Open Access Article
Open Access ArticleAl, P-co-doping and interface engineering synergistically boost the electrocatalytic performance of WS2/Ni3S2/NiS nanosheet heterostructure for efficient hydrogen evolution reaction†
Mghaib Al Shahrani,
Mabrook S. Amer *,
Ahmad A. Alsaleh,
Prabhakarn Arunachalam
*,
Ahmad A. Alsaleh,
Prabhakarn Arunachalam and
Abdullah M. Al-Mayouf
and
Abdullah M. Al-Mayouf *
*
Electrochemical Sciences Research Chair (ESRC), Chemistry Department, College of Science, King Saud University, P. O. Box 2455, Riyadh 11451, Saudi Arabia. E-mail: msamer@ksu.edu.sa; amayouf@ksu.edu.sa
First published on 14th October 2024
Abstract
The fabrication of earth-abundant electrocatalysts capable of facilitating hydrogen evolution reactions (HER) is essential for creating sustainable hydrogen fuel by water splitting. Here, we present a one-pot hydrothermal approach for producing aluminum and phosphorus co-doped NiS/Ni3S2/WS2 heterostructure hybrid frameworks on nickel foam. The optimal Al and Al, P/NiWS-b@NF catalyst exhibits high HER activity with overpotentials of 139 and 227 mV at current densities of 10 and 50 mA cm−2, respectively, thanks to the synergistic effect of the various constituents of the catalyst. What is more, it also exhibits a promising Tafel slope of 124 mV dec−1 and is electrocatalytically durable for 10 hours in 0.5 M H2SO4 solution. The high HER activity of Al, P/NiWS-b@NF could be explained by the large number of active sites of the hierarchical heterostructure and electron effects produced by the combination of interfacial and aluminum and phosphorus doping.
1. Introduction
Energy consumption growth poses a significant challenge regarding sustainability and environmental impact.1–3 Hydrogen has the potential to play a significant role as an energy carrier and the transition to a more sustainable energy future, as it is clean, efficient, renewable, and highly energy dense, making it an ideal energy storage and transportation option.4 The electrolysis of water is certainly considered one of the most sustainable techniques for producing hydrogen. The process involves splitting water molecules (H2O) into hydrogen and oxygen using electricity obtained from renewable energy sources such as solar and wind power, which has a thermodynamic potential of 1.23 V.4–7 However, water electrolysis for hydrogen production poses significant challenges due to the inherent dynamic overpotentials in HER and the oxygen evolution reaction (OER).8 Consequently, the design and optimization of electrocatalysts for HER and OER can contribute to achieving a reduction in overpotentials as well as enhanced reaction kinetics. Platinum-based materials are usually recognized as excellent catalysts for HER due to their outstanding catalytic activity and durability.9 Still, due to the high cost and limited availability of platinum, widespread commercial adoption of water electrolysis for hydrogen production remains a challenge.10,11 Recent research efforts are ongoing to explore a wide range of HER earth-abundant transition metal electrocatalysts, including metal alloys, metal oxides, nitrides, sulfides, selenides, and carbon-based materials.9,12–17 Among these catalysts, transition metal sulfide (TMS)-based electrocatalysts have emerged as promising materials for various electrochemical reactions, including water electrolysis for hydrogen production.18 However, the limitations associated with pure TMSs such as their unfavorable free energy of hydrogen adsorption (ΔGH*) values, inability to facilitate water dissociation, and their high resistance coupled with conductivity due to a large band gap, pose significant challenges for their use as effective electrocatalysts, particularly in the HER during water electrolysis. Due to these limitations, designing and fabricating high-efficiency and highly stable TMS catalysts for the HER is both an interesting challenge and an exciting opportunity.Catalysts have high activity due to their synergistic effects, which include electron transmission speed, reaction energy barrier, electroactive sites where reactions occur, and electroactive sites' capacity.19 As an example, surface and interface engineering can be applied to enhance the electrocatalyst performance. A number of methods, for example, can be used to prepare some vacancies, dislocations, and grain boundary defects by using (1) plasma treatment,20 (2) common doping methods can improve the catalyst's composition and electrical properties,21 and (3) heterogeneous structures can be designed to provide high energy interfaces for catalytic reactions.22 Interfacial effects play a big role in engineering electrocatalysts to be catalytically active, selective, and stable by regulating how adsorbents, intermediates, and electrons are bound, transformed, and transferred at the boundary of two domains.20,22–25 By modifying the interfacial characteristics of TMSs, low-dimensional materials could be prevented from aggregating, while charge and mass transfer can be accomplished efficiently.26,27 Further, interface engineering might enrich the active sites and promote electron redistribution, therefore regulating both the adsorption and desorption of intermediates and improving the efficiency of HER.28 Among the TMS electrocatalysts, tungsten disulfide (WS2) is widely used as an HER catalyst because of its high electrochemical efficiency.29–31 The development of heterostructures has been a key way to overcome WS2 electrocatalysts limitations by attaining electronic transformations, thereby improving efficiency and expanding active surface areas.32 Researchers have studied various WS2-based heterostructures for improving the role of WS2 as HER electrocatalysts, such as MoS2, CoSe2, and WSe2. Various WS2-based heterostructures, including MoS2, CoSe2, and WSe2, have been considered to enhance the characteristics of WS2 as an electrocatalyst for HER.33–35 Co-doping of metals over tungsten sulfide heterojunction catalysts for HER has only been reported in rare cases. It is therefore necessary to advance a facile approach for preparing tungsten sulfide heterojunctions to further improve HER activity.
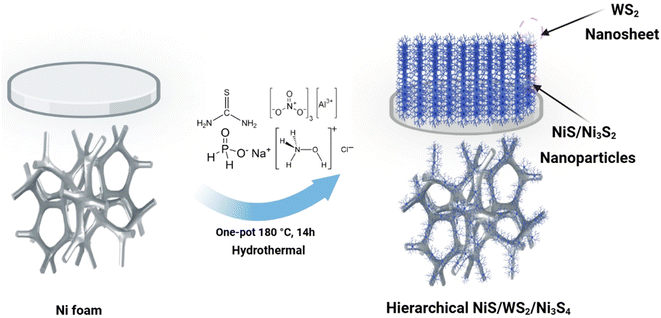
Herein, we propose a facial one-pot fabrication of Al, and P element co-doped into a hierarchical NiWS@NF heterostructure hybrid framework consisting of Ni-sulfides (NiS, and Ni3S2) nanoparticles and WS2 nanosheets supported on Ni-foam (NF) to promote HER kinetics on metal sulfides. Given the available hierarchical structures constructed for this study, it was found that the optimal Al, P/NiWS-b@NF catalyst had the superior catalytic features for the HER, exhibiting lower overpotentials of 139 and 227 mV at current densities of 10 and 50 mA cm−2, correspondingly. Moreover, it displays a favorable Tafel slope of 124 mV dec−1 in 0.5 M H2SO4 solution for the HER. Based on the obtained results, multi-interface collaboration, Al, and P doping are associated with superior performance in hierarchical NiWS@NF hybrid frameworks.
2. Experimental
2.1 Chemicals and materials
The regents were all analytical grade without additional purification. Sodium hypophosphite monohydrate (NaH2PO2·H2O ≥ 99.0%), aluminum nitrate hexahydrate (Al(NO3)3·6H2O ≥ 98.0%), and sodium tungstate dihydrate (Na2WO4·2H2O ≥ 99.0%) were acquired from Alfa Aesar and used as the P, Al, and W precursors, respectively. Thiourea ((NH2)2CS ≥ 99.0%), and hydroxylamine hydrochloride (NH2OH·HCl ≥ 98.0%) were purchased from Sigma-Aldrich and utilized as sulfur sources and reducing agents, respectively. Potassium hydroxide belts (KOH ≥ 85.0%), nitric acid (HNO3 68–70%), isopropanol (C3H8O ≥ 99.0%), and Nafion (C9HF17O5S 5%) were obtained from Sigma-Aldrich. Nickel foam (1.7 × 200 × 300 mm, NF ≥ 99.0%) was purchased from Xiamen Zopin New Material Limited Company.2.2 Synthesis of the hierarchical NiWS@NF, and Al, P/NiWS@NF hybrid framework
In general, commercial NF, which is available in an area of 2 × 5 cm2, is typically used as both a nickel source and template. NF was cleaned with 3 M HCl solution, acetone, and anhydrous ethanol in sequence for 15 min each through sonication. It was lastly dried in air and kept for future use. The NiWS@NF and Al, P/NiSW-x@NF electrocatalysts were prepared using a hydrothermal-directed method. Typically, in 25 mL DI water, 1 mM Na2WO4·2H2O, 1 mM NaH2PO2·H2O, 6 mM NH2CSNH2, and 2 mM NH2OH·HCl are dissolved by gentle magnetic stirring, and a clear solution is obtained. Following that, 25 mL of DI water including 0.2 mM Al(NO3)3·6H2O was submerged independently and introduced dropwise to the first mixture solution while stirring magnetically. After the transfer of the mixture to a 100 mL Teflon-lined autoclave, the NF was kept in the previous solution and heated at 180 °C for 14 hours. Upon the reaction being complete, Al, P/NiWS-x@NF was thoroughly rinsed using an ethanol and water mixture prior to getting vacuum-dehydrated using a furnace at 60 °C overnight.2.3 Synthesis of control samples
To produce nickel sulfide, tungsten sulfide nanostructures on NF (NiWS@NF), aluminum-doped tungsten sulfide nanostructures on NF (Al/NiWS@NF), and P-doped WS2 nanostructures on NF (P/NiWS@NF), the hydrothermal reaction was carried out similarly. To achieve this, the appropriate precursor salts were incorporated in the required molar ratios. After heating to 180 °C for 14 hours, they were dried overnight at 60 °C in a vacuum oven.2.4 Preparation of a Pt/C reference electrode on NF
The synthesized electrodes were compared to commercial electrodes using a Pt/C catalyst loaded onto NF substrates. Typically, 950 μL of water and isopropyl alcohol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were mixed with 5 mg of Pt on carbon (20 wt%) catalyst and 20 μL of Nafion (5 wt%). The solution was then stirred by sonication for 30 minutes. After spreading Pt/C ink to the raw NF surface in a 1 × 1 cm2 geometric area, the electrode was dried in a vacuum oven at 60 °C.
1) were mixed with 5 mg of Pt on carbon (20 wt%) catalyst and 20 μL of Nafion (5 wt%). The solution was then stirred by sonication for 30 minutes. After spreading Pt/C ink to the raw NF surface in a 1 × 1 cm2 geometric area, the electrode was dried in a vacuum oven at 60 °C.
2.5 Characterization
The high-resolution scanning electron microscope (HRSEM) photographs were observed on a Hitachi S4800 (Japan) that was functioned at 1.0 kV and 10 mA. To study the nanometric details of the samples and thin film deposited morphologies, a high-resolution transmission electron microscope JEOL 2100F microscope (Japan) at 200 kV is employed with an energy dispersive X-ray (EDX) detector. To conduct TEM analysis, samples were suspended in ethanol solution and mounted on a Cu grid with a holey carbon film. The crystallinity, purity, and phases of materials were measured using a MiniFlex-600 (Rigaku) with a Cu Kα irradiation technique (40 kV voltage, 15 mA current). An X-ray photoelectron spectrometer (Escalab 253, Thermo Fisher Scientific) with a monochromated MgKα X-ray source has been employed for X-ray photoelectron spectroscopy (XPS) analysis of the produced catalyst. To calibrate the binding energy (BE) scale, the C 1s component of aliphatic hydrocarbons has been used, with a BE value of 285.0 eV.2.6 Electrochemical measurements
A potentiostat (BioLogic SAS, model) was used to conduct electrochemical measurements on three electrodes, with NiWS@NF catalysts, Al, P/NiWS-x@NF catalysts working electrodes, and carbon graphite rods and saturated silver/silver chloride electrodes serving as counter and reference electrodes, respectively. HER activity of NiWS@NF and Al, P/NiWS-x@NF catalysts in 0.5 M H2SO4 was investigated using linear sweep voltammetry (LSV) at a scanning rate of 5 mV s−1 with a potential window varying from 0.20 to −0.6 VRHE. The LSV plots were analyzed without IR compensation. In electrochemical impedance spectroscopy (EIS) measurements, the same cell configuration was used at the chosen potential and at frequencies between 200 MHz and 100 Hz with amplitudes of 10 mV. All electrochemical analysis were carried out under normal atmospheric pressure and at 25 °C under nitrogen purge. Electrochemical surface area (ECSA) and double-layer capacitance (Cdl) were evaluated at various scanning speeds from 10 to 100 mV s−1 in non-faradaic areas.3. Results and discussion
3.1 Electrocatalysts preparation and characterization
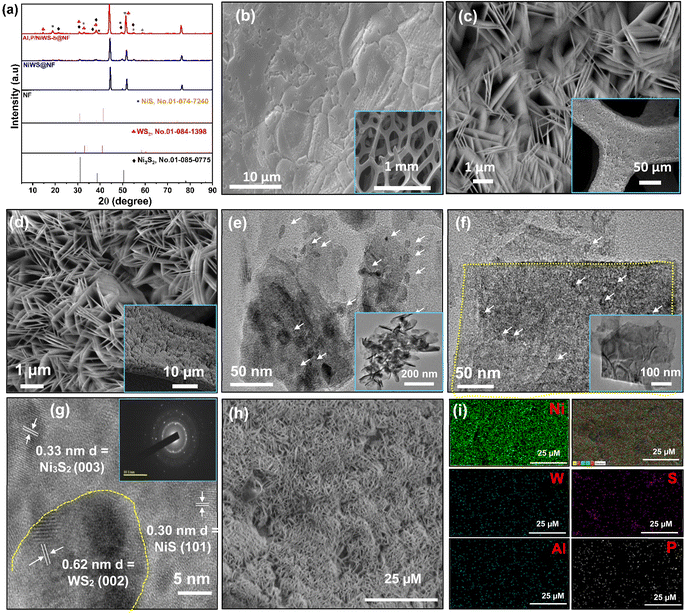
Several tungsten sulfide/nickel sulfide-based electrocatalysts were synthesized and studied for HERs. Various proportions of aluminum and/or phosphorus were incorporated into nickel sulfide and tungsten sulfide hybrid structures, which were termed Al, P/NiWS-a@NF (the same amount of Al to P), Al, P/NiWS-b@NF (Al < P), and Al, P/NiWS-c@NF (Al > P) (Table S1†). The second series includes pure NiWS@NF, and single-element doped electrocatalysts i.e. (Al/NiWS-d@NF, and P/NiWP-e@NF). Fig. 1 shows how the Al, P/NiWS-x@NF heterostructure was produced via a facial hydrothermal reaction. For the synthesis of NiWS@NF nanosheets, Ni foam having a porous structure served as the growth support and Ni source. Since metallic Ni has high reactivity, it readily reacts with thiourea to form trigonal T-NiS/T-Ni3S2 nanoparticles on NF. As a result of the highly reactive S2− on the T-NiS/T-Ni3S2 surface and the numerous nucleation sites provided by the nanoparticles, high-density hexagonal H-WS2 nanosheets were then formed on the T-NiS/T-Ni3S2 nanosheets using a hydrothermal method.22To identify the crystal structure of the obtained catalysts, the XRD measurement of fabricated NiWS@NF, Al/NiWS@NF, P/NiWS@NF, and Al, P@NiWS-x@NF, materials were recorded, as depicted in Fig. 2a and S1a.† In Fig. 2b, except for the indexed WS2 peaks of 2θ about 14.36, 32.77, 33.59, 35.94, 39.60, and 49.80° which correspond to the (002), (100), (101), (102), (103), (221), and (105) planes for hexagonal H-WS2 (card no. 01-084-1398), all of the characteristic peaks at the 2θ of 21.93, 31.13, 38.41, 38.69, 44.73, 50.24, 50.47, and 55.50° are extremely compatible with 101, 110, 021, 003, 202, 211, 113, and 122 planes of trigonal T-Ni3S2 phase (card no. 01-085-0775). Furthermore, other obvious peaks were also observed at the 2θ of trigonal phase T-NiS planes (card no. 01-074-7240), demonstrating that the nanocomposites are composed of WS2, Ni3S2, and NiS.36 It is evident from the observed sharp diffraction peaks that NiWS@NF materials doped with Al or P have greater crystallinity. Fig. S1b† displays a diffraction peak location shift after Al and/or P insertion in the pure NiWS@NF sample, which can be attributed to lattice expansion. There were no additional diffraction peaks observed, signifying that the bimetallic doping process does not change the crystalline phases. HR-SEM was used to evaluate the morphologies of various materials. As can be seen in Fig. 2b, the NF was composed of a porous Ni framework, and the inset of Fig. 2b shows the Ni skeleton's smooth surface. After the hydrothermal reaction, in situ grown NiWS@NF nanosheets having a mean diameter of 80 nm were formed (Fig. 2c and d). According to the HR-SEM images (inset of Fig. 2c and d), NiWS nanosheets had smooth surfaces. As shown in Fig. 2d, HR-SEM imaging reveals that the sample's surface appears to be comprised of nanoflakes–nanosheet assemblies. The top view of the framework in Fig. 2c and d confirms that the nanosheet assemblies are very uniform. Moreover, the top view of the Al, P/NiWSAlP-b@NF framework indicates that WS2 nanosheets and NiS/Ni3S2 nanoparticles are strongly linked, suggesting that NiS/WS2 and WS2/Ni3S4 interfaces are abundant. As a result of the in situ growth of NiWS nanosheets, there was very high structural stability, which prevented most of the bubbles that were generated during electrocatalysis from damaging electrocatalysts, thus promoting the stability of the HER catalyst.37 To further illustrate the locally assembled structure of the heterostructure frameworks, transmission electron microscopy was applied to both the NiWS@NF and Al, P/NiWS-d@NF heterostructures. Fig. 2e and f show the low-magnification TEM image of NiWS@NF, and Al, P/NiWS-b@NF heterostructure respectively. In Fig. 2e and f, the TEM image of NiWS@NF, and Al, P/NiWS-b@NF heterostructure, a large granular component, about a few tens of nanometers in size, is thought to be T-NiS/T-Ni3S2. It is easy to identify H-WS2 nanosheets by their distinguishable lateral lamellar stripes, ranging in size from a few nanometers to tens of nanometers (Fig. 2f and S2†). Furthermore, HR-TEM images of Fig. 2g reveal a distinct interface between H-WS2–T-NiS and T-NiS–T-Ni3S2, indicating heterojunction formation. As illustrated in Fig. 2g and S2d,† the electron diffraction patterns produced in the selected area of the sample confirm the presence of H-WS2, T-NiS, and T-Ni3S2. With the help of HR-TEM (Fig. 2g and S2d†), we have studied the structure of NiWS@NF as well as Al, P/NiWS-b@NF heterostructures. The hexagonal phase of the H-WS2 nanosheet's (002) crystalline plane has a lattice spacing of 0.62 nm.
A crystalline plane with a lattice spacing of 0.30 nm and 0.24 nm indicates the presence of planes (101) and (220) in the trigonal phase of T-NiS nanoparticles. The (220) plane, which has a lattice spacing of 0.33 nm, reveals the presence of the trigonal phase of the T-Ni3S2 crystal structure. To validate the elemental composition of the NiWS@NF, and Al, P/NiWS-b/NF samples, HR-SEM (Fig. S3a–f† and 2i) and energy dispersive X-ray (EDS, Fig. S4 and S5†) color mapping was performed. The elemental mapping analysis of NiWS@NF, and Al, P/NiWS-b@NF verified that the spatial distribution of W, Ni, S, and P elements was uniform, as shown in Fig. S3a–f† and 2i. Consequently, WS2 nanosheets and NiS/Ni3S2 nanoparticles were crosslinked to produce an exceptionally accessible and abundant heterogeneous interface in the Al, P/NiWS-b@NF framework. According to EDS analysis of Al, P/NiWS-b@NF sample, Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al and P had weight ratios of 60.33
Al and P had weight ratios of 60.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.34
26.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.22
11.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.81
0.81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.29 (Fig. S4†). This HR-SEM image, as well as an accompanying elemental map, confirmed the homogeneous dispersion of Ni
1.29 (Fig. S4†). This HR-SEM image, as well as an accompanying elemental map, confirmed the homogeneous dispersion of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al and P in the Al, P/NiWS-b@NF heterostructure. To reveal the surface elements, chemical states and composition, XPS spectrum analysis was performed on NiWS@NF and Al, P/NiWS-b@NF heterostructure catalysts. Fig. S6† illustrates the full XPS spectrum for NiWS@NF, P/NiWS@NF, Al/NiWS@NF, and Al, P/NiWS-b@NF which reveals W, Ni, S, Al, and P in the sample. As seen in Fig. 3a, the Ni 2p spectrum of NiWS@NF, and Al, P/NiWS-b@NF shows binding energies at about 853.1 eV, 870.5 eV, and 854.3 eV, 874.6 eV, corresponding to the spin–orbit characteristics of Ni2+ and Ni3+, respectively.37,38
Al and P in the Al, P/NiWS-b@NF heterostructure. To reveal the surface elements, chemical states and composition, XPS spectrum analysis was performed on NiWS@NF and Al, P/NiWS-b@NF heterostructure catalysts. Fig. S6† illustrates the full XPS spectrum for NiWS@NF, P/NiWS@NF, Al/NiWS@NF, and Al, P/NiWS-b@NF which reveals W, Ni, S, Al, and P in the sample. As seen in Fig. 3a, the Ni 2p spectrum of NiWS@NF, and Al, P/NiWS-b@NF shows binding energies at about 853.1 eV, 870.5 eV, and 854.3 eV, 874.6 eV, corresponding to the spin–orbit characteristics of Ni2+ and Ni3+, respectively.37,38
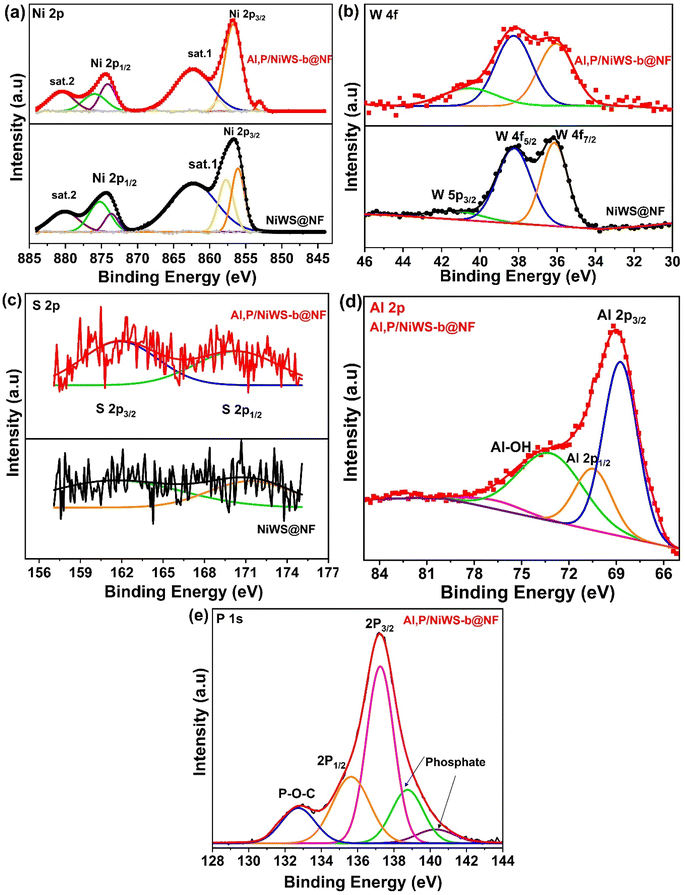 | ||
| Fig. 3 Elemental XPS spectra of (a) Ni 2p, (b) W (4f), (c) S 2p, (d) Al (2p), and (e) P 2p of NiWS@NF, and Al, P/NiWS-b@NF. | ||
There is an obvious shift in Ni 2p peak positions between Al, P/NiWS-b@NF compared to the pristine NiWS@NF, as a result of charge transfer through heterostructure interfaces. Fig. 3b shows XPS peaks for W 4f. A four-component spectrum can be deconvoluted from the W 4f spectrum. The peaks at 36.20 eV and 38.22 eV agree to W 4f7/2 and W 4f5/2 in H-WS2.36,37 In addition, the peak at 41.05 eV is expected to originate from oxidation species such as W 5p3/2. There is a positive shift in W 4f7/2 from 36.20 eV for pure NiWS@NF to 36.0 eV for Al, P/NWS-b@NF (Fig. 3b). The spectra of S 2p are revealed in Fig. 3c. There are two peaks at binding energies of 161.92 and 170.88 eV that belong to the S 2p3/2 and S 2p1/2 components of S in NiWS@NF, and Al, P/NiWS-b@NF, respectively.37,38 Fig. 3d displays the high-resolution peaks of XPS of Al 2p in Al, P/NiWS-b@NF. BE signals at 70.55 eV and 68.81 eV reveal the characteristic peaks of Al3+ states, which are Al 2p3/2 and Al 2p1/2 respectively.39,40 The spectrum of P 2p is shown in Fig. 3e. As shown in Fig. 3e, air-oxidized phosphorus could have produced two peaks with high binding energies (138.74 and 140.31 eV). Moreover, existing two peaks were situated at 135.63 eV and 137.19 eV, which may be read as the BEs of P 2p1/2 and P 2p3/2, accordingly, that corroborate the existence of an interaction across nonmetal/metal and P in Al, P/NiWS-b@NF.34,35 The results demonstrate the composition of elements, proving the effective creation of nanosheets in Al, P/NiWS-b@NF heterojunction frameworks using a simple hydrothermal self-organized procedure. Furthermore, the chemical state changes in Ni, and W elucidate the development of highly coupled interfaces between H-WS2, and T-NiS/T-Ni3S2 via powerful electronic interactions. In this way, metal sulfides can be manipulated to control the adsorption and desorption energies of reaction intermediates by manipulating their frontier orbital energies.41–45
3.2 Electrochemical HER performance
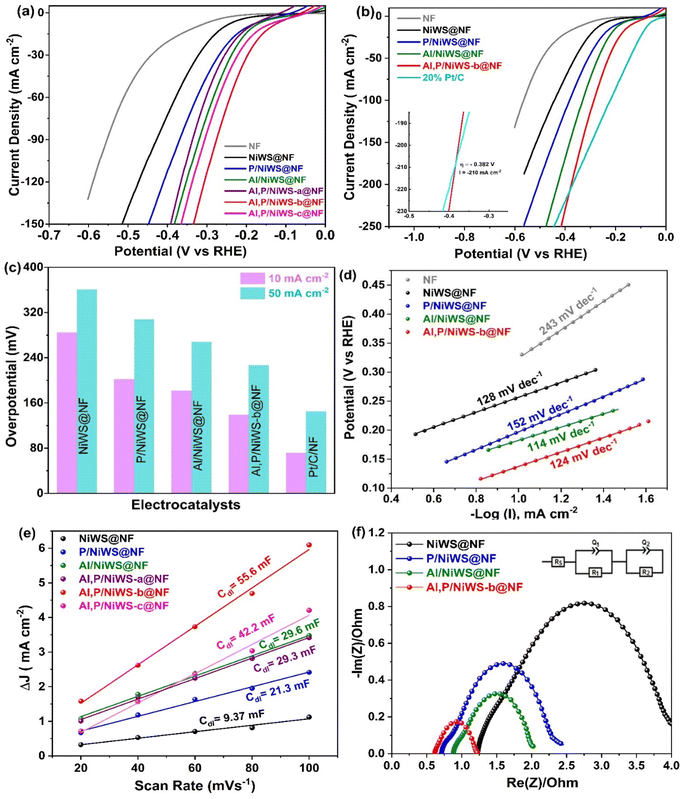
Al, P/NiWS-x@NF electrocatalysts displays the above advantages, such as a heterointerface and high mass and charge transfer capabilities, which indicates it may be a good electrocatalyst for HER. A conventional three-electrode–electrolyte medium containing N2-saturated 0.5 M H2SO4 solution and graphite rod as the counter electrode was used to evaluate the catalytic features of all the as-made electrocatalysts. According to Fig. S8a and b† HER performance was compared at Al, P/NiWS-b@NF growth times of 6 h, 10 h, 14 h, and 18 h. It was evident that Al, P/NiWS-b@NF 14 h demonstrated the best HER activity. During the 14 hour growth process, H-WS2 nanosheet density increased continuously, enabling more active edge sites. Based on Fig. 4a, the polarization curves clearly reveal that Al, P/NiWS-b@NF has the same activity as commercial Pt/C (20 wt%) at low potential, and even becomes preferable to commercial Pt/C when current density reaches 210 mA cm−2 (inset in Fig. 4b, left). In addition, Al, P/NiWS-b@NF catalyst reveal improved HER performance at current densities of 10 and 50 mA cm−2, which are much lower than those of Al, P/NiWS-a@NF, Al, P/NiWS-c@NF, NiNW@NF, Al/NiNW@NF, P/NiNW@NF and NF (Fig. 4a and b), due to the highly exposed and rich hetero-interfaces in the T-NiS/H-WS2/T-Ni3S2 framework, which encourages HER. As P and Al are introduced into NiWS@NF, hierarchical nanosheet heterostructures are formed and the elemental composition is modulated to enhance HER activity. Fig. 4c shows Al, P/NiWS-b@NF exhibiting an overpotential of 139, and 227 mV to afford 10, and 50 mA cm−2 current density, much lesser than Al/NiWS@NF (182, and 268 mV), P/NiWS@NF (202, 308 mV), and NiWS@NF F (285, and 361 mV). A smaller overpotential at Al, P/NiWS-b/NF indicates fast HER kinetics and high catalytic activity, as illustrated in Fig. 4c.Compared to NiWS@NF catalyst electrodes, Al, P/NiWS-b@NF exhibit superior HER activities, indicating that a heterostructure formed by NiS/Ni3S2 and Al, P/NiWS should be able to enhance WS2 HER. Thus, the improved electrocatalytic features of the synthesized heterostructures can be attributed to the synergistic interactions between the different dopants and host materials. By introducing Al and P, electronic conductivity and active site availability are improved, resulting in higher HER activity. Additionally, the unique morphology and high surface area of the NiS/Ni3S2/WS2 heterostructures further facilitate efficient hydrogen production. As part of the analysis of the HER kinetics, Tafel plots and a Tafel slope value were also utilized (Fig. 4d) to regulate the next step of the HER using the Tafel equation; η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j.46 In general, HER involves two stages, hydrogen adsorption and hydrogen desorption. Hydrogen adsorption occurs through the Volmer reaction, H+ + M + e− → M–Hads (where stands for active site and H represents absorbed hydrogen) and desorption occurs through either Heyrovsky, M–Hads + H+ + e− → M + H2 or Tafel reactions, 2M–Hads → 2M + H2 in an acidic medium. As reported, the Volmer, Heyrovsky, and Tafel reactions possess Tafel slopes of 120, 40, and 30 mV dec−1, respectively.47 Commercial 20% Pt/C had a Tafel slope of 54 mV dec−1, which is similar to what has been reported.48 It follows that Al/NiWS@NF and Al, P/NiWS-b/NF have the lowest Tafel slope values of 114 and 124, respectively, and follow the order NF (243 mV dec−1) > P/NWS@NF, (152 mV dec−1) > NiWS@NF, (128 mV dec−1). According to Tafel slope values for Al, P/NiWS-b/NF in an acidic medium, HER is processed via the Heyrovsky mechanism.49 In other words, the smaller Tafel values indicate more efficient kinetics and greater electrocatalytic features of Al, P/NiS/WS2/Ni3S4 towards the HER. Furthermore, the electrochemical double-layer capacitances (Cdl) measured in non-faradaic conditions were examined to determine the ECSA for the developed electrode materials (Fig. S7† and 4e). In Fig. 4e, Al, P/NiWS-b@NF revealed a significantly higher Cdl (55.6 mF cm−2) than Al, P/NiWS-c@NF (42.2 mF cm−2), Al, P/NiWS-a@NF (29.3 mF cm−2), P/NiWS@NF (21.3 mF cm−2), Al/NiWS@NF (29.6 mF cm−2), and NiWS@NF (9.37 mF cm−2). According to Cdl values, Al, P/NiWS-b@NF exhibits a strong increase in ESCA, indicating that Al and P doping can increase ESCA and increase HER accessibility. It validates that the Ni3S2/WS2 heterostructure has a higher exposure to efficient active sites, which contributes to its exceptional HER features as seen in Fig. 4a. Furthermore, the crosslinked H-WS2 nanosheet (Fig. 2f, HR-TEM images) as well as the abundant T-NiS/T-WS2 and H-WS2/T-Ni3S2 heterojunction in Al, P/NiWS-b@NF are regarded as major contributors to the increase in the ECSA value of Al, P/NiWS-b@NF. Electrocatalyst capacitance and interfacial properties at active state are investigated by EIS measurements (Fig. 4f, and Table 1). The smallest charge transfer resistance (Rct) was obtained for Al, P/NiWS-b@NF (0.683 ohm) compared to the reference's catalysts (Table 1) suggesting that electron transfer kinetics are much faster at the electrode–electrolyte interface. Considering the lowest Rct value for Al, P/NiWS-b@NF, it implies that there is a higher intrinsic conductivity present in the hybrid framework that is responsible for rapid electron transport kinetics in the electrocatalytic HER. By creating a heterointerface between Ni3S2 and WS2, the electronic structure of the heterostructure was modulated, boosting the charge transference and improving the catalytic features of the Ni3S2/WS2 heterostructure. Therefore, Al, P/NiWS-b@NF could be credited with promoting HER activity for the following reasons: (a) defects in Al, P/NiWS-b@NF provided active sites and a high charge transfer rate between Ni3S2/WS2 heterojunctions, resulting in rapid NiWS-b@NF catalytic kinetics. Consequently, the HER activity was further accelerated with a small Tafel slope after a low overpotential was achieved. Due to its thin petal-like structure, Ni3S2/WS2 exposes more active sites with a large ECSA and reduces charge transfer distance, which increases reactant accessibility. Lastly, the co-existence of WS2 and Ni3S2 phases and the co-doping of Al, P ions at the heterointerface resulted in numerous lattice defects that enhanced hydrogen adsorption. This dual-doping strategy not only increases the number of active sites but also optimizes the electronic properties of the material, resulting in a more efficient HER.
j.46 In general, HER involves two stages, hydrogen adsorption and hydrogen desorption. Hydrogen adsorption occurs through the Volmer reaction, H+ + M + e− → M–Hads (where stands for active site and H represents absorbed hydrogen) and desorption occurs through either Heyrovsky, M–Hads + H+ + e− → M + H2 or Tafel reactions, 2M–Hads → 2M + H2 in an acidic medium. As reported, the Volmer, Heyrovsky, and Tafel reactions possess Tafel slopes of 120, 40, and 30 mV dec−1, respectively.47 Commercial 20% Pt/C had a Tafel slope of 54 mV dec−1, which is similar to what has been reported.48 It follows that Al/NiWS@NF and Al, P/NiWS-b/NF have the lowest Tafel slope values of 114 and 124, respectively, and follow the order NF (243 mV dec−1) > P/NWS@NF, (152 mV dec−1) > NiWS@NF, (128 mV dec−1). According to Tafel slope values for Al, P/NiWS-b/NF in an acidic medium, HER is processed via the Heyrovsky mechanism.49 In other words, the smaller Tafel values indicate more efficient kinetics and greater electrocatalytic features of Al, P/NiS/WS2/Ni3S4 towards the HER. Furthermore, the electrochemical double-layer capacitances (Cdl) measured in non-faradaic conditions were examined to determine the ECSA for the developed electrode materials (Fig. S7† and 4e). In Fig. 4e, Al, P/NiWS-b@NF revealed a significantly higher Cdl (55.6 mF cm−2) than Al, P/NiWS-c@NF (42.2 mF cm−2), Al, P/NiWS-a@NF (29.3 mF cm−2), P/NiWS@NF (21.3 mF cm−2), Al/NiWS@NF (29.6 mF cm−2), and NiWS@NF (9.37 mF cm−2). According to Cdl values, Al, P/NiWS-b@NF exhibits a strong increase in ESCA, indicating that Al and P doping can increase ESCA and increase HER accessibility. It validates that the Ni3S2/WS2 heterostructure has a higher exposure to efficient active sites, which contributes to its exceptional HER features as seen in Fig. 4a. Furthermore, the crosslinked H-WS2 nanosheet (Fig. 2f, HR-TEM images) as well as the abundant T-NiS/T-WS2 and H-WS2/T-Ni3S2 heterojunction in Al, P/NiWS-b@NF are regarded as major contributors to the increase in the ECSA value of Al, P/NiWS-b@NF. Electrocatalyst capacitance and interfacial properties at active state are investigated by EIS measurements (Fig. 4f, and Table 1). The smallest charge transfer resistance (Rct) was obtained for Al, P/NiWS-b@NF (0.683 ohm) compared to the reference's catalysts (Table 1) suggesting that electron transfer kinetics are much faster at the electrode–electrolyte interface. Considering the lowest Rct value for Al, P/NiWS-b@NF, it implies that there is a higher intrinsic conductivity present in the hybrid framework that is responsible for rapid electron transport kinetics in the electrocatalytic HER. By creating a heterointerface between Ni3S2 and WS2, the electronic structure of the heterostructure was modulated, boosting the charge transference and improving the catalytic features of the Ni3S2/WS2 heterostructure. Therefore, Al, P/NiWS-b@NF could be credited with promoting HER activity for the following reasons: (a) defects in Al, P/NiWS-b@NF provided active sites and a high charge transfer rate between Ni3S2/WS2 heterojunctions, resulting in rapid NiWS-b@NF catalytic kinetics. Consequently, the HER activity was further accelerated with a small Tafel slope after a low overpotential was achieved. Due to its thin petal-like structure, Ni3S2/WS2 exposes more active sites with a large ECSA and reduces charge transfer distance, which increases reactant accessibility. Lastly, the co-existence of WS2 and Ni3S2 phases and the co-doping of Al, P ions at the heterointerface resulted in numerous lattice defects that enhanced hydrogen adsorption. This dual-doping strategy not only increases the number of active sites but also optimizes the electronic properties of the material, resulting in a more efficient HER.
| Electrocatalyst | η (mV vs. RHE) @ J = −10 mA cm−2 | η (mV vs. RHE) @ J = −50 mA cm−2 | Tafel slope (mV dec−1) | Cdl (mF cm−2) | Rct (Ω cm2) |
|---|---|---|---|---|---|
| NF | 322 | 494 | 283 | — | — |
| NiWS@NF | 285 | 361 | 128 | 9 | 3.20 |
| P/NiWS@NF | 202 | 308 | 152 | 21 | 3.19 |
| Al/NiWS@NF | 3 | 182 | 114 | 30 | 2.43 |
| Al, P/NiWS-a@NF | 195 | 288 | — | 29 | — |
| Al, P/NiWS-b@NF | 139 | 227 | 124 | 56 | 0.68 |
| Al, P/NiWS-c@NF | 154 | 256 | — | 42 | — |
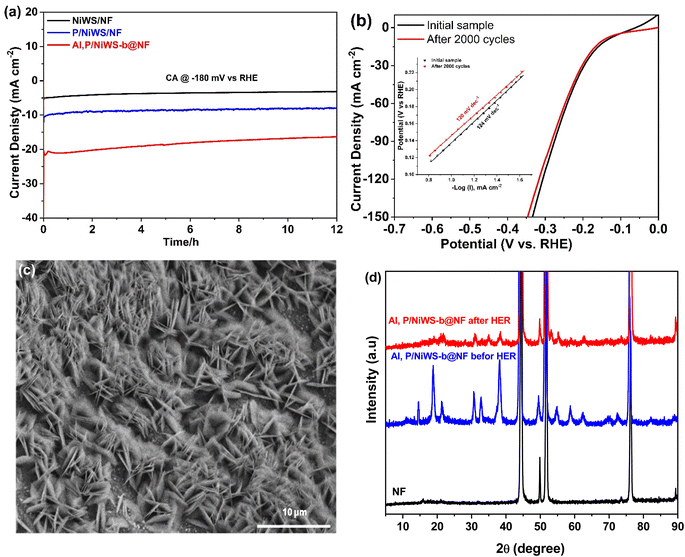
Further, electrocatalyst stability is important for practical applications. The long-term catalytic stability and durability of the Al, P/NiWS-b@NF, Al/NiWS@NF, P/NiWS@NF, NiWS@NF, and Pt/C@NF (20% wt) were assessed by chronoamperometric analysis at −180 mVRHE (Fig. 5a). According to Fig. S9,† commercial 20% Pt/C showed a 59.40% drop in current density after continuous electrocatalysis for 12 h in 0.5 M H2SO4 electrolyte. Current loss can be caused by several factors during a long-term stability test. Long-term immersion in a solution and the presence of gas bubbles could lead to the creation of adsorption products that would block activity or result in peeling away of low-dimensional nanostructures. Fig. 5a depicts the 12 hour chronopotentiometric durability test at a cathodic current density of 20 mA cm−2. The fact that there were no substantial fluctuations in starting current during the test indicates good stability.
To further examine the durability of the Al, P/NiWS-b@NF electrodes, a deterioration test in 0.5 M H2SO4 was carried out with potential sweeps from −0.7 to +0.2 V for 2000 cycles. As demonstrated in Fig. 5b, the LSV curves prior to and following 2000 cycles show a nearly negligible drop in current density, with a slight change of only around 10 mV at current density of 20 mA cm−2, which reveals a good stability of the Al, P/NiWS-b@NF catalyst, agreeing with Tafel plot measurements, where no increase in the Tafel slope was exhibited after cycling (inset in Fig. 5b, left). The morphology, crystal structure, and stability of Al, P/NiWS-b@NF samples were investigated using HR-SEM and XRD after the 12 h chronoamperometry test. As shown in Fig. 5c, the hierarchical and morphological structure of Al, P/NiWS@NF is stable after long-term testing. Furthermore, the XRD pattern showed that the Al, P/NiWS-b@NF heterostructure retained its crystal structure (Fig. 5d). The reduction in WS2 peak intensity might be attributed to the exfoliation of WS2 nanosheets. It has been shown that Al, P/NiWS-b@NF electrodes are both a promising and cost-effective option for HER applications. A comparison of the electrocatalytic properties of Al, P/NiWS@NF with other catalysts that have been reported in the literature can be found in Table 2. Particularly, the HER activity of our catalyst was significantly higher than that of other catalysts, resulting in a lower overpotential and a higher current density. Several synergies in the elaborate morphological structure, along with electronic and interfacial interactions, are responsible for the superior activity of Al, P/NiWS@NF. Strongly coupled WS2 nanosheets and NiS/Ni3S4 nanoparticles combined in a hierarchical framework provide maximum exposure to active heterogeneous interfaces as well as efficient mass and electron transfer. It has been recognized that in situ aluminum and phosphorus introductions are highly effective strategies for activating the inert basal plane and promoting the edge sites' activity of 2D WS2/MoS2 and other metal sulfides.49–52 As a result of the P dopant, metal sulfides can display an increase in valence bandwidth as well as higher density of states around Fermi levels, thereby enhancing conductivity.20,52 Additionally, Al decoration of metal sulfides, such as MoS2, may lead to a reduction in bandgaps, hence improving the intrinsic conductivity of nanodomains and allowing the conjunction of positive protons and catalysts, resulting in enhanced HER performance.53,54 Based on understanding the active origin of the Al, P/NiWS-b@NF catalyst, a rough estimation of its turnover frequency (TOF) was made, one of the most important criteria for evaluating an electrocatalyst's intrinsic performance. A further verification of the mass activities was carried out to determine the intrinsic characteristics of Al, P/NiWS-b@NF. In Note S1,† the specific calculation process for TOF and mass activities is described. By adding Al, P doping to NiWS-b@NF, mass activity increased from 112 A g−1 at 0.3 V vs. RHE to 575 A g−1 at 0.3 V vs. RHE (Table S3†). The acquired parameters are clearly in agreement with the CV, Tafel, and EIS results. As the benchmark HER catalyst, Pt/C displayed the TOF values of 0.772 s−1 at overpotentials of 0.300 V, respectively, in the 0.5 M H2SO4 electrolyte. Al, P/NiWS-b@NF catalysts required overpotentials of 0.300 V to achieve TOF values of 0.8 s−1, which is greater than that required for other investigated electrocatalysts in the same electrolyte, shown in Table S1.† It is evident that the Al, P/NiWS-b@NF electrodes perform significantly better than the investigated electrocatalysts. These results evidence that Al, P/NiWS-b@NF strongly enhanced the electrochemical performance of electrochemical HERs. Overall, Al, P/NiWS-b@NF electrode materials have a high potential for storage of energy and conversion devices due to their increased HER properties.
| Electrocatalyst | Electrode reaction | Electrolyte | Overpotential (mV) at −10 mA cm−2 | Tafel slope (mV dec−1) | Substrate/preparation method | Ref. |
|---|---|---|---|---|---|---|
| WS2NS2 | HER | 0.5 M H2SO4 | −150 | 138 | Glassy carbon/mechanical activation method | 55 |
| FexSy/WS2 Ns | HER | 0.5 M H2SO4 | −118 | 87 | Glassy carbon/hydrothermal method | 56 |
| MoS2/WS2 | HER | 0.5 M H2SO4 | −280 | 53 | Glassy carbon/hydrothermal method | 57 |
| NiS–NiS2–Ni3S4 | HER | 1 M KOH | −68 | 79 | Nickel foam/hydrothermal sulfidation method | 58 |
| Fe-doped WS2 | HER | 0.5 M H2SO4 | −166 | 82.2 | Carbon cloth/solvothermal method | 59 |
| Ni3S2@Ni2P/MoS2/NF | HER | 1 M KOH | −160 | 103.6 | Nickel foam/hydrothermal phosphating method | 60 |
| Ni/Ni3S2/SC NSAs | HER | 0.5 M H2SO4 | −90 | 81 | Carbon cloth/hydrothermal method | 61 |
| Ni3S2@NiSe/NF | HER | 0.5 M H2SO4 | −103 | 74.2 | Nickel foam/thermal diffusion method | 62 |
| Al, P/NiWS-b@NF | HER | 0.5 M H2SO4 | −136 @ 10 mA cm−2 | 124 | Nickel foam/hydrothermal method | This work |
| −227 @ 50 mA cm−2 |
4. Conclusion
In conclusion, the hydrothermal approach was employed for constructing an Al, P/NiWS-x@NF heterostructure on NF. Because of the advantageous heterostructural properties, particularly the nanoparticles/nanosheets structured as well as optimizing Al, and P co-doping concentrations, Al, P/NiWS-b@NF showed excellent electrocatalytic performance towards HER with a small Tafel slope (∼128 mV dec−1), and an overpotential of only 136 mV to obtain a current density of 10 mA cm−2 in acidic media. Based on the experimental results, the combination of Al and P co-doping with NiS/Ni3S2/WS2 heterostructure led to superb acidic HER performance and exceptional durability. A hierarchical structure facilitates fast charge/mass transport and maximizes active heterointerface accessibility. The present work demonstrates a method for boosting hydrogen desorption by synergistically combining multiple components, thus accelerating the H2 evolution.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Mghaib Al Shahrani: data curation, formal analysis, investigation. Mabrook S. Amer: conceptualization, writing – review & editing, data curation, supervision. Ahmad A. Alsaleh: data curation, writing – review & editing, Prabhakarn Arunachalam: data curation, writing – review & editing, Abdullah M. Al-Mayouf: supervision, funding acquisition, project administration.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the Researchers Supporting Project number (RSPD2024R540) at King Saud University, Riyadh, Saudi Arabia.References
- H. B. Gray, Nat. Chem., 2009, 1, 7 CrossRef CAS.
- V. R. Stamenkovic, D. Strmcnik, P. P. Lopes and N. M. Markovic, Nat. Mater., 2017, 16, 57–69 CrossRef CAS PubMed.
- M. S. Dresselhaus and I. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS.
- D. Niblett, M. Delpisheh, S. Ramakrishnan and M. Mamlouk, J. Power Sources, 2024, 592, 233904 CrossRef CAS.
- B. You and Y. Sun, Acc. Chem. Res., 2018, 51, 1571–1580 CrossRef CAS.
- P. Arunachalam, M. S. Amer, A. M. Al-Mayouf and A. A. Alsaleh, ChemCatChem, 2024, 16(14), e202400312 CrossRef CAS.
- P. Arunachalam, M. N. Shaddad, M. S. Amer, A. M. Alsalman and J. Madhavan, Environ. Sci.: Nano, 2024, 11(6), 2668–2682 RSC.
- M. S. Amer, P. Arunachalam, A. M. Al-Mayouf, A. A. AlSaleh and Z. A. Almutairi, Environ. Res., 2023, 236, 116818 CrossRef CAS PubMed.
- J. Li, J. Zhang, J. Zhang, K. M. Pan, H. Xu, H. Chen, G. Liu, N. Wu, C. Yuan and X. Liu, J. Mater. Chem. A, 2023, 11, 19812–19844 RSC.
- M. S. Faber and S. Jin, Energy Environ. Sci., 2014, 7, 3519–3542 RSC.
- Y. Jiao, Y. Zheng, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2015, 44, 2060–2086 RSC.
- Y. Jiao, Y. Zheng, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2015, 44, 2060–2086 RSC.
- T. Ling, T. Zhang, B. Ge, L. Han, L. Zheng, F. Lin, Z. Xu, W. B. Hu, X. W. Du and K. Davey, Adv. Mater., 2019, 31, 1807771 CrossRef PubMed.
- J. Xie and Y. Xie, Chem.–Eur. J., 2016, 22, 3588–3598 CrossRef CAS PubMed.
- X. Y. Yu and X. W. Lou, Adv. Energy Mater., 2018, 8, 1701592 CrossRef.
- M. S. Amer, P. Arunachalam, A. M. Al-Mayouf, A. A. Alsaleh, M. Alshalwi and Z. A. Almutairi, ACS Appl. Energy Mater., 2023, 6, 11718–11731 CrossRef CAS.
- L. Zhang, J. Xiao, H. Wang and M. Shao, ACS Catal., 2017, 7, 7855–7865 CrossRef CAS.
- Y. Guo, T. Park, J. W. Yi, J. Henzie, J. Kim, Z. Wang, B. Jiang, Y. Bando, Y. Sugahara and J. Tang, Adv. Mater., 2019, 31, 1807134 CrossRef PubMed.
- C. Huang, X. Miao, C. Pi, B. Gao, X. Zhang, P. Qin, K. Huo, X. Peng and P. K. Chu, Nano Energy, 2019, 60, 520–526 CrossRef CAS.
- D. Wu, S. Xu, M. Li, C. Zhang, Y. Zhu, Y. Xu, W. Zhang, R. Huang, R. Qi and L. Wang, J. Mater. Chem. A, 2015, 3, 16695–16707 RSC.
- L.-L. Feng, G. Yu, Y. Wu, G.-D. Li, H. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS PubMed.
- Y. Liu, H. Zhang, W. Song, Y. Zhang, Z. Hou, G. Zhou, Z. Zhang and J. Liu, Chem. Eng. J., 2023, 451, 138905 CrossRef CAS.
- H. Wang, X. Xu, B. Ni, H. Li, W. Bian and X. Wang, Nanoscale, 2017, 9, 15895–15900 RSC.
- S. Chandrasekaran, L. Yao, L. Deng, C. Bowen, Y. Zhang, S. Chen, Z. Lin, F. Peng and P. Zhang, Chem. Soc. Rev., 2019, 48, 4178–4280 RSC.
- Q. Lu, H. Wu, X. Zheng, Y. Cao, J. Li, Y. Wang, H. Wang, C. Zhi, Y. Deng and X. Han, Adv. Energy Mater., 2022, 12, 2202215 CrossRef CAS.
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong, S. Liu, X. Zhuang and X. Feng, Angew. Chem., 2016, 128, 6814–6819 CrossRef.
- P. Zhai, Y. Zhang, Y. Wu, J. Gao, B. Zhang, S. Cao, Y. Zhang, Z. Li, L. Sun and J. Hou, Nat. Commun., 2020, 11, 5462 CrossRef CAS PubMed.
- Q. Xu, J. Zhang, H. Zhang, L. Zhang, L. Chen, Y. Hu, H. Jiang and C. Li, Energy Environ. Sci., 2021, 14, 5228–5259 RSC.
- S. X. Leong, C. C. Mayorga-Martinez, X. Chia, J. Luxa, Z. Sofer and M. Pumera, ACS Appl. Mater. Interfaces, 2017, 9, 26350–26356 CrossRef CAS.
- X. Guo, J. Ji, Q. Jiang, L. Zhang, Z. Ao, X. Fan, S. Wang, Y. Li, F. Zhang, G. Zhang and W. Peng, ACS Appl. Mater. Interfaces, 2017, 9, 30591–30598 CrossRef CAS.
- L. Sun, M. Gao, Z. Jing, Z. Cheng, D. Zheng, H. Xu, Q. Zhou and J. Lin, Chem. Eng. J., 2022, 429, 132187 CrossRef CAS.
- Y. Gu, A. Wu, Y. Jiao, H. Zheng, X. Wang, Y. Xie, L. Wang, C. Tian and H. Fu, Angew. Chem., Int. Ed., 2021, 60(12), 6673–6681 CrossRef CAS PubMed.
- B. Rehman, K. M. M. D. K. Kimbulapitiya, M. Date, C. T. Chen, R. H. Cyu, Y. R. Peng and Y. L. Chueh, ACS Appl. Mater. Interfaces, 2024, 16, 32490–32502 CrossRef CAS.
- H. Chen, M. Hu, X. Wang, X. Xu, P. Jing, B. Liu and J. Zhang, Small Struct., 2023, 4, 2300026 CrossRef CAS.
- S. Ponnada, M. S. Kiai, D. B. Gorle, R. S. Bose, V. Rajagopal, B. Saini and R. K. Sharma, Catal. Sci. Technol., 2022, 12(14), 4413–4441 RSC.
- H. Wang, M. Ma, J. Li, Z. Zhang, W. Zhou and H. Liu, J. Mater. Chem. A, 2021, 9, 25539–25546 RSC.
- H. Liu, Z. Liu, F. Wang and L. Feng, Chem. Eng. J., 2020, 397, 125507 CrossRef CAS.
- C. Dai, B. Li, J. Li, B. Zhao, R. Wu, H. Ma and X. Duan, Nano Res., 2020, 13, 2506–2511 CrossRef CAS.
- K. Tang, X. Wang, Q. Li and C. Yan, Adv. Mater., 2018, 30, 1704779 CrossRef PubMed.
- X. Lu, R. Liu, Q. Wang and C. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 40014–40021 CrossRef CAS.
- Y. Yang, H. Yao, Z. Yu, S. M. Islam, H. He, M. Yuan, Y. Yue, K. Xu, W. Hao and G. Sun, J. Am. Chem. Soc., 2019, 141, 10417–10430 CrossRef CAS.
- P. Zhou, P. K. Wong, P. Niu, M. Chen, C. T. Kwok, Y. Tang, R. Li, S. Wang and H. Pan, Sci. China Mater., 2023, 66, 1033–1041 CrossRef CAS.
- M. Wang, W. Zhang, F. Zhang, Z. Zhang, B. Tang, J. Li and X. Wang, ACS Catal., 2019, 9, 1489–1502 CrossRef CAS.
- B. He, C.-Q. Peng, F. Ye, H.-W. Gao, Y. Wang, Y.-W. Tang, Q.-L. Hao, H.-K. Liu and Z. Su, CrystEngComm, 2021, 23, 3861–3869 RSC.
- Z. Fang, L. Peng, Y. Qian, X. Zhang, Y. Xie, J. J. Cha and G. Yu, J. Am. Chem. Soc., 2018, 140, 5241–5247 CrossRef CAS.
- Z. Liu, K. Wang, Y. Li, S. Yuan, G. Huang, X. Li and N. Li, Appl. Catal., B, 2022, 300, 120696 CrossRef CAS.
- P. Yu, F. Wang, T. A. Shifa, X. Zhan, X. Lou, F. Xia and J. He, Nano Energy, 2019, 58, 244–276 CrossRef CAS.
- C. Wang, T. Wang, J. Liu, Y. Zhou, D. Yu, F. Han, Q. Li, J. Chen and Y. Huang, Energy Environ. Sci., 2018, 11, 2467–2475 RSC.
- P. Liu, J. Zhu, J. Zhang, P. Xi, K. Tao, D. Gao and D. Xue, ACS Energy Lett., 2017, 2, 745–752 CrossRef CAS.
- D. Ren, Z. Liang, Y. H. Ng, P. Zhang, Q. Xiang and X. Li, Chem. Eng. J., 2020, 390, 124496 CrossRef CAS.
- L. Sun, M. Gao, Z. Jing, Z. Cheng, D. Zheng, H. Xu, Q. Zhou and J. Lin, Chem. Eng. J., 2022, 429, 132187 CrossRef CAS.
- D. R. Paudel, U. N. Pan, T. I. Singh, C. C. Gudal, N. H. Kim and J. H. Lee, Appl. Catal., B, 2021, 286, 119897 CrossRef CAS.
- J. Jian, Y. Li, H. Bi, X. Wang, X. Wu and W. Qin, ACS Sustain. Chem. Eng., 2020, 8, 4547–4554 CrossRef CAS.
- J. Jian, H. Kang, X. Qiao, K. Cui, Y. Liu, Y. Li, W. Qin and X. Wu, ACS Sustain. Chem. Eng., 2022, 10, 10203–10210 CrossRef CAS.
- Z. Wu, B. Fang, A. Bonakdarpour, A. Sun, D. P. Wilkinson and D. Wang, Appl. Catal., B, 2012, 125, 59–66 CrossRef CAS.
- H. Chen, Y. Li, H. Huang, Q. Kang and T. Ma, Energy Fuels, 2022, 36, 4888–4894 CrossRef CAS.
- D.-z. WANG, Y. Liu-yi-yi, R.-q. LIU, G. Ting, F. Hao and Z.-z. WU, Trans. Nonferrous Met. Soc. China, 2023, 33, 1540–1549 CrossRef CAS.
- H. Wang, W. Zhang, X. Zhang, S. Hu, Z. Zhang, W. Zhou and H. Liu, Nano Res., 2021, 14, 4857–4864 CrossRef CAS.
- X. Pu, J. Qian, J. Li, D. Gao and R. Zhang, FlatChem, 2021, 29, 100278 CrossRef CAS.
- X. Yu, S. Xu, Z. Wang, S. Wang, J. Zhang, Q. Liu, Y. Luo, Y. Du, X. Sun and Q. Wu, Dalton Trans., 2021, 50, 15094–15102 RSC.
- Y. Li, D. M. Patel, C. S. Tsang, R. Zhang, M. Liu, G. S. Hwang and L. Y. S. Lee, Adv. Mater. Interfaces, 2021, 8, 2001665 CrossRef CAS.
- N. Shaikh, I. Mukhopadhyay and A. Ray, J. Mater. Chem. A, 2022, 10, 12733–12746 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05868b |
| This journal is © The Royal Society of Chemistry 2024 |