Open Access Article
Open Access ArticleEnhanced desulfurization performance of model fuel by Cu–ZnO/TiO2 heterostructure†
Jiaxin Xu,
Yongjie Zheng *,
Jingzhi Tian,
Yunpeng Zhao and
Heshan Zheng
*,
Jingzhi Tian,
Yunpeng Zhao and
Heshan Zheng
Qiqihar University, Qiqihar 161006, P. R. China. E-mail: zyj1964@163.com; Fax: +86-452-2738152
First published on 18th November 2024
Abstract
A facile hydrothermal approach was employed to synthesize a novel Cu–ZnO/TiO2 Z-heterojunction with a high density of defects, which was then utilized for the oxidative desulfurization process, demonstrating excellent photodegradation performance. The results showed that by adjusting components such as Cu, ZnO, and TiO2, the removal efficiency of DBT reached 88.12% within a duration of 240 min. In the 5 repeated experiments, 7.5%Cu–ZnO/TiO2 still exhibited high stability and could be reused. The improved photocatalytic performance of the 7.5%Cu–ZnO/TiO2 composite can be attributed to its high light absorption capability and well-matched energy levels, which are due to the abundant presence of imperfections. The adoption of a Z-heterojunction has enabled efficient separation and transfer of photo-generated electrons and holes (e−/h+), thereby reducing the probability of charge carrier recombination.
1 Introduction
In the combustion process of automobile engines, due to the oxidation reaction of organic sulfide, a large amount of SOX is produced, which is the main cause of acid rain. As a result, environmental concerns have compelled refiners to develop effective desulfurization technologies for the removal of these sulfur-containing compounds. HDS is commonly used for industrial desulfurization, but it faces challenges in removing organic sulfides with steric hindrance.1,2 PODS is a cost-effective and efficient method for desulfurization that eliminates the need for hydrogen gas, while also exhibiting high oxidative activity towards difficult-to-dissolve sulfur compounds like dibenzothiophene (DBT).The wide band gap and limited utilization of visible light of TiO2 and ZnO affect their overall efficiency.3–5 Therefore, people have been focusing on improving the activity of photocatalyst through morphological regulation, metal or non-metal doping, defect engineering, etc.6–10 In particular, heterojunction construction with other semiconductors with advantages in bandgap and photocatalytic activity can effectively promote photogenerated charge transfer, inhibit carrier recombination, and improve light utilization efficiency. Since the conduction potential and valence band potential of ZnO are slightly lower than those of TiO2, when ZnO and TiO2 form a type II heterojunction, charge can be effectively transferred from ZnO to TiO2.11 Additionally, hole transfer occurs in the opposite direction, which aids in achieving efficient electron–hole separation.12–14 The ZnO–TiO2 composite nanomaterials were synthesized using the co-precipitation method, as reported by Lachom et al.15 The reaction rate constant of methyl orange was found to be 1.86 × 10−2 min−1, demonstrating a significant improvement in efficiency compared to pure TiO2 and ZnO particles. Cheng et al. selectively deposited amorphous TiO2 onto the tips of ZnO nanorods, resulting in a ZnO/TiO2 hybrid nanostructure leads to the manifestation of remarkably improved photocatalytic performance in comparison to that of individual ZnO nanorods and amorphous TiO2 nanoparticles.16 Song et al. discovered that ZnO nanorods were grafted onto electrospun TiO2 nanofibers using hydrothermal technology, and silver metal nanoparticles were uniformly dispersed on the surface to create a ternary photocatalyst.17 The presence of the heterostructure and Schottky barrier significantly enhances the photocatalytic activity, thereby effectively improving the antibiotic effect.
Anatase type TiO2 has higher reactivity on the (001) crystal surface, but at present most crystals are mainly exposed on the (101) surface, which has lower surface energy. By employing titanium tetrafluoride as the primary ingredient and HF as the regulating agent, Yang et al. effectively produced anatase TiO2 with a surface exposure percentage of 47% on the (001) plane.18 However, the catalysis of fluoride makes the photocatalyst highly toxic and corrosive, and it is difficult to remove fluorine-containing compounds. Zhao et al. synthesized a stable mesoporous anatase type TiO2 nanosphere by hydrothermal method, and effectively stabilized the (001) plane by using sulfuric acid as an inducer and capping agent.19 The interaction between sulfuric acid and the (001) plane exhibits a relatively weak affinity, which facilitates the easy removal of the sulfuric acid capping agent through washing.
The paper successfully synthesized anatase TiO2 nanospheres with a (001) crystal surface without fluorine, and grafted ZnO nanoneedles onto the surface to construct a photocatalytic material with ternary Z-type heterojunction. The photocatalytic activity of (Cu–ZnO/TiO2) was evaluated, and its stability and repeatability were verified through systematic studies of chemical composition, structure, morphology, and physicochemical properties. Additionally, free radical trapping experiments were conducted to explore the possible photocatalytic mechanism.
2 Experiment
2.1 Materials and chemical reagents
Tetrabutyl titanate (TBOT, 98.0%) and zinc acetate (99.0%) are offered by Tianjin Kemi Ou Chemical Reagent Co., Ltd. The copper nitrate (99.0%) is provided by Tianjin Guangfu Fine Chemical Research Institute. Sodium hydroxide (96.0%) is provided by Tianjin Damao Chemical Reagent Factory. Sulfuric acid (98.0%) is provided by Liaoning Quanrui Reagent Co., Ltd. The dibenzothiophene (DBT, 99.0%), benzothiophene (BT, 99.0%), thiophene (T, 99.0%), and n-octane (96.0%) are from Shanghai Maclin Biochemical Technology Co., Ltd. Anhydrous ethanol (99.8%) is provided by Tianjin Kai Tong Chemical Reagent Co., Ltd.2.2 Synthesis of materials
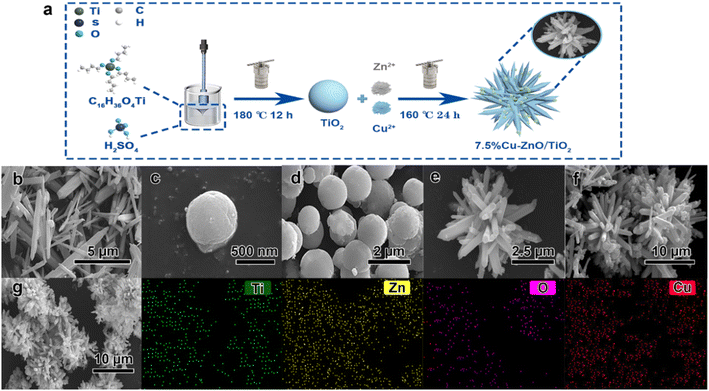
Preparation of Cu–ZnO/TiO2 composite materials: the synthesis of the Cu–ZnO/TiO2 nanocomposite material was achieved using a simple hydrothermal technique, as illustrated in Fig. 1(a). In the aforementioned B solution, Cu2+ is incorporated in varying proportions of 2.5%, 5.0%, 7.5%, 10.0%, and 12.5% relative to the mass of zinc while maintaining constant experimental conditions, to synthesize Cu–ZnO/TiO2 ternary photocatalysts denoted as y%Cu–ZnO/TiO2.
2.3 Photodegradation of organic sulfides
The present study utilized an n-octane solvent containing dibenzothiophene (DBT) as a representative oil model to assess the photocatalytic degradation activity of sulfur-containing organic compounds. In a typical desulfurization experiment, 0.10 g of photocatalyst was uniformly dispersed within a quartz tube containing 50 ml of simulated oil with a concentration of 10 mg L−1. Firstly, samples were collected after 30 min of stirring under dark conditions to achieve adsorption–desorption equilibrium. Subsequently, the samples were exposed to ultraviolet light and sampled at 30 min intervals throughout the reaction process, with approximately 3 ml of sample collected each time. To ensure accurate data analysis, it is imperative to promptly centrifuge and separate the sample after completing the reaction, and then transfer it into a properly labeled sample vial. A quantitative analysis was conducted on the filtrate using a UV-9000 ultraviolet-visible spectrophotometer to estimate the degradation efficiency. The concentration of DBT in the supernatant is determined by establishing a correlation between absorbance and concentration. The influence of degradation parameters, such as the initial concentration of DBT, catalyst dosage, and catalyst composition ratio on degradation efficiency was investigated and optimized through single-factor experiments. Finally, the degradation efficiency is calculated based on eqn (1):20| Degradation rate (%) = [(C0 − Ct)/C0] × 100 = [(A0 − At)/A0] | (1) |
In this context, C0 and Ct represent the initial concentration and real-time concentration (mg L−1) of DBT at time t (min), while A0 and At correspond to the absorbance of DBT at the beginning point and different time intervals (t), respectively. The breakdown of organic pollutants using photocatalysis generally follows a kinetic model that resembles pseudo-first-order kinetics, as indicated by previous research findings. Therefore, this study examined the kinetics characteristics of DBT photocatalytic degradation using the pseudo-first-order kinetic equation.
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ct/C0 = −kt Ct/C0 = −kt
| (2) |
In this equation, k represents the apparent rate constant (min−1); the parameters C0 and Ct are analogous to those in eqn (1), while t denotes the duration of the reaction (min).
The experiment evaluated the role of active substances in the PODS system by trapping superoxide radicals, hydroxyl radicals, holes and electrons.
3 Results and discussion
3.1 Physical and chemical properties
After conducting SEM analysis (Fig. 1), it was observed that the synthesized microstructure of ZnO consists of nanoneedle, while TiO2 nanoparticles were uniformly dispersed in a spherical shape with a rough surface, exhibiting a diameter ranging from 400 to 600 nm. In the composite catalyst of 7.5%Cu–ZnO/TiO2, the TiO2 nanospheres act as the core and are decorated with needle-like ZnO nanoneedles. EDS spectrum analysis confirmed the successful integration of all components in the ternary photocatalyst.The XPS spectrum measurement reveals the presence of four elements, namely O, Ti, Zn, and Cu in 7.5%Cu–ZnO/TiO2 in Fig. 3(a–e). In the case of pure TiO2, the energy peaks detected at 458.78 eV and 464.48 eV can be ascribed to the orbitals of Ti 2p3/2 and Ti 2p1/2, respectively. Moreover, the observed spacing between these peaks measuring 5.70 eV strongly indicates that titanium exists in its +4 oxidation state.21–23 In the ZnO/TiO2 and 7.5%Cu–ZnO/TiO2 samples, the observation of shoulder bands attributed to Ti3+ 2p1/2 and Ti3+ 2p3/2 suggests that oxygen vacancies have been incorporated into the composite photocatalysts.24,25 Previous studies have demonstrated the crucial role played by the oxidation state of titanium in determining the catalytic efficiency of compounds that incorporate this element. As the reaction progresses, there is an observed augmentation in the Ti3+/Ti4+ ratio, leading to an enhancement in the photocatalytic effect of composite catalysts.26 The Zn 2p spectrum reveals energy bands at 1022.18 eV and 1045.28 eV for Zn 2p3/2 and Zn 2p1/2, respectively, exhibiting a binding energy difference of 23.1 eV between the two orbitals. Consequently, it can be inferred that zinc predominantly exists in the form of Zn2+ and readily forms stable chemical bonds (Zn–O bonds) with oxygen atoms.27,28 The measured binding energies of Cu 2p3/2 and Cu 2p1/2 are determined to be 931.18 eV and 950.98 eV, respectively, indicating a consistent energy difference of 19.8 eV between the two orbitals. This value corresponds to the standard spin–orbit components associated with Cu 2p.29 There are two main peaks at 931.28 and 951.28 eV, and further deconvolution reveals four peaks. The peaks at 931.18 eV and 950.98 eV are generated by Cu0, and the peaks at 932.28 eV and 951.98 eV are generated by Cu2+. In the spectrum of 7.5%Cu–ZnO/TiO2, three distinct peaks can be observed, representing oxygen species associated with the crystal lattice (OL), oxygen vacancies (OV), and adsorbed oxygen molecules (Oad). The 7.5%Cu–ZnO/TiO2 catalyst in the three comparative samples exhibits a significantly increased presence of oxygen vacancies, which undoubtedly plays a pivotal role in augmenting the catalyst's activity. In summary, this comprehensive XPS analysis provides a meticulous understanding of the elemental composition and chemical intricacies, validating the intricate interplay among doping, defect formation, and enhanced catalytic performance in 7.5%Cu–ZnO/TiO2 nanomaterials.
The crystal structures of ZnO, TiO2, ZnO/TiO2 and 7.5%Cu–ZnO/TiO2 nanocomposites were studied by XRD. The X-ray diffraction (XRD) patterns of ZnO exhibit a strong similarity to the hexagonal phase of wurtzite, which is represented by JCPDS no. 36-1451. The XRD patterns of TiO2 closely resemble the anatase structure as indicated by JCPDS no. 21-1272. For 7.5%Cu–ZnO/TiO2 samples, XRD shows only the characteristic peaks of anatase TiO2 (101), (103), (200), (105), (211) and (204) and sphalerite hexagonal ZnO high intensity peaks of (100), (002), (101), (102), (110), (103), (200), (112) and (201) crystal faces. The absence of diffraction peaks at 2θ values of 27° and 31° suggests the exclusive presence of the pure anatase phase of TiO2, indicating no evidence for either rutile or brookite phases.30 After observing the diffraction peak at a 2θ angle of 37.9° for the (004) plane and identifying the appearance of the diffraction peak at a 2θ angle of 48.2° for the (200) crystal plane, it was inferred that there was growth in crystalline structure along the (001) orientation. By XRD analysis, it is obvious that no traces of copper, copper-containing oxides and binary titanium–copper phases are observed in the atlas.31,32 It may be because the diffraction peaks of TiO2 and Cu overlap or Cu exists in trace form, which is lower than the detection limit of XRD method.33,34
In Fig. 2(g), the absorption peaks observed at 3442 cm−1 and 1649 cm−1 can be attributed to the vibrational motions of O–H bonds, specifically involving stretching and bending movements, induced by the presence of water adsorbed on the catalyst's surface.35 The observed peaks in the spectral ranges at 665 cm−1 and 1090 cm−1 can be attributed to the stretching vibrations of Ti–O bonds and the bending vibrations of the Ti–O–Ti framework, respectively. The stretching vibration of Zn–O bonds is manifested by a peak observed at 499 cm−1 in pure ZnO. However, in the composite catalyst of 7.5%Cu–ZnO/TiO2, this peak undergoes a redshift to 481 cm−1.36 The spectral curves obtained from FT-IR analysis of ZnO/TiO2 and 7.5%Cu–ZnO/TiO2 exhibit remarkable similarities. The absence of a prominent peak at 660 cm−1, associated with the stretching vibration of Cu–O, suggests an extremely low copper concentration that remains undetectable through infrared spectroscopy. The results obtained from XPS analysis are consistent with this finding. The peak shape and position of each catalyst in the figure demonstrate a high degree of consistency, indicating that the modified catalysts effectively retain the original crystal structure of TiO2. The absence of any spurious peaks following modification suggests that the composite material possesses a well-defined crystalline structure without any impurities, which aligns with the XRD findings.
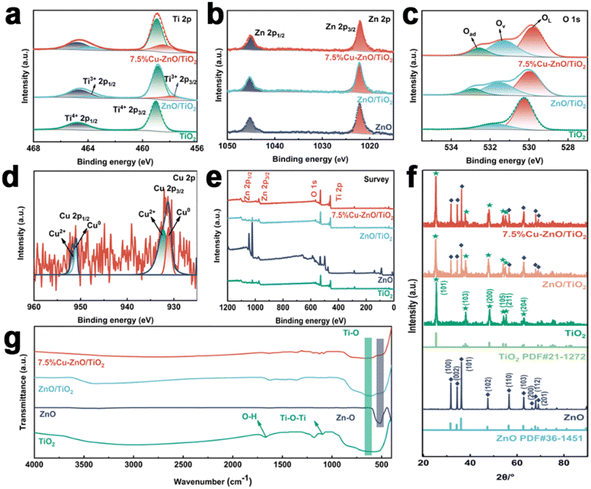 | ||
| Fig. 2 (a–d) X-ray photoelectron spectroscopy (XPS) spectra with enhanced resolution for Ti 2p, Zn 2p, O 1s, and Cu 2p, (e) XPS full spectrum, (f) XRD pattern, (g) the FT-IR spectrum. | ||
The absorption characteristics of TiO2, ZnO, ZnO/TiO2, and 7.5%Cu–ZnO/TiO2 were observed to be particularly strong in the wavelength range of 200–400 nm as shown in Fig. 3(a). This observation suggests that the prepared photocatalyst primarily exhibits adsorption capacity in the ultraviolet region. The light response intensity in the ultraviolet region of the 7.5%Cu–ZnO/TiO2 composite material demonstrates a comparatively reduced intensity when compared to that observed for pure TiO2. Moreover, there is a noticeable shift in the light response range from 389 nm to approximately 459 nm, indicating that the integration of TiO2 and ZnO through a heterojunction significantly enhances the efficiency of light energy utilization. The central peak position of four-coordinated titanium is observed at 220 nm, while the central peak position of six-coordinated titanium falls within the range of 240 nm and 300 nm.37,38 The graph indicates that Ti primarily exists in a six-coordinated state within the composite photocatalyst. In order to calculate the band gap width of photocatalyst, the Kubelka–Munk formula is used:39,40
| (αhν)1/2 = A(hν − Eg) | (3) |
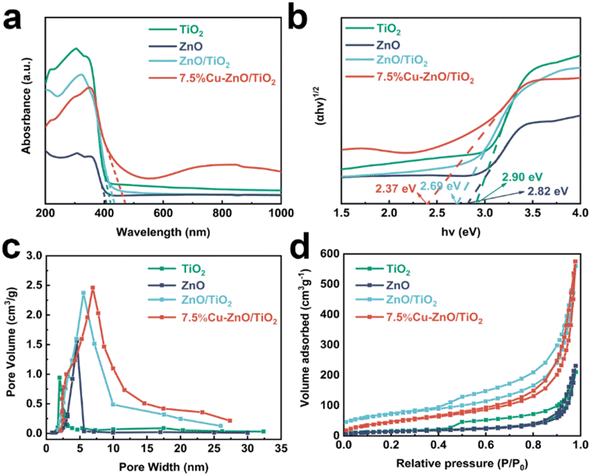 | ||
| Fig. 3 (a) Ultraviolet-visible diffuse reflectance spectroscopy, (b) optical bandgap energy, (c) N2 adsorption–desorption isotherm curve, (d) pore size distribution graph. | ||
In the formula, A, α, h, ν and Eg are respectively proportionality constant, absorption coefficient, Planck constant, incident light frequency and band gap width. As can be seen from Fig. 3(b), the bandgap widths of TiO2, ZnO, ZnO/TiO2 and 7.5%Cu–ZnO/TiO2 are 2.90 eV, 2.82 eV, 2.69 eV and 2.37 eV, respectively. The composite catalyst exhibits the presence of heterojunctions, which leads to a narrower bandgap width in 7.5%Cu–ZnO/TiO2. This promotes the movement of electrons across energy barriers, stimulates the generation of hole–electron pairs, enhances light absorption and utilization, thereby improving the photocatalytic desulfurization performance of the sample. The confirmation of the hypothesis was achieved by conducting experiments that utilized a pair of catalysts for ultraviolet light-induced photocatalytic desulfurization.
As shown in Fig. 3(c), the photocatalysts TiO2, ZnO, ZnO/TiO2, and 7.5%Cu–ZnO/TiO2 exhibited type IV isotherms with H3 hysteresis loops, indicating the presence of mesopores on their surfaces.41 The pore distribution depicted in Fig. 3(d) further confirms this point and indicates a relatively even distribution. The homogeneity of this structure suggests an increased availability of catalytic sites, diffusion pathways, and overall surface reactivity. The calculated results for the specific surface area and pore parameters of the prepared photocatalysts are presented in summarized Table 1.† The BET surface areas of TiO2, ZnO, ZnO/TiO2, and 7.5%Cu–ZnO/TiO2 are recorded as 38.21 m2 g−1, 40.15 m2 g−1, 73.612 m2 g−1, and 80.159 m2 g−1, in Table 1, respectively. It is evident that the specific surface area of 7.5%Cu–ZnO/TiO2 exceeds that of TiO2, ZnO, and ZnO/TiO2, suggesting an enhancement in the specific surface area resulting from the incorporation of a ternary catalyst composite. This leads to an increased quantity of active sites present on the surface, resulting in enhanced adsorption of compounds containing sulfur and thus improving the photocatalytic performance. Furthermore, the observed increase in specific surface area and pore size indirectly indicates an enhanced interface contact between Cu, ZnO, and TiO2.
| Catalyst | BET surface area (m2 g−1) | Average pore size (nm) | Pore volume (m2 g−1) |
|---|---|---|---|
| TiO2 | 38.21 | 3.055 | 0.047 |
| ZnO | 40.15 | 22.74 | 0.086 |
| ZnO/TiO2 | 73.612 | 17.357 | 0.218 |
| 7.5%Cu–ZnO/TiO2 | 80.159 | 24.226 | 0.201 |
3.2 Photocatalytic performance
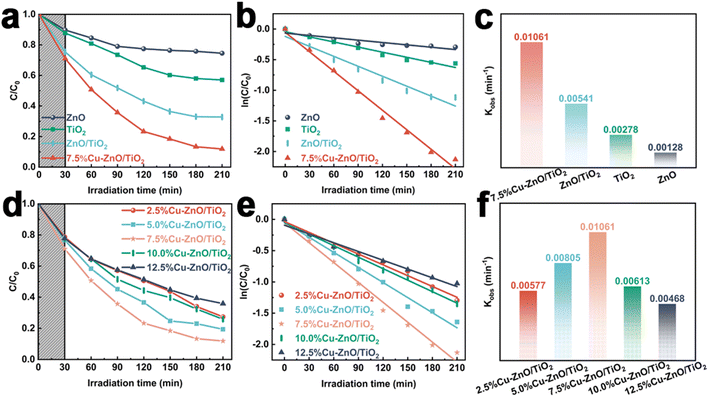
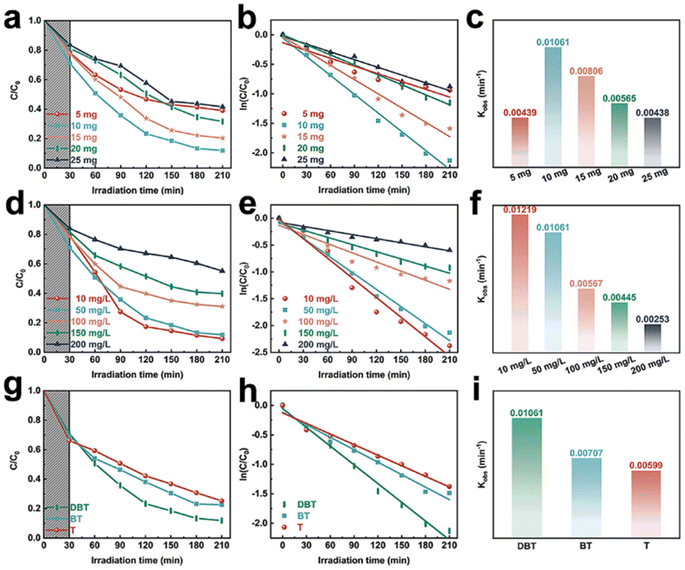
In this research, we conducted an analysis on the physicochemical characteristics of a newly developed photocatalyst. Additionally, we evaluated its efficacy in degrading a persistent DBT-simulated pollutant under UV-visible light exposure for a duration of 210 minutes. The original ZnO and TiO2, as depicted in Fig. 4(a), exhibited removal rates of 25.48% and 42.96% for DBT, indicating their relatively low photocatalytic activity. However, the binary composite system of ZnO/TiO2 demonstrated a significantly enhanced photocatalytic degradation rate of 67.24%, surpassing that of pure ZnO and TiO2. The degradation rate of 7.5%Cu–ZnO/TiO2 is the highest, reaching approximately 88.12%. The main explanation for the high rate of photocatalytic degradation in Cu–ZnO/TiO2 is as follows:42–44 (a) the construction of Z-type heterojunctions facilitates the efficient separation and transport of photo-generated electrons and holes. (b) Copper, being an effective conductor for the transportation of electrons and holes, exhibits the capability to promote the separation and transfer of electron–hole pairs produced by light, thereby augmenting light absorption. (c) The presence of oxygen defects provides numerous sites for photocatalytic reactions during the process. (d) Compared to binary ZnO/TiO2 photocatalysts and monolithic ZnO or TiO2, ternary nanocomposites demonstrate an increased surface area. The experimental section presents a detailed analysis of the photodegradation process of DBT, employing the pseudo-first-order kinetic model for assessment. It is evident that there exists a significant linear relationship between the natural logarithm of (Ct/C0) and the reaction duration (t), indicating adherence to a pseudo-first-order kinetic equation (Fig. 4(b)). Fig. 4(c) presents a histogram that illustrates the values of the apparent rate constant (k) derived from Fig. 4(b). Significantly, the 7.5%Cu–ZnO/TiO2 exhibits a remarkable DBT removal efficiency of 0.01061 min−1, surpassing ZnO by a factor of 8.23 (0.00128 min−1), TiO2 by a factor of 3.82 (0.00278 min−1), and ZnO/TiO2 by a factor of 1.96 (0.00541 min−1). The research mentioned above suggests that the combined utilization of Cu, ZnO, and TiO2 can greatly improve the effectiveness of photocatalytic oxidation in desulfurization. Additionally, as the percentage of Cu mass in Cu–ZnO/TiO2 increases, there is a distinct trend of initial enhancement followed by a decline in the photocatalytic degradation rate of DBT (Fig. 4(d)). This occurrence can be ascribed to the effective transmission of interface charges and the extended duration of electron–hole transfer, which effectively enhance the photocatalytic efficacy of the catalyst under optimal Cu loading conditions. However, an excessive amount of copper loading leads to a decline in photo performance as it hampers the efficient generation of electron–hole pairs and promotes an increased recombination rate. It is noteworthy that the degradation rate of the photocatalyst (7.5%Cu–ZnO/TiO2), which was formed with a mass fraction of 7.5%Cu in Cu–ZnO/TiO2 reached 88.12%. In comparison, the photocatalytic degradation rates of Cu–ZnO/TiO2 with different concentrations (2.5%, 5.0%, 10.0%, and 12.5%) were measured as follows: 72.62%, 80.62%, 74.12%, and 64.12%, respectively (Fig. 4(d)). In addition, the photocatalytic reactions demonstrate a kinetic process that exhibits pseudo-first-order behavior (Fig. 4(e)). The rate constants (K values) for these reactions are as follows: 0.00577 min−1 (2.5%Cu–ZnO/TiO2), 0.00805 min−1 (5.0%Cu–ZnO/TiO2), 0.01061 min−1 (7.5%Cu–ZnO/TiO2), 0.00613 min−1 (10.0%Cu–ZnO/TiO2), and 0.00468 min−1 (12.5%Cu–ZnO/TiO2). Consequently, the optimal photocatalyst is determined to be the one with a composition of 7.5%Cu–ZnO/TiO2.Factors such as catalyst dosage, sulfide concentration, and types of sulfides were studied to investigate and optimize the conditions for enhancing the efficiency of DBT removal through photocatalysis. The optimal catalyst dosage for achieving the highest photocatalytic degradation efficiency is found to be 10 mg, exhibiting an impressive rate of 88.12% as depicted in Fig. 5(a). However, in cases where the catalyst concentration falls below a certain threshold, augmenting the quantity of photocatalyst can enhance both the effective specific surface area and the quantity of active sites. As a result, there is an enhanced production of hydroxyl groups and superoxide radicals, leading to a substantial enhancement in photocatalytic performance. The opacity of the suspension increases further when the dosage of photocatalyst exceeds 10 mg due to the effects of light shielding and scattering, resulting in a decrease in degradation efficiency. The corresponding k values for different concentrations of DBT are 0.00439 min−1 (5 mg), 0.01061 min−1 (10 mg), 0.00806 min−1 (15 mg), 0.00565 min−1 (20 mg), and 0.00438 min−1 (25 mg) respectively, as shown in Fig. 5(b). As depicted in Fig. 5(d), within the 7.5%Cu–ZnO/TiO2 system, an increase in DBT concentration from 10 mg L−1 to 200 mg L−1 results in a decrease in its degradation rate from 88.12% to 44.87%, highlighting the significant impact of initial DBT concentration on pollutant removal efficiency. There are two primary factors that contribute to this phenomenon:45,46 (a) the elevation of DBT concentration in the water leads to a reduction in light transmittance, thereby diminishing the number of effective photons. (b) During the degradation process of high-concentration DBT solutions, a substantial quantity of intermediates is generated, which compete with DBT compete with DBT compounds for the process of adsorption and photodegradation. Meanwhile, the aforementioned photodegradation process also follows a pseudo-first-order kinetic model (Fig. 5(e)), with corresponding rate constants (Fig. 5(f)) of 0.01219 min−1 (10 mg L−1), 0.01061 min−1 (50 mg L−1), 0.00567 min−1 (100 mg L−1), 0.00445 min−1 (150 mg L−1), and 0.00253 min−1 (200 mg L−1). Significant variations in the degradation efficiency of 50 mg DBT, BT (benzothiophene), and T (thiophene) were observed after degradation using a 10 mg 7.5%Cu–ZnO/TiO2 photocatalyst. The photocatalyst demonstrates distinct variations in removal efficiency for three different sulfur-containing compounds, as depicted in Fig. 5(g). It achieves the highest degradation efficiency of 88.12% for DBT, while BT and T demonstrate degradation efficiencies of 77.38% and 74.78%, respectively. The degradation of three distinct compounds containing sulfur also follows a kinetic model that can be approximated as pseudo-first-order (Fig. 5(h)). The corresponding rate constants for DBT, BT, and T are measured to be 0.01061 min−1, 0.00707 min−1, and 0.00599 min−1 respectively (Fig. 5(i)).
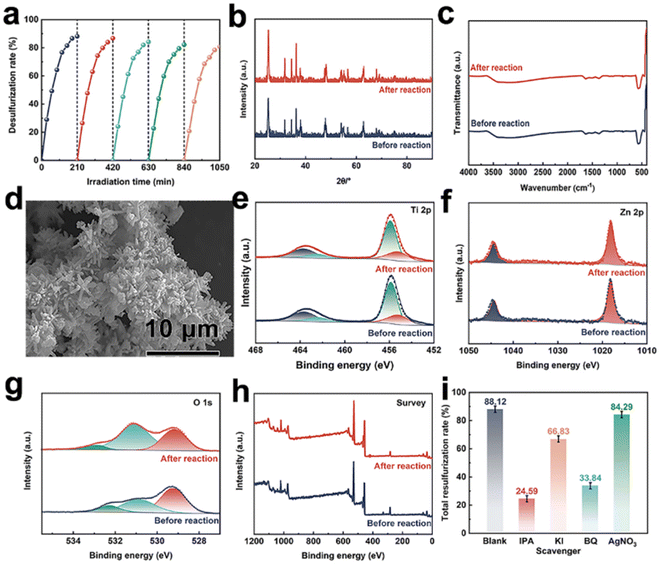
After conducting 5 consecutive photocatalytic experiments for DBT removal, the reusability and stability of 7.5%Cu–ZnO/TiO2 were assessed under identical conditions, which are crucial factors to consider in practical applications. After 5 cycles, as depicted in Fig. 6(a), the degradation rate of the 7.5%Cu–ZnO/TiO2 photocatalyst remains at 80.69%, indicating its inherent stability. The degradation rate has exhibited a slight decrease, potentially attributed to losses incurred during the process of photocatalyst recovery. Furthermore, the XRD, FT-IR, and SEM results (Fig. 6(b–d)) demonstrate that there were no noticeable changes in the crystallographic planes, characteristic peaks or morphology after five cycles of testing. This provides additional validation for the exceptional stability and reproducibility of 7.5%Cu–ZnO/TiO2. After conducting further evaluation of the stability of 7.5%Cu–ZnO/TiO2 using XPS, a comprehensive energy spectrum analysis was performed for each individual element. The elemental composition of 7.5%Cu–ZnO/TiO2 remained unchanged during the photocatalytic reaction. No noticeable alterations were detected in the Ti 2p and Zn 2p spectra, as well as in the XPS full spectrum before and after the light reaction (see Fig. 6(e–h)). In the O 1s spectrum, both the concentration of oxygen vacancies (Ov) and the total oxygen content exhibited significant increases, which can be attributed to the adsorption of oxygen on the surface of 7.5%Cu–ZnO/TiO2 after testing.47 The comprehensive characterization conducted after testing confirmed minimal changes in the 7.5%Cu–ZnO/TiO2 photocatalyst before and after light reaction, providing compelling evidence of its exceptional stability and reproducibility.
3.3 Possible photocatalytic mechanisms
Based on the analysis of the physical and chemical properties of TiO2, ZnO, ZnO/TiO2, and specifically 7.5%Cu–ZnO/TiO2, a potential mechanism for DBT photodegradation by 7.5%Cu–ZnO/TiO2 was proposed. The photocatalytic performance of the composite material can be significantly improved due to the effective suppression of electron–hole recombination, which is achieved by promoting efficient charge transfer among individual semiconductors in the 7.5%Cu–ZnO/TiO2 nanocomposite material. Hence, acquiring a comprehensive comprehension of the band edges of individual materials within the 7.5%Cu–ZnO/TiO2 system holds immense importance in accurately grasping its underlying mechanism. The formula is used to calculate the conduction band (CB) and valence band (VB) of TiO2 and ZnO:48–50| ECB = X − EC − 0.5Eg | (4) |
| EVB = X − EC + 0.5Eg | (5) |
In the given equation, the CB and VB edge potentials are denoted by ECB and EVB respectively. X represents electronegativity, with ZnO having an electronegativity of 5.94 eV and TiO2 having an electronegativity of 5.89 eV; the band gap energy is denoted as Eg, with values of 2.82 eV for ZnO and 2.90 eV for TiO2; EC denotes the energy of free electrons relative to hydrogen (4.5 eV). After performing the necessary calculations, it was determined that ZnO exhibits a CB potential of 0.03 eV and a VB potential of 2.85 eV, while TiO2 displays a CB potential of −0.06 eV and a VB potential of 2.84 eV.
To investigate the function of 7.5%Cu–ZnO/TiO2 in the PODS system, experiments were conducted to determine the active components and evaluate their capability for free radical elimination. Different substances, including isopropyl alcohol (IPA), potassium iodide (KI), benzoquinone (BQ), and silver nitrate (AgNO3), were utilized as scavengers to capture hydroxyl radicals (˙OH), holes (h+), superoxide radicals (˙O2−), and electrons (e−).51,52 The specific experimental procedure was consistent with that of the photocatalytic desulfurization process. The desulfurization rate exhibits a descending trend, as shown in Fig. 6(i), with the following order of decrease: blank sample, AgNO3, KI, BQ, and IPA. In the absence of any scavengers, the desulfurization efficiency of 7.5%Cu–ZnO/TiO2 reaches 88.12% after 210 min. However, the addition of KI and AgNO3 to the target sulfide does not significantly affect the degradation of DBT by 7.5%Cu–ZnO/TiO2, indicating that neither h+ nor e− are active species in this system. In contrast, the addition of IPA and BQ significantly reduces the desulfurization efficiency of 7.5%Cu–ZnO/TiO2 towards DBT, with reductions of 63.53% and 54.28%, respectively. This emphasizes the significant contributions of both ·OH and ˙O2− in the elimination mechanism of DBT by 7.5%Cu–ZnO/TiO2, with ˙OH demonstrating superior efficacy compared to ˙O2−.
Based on the experimental findings and relevant literature, Fig. 7 depicts the photocatalytic mechanism exhibited by 7.5%Cu–ZnO/TiO2.15,53–55 In general, ZnO/TiO2 exhibits a type II heterojunction between TiO2 and ZnO, which facilitates the efficient degradation of DBT. In the II-type ZnO/TiO2 heterojunction, TiO2 predominantly captures electrons while ZnO accumulates holes, effectively inhibiting charge carrier recombination and thus enhancing photocatalytic performance. However, the research focus has shifted towards Z-type heterojunctions due to the Coulomb repulsion effect among photo-generated electrons. The electron migration effect is enhanced in a Z-type heterojunction, which promotes efficient charge transfer between semiconductor interfaces. In the proposed nano-composite photocatalyst, Cu facilitates electron migration between ZnO and TiO2, thereby accelerating interfacial electron transfer in the 7.5%Cu–ZnO/TiO2 composite catalyst and significantly enhancing its photocatalytic activity.
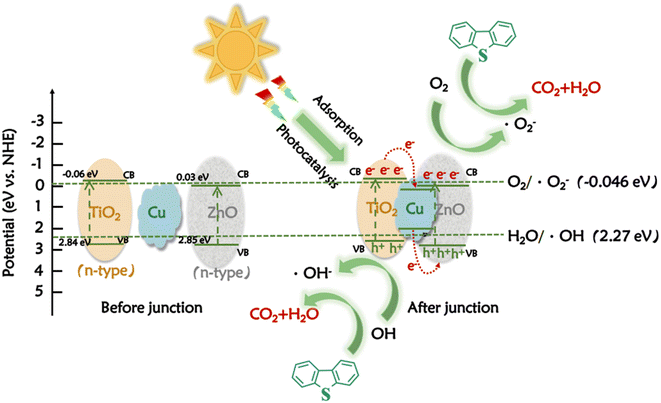 | ||
| Fig. 7 The potential mechanism underlying the photocatalytic decomposition of DBT using 7.5%Cu–ZnO/TiO2 is being studied. | ||
First, the sulfide in the oil phase is adsorbed on the surface of the adsorption site of the composite catalyst under the action of polarity. Subsequently, the oxidation reaction of the sulfide occurs at the catalytic site of the composite catalyst, that is, under the irradiation of ultraviolet light, e− excites from the valence band to the conduction band, resulting in photogenerated electron–hole pairs. e− reacts with molecular oxygen to form ˙O2−, which further reacts with H2O or OH− to form ˙OH radical. At the same time, h+ reacts with dissolved water to produce highly oxidizing ˙OH. Hydroxyl radical (˙OH) and superoxide radical (˙O2−) were responsible for the degradation of DBT. In addition, in the 7.5%Cu–ZnO/TiO2 composite photocatalyst, the Fermi level of Cu exhibits a higher position compared to the conduction band (CB) level of TiO2, thereby facilitating effective electron transfer from the CB level of TiO2 to Cu. At the same time, there is a difference in the Fermi level between Cu and the VB level of ZnO, which facilitates the movement of electrons from accumulated Cu to the VB level of ZnO. This procedure effectively suppresses the presence of photo-induced vacancies in ZnO and enhances the efficiency of segregating photo-generated electrons in ZnO, resulting in a significant improvement in its reduction potential. Therefore, the 7.5%Cu–ZnO/TiO2 nanocomposite photocatalyst exhibits excellent photocatalytic activity and can effectively promote the degradation of DBT.
4 Conclusion
In this paper, Z-type heterostructures with a large number of defects were successfully constructed using a simple hydrothermal synthesis method and applied to DBT photocatalysts that are difficult to degrade. By adjusting the mass percentage of Cu and the operating parameters of Cu–ZnO/TiO2 photocatalyst, the best photocatalytic activity was achieved, resulting in a removal rate of DBT as high as 88.12%. The 7.5%Cu–ZnO/TiO2 catalyst exhibited good repeatability and stability, and the improvement in photocatalytic performance can be attributed to the construction of Z-type heterojunctions and the utilization of Cu as a charge transport channel. This provides a new approach for degrading DBT using titanium-based photocatalytic materials.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Conflicts of interest
The authors declare that they are aware of any competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This manuscript is submitted without any conflicts of interest and has been published with the consent of all authors. On behalf of my co-authors, I would like to state that the work described is original research that has not been previously published and is not being considered for publication elsewhere, either in whole or in part. The listed authors have agreed to the attached manuscript.Acknowledgements
The authors are grateful for the Project supported by the National Natural Science Foundation of China (Grant no. 52172092).References
- X. Zhou, T. Wang, H. Liu, X. Gao, C. Wang and G. Wang, Desulfurization through photocatalytic oxidation: a critical review, ChemSusChem, 2021, 14(2), 492–511 CrossRef CAS PubMed.
- K. Chen, X.-M. Zhang, X.-F. Yang, M.-G. Jiao, Z. Zhou, M.-H. Zhang, D.-H. Wang and X.-H. Bu, Electronic structure of heterojunction MoO2/g-C3N4 catalyst for oxidative desulfurization, Appl. Catal., B, 2018, 238, 263–273 CrossRef CAS.
- R. Wang, X. Xie, C. Xu, Y. Lin, D. You, J. Chen, Z. Li, Z. Shi, Q. Cui and M. Wang, Bi-piezoelectric effect assisted ZnO nanorods/PVDF-HFP spongy photocatalyst for enhanced performance on degrading organic pollutant, Chem. Eng. J., 2022, 439, 135787 CrossRef CAS.
- M. Li, P. Li, L. Zhang, M. Chen, J. Tang, C. Qin, S. L. J. Lee and S. Lin, Facile fabrication of ZnO decorated ZnFe-layered double hydroxides@biochar nanocomposites for synergistic photodegradation of tetracycline under visible light, Chem. Eng. J., 2022, 434, 134772 CrossRef CAS.
- S. Y. Sawant, M. S. Sayed, T. H. Han, M. R. Karim, J.-J. Shim and M. H. Cho, Bio-synthesis of finely distributed Ag nanoparticle-decorated TiO2 nanorods for sunlight-induced photoelectrochemical water splitting, J. Ind. Eng. Chem., 2019, 69, 48–56 CrossRef CAS.
- S. G. Kumar and R. Kavitha, Lanthanide ions doped ZnO based photocatalysts, Sep. Purif. Technol., 2021, 274, 118853 CrossRef.
- Y. Wan, J. Li, J. Ni, C. Wang, C. Ni and H. Chen, Crystal-facet and microstructure engineering in ZnO for photocatalytic NO oxidation, J. Hazard. Mater., 2022, 435, 129073 CrossRef CAS.
- P. Bharathi, S. Harish, J. Archana, M. Navaneethan, S. Ponnusamy, C. Muthamizhchelvan, M. Shimomura and Y. Hayakawa, Enhanced charge transfer and separation of hierarchical CuO/ZnO composites: the synergistic effect of photocatalysis for the mineralization of organic pollutant in water, Appl. Surf. Sci., 2019, 484, 884–891 CrossRef CAS.
- Y. Poo-arporn, S. Kityakarn, A. Niltharach, M. F. Smith, S. Seraphin, M. Wörner and A. Worayingyong, Photocatalytic oxidation of thiophene over cerium doped TiO2 thin film, Mater. Sci. Semicond. Process., 2019, 93, 21–27 CrossRef CAS.
- X. Li, Y. Mao, K. Leng, G. Ye, Y. Sun and W. Xu, Enhancement of oxidative desulfurization performance over amorphous titania by doping MIL-101 (Cr), Microporous Mesoporous Mater., 2017, 254, 114–120 CrossRef CAS.
- R. Daghrir, P. Drogui and D. Robert, Modified TiO2 for environmental photocatalytic applications: a review, Ind. Eng. Chem. Res., 2013, 52(10), 3581–3599 CrossRef CAS.
- F. Kayaci, S. Vempati, C. Ozgit-Akgun, I. Donmez, N. Biyikli and T. Uyar, Selective isolation of the electron or hole in photocatalysis: ZnO–TiO2 and TiO2–ZnO core–shell structured heterojunction nanofibers via electrospinning and atomic layer deposition, Nanoscale, 2014, 6(11), 5735–5745 RSC.
- T. D. N. Thi, L. H. Nguyen, X. H. Nguyen, H. V. Phung, T. H. T. Vinh, P. Van Viet, N. Van Thai, H. N. Le, D. T. Pham and H. T. Van, Enhanced heterogeneous photocatalytic perozone degradation of amoxicillin by ZnO modified TiO2 nanocomposites under visible light irradiation, Mater. Sci. Semicond. Process., 2022, 142, 106456 CrossRef.
- E. Vasilaki, N. Katsarakis, S. Dokianakis and M. Vamvakaki, rGO functionalized ZnO–TiO2 core–shell flower-like architectures for visible light photocatalysis, Catalysts, 2021, 11(3), 332 CrossRef CAS.
- V. Lachom, P. Poolcharuansin and P. Laokul, Preparation, characterizations and photocatalytic activity of a ZnO/TiO2 nanocomposite, Mater. Res. Express, 2017, 4(3), 035006 CrossRef.
- C. Cheng, A. Amini, C. Zhu, Z. Xu, H. Song and N. Wang, Enhanced photocatalytic performance of TiO2–ZnO hybrid nanostructures, Sci. Rep., 2014, 4(1), 4181 CrossRef.
- J. Song, G. Sun, J. Yu, Y. Si and B. Ding, Construction of ternary Ag@ ZnO/TiO2 fibrous membranes with hierarchical nanostructures and mechanical flexibility for water purification, Ceram. Int., 2020, 46(1), 468–475 CrossRef CAS.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Anatase TiO2 single crystals with a large percentage of reactive facets, Nature, 2008, 453(7195), 638–641 CrossRef CAS PubMed.
- Z. Zhao, Z. Sun, H. Zhao, M. Zheng, P. Du, J. Zhao and H. Fan, Phase control of hierarchically structured mesoporous anatase TiO2 microspheres covered with {001} facets, J. Mater. Chem., 2012, 22(41), 21965–21971 RSC.
- S. U. Hassan, H. Khalid, S. Shafique, M. A. Farid, M. H. Saeed, Z. Ali, M. S. Nazir, M. Hussain and Y.-K. Park, Investigating catalytic oxidative desulfurization of model fuel using hollow PW12/TiO2@MgCO3 and performance optimization via Box–Behnken design, Chemosphere, 2023, 339, 139662 CrossRef CAS.
- X. Chen, J. Zheng, L. Li and W. Chu, Strategy for enhanced performance of silicon nanoparticle anodes for lithium-ion batteries, RSC Adv., 2022, 12(28), 17889–17897 RSC.
- Y. Li, F. Zhang, K. Xu, Z. Yang and Z. Dong, The synergistic enhancement of photocatalytic activity by Fe3O4–TiO2 nanosheets for rhodamine B degradation, Colloids Surf., A, 2024, 700, 134803 CrossRef CAS.
- Z. Li, Y. Chu, T. Mi, J. Zhao and F. Meng, Enhanced photocatalytic H2 evolution over conjugated polymer/Au@TiO2 ternary heterojunction under visible light: Enriched photons input from polymers, Colloids Surf., A, 2024, 692, 133991 CrossRef CAS.
- M. Xu, S. He, H. Chen, G. Cui, L. Zheng, B. Wang and M. Wei, TiO2–x-modified Ni nanocatalyst with tunable metal–support interaction for water–gas shift reaction, ACS Catal., 2017, 7(11), 7600–7609 CrossRef CAS.
- J. Ji, Two-Stage Ultraviolet Degradation of Perovskite Solar Cells Induced by the Oxygen Vacancy-Ti4+ States, iScience, 2020, 23(4), 101013 CrossRef CAS PubMed.
- R. Nawaz, C. F. Kait, H. Y. Chia, M. H. Isa, L. W. Huei, N. T. Sahrin and N. Khan, Manipulation of the Ti3+/Ti4+ ratio in colored titanium dioxide and its role in photocatalytic degradation of environmental pollutants, Surf. Interfaces, 2022, 32, 102146 CrossRef CAS.
- G. Dong, W. Chi, D.-f. Chai, Z. Zhang, J. Li, M. Zhao, W. Zhang, J. Lv and S. Chen, A novel Ag3BiO3/ZnO/BC composite with abundant defects and utilizing hemp BC as charge transfer mediator for photocatalytic degradation of levofloxacin, Appl. Surf. Sci., 2023, 619, 156732 CrossRef CAS.
- Z. Guo, H. Hou, J. Zhang, P. Cai and J. Lin, Prominent roles of Ni (OH)2 deposited on ZnIn2S4 microspheres in efficient charge separation and photocatalytic H2 evolution, RSC Adv., 2021, 11(20), 12442–12448 RSC.
- C. K. Swamy, A. Hezam, A. M. Ramesh, D. H. Ramakrishnegowda, D. K. Purushothama, J. Krishnegowda and S. Shivanna, Microwave hydrothermal synthesis of copper induced ZnO/gC3N4 heterostructure with efficient photocatalytic degradation through S-scheme mechanism, J. Photochem. Photobiol., A, 2021, 418, 113394 CrossRef.
- L. Gu, J. Wang, H. Cheng, Y. Du and X. Han, Synthesis of nano-sized anatase TiO2 with reactive {001} facets using lamellar protonated titanate as precursor, Chem. Commun., 2012, 48(55), 6978–6980 RSC.
- W. Q. Fang, J. Z. Zhou, J. Liu, Z. G. Chen, C. Yang, C. H. Sun, G. R. Qian, J. Zou, S. Z. Qiao and H. G. Yang, Hierarchical structures of single-crystalline anatase TiO2 nanosheets dominated by {001} Facets, Chem.–Eur. J., 2011, 17(5), 1423 CrossRef CAS PubMed.
- Y. Pang, G. Xu, Q. Feng, J. Lv, Y. Qin, Y. Zhang, Z. Zheng and Y. Wu, Crystalline orientation preference for TiO2 nanotube arrays with efficient photoelectrochemical properties, Phys. Lett. A, 2018, 382(38), 2759–2762 CrossRef CAS.
- B. Xu, L. Dong and Y. Chen, Influence of CuO loading on dispersion and reduction behavior of CuO/TiO2 (anatase) system, J. Chem. Soc., Faraday Trans., 1998, 94(13), 1905–1909 RSC.
- A. Machín, M. Cotto, J. Ducongé, J. Arango, C. Morant and F. Márquez, Synthesis and characterization of Au@TiO2 NWs and their catalytic activity by water splitting: a comparative study with Degussa P25. Am, J. Eng. Appl. Sci., 2017, 10, 298–311 Search PubMed.
- K. S. Babu and V. Narayanan, Hydrothermal synthesis of hydrated zinc oxide nanoparticles and its characterization, Chem. Sci. Trans., 2013, 2(S1), S33–S36 Search PubMed.
- M. Gholami, M. Shirzad-Siboni, M. Farzadkia and J.-K. Yang, Synthesis, characterization, and application of ZnO/TiO2 nanocomposite for photocatalysis of a herbicide (Bentazon), Desalin. Water Treat., 2016, 57(29), 13632–13644 CrossRef CAS.
- T. Blasco, M. Camblor, A. Corma and J. Perez-Pariente, The state of Ti in titanoaluminosilicates isomorphous with zeolite. beta, J. Am. Chem. Soc., 1993, 115(25), 11806–11813 CrossRef CAS.
- F. A. Tantray, A. Agrawal, M. Gupta, J. T. Andrews and P. Sen, Effect of oxygen partial pressure on the structural and optical properties of ion beam sputtered TiO2 thin films, Thin Solid Films, 2016, 619, 86–90 CrossRef CAS.
- S. A. Ansari, M. M. Khan, M. O. Ansari and M. H. Cho, Gold nanoparticles-sensitized wide and narrow band gap TiO2 for visible light applications: a comparative study, New J. Chem., 2015, 39(6), 4708–4715 RSC.
- Y. Liu, J.-z. Tian, X. Hao, Y.-j. Zheng, T. Jing, Y.-p. Zhao and W.-l. Yang, Preparation of TiO2/porous glass-H with the coupling of photocatalysis oxidation–adsorption system in the initial position and its desulfurization performance on model fuel, RSC Adv., 2021, 11(46), 28508–28520 RSC.
- D. Qiao, Z. Li, J. Duan and X. He, Adsorption and photocatalytic degradation mechanism of magnetic graphene oxide/ZnO nanocomposites for tetracycline contaminants, Chem. Eng. J., 2020, 400, 125952 CrossRef CAS.
- L. Li, C.-G. Niu, H. Guo, J. Wang, M. Ruan, L. Zhang, C. Liang, H.-Y. Liu and Y.-Y. Yang, Efficient degradation of Levofloxacin with magnetically separable ZnFe2O4/NCDs/Ag2CO3 Z-scheme heterojunction photocatalyst: vis-NIR light response ability and mechanism insight, Chem. Eng. J., 2020, 383, 123192 CrossRef CAS.
- S. Meng, J. Zhang, S. Chen, S. Zhang and W. Huang, Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers, Appl. Surf. Sci., 2019, 476, 982–992 CrossRef CAS.
- B. Jaleh, M. Gandomi Rouzbahani, K. Abedi, S. Azizian, H. Ebrahimi, M. Nasrollahzadeh and R. S. Varma, Photocatalytic decomposition of VOCs by AC–TiO2 and EG–TiO2 nanocomposites, Clean Technol. Environ. Policy, 2019, 21, 1259–1268 CrossRef CAS.
- Y.-C. Chan, J.-N. Chen and M.-C. Lu, Intermediate inhibition in the heterogeneous UV-catalysis using a TiO2 suspension system, Chemosphere, 2001, 45(1), 29–35 CrossRef CAS.
- Z. Lin, X. Jiang, W. Xu, F. Li, X. Chen, H. Wang, S. Liu and X. Lu, The effects of water, substrate, and intermediate adsorption on the photocatalytic decomposition of air pollutants over nano-TiO2 photocatalysts, Phys. Chem. Chem. Phys., 2024, 26, 662–678 RSC.
- J. Zhang, H. B. Yang, D. Zhou and B. Liu, Adsorption energy in oxygen electrocatalysis, Chem. Rev., 2022, 122(23), 17028–17072 CrossRef CAS.
- R. Nadarajan, W. A. W. A. Bakar, R. Ali and R. Ismail, Effect of structural defects towards the performance of TiO2/SnO2/WO3 photocatalyst in the degradation of 1, 2-dichlorobenzene, J. Taiwan Inst. Chem. Eng., 2016, 64, 106–115 CrossRef CAS.
- Y. Zhang, Q. Shao, H. Jiang, L. Liu, M. Wu, J. Lin, J. Zhang, S. Wu, M. Dong and Z. Guo, One-step co-precipitation synthesis of novel BiOCl/CeO2 composites with enhanced photodegradation of rhodamine B, Inorg. Chem. Front., 2020, 7(6), 1345–1361 RSC.
- J. Tian, Q. Shao, J. Zhao, D. Pan, M. Dong, C. Jia, T. Ding, T. Wu and Z. Guo, Microwave solvothermal carboxymethyl chitosan templated synthesis of TiO2/ZrO2 composites toward enhanced photocatalytic degradation of Rhodamine B, J. Colloid Interface Sci., 2019, 541, 18–29 CrossRef CAS PubMed.
- X. Li, F. Li, X. Lu, S. Zuo, C. Yao and C. Ni, Development of Bi2W1–xMoxO6/Montmorillonite nanocomposite as efficient catalyst for photocatalytic desulfurization, J. Alloys Compd., 2017, 709, 285–292 CrossRef CAS.
- X. Lu, X. Li, F. Chen, Z. Chen, J. Qian and Q. Zhang, Biotemplating synthesis of N-doped two-dimensional CeO2–TiO2 nanosheets with enhanced visible light photocatalytic desulfurization performance, J. Alloys Compd., 2020, 815, 152326 CrossRef CAS.
- B. Arjun Kumar, T. Elangovan, D. Karthigaimuthu, D. Aravinth, G. Ramalingam, F. Ran and S. Sangaraju, CdSe Quantum Dots Bedecked on ZnO/TiO2/CuO Ternary Nanocomposite for Enhanced Photocatalytic and Photovoltaic Applications, Langmuir, 2023, 39(45), 15864–15877 CrossRef CAS PubMed.
- V. K. Landge, C.-M. Huang, V. S. Hakke, S. H. Sonawane, S. Manickam and M.-C. Hsieh, Solar-energy-driven Cu-ZnO/TiO2 nanocomposite photocatalyst for the rapid degradation of Congo Red azo dye, Catalysts, 2022, 12(6), 605 CrossRef CAS.
- X. Zheng, D. Li, X. Li, J. Chen, C. Cao, J. Fang, J. Wang, Y. He and Y. Zheng, Construction of ZnO/TiO2 photonic crystal heterostructures for enhanced photocatalytic properties, Appl. Catal., B, 2015, 168, 408–415 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra06833e |
| This journal is © The Royal Society of Chemistry 2024 |