Open Access Article
Open Access ArticleCombined application of resveratrol and a ryegrass endophyte in PAH-contaminated soil remediation and its impact on soil microbial communities†
Jiawei Zhaoa,
Li Lu *a,
Qiwei Chaiab,
Wei Jina,
Min Zhua,
Shengqi Qia,
Jiali Shentua,
Yuyang Longa and
Dongsheng Shena
*a,
Qiwei Chaiab,
Wei Jina,
Min Zhua,
Shengqi Qia,
Jiali Shentua,
Yuyang Longa and
Dongsheng Shena
aZhejiang Provincial Key Laboratory of Solid Waste Treatment and Recycling, Zhejiang Engineering Research Center of Non-ferrous Metal Waste Recycling, School of Environmental Science and Engineering, Zhejiang Gongshang University, Hangzhou, 310012, China. E-mail: LL0106@zjgsu.edu.cn
bZhoushan Municipal Ecology and Environment Bureau, Zhoushan, China
First published on 8th October 2024
Abstract
The unique capacity of certain plant endophytes to degrade organic pollutants has garnered considerable interest in recent years. However, it remains uncertain whether endophytes can maintain high degradation activity after in vitro culture and whether they can be used directly in the remediation of contaminated soils. This study reveals that resveratrol, a plant secondary metabolite, selectively boosts the degradation of polycyclic aromatic hydrocarbons (PAHs) by endophytic Methylobacterium extorquens C1 (C1) in vitro, while exerting negligible effects on the activity of indigenous soil bacteria. For the first time, a combined application of C1 and resveratrol was employed in the remediation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil. The findings indicate that the sole use of resveratrol failed to promote the removal of PAHs by indigenous soil microorganisms, whereas sole application of C1 boosted Methylobacterium-related PAH-degrading bacterial abundance, enhancing PAH removal, yet concurrently reduced overall soil microbial diversity. The combination of resveratrol and C1 not only stimulated the PAH removal but also mitigated the impact of C1 on the soil microbial community structure when C1 was applied individually. Specifically, the optimal removal efficacy was achieved with a treatment combination of 5 mg kg−1 resveratrol and 1.2 × 107 CFU kg−1 of C1, leading to a 130% and 231% increase in the removal of phenanthrene and acenaphthene, respectively, over a 15 days period. This study proposes a novel approach for the bioremediation of organic-contaminated soil by using the specific biological response of plant endophytic bacteria to secondary metabolites.
1 Introduction
Microbial phytoremediation is an eco-friendly and cost-effective approach for addressing organic soil contamination.1–3 This method harnesses the combined strengths of phytoremediation, which involves the use of plants to remove pollutants, and microbial remediation, where microorganisms breakdown contaminants. The synergy between plants and microorganisms is central to this process, particularly in the degradation of complex pollutants such as polycyclic aromatic hydrocarbons (PAHs). Endophytes are microbes that colonize plant tissues, play critical roles in the microbial phytoremediation of contaminated soils and have drawn increasing attention.4Different types of endophytes capable of degrading organic pollutants, such as Enterobacter, Paenibacillus, and Pseudomonas, have been screened.5–14 Endophytes screened from plants often show better degradation characteristics than local soil microorganisms do under specific conditions (e.g., plant apoplasts),4 and current studies have focused mostly on the inoculation of isolated endophytes into specific remediation plants to obtain better phytoremediation performance.7–11 In addition, endophyte-colonized plants presented increased growth and stress resistance. For example, Khan et al.7 reported that inoculation of endophytic Pseudomonas putida PD1 in plants not only promoted plant growth but also increased phenanthrene removal in the soil–plant system by 25–40%. Baoune et al.10 inoculated maize with Streptomyces sp. and reported that the biomass, root length, and stem length of plants, as well as petroleum hydrocarbon removal, were significantly greater than those of noninoculated plants.
The efficiency of combined plant-endophyte remediation technology often becomes a bottleneck restricting its application because of the uncertainty of the colonization rate of endophytes in remediated plants; the long growth cycle of plants; and their susceptibility to soil, climate, and other conditions. If an endophyte with high degradation activity can be easily and efficiently cultured in vitro and then directly applied to soil, it will be possible to regulate the active biomass of the endophyte, thereby facilitating soil remediation. However, it is still questionable whether endophytes can maintain high degradation activity after in vitro culture and whether they can be directly applied to remediate contaminated soils. We believe that simulating the special chemical environment of the intercellular spaces (apoplasts) of plant tissues where endophytes reside may be an effective solution to this problem.
Endophytes reside in the intercellular spaces of plant tissues, where high concentrations of nutrients and some plant secondary metabolites (SPMEs) support their survival, proliferation, and metabolism of special substances.15–17 Therefore, a similar composite of nutrients and/or SPMEs may facilitate the growth and metabolic activity of endophytes in vitro. Our previous study revealed that phytoalexins, as a special class of SPMEs, can promote the degradation of PAHs in aqueous solution by an endophyte at very low concentrations.18 Therefore, this chemically enhanced microbial technology may be a potential remediation solution for soils contaminated with PAHs.
In this study, the use of ryegrass endophytes and resveratrol in combination or alone to remediate soils contaminated with PAHs was explored, and how different treatments remediate soils and affect soil microbial communities was discussed, aiming to provide new possibilities for the chemically enhanced bioremediation of soils contaminated with organic pollutants.
In this investigation, we systematically explored the efficacy of ryegrass endophytes and resveratrol, both individually and in conjunction, for the bioremediation of soils contaminated with PAHs. The focus extended beyond the mere assessment of contaminant removal effectiveness to encompass the comprehensive analysis of the impact of these treatments on soil microbial communities. The overarching aim was to provide new possibilities for the chemically enhanced bioremediation of soils contaminated with organic pollutants.
2 Materials and methods
2.1 Chemicals and materials
Acenaphthene (ACE) and phenanthrene (PHE) (>98%) were purchased from Aldrich Chemical Company. Resveratrol (analytical grade) was purchased from Aladdin Company. HPLC-grade methanol, acetonitrile, n-hexane, and dichloromethane were obtained from Ourchem Company.The experimental soil was collected from Hangzhou City, Zhejiang Province, China; it was artificially contaminated with PAHs and aged for one month. Ryegrass was grown for two months in artificially contaminated soil. Methylobacterium extorquens C1 (C1), an endophytic strain of PAH-degrading bacteria, was isolated from ryegrass.18 Pseudomonas aeruginosa J1 (J1), which is a representative indigenous soil bacteria and nonendophyte, was isolated from experimental soil.19 The detail information of the bacterial strains are provided in ESI material M1.†
2.2 Effects of resveratrol on endophytes and nonendophytes for PAH degradation
The processes of PAH degradation by C1 and J1 were investigated under different concentrations of resveratrol. The two strains were cultured in MSM containing PAHs and resveratrol. The concentrations of resveratrol varied from 0.03 to 3.00 mg L−1. Every treatment was tested in triplicate. At 12, 24, 36, 48, and 72 hours, samples were taken, and the OD600 of the cultures and the concentration of PAHs were analyzed. An HPLC 1260 (Agilent, USA) was used to detect ACE and PHE concentrations. The specific operating conditions are shown in ESI material M2.†2.3 Application of resveratrol and C1 in the remediation of PAH-contaminated soils
PAHs were dissolved with methanol and added to the soil, resulting in concentrations of PHE and ACE in the soil of 50 mg kg−1. After that, the soil was placed in the shade and aged for one month. During this period, an appropriate amount of water was periodically added to the soil to maintain a consistent moisture content of 15%.Two hundred grams of the contaminated soil was placed in plastic pots (10 cm diameter). We set up 4 groups: (1) a resveratrol solution (30 mL) was added to the soil (R), and the concentrations of resveratrol in the soil were 0.15–5.00 mg kg; (2) a bacterial suspension of strain C1 (30 mL, OD600 = 0.02) was added to the soil (B); (3) a resveratrol solution (15 mL) and a bacterial suspension of strain C1 (15 mL, OD600 = 0.04) were added to the soil (RB), and the concentrations of resveratrol in the soil were 0.15–5.00 mg kg; and (4) distilled water (30 mL) was added to the soil (CK). Each group was analyzed in a bipartite manner. The mixture was maintained at 25 °C for 12 hours during the day and night, after which it was watered daily. After 15 days of processing, samples were collected to analyze the concentration of residual PAH in the soil and changes in the soil microbial communities.
2.4 Analysis of PAHs in soil
One gram of soil was mixed with 1 g of anhydrous Na2SO4 in a glass tube and extracted via sonication with 10 mL of dichloromethane at 25 °C. Then, the glass tube was centrifuged (3500 rpm, 15 min), and 3 mL of the supernatant was passed through a 4 g silica gel column (200–300 mesh) for purification. The eluent used was a mixed solvent of 10 mL of n-hexane and dichloromethane with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, 30 μL of dimethyl sulfoxide was added to the eluate, which was blown to near dryness on a nitrogen gasifier and brought up to 1.0 mL by adding 970 μL of acetonitrile. PAHs were analyzed by high-performance liquid chromatography (HPLC) after passing through a 0.22 μm organic phase filter.
1. Then, 30 μL of dimethyl sulfoxide was added to the eluate, which was blown to near dryness on a nitrogen gasifier and brought up to 1.0 mL by adding 970 μL of acetonitrile. PAHs were analyzed by high-performance liquid chromatography (HPLC) after passing through a 0.22 μm organic phase filter.
2.5 Analysis of soil biodiversity
A PowerSoil DNA Isolation Kit (MO BIO, cat. no. 12888) was used to isolate genomic DNA from 0.25 g of soil, which was then PCR-amplified and sequenced (ESI material M3†). UPARSE software (UPARSE V8.1.1861) was used to analyze the sequences. Alpha diversity and beta diversity was used to analyze the complexity of species diversity in the soil samples.3 Results and discussion
3.1 Comparison of the effects of resveratrol on PAH degradation by plant endophytic bacteria and soil indigenous microorganisms
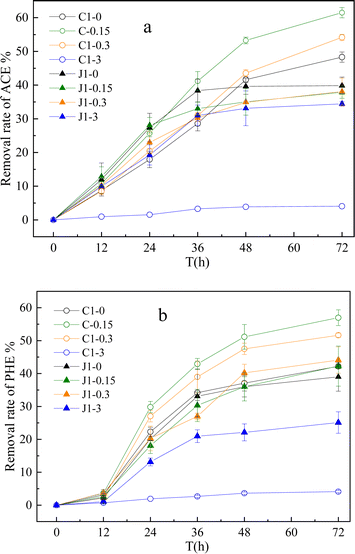
Our previous research revealed that secondary metabolites of plants, such as resveratrol, can promote the degradation of PAHs in aqueous solutions by the plant endophytic bacterium Methylobacterium extorquens C1.18 To further explore the possibility of using resveratrol in the remediation of contaminated soil, we studied the effect of resveratrol on a native PAH-degrading bacteria, Pseudomonas aeruginosa J1 (J1), which was isolated from the soil used in our experiment. The effects of resveratrol on the degradation of PAHs by the two strains were compared.As depicted in Fig. 1, resveratrol concentrations ranging from 0 to 0.3 mg L−1 facilitated the growth of the C1 strain, with the most pronounced effect occurring at 0.15 mg L−1. However, this beneficial effect reversed at a higher concentration of 3.0 mg L−1, where resveratrol inhibited the growth of the C1 strain. Within the tested range of 0–3.0 mg L−1, resveratrol impeded the growth of the J1 strain, with the negative impact intensifying as the concentration of resveratrol increased. As shown in Fig. 2, the influence of resveratrol on the degradation of PAHs by C1 and J1 differed significantly. For C1, the addition of resveratrol within the 0–0.3 mg L−1 range significantly accelerated the degradation of PHE and ACE, achieving a maximal removal rate of 60% at 0.15 mg L−1 resveratrol and an increase of approximately 30% compared with the absence of resveratrol. Conversely, for J1, resveratrol within the 0–0.30 mg L−1 range had no discernible effect on PAH degradation activity, and a concentration of 3.0 mg L−1 resveratrol significantly inhibited the degradation of PAHs by J1. Additionally, even for C1, the introduction of high resveratrol concentrations, such as 3.0 mg L−1, was found to exert an inhibitory effect on PAH degradation.
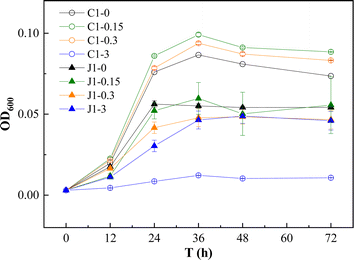 | ||
| Fig. 1 Effects of resveratrol on the growth of strains C1 and J1. ○ represents strain C1, and △ represents strain J1. The number represents the concentration of resveratrol (mg L−1). | ||
Compared with nonendophyte J1, resveratrol at 0–0.30 mg L−1 had a specific activating effect on ryegrass endophyte C1. In this study, the concentration of resveratrol that effectively promoted PAH degradation by endophytes was close to that observed in some plant sprouts (0.24–1.05 mg L−1).20 Therefore, we speculate that the enhanced ability of C1 to degrade PAHs after exposure to resveratrol may reflect a specific response of endophytes to the chemical environment of the plant apoplast. This may be similar to the positive effects of plant metabolites on microorganisms that some studies have noted. Techer et al.21 evaluated the microbial biomass and PAH-degrading activity of bacteria in the presence of root exudates and concluded that secondary metabolites in root exudates can increase the activity of PAH metabolism. Garces Mejia et al.22 reported that some plant secondary metabolites could promote PAH degradation by rhizosphere bacteria, but some also inhibited the degradation activity of bacteria. In the plant-endophyte symbiosis system, the mutual stimulation and feedback and underlying mechanism of cometabolizing pollutants need to be further studied.
3.2 PAH removal effectiveness from soil after application of resveratrol and C1
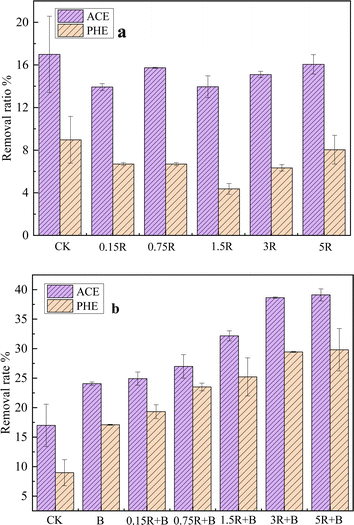
On the basis of the promotional effect of resveratrol on the degradation of PAHs by C1, we applied resveratrol and C1, either individually or concurrently, in the bioremediation of soil contaminated with PAHs. As shown in Fig. 3a, the addition of resveratrol alone did not promote PAH removal in soils under the present experimental conditions. For the treatment with 0.15–5 mg kg−1 of resveratrol alone, the removal ratios of PAHs were all lower than those of the treatment without the addition of resveratrol (CK). These results indicated that resveratrol did not promote or even inhibited the PAH-degrading activity of indigenous bacteria.However, as depicted in Fig. 3b, after culturing strain C1 in soil for 15 days, the removal rates of PHE and ACE in the soil reached 24.0% and 17.1%, respectively, representing increases of 41.2% and 90.0% compared with those of the CK. Upon simultaneous addition of resveratrol and C1 to the soil, the removal rate of PAH increased with increasing resveratrol concentration within the range of 0–5 mg kg−1. Specifically, at a resveratrol concentration of 5 mg kg−1, the removal rates of PHE and ACE in the soil were 39.1% and 29.8%, respectively, exceeding the removal rates achieved with C1 alone by 62.9% and 74.3% and those of the CK group by 130% and 231%, respectively. The above experimental results indicate that the combination of resveratrol and C1 could effectively remediate PAH-contaminated soils.
A comparison of the experimental groups with individual resveratrol and resveratrol combined with C1 revealed that the activating effect of resveratrol on the microbial degradation of PAHs is selective and that the response of C1 from the ryegrass endophyte to resveratrol is different from that of common indigenous soil bacteria. This finding is consistent with the abovementioned effects of resveratrol on the PAH-degrading ability of C1 and J1.
3.3 Effects of resveratrol on soil microbial diversity
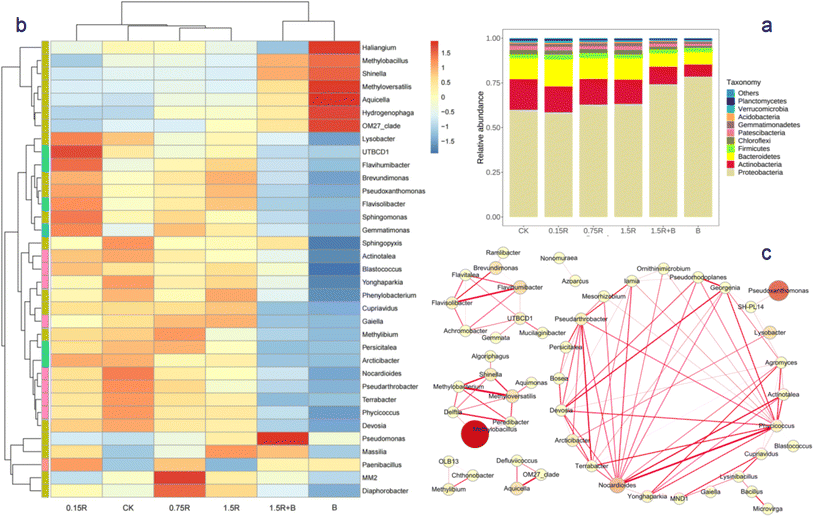
The abundance of bacteria in the soil under each treatment at the phylum level after 15 days of treatment is shown in Fig. 4a. In the CK group, the relative abundance of Proteobacteria was the highest at 60%, followed by that of Actinobacteria and Bacteroidetes at 16.5% and 12%, respectively. When only strain C1 (B) was added to the soil, the relative abundance of Proteobacteria significantly increased, and those of Actinobacteria and Bacteroidetes significantly decreased compared with those in the CK. When only 0.15–0.75 mg kg−1 resveratrol was added (0.15R and 0.75R), compared with that in the CK, the relative abundance of Proteobacteria remained unchanged, but that of Actinobacteria decreased. When the resveratrol concentration reached 1.5 mg kg−1 (1.5R), the relative abundance of bacterial phyla greatly changed, that of Proteobacteria increased, and that of Actinobacteria decreased. When resveratrol and strain C1 were mixed and added to the soil (1.5R + B), the relative abundance of Proteobacteria was much greater than that in the 1.5R group, and the relative abundance of Actinobacteria still decreased.To further analyze the changes in bacterial genera in the soil on the 15th day, a clustering heatmap of the relative abundance of the top 35 genera is shown in Fig. 4b, and the interspecies relationships were analyzed via Spearman's rank correlation analysis (Fig. 4c) on the basis of the changes in abundance of species at the genus level in various treated soil samples. The results indicated that at the genus level, in addition to the genera Methylobacillus and Methyloversatilis related to Methylobacterium, three typical PAH-degrading bacterial genera, namely, Massilia,23 Pseudomonas24 and Sphingomonas,25 were detected. When only strain C1 was added, the abundances of genera related to Methylobacterium, such as Methylobacillus and Methyloversatilis, clearly increased significantly. The relative abundances of Massilia and Pseudomonas in the groups treated with only resveratrol (0.15R, 0.75R and 1.5R) were greater than those in the CK group and increased with increasing resveratrol dose. When resveratrol and C1 were mixed into the soil (1.5R + B), not only were the genera related to Methylobacterium significantly increased, but the relative abundances of Massilia and Pseudomonas also increased, which was consistent with the highest removal ratio of PAH after 15 days of the 1.5R + B treatment.
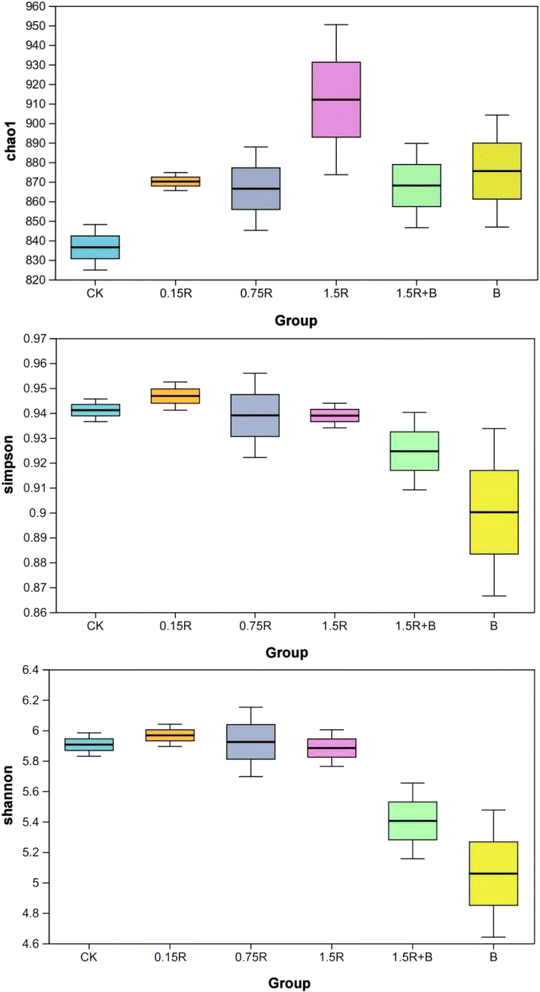
As shown in Fig. 5, the alpha diversity index was used to analyze the differences between the different treatment groups. For all the treatments, regardless of whether resveratrol or C1 was added to the soil alone or together, the Chao1 index was greater than that for the CK, indicating that there was an increase in microbial richness. When resveratrol was added alone (0.15R, 0.75R and 1.5R), the Chao1 index increased significantly compared with that of the CK, but the Shannon and Simpson indices did not change significantly. This indicated that when resveratrol was employed alone, the microbial community richness in the soil increased, but the soil microbial diversity did not change much. Luo et al.26 reported a similar effect of SPMEs (carvone, isoprene, limonene, naringin, and coumarin) on the microbial community structure in PCB-contaminated soil.
Compared with those in the CK treatment, in the C1 alone treatment (B), the Chao1 index increased, but the Simpson and Shannon indices decreased, which indicates that the addition of exogenous C1 led to the suppression of other species. This unbalanced competition phenomenon has been reported in several previous papers.27,28 However, when a mixture of resveratrol and C1 (1.5R + B) was added to the soil, compared with that in the treatment with C1 alone (B), the soil microbial diversity increased again. To some extent, the presence of resveratrol mitigated the impact of C1 addition on the original soil microbial structure.
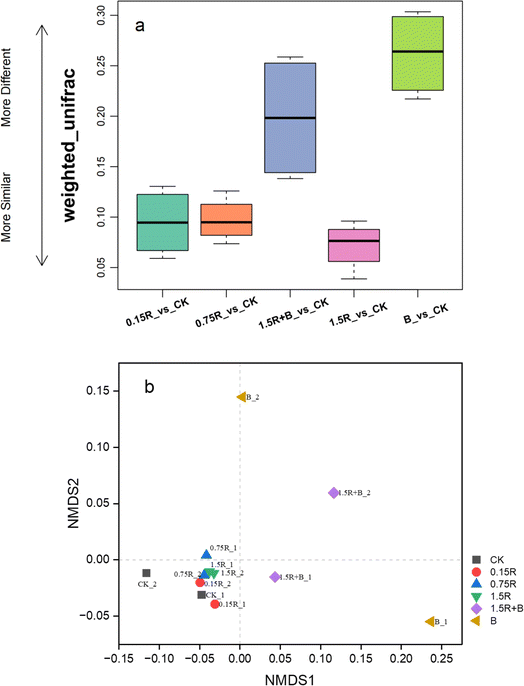
The results of the beta diversity analysis further revealed the differences in the structure of the soil microbial communities under various treatments. As illustrated in Fig. 6a, the weighted UniFrac distances between the soil samples treated with only C1 and the untreated soil (CK) were the greatest. The soils treated solely with resveratrol presented the smallest weighted UniFrac distances from the CK soil. The weighted UniFrac distance between the cotreated soil (1.5R + B) and the CK soil was smaller than that between the C1-treated soil (B) and the CK soil. The nonmetric multidimensional scaling (NMDS) analysis (Fig. 6b) also revealed that the points representing soil treated solely with C1 were most distinctly separated from all other points. In contrast, the points corresponding to soil cotreated with C1 and resveratrol exhibited a less distinct separation from those representing untreated soil (CK). The results demonstrated the most significant variation in microbial community structure between soil treated solely with C1 and untreated soil. In contrast, the individual addition of resveratrol did not significantly affect the soil microbial community structure, and the presence of resveratrol mitigated the impact of C1 on the soil microbial community to some extent.
In summary, the use of resveratrol alone in these experiments has certain benefits for some indigenous PAH-degrading bacteria and is beneficial for the richness of microorganisms in the soil. However, it did not have a significant effect on the degradation of PAHs in the soil. The addition of C1 alone significantly increased the abundances of genera related to Methylobacterium and effectively promoted the degradation and removal of PAHs in soil but also led to the suppression of other species. When C1 and resveratrol were used at the same time, resveratrol not only promoted the degradation of PAH by C1 but also mitigated the impact of C1 addition on soil microbial diversity. Therefore, the combined application of C1 and resveratrol is the optimal solution in terms of remediation effects and ecological impacts. The combined application of such plant secondary metabolites and endophytic bacteria to PAH-contaminated soil for remediation has broad prospects.
4 Conclusion
The combination of resveratrol and plant endophytes was used to remediate PAH-contaminated soil, and a good remediation effect was achieved. Studies have shown that the removal of PAH in soil was the best when C1 and resveratrol were added at concentrations of 2.4 × 107 CFU mg−1 and 5 mg kg−1, respectively. After 15 days, the removal effects of ACE and PHE in the soil reached 39.1% and 29.8%, respectively. Compared with the control (CK) without C1 and resveratrol added, the C1 and resveratrol combinations increased the removal of acenaphthene and phenanthrene in the soil by 130% and 231%, respectively. The analysis of the PAH content and the microbial community structure in the soil revealed that the abundance of Methylobacterium-related PAH-degrading bacteria in the soil increased significantly after inoculation with only strain C1 but decreased the soil microbial diversity. The combined application of resveratrol not only promoted PAH degradation by Methylobacterium-related PAH-degrading bacteria but also mitigated the impact of C1 addition on the soil microbial community structure at the same time.Data availability
All data generated or analyzed during this study are included in this published article.Author contributions
Jiawei Zhao: investigation, writing – original draft. Li Lu: conceptualization, investigation, writing – review & editing. Qiwei Chai: investigation, methodology. Wei Jin: investigation, methodology. Min Zhu: methodology. Shengqi Qi: writing – review & editing. Jiali Shentu: resources, supervision. Yuyang Long: resources, supervision. Dongsheng Shen: resources, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was supported by the Fundamental Research Funds of Zhejiang Gongshang University (No. XRK23003).Notes and references
- S. Gan, E. V. Lau and H. K. Ng, J. Hazard. Mater., 2009, 172(2–3), 532–549, DOI:10.1016/j.jhazmat.2009.07.118.
- S. Kuppusamy, P. Thavamani, K. Venkateswarlu, Y. B. Lee, R. Naidu and M. Megharaj, Chemosphere, 2017, 168, 944–968, DOI:10.1016/j.chemosphere.2016.10.115.
- M. García-Sánchez, Z. Košnář, F. Mercl, E. Aranda and P. Tlustos, Ecotoxicol. Environ. Saf., 2018, 147, 165–174, DOI:10.1016/j.ecoenv.2017.08.012.
- M. Afzal, Q. M. Khan and A. Sessitsch, Chemosphere, 2014, 117, 232–242, DOI:10.1016/j.chemosphere.2014.06.078.
- S. D. Siciliano, N. Fortin, A. Mihoc, G. Wisse, S. Labelle, D. Beaumier, D. Ouellette, R. Roy, L. G. Whyte, M. K. Banks, P. Schwab, K. Lee and C. W. Greer, Appl. Environ. Microbiol., 2001, 67(6), 2469–2475, DOI:10.1128/AEM.67.6.2469-2475.2001.
- Y. Chen, X. G. Xie, C. G. Ren and C. C. Dai, Bioresour. Technol., 2013, 129, 568–574, DOI:10.1016/j.biortech.2012.11.100.
- Z. Khan, D. Roman, T. Kintz, M. Delas Alas, R. Yap and S. Doty, Environ. Sci. Technol., 2014, 48(20), 12221–12228, DOI:10.1021/es503880t.
- K. Sun, J. Liu, Y. Z. Gao, L. Jin, Y. J. Gu and W. Q. Wang, Sci. Rep., 2014, 4, 5426, DOI:10.1038/srep05462.
- J. Wang, J. Liu, W. T. Ling, Q. G. Huang and Y. Z. Gao, Sci. Total Environ., 2017, 598, 471–478, DOI:10.1016/j.scitotenv.2017.04.126.
- H. Baoune, J. Daniel Aparicio, A. Acuña, A. O. El Hadj-khelil, L. Sanchez, M. Alejandra Polti and A. Alvarez, Ecotoxicol. Environ. Saf., 2019, 184, 109591, DOI:10.1016/j.ecoenv.2019.109591.
- S. Chen, Z. Ma, S. Y. Li, M. G. Waigi, J. D. Jiang, J. Liu and W. T. Ling, Environ. Int., 2019, 132, 105081, DOI:10.1016/j.envint.2019.105081.
- H. Jabeen, S. Iqbal, F. Ahmad, M. Afzal and S. Firdous, Int. J. Phytorem., 2016, 18(2), 126–133, DOI:10.1080/15226514.2015.1073666.
- X. Zhu, L. Jin, K. Sun, S. Li, W. Ling and X. Li, Int. J. Environ. Res. Public Health, 2016, 13(7), 633, DOI:10.3390/ijerph13070633.
- W. Q. Fu, M. Xu, K. Sun, L. Y. Hu, W. Cao, C. C. Dai and Y. Jia, Chemosphere, 2018, 203, 160–169, DOI:10.1016/j.chemosphere.2018.03.164.
- M. A. Karas, S. Wdowiak-Wrobel and W. Sokolowski, Int. J. Mol. Sci., 2021, 22(17), 9557, DOI:10.3390/ijms22179557.
- A. Kour, A. S. Shawl, S. Rehman, P. Sultan, P. H. Qazi, P. Suden, R. K. Khajuria and V. Verma, World J. Microbiol. Biotechnol., 2008, 24, 1115–1121, DOI:10.1007/s11274-007-9582-5.
- L. Musilova, J. Ridl, M. Polivkova, T. Macek and O. Uhlik, Int. J. Mol. Sci., 2016, 17(8), 1205, DOI:10.3390/ijms17081205.
- L. Lu, Q. W. Chai, S. Y. He, C. P. Yang and D. Zhang, Environ. Pollut., 2019, 253, 872–881, DOI:10.1016/j.envpol.2019.07.097.
- L. Lu, A. N. Li, X. Q. Ji, S. Y. He and C. P. Yang, Environ. Sci. Pollut. Res., 2021, 28(4), 4807–4814, DOI:10.1007/s11356-020-10830-z.
- A. Limmongkon, P. Janhom, A. Amthong, M. Kawpanuk, P. Nopprang, J. Poohadsuan, T. Somboon, S. Saijeen, D. Surangkul, M. Srikummool and T. Boonsong, Asian Pac. J. Trop. Biomed., 2017, 7(4), 332–338, DOI:10.1016/j.apjtb.2017.01.002.
- D. Techer, M. D'Innocenzo, P. Laval-Gilly, S. Henry, A. Bennasroune, C. Martinez-Chois and J. Falla, Appl. Soil Ecol., 2012, 62, 142–146, DOI:10.1016/j.apsoil.2012.06.009.
- A. C. Garces Mejia, N. J. Pino and G. A. Penuela, Environ. Eng. Sci., 2018, 35(3), 203–209, DOI:10.1089/ees.2017.0156.
- H. P. Gu, K. Yan, Q. You, Y. Z. Chen, Y. H. Pan, H. Z. Wang, L. S. Wu and J. M. Xu, Sci. Total Environ., 2021, 781, 146655, DOI:10.1016/j.scitotenv.2021.146655.
- J. M. Ma, E. R. Rene, Z. Y. Chen and W. F. Ma, J. Hazard. Mater., 2022, 424, 127500, DOI:10.1016/j.jhazmat.2021.127500.
- S. Shokrollahzadeh, F. Azizmohseni, F. Golmohammad, H. Shokouhi and F. Khademhaghighat, Bioresour. Technol., 2008, 99(14), 6127–6133, DOI:10.1016/j.biortech.2007.12.034.
- W. S. Luo, E. M. D'Angelo and M. S. Coyne, Soil Biol. Biochem., 2007, 39(3), 735–743, DOI:10.1016/j.soilbio.2006.09.019.
- Z. M. Dai, A. Enders, J. L. M. Rodrigues, K. L. Hanley, P. C. Brookes, J. M. Xu and J. Lehmann, Soil Biol. Biochem., 2018, 126, 159–167, DOI:10.1016/j.soilbio.2018.09.001.
- M. Yu, W. Su, L. Huang, S. J. Parikh, C. Tang, R. A. Dahlgren and J. M. Xu, Soil Biol. Biochem., 2021, 162, 108420, DOI:10.1016/j.soilbio.2021.108420.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05648e |
| This journal is © The Royal Society of Chemistry 2024 |