Open Access Article
Open Access ArticleHigh-efficiency detection of primary amine-based chiral molecules by a facile aldimine condensation reaction†
Yang Yu‡
 *a,
Aiyan Shi‡b,
Tongtong Wang
*a,
Aiyan Shi‡b,
Tongtong Wang a,
Tiefeng Wanga and
Fei Xua
a,
Tiefeng Wanga and
Fei Xua
aCollege of Advanced Materials Engineering, Jiaxing Nanhu University, 314001, Jiaxing, P. R. China. E-mail: yuyang@jxnhu.edu.cn
bSmart Materials for Architecture Research Lab, Innovation Center of Yangtze River Delta, Zhejiang University, Jiaxing, 314100, P. R. China
First published on 8th October 2024
Abstract
Detection of chiral molecules in a high-efficiency way is very important to meet the demands for chiral analysis in drug testing, asymmetric synthesis, etc. Herein, we have developed a novel route to realize the rapid determination of concentration and configuration of primary amine-based chiral molecules. An aldehyde functionalized acid & base-sensitive fluorane dye (R–C) was used as the active agent to be reacted with the chiral molecules through an aldimine condensation reaction. After the mixing operation, concentration and configuration of the detected chiral molecule could be facilely read from the UV-vis absorption spectra and CD spectra, respectively.
Detection of chiral molecules (concentration and configuration) is of great significance in asymmetric synthesis, pharmaceutical analysis, etc.1–7 With the constant progress of science and technology, numerous methods for chiral molecular detection have been developed, such as nuclear magnetic resonance (NMR),8,9 chiral chromatography10,11 and spectral sensing assays,12–16 etc. Among the current detection schemes, spectral analysis has been regarded as one of the most competitive qualitative/quantitative analysis techniques, due to its unique advantages of facile operation, intuitive observation and low solvent consumption.17–20
Since most common chiral molecules (analytes) haven't the ability of absorption in ultraviolet (UV) and visible (vis) bands, traditional absorption spectral instruments cannot detect any identifiable signal.21,22 To solve this problem, the chiral analytes have been used to produce specific absorption characteristics by linking them with some suitable chromophores through covalent or non-covalent interactions.23,24 Based on this method, the absorption intensity detected by the UV-vis absorption spectrometer can be used to determine the concentration of chiral analytes.25 And, the circular dichroism (CD) absorption signal measured by CD spectrometer can identify the configuration of chiral analytes directly.26–31
So far, various functional materials have been developed to be combined with chiral analytes, so that to generate recognizable absorption signals.32,33 For example, detected chiral signal could be generated after the interaction between the chiral analytes and some polymers34 or supramolecular assemblies35 (hydrogen bonds, electrostatic interaction, etc.). In this way, high-sensitivity detection can be realized, but the poor repeatability cannot be ignored and applicable range of detective concentration is usually narrow. In another kind of material system, some organic molecules36/oligomers37 with active functional groups or some specific ionic compounds38 can react with chiral analytes to form new products with the formation of apparent spectral absorption. With this method, the repeatability and applicability of the detection on chiral analytes could be ensured.39 However, the detection sensitivity and the operability still need to be improved.40,41 According to the material design principle, exploring a functional molecule with sensitive reaction sites, easy synthesis processes and high molar absorption efficiencies are the key parameters to optimize the detection sensitivity and promote the application.
Therefore, in this work, we have designed a novel fluorane dye-based molecular sensor (R–C, 6′-(diethylamino)-3′-hydroxy-3-oxo-3H-spiro[isobenzofuran-1,9′-xanthene]-4′-carbaldehyde) with the functional group of aldehyde to detect the primary amines-based chiral molecules (Scheme 1). Based on the aldimine condensation reaction,42–47 the chiral analytes could react with the R–C to form new chiral products. And, due to the exhibited acidity or alkalinity of chiral analytes, the new product has a strong absorption in vis band induced by the ring-open pattern of pH-sensitive dye48–50 (R–C). According to this mechanism, the concentration of chiral analytes could be facilely determined by the UV-vis absorption spectra, while the configuration could be easily read from the CD spectra.
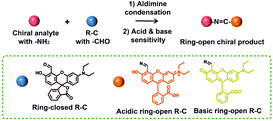 | ||
| Scheme 1 Schematic diagram of the chiral detection mechanism between R–C and the chiral molecules with –NH2. | ||
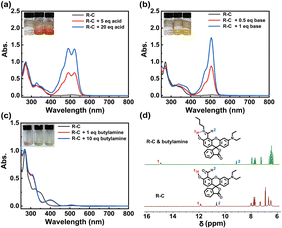
Firstly, the R–C was prepared through a two-step organic synthesis process (Scheme S1 and Fig. S1†). And, its acid & base-responsive property was researched. As shown in Fig. 1a and b, the R–C was transparent without any absorption at vis band. When R–C was treated with acid/base (trifluoroacetic acid, TFA/tetrabutyl ammonium hydroxide, TBAOH), the characteristic absorption spectra appeared with the formation of various colors (orange in acidic state, deep yellow in alkaline state). This meant that the R–C has the potential to identify the chiral analytes which could exhibit acidity or alkalinity.
Apart from the absorption ability, the formation of new chiral product is another necessary condition for the chiral analytes, especially for the absolute configuration. Thus, the reaction feasibility between the R–C and the compounds with primary amines (–NH2) has been verified (Fig. 1c). The appeared change of UV-vis absorption spectra in the mixture of R–C and the primary amines-based chiral molecule (taking the butylamine as the example) meant the formation of a new product, compared with the UV-vis absorption spectra of R–C and butylamine (BA), respectively. In addition, from the results of 1H nuclear magnetic resonance (NMR) spectra (no. 1 and no. 2 hydrogen (H) had apparent shifts due to the presence of intramolecular hydrogen bond), it could be concluded that a “Schiff base” structure was generated by the condensation reaction between R–C and BA (Fig. 1d and S2†). Therefore, the R–C had a potential to be used as a sensitive sensor for the detection of primary amines-based chiral molecules which could exhibited the property of acid or base, according to the above experimental results.
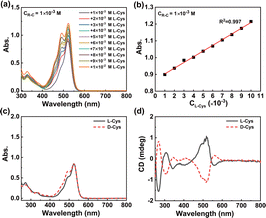
After the feasibility verification, we then researched the detection effects of R–C for the primary amines-based chiral molecules. Since the most amino acids have –NH2 and the ability of exhibiting acidity or alkalinity, they were suitable to be reacted with R–C as the detected chiral compounds. Taking the L-cysteine (L-Cys) as an example of chiral analyte, the detection of its concentration and configuration were validated. As shown in Fig. 2a, b and S3,† a linear response (R2 >99.7%) in the absorption intensity change could be observed with the addition of L-Cys (1 × 10−3 M–1 × 10−2 M) in the solution of 1 × 10−3 M R–C in acetonitrile (CH3CN). This meant that the R–C could be used as a sensor to detect the L-Cys according to a specific linear relationship. In addition, by changing the concentration of R–C, L-Cys with other concentration range could be detected effectively (Fig. S4†).
Based on the experimental results, it could be concluded that the detection range of L-Cys could be from 4 × 10−4 M to 1 × 10−2 M. And, even the concentration of L-Cys was as low as 3 × 10−4 M, it could be detected by the R–C with an apparent absorption change in the visible band, indicating the high sensitivity of our prepared system (Fig. S5†). In addition, the limit of quantitation (LOQ) was calculated as 1.14 × 10−4 M, which further proved this viewpoint.
Apart from the concentration of the chiral analyte, the configuration is also very important to be determined. According to the aldimine condensation reaction, the L-Cys could be combined with R–C through a covalent bond (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), accompanied by the formation of the new chiral reaction product. Thus, we measured chiral absorption property of the mixture systems composed of R–C and L-Cys/D-Cys (Fig. 2c and d). Specific absorption of acidic ring-open R–C was observed, accompanied by the appearance of totally symmetrical CD signals, indicating that the new chiral products with specific CD absorption features were prepared effectively. Therefore, according to the above results, R–C could be applied as an effective qualitative and quantitative detection material for the typical primary amines-based chiral molecules with acidity or alkalinity.
N–), accompanied by the formation of the new chiral reaction product. Thus, we measured chiral absorption property of the mixture systems composed of R–C and L-Cys/D-Cys (Fig. 2c and d). Specific absorption of acidic ring-open R–C was observed, accompanied by the appearance of totally symmetrical CD signals, indicating that the new chiral products with specific CD absorption features were prepared effectively. Therefore, according to the above results, R–C could be applied as an effective qualitative and quantitative detection material for the typical primary amines-based chiral molecules with acidity or alkalinity.
Then, the reaction mechanism was researched in detail. The reaction product prepared using the combination of R–C and L-Cys was determined by the 1H NMR spectra. As revealed in Fig. 3, with the addition of L-Cys, no. 2 H exhibited an apparent shift, which meant the formation of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. It was similar with the reaction result between R–C and BA (Fig. 1d), indicating the successful occurrence of aldimine condensation reaction. In addition, compared with the 1H NMR spectra of acidified and alkalized R–C, the L-Cys showed similar results in the chemical shifts of H with acidified R–C (Fig. S6†). Thus, the absorption change was due to the production of acidified ring-open R–C grafted with L-Cys. The similar spectral absorption changing results proved this viewpoint (Fig. 1a and 2a).
C. It was similar with the reaction result between R–C and BA (Fig. 1d), indicating the successful occurrence of aldimine condensation reaction. In addition, compared with the 1H NMR spectra of acidified and alkalized R–C, the L-Cys showed similar results in the chemical shifts of H with acidified R–C (Fig. S6†). Thus, the absorption change was due to the production of acidified ring-open R–C grafted with L-Cys. The similar spectral absorption changing results proved this viewpoint (Fig. 1a and 2a).
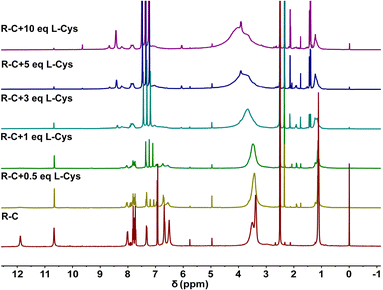 | ||
| Fig. 3 1H NMR spectra of 1.2 × 10−2 M R–C and when it was mixed with various eq. L-Cys in deuterated dimethyl sulfoxide (DMSO). | ||
In addition, the apparent molecular ion peak of the mass spectral result of the mixture of R–C and L-Cys kept the similar with the molecular weight of their condensation product (Fig. S7†). This result could further confirm that the chiral sensing mechanism in this system was based on the condensation reaction between R–C and L-Cys. According to these experimental results, the reaction process was depicted in Fig. S8.† R–C with –CHO was reacted by L-Cys with –NH2 to produce a new chiral product composed of acidic ring-open R–C and L-Cys, accompanied with apparent absorption changes in UV-vis and CD spectra.
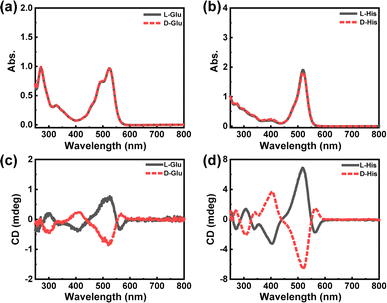
Based on the design concept and reaction mechanism, the primary amines-based chiral molecules exhibiting acidity or alkalinity could react with R–C to form a chiral product with strong absorption in the visible band. This meant that most amino acids could be detected in this way. Thus, two typical amino acids (L/D-glutamic acid, L/D-Glu; L/D-histidine, L/D-His) were tried here. As demonstrated in Fig. 4, apparent absorption changes and symmetric CD spectra could be induced by the addition of various chiral analytes with the opposite configurations. Considering the features of this system, other chiral molecules with both primary amines and acidity/alkalinity, such as aniline derivatives, saccharides, etc. could also be facilely detected in this way. As shown in Fig. S9,† a typical compound with –NH2 ((S)-(−)-α-methylbenzylamine, S(−)-α-MBA) was taken as the chiral analyst to be reacted with R–C. From the spectral measurement result, the concentration of S(−)-α-MBA could be determined by the absorption value effectively, which was similar with the detection results of L-Cys. This phenomenon indicated the great scalability of this detection system.
In conclusion, we have efficiently developed a facile method to achieve the rapid detection (concentration & configuration) of the primary amines-based chiral molecules exhibiting acidity or alkalinity. Through a facile aldimine condensation reaction, a pH-sensitive dye (R–C) could react with chiral analytes, which could exhibit the property of acid or base, so that to form a new chiral product with strong absorption effects in the visible band. The optical changes could be easily detected by the UV-vis absorption and CD spectra. It is worth mentioning that the R–C was only a typical representative molecule, which could be used as an effective chiral sensor. According to the features of various chiral analytes, different chiral molecular sensors could be designed and prepared according to the chiral sensing mechanism proposed in this work. In a word, this unique exploration opens a new route to achieve the high-efficiency chiral detection and undoubtedly will promote the development of the novel chiral analysis materials.
Experimental section
Experimental methods are available in the ESI.†Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its ESI materials].†Author contributions
Yang Yu: conceptualization, methodology, writing – original draft, resources, funding acquisition; Aiyan Shi: validation, software, data curation, investigation, writing – review & editing; Tongtong Wang: writing – review editing, funding acquisition; Tiefeng Wang: project administration, resources; Fei Xu: data curation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the General Scientific Research Project of Department of Education of Zhejiang Province (Y202454574), Youth Science and Technology Talent Special Project of Jiaxing (2024AY40017), the 2022 Ministry of Education “Chunhui Program” Co-Operative Scientific Research Project of Ministry of Education of China (No. HZKY20220195), the Public Welfare Research Plan of Jiaxing (2023AY11016), and the Start-Up Funds of Jiaxing Nanhu University (No. QD61220019 and No. QD61220017) for their financial supports.Notes and references
- Y. Liu, Z. Wu, D. W. Armstrong, H. Wolosker and Y. Zheng, Nat. Rev. Chem, 2023, 7, 355–373 CrossRef PubMed.
- H.-L. Qian, S.-T. Xu and X.-P. Yan, Anal. Chem., 2023, 95, 304–318 CrossRef PubMed.
- M. L. Solomon, A. A. E. Saleh, L. V. Poulikakos, J. M. Abendroth, L. F. Tadesse and J. A. Dionne, Acc. Chem. Res., 2020, 53, 588–598 CrossRef PubMed.
- C. Hao, L. Xu, H. Kuang and C. Xu, Adv. Mater., 2020, 32, 1802075 CrossRef CAS PubMed.
- P. Anzenbacher Jr, S. M. George and A. R. Sartori, Chem, 2024, 10, 2345–2348 Search PubMed.
- E. G. Shcherbakova, T. D. James and P. Anzenbacher Jr, Nat. Protoc., 2020, 15, 2203–2229 CrossRef CAS.
- S. Sheykhi, L. Mosca, M. Pushina, K. Dey and P. Anzenbacher Jr, Chem. Commun., 2020, 56, 8964–8967 RSC.
- L. T. Kuhn, K. Motiram-Corral, T. J. Athersuch, T. Parella and M. Pérez-Trujillo, Angew. Chem., Int. Ed., 2020, 59, 23615–23619 CrossRef CAS PubMed.
- G. Gu, Z. Xu, L. Wen, J. Liang, C. Wang, X. Wan and Y. Zhao, JACS Au, 2023, 3, 1348–1357 CrossRef CAS.
- D. Wu, C. Ma, G.-C. Fan, F. Pan, Y. Tao and Y. Kong, J. Sep. Sci., 2022, 45, 325–337 CrossRef CAS PubMed.
- W. Wang, Y. Zhang, B. Tang, H. Hou, S. Tang and A. Luo, J. Chromatogr. A, 2022, 1675, 463150 CrossRef CAS.
- M. Quan, X.-Y. Pang and W. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202201258 CrossRef CAS PubMed.
- F. Fan, C. Zhong, Z. Zhang, S. Li and S. Chang, Nanoscale Adv., 2021, 3, 4790–4798 RSC.
- L. Pu, Chem. Commun., 2022, 58, 8038–8048 RSC.
- Y. Chen, F. Zhao, J. Tian, L. Jiang, K. Lu, Y. Jiang, H. Li, S. Yu, X. Yu and L. Pu, J. Org. Chem., 2021, 86, 9603–9609 CrossRef CAS.
- L. Pu, Angew. Chem., Int. Ed., 2020, 59, 21814–21828 CrossRef CAS PubMed.
- J. H. Han, Y.-C. Lim, R. M. Kim, J. Lv, N. H. Cho, H. Kim, S. D. Namgung, S. W. Im and K. T. Nam, ACS Nano, 2023, 17, 2306–2317 CrossRef CAS.
- H. Zhu, Q. Li, Z. Gao, H. Wang, B. Shi, Y. Wu, L. Shangguan, X. Hong, F. Wang and F. Huang, Angew. Chem., Int. Ed., 2020, 59, 10868–10872 CrossRef CAS PubMed.
- R. M. Kim, J.-H. Huh, S. Yoo, T. G. Kim, C. Kim, H. Kim, J. H. Han, N. H. Cho, Y.-C. Lim, S. W. Im, E. Im, J. R. Jeong, M. H. Lee, T.-Y. Yoon, H.-Y. Lee, Q.-H. Park, S. Lee and K. T. Nam, Nature, 2022, 612, 470–476 CrossRef PubMed.
- S. A. Valenzuela, H. S. N. Crory, C.-Y. Yao, J. R. Howard, G. Saucedo, A. P. d. Silva and E. V. Anslyn, Angew. Chem., Int. Ed., 2021, 60, 13819–13823 CrossRef.
- D. S. Hassan and C. Wolf, Nat. Commun., 2021, 12, 6451 CrossRef PubMed.
- A. Prabodh, D. Bauer, S. Kubik, P. Rebmann, F. G. Klärner, T. Schrader, L. D. Bizzini, M. Mayor and F. Biedermann, Chem. Commun., 2020, 56, 4652–4655 RSC.
- S. Huang, H. Yu and Q. Li, Adv. Sci., 2021, 8, 2002132 CrossRef.
- G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2008, 108, 1–73 CrossRef.
- C. Wolf and K. W. Bentley, Chem. Soc. Rev., 2013, 42, 5408–5424 RSC.
- Y. Yu, G. Yang, S. Zhang, M. Liu, S. Xu, C. Wang, M. Li and S. X.-A. Zhang, ACS Nano, 2022, 16, 148–159 CrossRef PubMed.
- Y. Xiao, A. Shi, G. Yang, Y. Yu, Q. Nie, S. Qi, C. Xiang and T. Zhang, Small, 2024, 2404913 CrossRef.
- J. R. Howard, J. R. Shuluk, A. Bhakare and E. V. Anslyn, Chem, 2024, 10, 2074–2088 Search PubMed.
- A. Sripada, F. Y. Thanzeel and C. Wolf, Chem, 2022, 8, 1734–1749 Search PubMed.
- J. R. Howard, A. Bhakare, Z. Akhtar, C. Wolf and E. V. Anslyn, J. Am. Chem. Soc., 2022, 144, 17269–17276 CrossRef PubMed.
- J. Lim, M. Guo, S. Choi, S. J. Miller and E. V. Anslyn, Chem. Sci., 2023, 14, 5992–5999 RSC.
- J. S. S. K. Formen, J. R. Howard, E. V. Anslyn and C. Wolf, Angew. Chem., Int. Ed., 2024, 63, e202400767 CrossRef PubMed.
- J. Guo, x. Hou, J. Hu, Y. Geng, M. Li, H. Wang, l. Wang and Q. Luo, Chem. Commun., 2023, 59, 9157–9166 RSC.
- S. Ma, B. Zhao and J. Deng, ACS Cent. Sci., 2023, 9, 1409–1418 CrossRef.
- J.-Z. Liu, X.-Y. Chai, J. Huang, R. S. Li, C. M. Li, J. Ling, Q.-E. Cao and C. Z. Huang, Anal. Chem., 2024, 96, 4282–4289 CrossRef.
- C. Wolf and K. W. Bentley, Chem. Soc. Rev., 2013, 42, 5408–5424 RSC.
- Y. Wang, Y. Chen, H. Bian, Y. Sun, L. Zhu and D. Xia, Sens. Actuators, B, 2021, 341, 130044 CrossRef.
- D. Wu, Q. Yin, P. Cai, X. Zhao and Y. Pan, Anal. Chim. Acta, 2017, 962, 97–103 CrossRef PubMed.
- L. A. Warning, A. R. Miandashti, L. A. McCarthy, Q. Zhang, C. F. Landes and S. Link, ACS Nano, 2021, 15, 15538–15566 CrossRef.
- M. Hu, H.-T. Feng, Y.-X. Yuan, Y.-S. Zheng and B. Z. Tang, Coord. Chem. Rev., 2020, 416, 213329 CrossRef.
- M. M. Moein, Talanta, 2021, 224, 121794 CrossRef.
- G. Yang, Y. Yu, B. Yang, T. Lu, Y. Cai, H. Yin, H. Zhang, N. Zhang, L. Li, Y.-M. Zhang and S. X.-A. Zhang, Angew. Chem., Int. Ed., 2021, 60, 2018–2023 CrossRef.
- G. Yang, Z. Yao, X. Yang, Y. Xie, P. Duan, Y.-M. Zhang and S. X.-A. Zhang, Adv. Sci., 2022, 9, 2202636 CrossRef CAS PubMed.
- A. Bade, P. Yadav, L. Zhang, R. N. Bypaneni, M. Xu and T. E. Glass, Angew. Chem., Int. Ed., 2024, e202406401 Search PubMed.
- M. R. Smith, L. Zhang, Y. Jin, M. Yang, A. Bade, K. D. Gillis, S. Jana, R. N. Bypaneni, T. E. Glass and H. Lin, ACS Cent. Sci., 2023, 9, 980–991 CrossRef PubMed.
- L. Zhang, X. A. Liu, K. D. Gillis and T. E. Glass, Angew. Chem., Int. Ed., 2019, 58, 7611–7614 CrossRef PubMed.
- C. Yin, F. Huo, N. P. Cooley, D. Spencer, K. Bartholomew, C. L. Barnes and T. E. Glass, ACS Chem. Neurosci., 2017, 8, 1159–1162 CrossRef PubMed.
- J. Li, G. Yang, A. Shi, D. Han, M. Han, C. Xiang, T. Zhang and H. Wang, Adv. Opt. Mater., 2024, 12, 2400156 CrossRef.
- A. Shi, H. Wang, G. Yang, C. Gu, C. Xiang, L. Qian, J. W. Y. Lam, T. Zhang and B. Z. Tang, Angew. Chem., Int. Ed., 2024, 63, e202409782 Search PubMed.
- J. Sun, M. Han, G. Yang, Y. Wang, W. Fang, A. Shi, C. Xiang, J. Wang, T. Zhang and H. Wang, Adv. Opt. Mater., 2024, 2402080 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary figure for devices. See DOI: https://doi.org/10.1039/d4ra06291d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |