirox, and 6-mercaptopurine to the nitrogen atom of the Al12N12 nanocage. All the drug molecules were found to be chemisorbed on the B12N12 and Al12B12 nanocages. The adsorption distances are in the range of 1.50 (ciclopirox/B12N12 system) to 2.03 Å (isoniazid/Al12N12 system). All the Eads values were negative, indicating the exothermic nature of the adsorption process. The Eads values are in the range of −21.85 (nitrosourea/B12N12 system) to −84.81 kcal mol−1 (ciclopirox/Al12N12 system). A key finding is that the Eads values of the drug/Bl12N12 system are linearly correlated with the MESP Vmin values of the drug molecules. The transfer of the hydrogen atom from the drug molecules to the nitrogen atom of the A12N12 nanocage leads to relatively high Eads values.In all cases, the ΔVmin-C values are negative, implying that the B12N12 and Al12N12 nanocages become electron-rich upon adsorption of the drug molecules. We found significant changes in the reactivity parameters such as μ and η of the nanocages due to the chemisorption process. In general, the QTAIM results indicate the presence of partial covalent interactions between the drug molecules and the nanocages. However, the QTAIM results indicate the presence of noncovalent interactions for the 5-fluorouracil/Al12N12, nitrosourea/Al12N12, pyrazinamide/Al12N12, sulfanilamide/Al12N12, and ciclopirox/Al12N12 systems. We also investigated the effect of water on the interaction between the drug molecules and the nanocages. A more negative ΔGW indicates that the adsorption is more exergonic for the ciclopirox/Al12N12 complex in water.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is based upon work supported by the King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) under Award No. ORFS-2022-CRG11-5028. R. G. S. N. and A. K. N. N. would like to thank KAUST for providing computational resources of the Shaheen II supercomputer.References
- F. Casale, R. Canaparo, L. Serpe, E. Muntoni, C. D. Pepa, M. Costa, L. Mairone, G. P. Zara, G. Fornari and M. Eandi, Pharmacol. Res., 2004, 50, 173–179 CrossRef CAS PubMed.
- R. B. Weiss and B. F. Issell, Cancer Treat. Rev., 1982, 9, 313–330 CrossRef CAS PubMed.
- W. Shi, X. Zhang, X. Jiang, H. Yuan, J. S. Lee, C. E. Barry 3rd, H. Wang, W. Zhang and Y. Zhang, Science, 2011, 333, 1630–1632 CrossRef CAS PubMed.
- K. Johnsson, D. S. King and P. G. Schultz, J. Am. Chem. Soc., 1995, 117, 5009–5010 CrossRef CAS.
- L. duBouchet, M. R. Spence, M. F. Rein, M. R. Danzig and W. M. McCormack, Sex. Transm. Dis., 1997, 24, 156–160 CrossRef CAS PubMed.
- A. Vora, C. D. Mitchell, L. Lennard, T. O. Eden, S. E. Kinsey, J. Lilleyman and S. M. Richards, Lancet, 2006, 368, 1339–1348 CrossRef CAS PubMed.
- A. K. Gupta and T. Plott, Int. J. Dermatol., 2004, 43, 3–8 CrossRef CAS PubMed.
- C. J. Bailey and R. C. Turner, N. Engl. J. Med., 1996, 334, 574–579 CrossRef CAS.
- F. A. Davis, D. Stefoski and J. Rush, Ann. Neurol., 1990, 27, 186–192 CrossRef CAS.
- J. P. Kelly, Drug Test. Anal., 2011, 3, 439–453 CrossRef CAS PubMed.
- R. Bakry, R. M. Vallant, M. Najam-ul-Haq, M. Rainer, Z. Szabo, C. W. Huck and G. K. Bonn, Int. J. Nanomed., 2007, 2, 639–649 CAS.
- J. Chen, S. Chen, X. Zhao, L. V. Kuznetsova, S. S. Wong and I. Ojima, J. Am. Chem. Soc., 2008, 130, 16778–16785 CrossRef CAS PubMed.
- C. L. Weaver, J. M. LaRosa, X. Luo and X. T. Cui, ACS Nano, 2014, 8, 1834–1843 CrossRef CAS PubMed.
- N. Panwar, A. M. Soehartono, K. K. Chan, S. Zeng, G. Xu, J. Qu, P. Coquet, K.-T. Yong and X. Chen, Chem. Rev., 2019, 119, 9559–9656 CrossRef CAS.
- N. Saikia and R. C. Deka, J. Mol. Model., 2013, 19, 215–226 CrossRef CAS.
- M. S. Hoseininezhad-Namin, P. Pargolghasemi, S. Alimohammadi, A. S. Rad and L. Taqavi, Phys. E, 2017, 90, 204–213 CrossRef CAS.
- F. Safdari, H. Raissi, M. Shahabi and M. Zaboli, J. Inorg. Organomet. Polym. Mater., 2017, 27, 805–817 CrossRef CAS.
- M. Vatanparast and Z. Shariatinia, J. Mol. Graphics Modell., 2019, 89, 50–59 CrossRef CAS PubMed.
- S. N. Ema, M. A. Khaleque, A. Ghosh, A. A. Piya, U. Habiba and S. U. D. Shamim, RSC Adv., 2021, 11, 36866–36883 RSC.
- M. D. Mohammadi, H. Y. Abdullah, V. Kalamse and A. Chaudhari, Comput. Theor. Chem., 2022, 1212, 113699 CrossRef.
- C. Rungnim, R. Chanajaree, T. Rungrotmongkol, S. Hannongbua, N. Kungwan, P. Wolschann, A. Karpfen and V. Parasuk, J. Mol. Model., 2016, 22, 85 CrossRef.
- M. Yahyavi, F. Badalkhani-Khamseh and N. L. Hadipour, Chem. Phys. Lett., 2020, 750, 137492 CrossRef.
- S. A. S. Al-Zuhairy, M. M. Kadhim, M. Hatem Shadhar, N. A. Jaber, H. Abdulkareem Almashhadani, A. Mahdi Rheima, M. N. Mousa and Y. Cao, Inorg. Chem. Commun., 2022, 142, 109617 CrossRef.
- M. K. Hazrati, Z. Javanshir and Z. Bagheri, J. Mol. Graphics Modell., 2017, 77, 17–24 CrossRef PubMed.
- M. Li, Y. Wei, G. Zhang, F. Wang, M. Li and H. Soleymanabadi, Phys. E, 2020, 118, 113878 CrossRef.
- M. D. Esrafili and A. A. Khan, RSC Adv., 2022, 12, 3948–3956 RSC.
- R. Padash, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, M. Mirmotahari, H. Ehrlich, A. S. Rad and M. Peyravi, Appl. Phys. A, 2018, 124, 582 CrossRef.
- S. A. Aslanzadeh, Mol. Phys., 2019, 117, 531–538 CrossRef CAS.
- A. S. Ghasemi, M. R. Taghartapeh, A. Soltani and P. J. Mahon, J. Mol. Liq., 2019, 275, 955–967 CrossRef CAS.
- M. Noormohammadbeigi, S. Kamalinahad, F. Izadi, M. Adimi and A. Ghasemkhani, Mater. Res. Express, 2019, 6, 1250g1252 Search PubMed.
- F. Azarakhshi, S. Shahab, S. Kaviani and M. Sheikhi, Lett. Org. Chem., 2021, 18, 640–655 CrossRef CAS.
- S. Kaviani, S. Shahab, M. Sheikhi, V. Potkin and H. Zhou, Comput. Theor. Chem., 2021, 1201, 113246 CrossRef CAS.
- H. R. A. El-Mageed, F. M. Mustafa and M. K. Abdel-Latif, J. Biomol. Struct. Dyn., 2022, 40, 226–235 CrossRef CAS.
- M. J. Saadh, T. E. Sánchez Herrera, A. Mohammed Dhiaa, O. Villacrés Cáceres, L. M. Flores Fiallos, B. S. Rojas Oviedo, A. A. Omran, M. N. Hawas and A. Elawady, Mol. Phys., 2024, e2314132 CrossRef.
- T. Oku, A. Nishiwaki and I. Narita, Sci. Technol. Adv. Mater., 2004, 5, 635–638 CrossRef CAS.
- C. Balasubramanian, S. Bellucci, P. Castrucci, M. De Crescenzi and S. V. Bhoraskar, Chem. Phys. Lett., 2004, 383, 188–191 CrossRef CAS.
- C. Liu, Z. Hu, Q. Wu, X. Wang, Y. Chen, H. Sang, J. Zhu, S. Deng and N. Xu, J. Am. Chem. Soc., 2005, 127, 1318–1322 CrossRef CAS PubMed.
- H.-S. Wu, F.-Q. Zhang, X.-H. Xu, C.-J. Zhang and H. Jiao, J. Phys. Chem. A, 2003, 107, 204–209 CrossRef CAS.
- R. Geetha Sadasivan Nair, A. K. Narayanan Nair and S. Sun, Energy Fuels, 2023, 37, 14053–14063 CrossRef CAS.
- R. Geetha Sadasivan Nair, A. K. Narayanan Nair and S. Sun, J. Mol. Liq., 2023, 389, 122923 CrossRef CAS.
- C. H. Suresh, G. S. Remya and P. K. Anjalikrishna, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1601 CAS.
- R. Geetha Sadasivan Nair, A. K. Narayanan Nair and S. Sun, New J. Chem., 2024, 48, 8093–8105 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone and G. A. Petersson, et al., Gaussian 16. Rev. B.01, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- C. H. Suresh, G. S. Remya and P. K. Anjalikrishna, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1601 Search PubMed.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Acc. Chem. Res., 2008, 41, 157–167 CrossRef CAS.
- H. Chermette, J. Comput. Chem., 1999, 20, 129–154 CrossRef.
- R. F. Bader, Chem. Rev., 1991, 91, 893–928 Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef.
- M. Ziółkowski, S. J. Grabowski and J. Leszczynski, J. Phys. Chem. A, 2006, 110, 6514–6521 CrossRef.
- A. L. Pereira Silva and J. d. J. G. Varela Júnior, Inorg. Chem., 2023, 62, 1926–1934 CrossRef.
- E. Vessally, M. D. Esrafili, R. Nurazar, P. Nematollahi and A. Bekhradnia, Struct. Chem., 2017, 28, 735–748 CrossRef.
- N. Wazzan, K. A. Soliman and W. S. A. Halim, J. Mol. Model., 2019, 25, 265 CrossRef.
- H. Zhu, C. Zhao, Q. Cai, X. Fu and F. R. Sheykhahmad, Inorg. Chem. Commun., 2020, 114, 107808 CrossRef.
- M. Sheikhi, Y. Ahmadi, S. Kaviani and S. Shahab, Struct. Chem., 2021, 32, 1181–1196 CrossRef.
- M. Aghaei, M. Ramezanitaghartapeh, M. Javan, M. S. Hoseininezhad-Namin, H. Mirzaei, A. S. Rad, A. Soltani, S. Sedighi, A. N. K. Lup and V. Khori, Spectrochim. Acta, Part A, 2021, 246, 119023 CrossRef PubMed.
- J. S. Al-Otaibi, Y. S. Mary and Y. S. Mary, J. Mol. Model., 2022, 28, 98 CrossRef PubMed.
- L. Hitler, J. F. Eze, A. D. Nwagu, H. O. Edet, T. O. Unimuke, E. A. Eno, V. N. Osabor and A. S. Adeyinka, ChemistrySelect, 2023, 8, e202203607 CrossRef.
- A. A. Pisu, F. Siddi, G. Cappellini and R. Cardia, RSC Adv., 2023, 13, 22481–22492 RSC.
- A.-s. S. Rady, N. A. Moussa, L. A. Mohamed, P. A. Sidhom, S. R. Sayed, M. K. Abd El-Rahman, E. Dabbish, T. Shoeib and M. A. Ibrahim, Heliyon, 2023, 9, e18690 CrossRef PubMed.
- H. R. A. El-Mageed and M. A. A. Ibrahim, J. Mol. Liq., 2021, 326, 115297 CrossRef.
- H. R. Abd El-Mageed and H. S. Abbas, J. Biomol. Struct. Dyn., 2022, 40, 9464–9483 CrossRef.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef.
- B. Galabov, S. Ilieva, G. Koleva, W. D. Allen, H. F. Schaefer III and P. v. R. Schleyer, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2013, 3, 37–55 Search PubMed.
- K. Chukwuemeka, H. Louis, I. Benjamin, P. A. Nyong, E. U. Ejiofor, E. A. Eno and A.-L. E. Manicum, ACS Appl. Bio Mater., 2023, 6, 1146–1160 CrossRef.
- S. Wu, L. Li, Q. Liang, H. Gao, D. Hu, T. Tang and Y. Tang, New J. Chem., 2023, 47, 11478–11491 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional details of DFT analysis are provided. See DOI: https://doi.org/10.1039/d4ra05586a |
| This journal is © The Royal Society of Chemistry 2024 |