Open Access Article
Open Access ArticleDeep eutectic solvent as a green catalyst for the one-pot multicomponent synthesis of 2-substituted benzothiazole derivatives†
Vy Anh Truong‡
 ab,
Minh Hai Tran‡ab,
Trinh Hao Nguyen
ab,
Minh Hai Tran‡ab,
Trinh Hao Nguyen abc and
Hai Truong Nguyen
abc and
Hai Truong Nguyen *ab
*ab
aDepartment of Organic Chemistry, Faculty of Chemistry, University of Science, Ho Chi Minh City, Vietnam. E-mail: ngthai@hcmus.edu.vn; Tel: +84-908-108-824
bVietnam National University, Ho Chi Minh City, Vietnam
cFaculty of Interdisciplinary Science, University of Science, Ho Chi Minh City, Vietnam
First published on 13th December 2024
Abstract
The need for diverse essential chemicals and resources has markedly risen alongside the advancement of civilization. Regrettably, many toxic solvents used in chemical laboratories and industrial settings pose significant risks to the health of researchers and intensify environmental pollution. Deep eutectic solvents (DESs), serving as an alternative to ionic liquids, provide superior environmental benefits and have garnered significant interest in chemical research. DESs have garnered increasing interest in the field of chemistry for their use as catalysts and solvents. Benzothiazole is an organic molecule with a heterocyclic nucleus (thiazole) that possesses a wide range of biological activities. In this study, we established [CholineCl][Imidazole]2 as an efficient catalyst for the one-pot multicomponent synthesis of 2-substituted benzothiazole derivatives using conventional heating under solvent-free conditions. Its reactivity remains stable with a maximum yield of 78% for 2-phenylbenzo[d]thiazole, and using a solvent that is both environmentally safe and compatible with the reusability of the [CholineCl][Imidazole]2 catalyst, the reaction time can be effectively decreased.
1. Introduction
Elemental sulfur, which exists as a yellow solid, has been known for its huge reserves and easy extraction from its ores.1 Sulphur and its heterocyclic compounds offer unique applications not only in industry but also in scientific research.2 In addition to its low cost, other noticeable features of this interesting element are its non-volatility and stability under room conditions.3 Similar to molten salts, sulfur, at temperatures above its melting point, can act as a reaction medium in some cases.4 Owing to these properties, sulfur can be applied in the synthesis of some biological compounds containing retroelements.5,6 The benefits of elemental sulfur are enhanced by its user-friendliness, which is characterized by its non-toxic, non-volatile, odorless, non-hygroscopic, bench-stable, and free-flowing nature.7 Consequently, the advancement of techniques for the direct incorporation of sulfur into organic frameworks from elemental sulfur is of considerable importance from both theoretical and practical perspectives, particularly within a multicomponent framework.8 Nguyen et al. demonstrated that sulfur acted as a polyvalent reagent in multicomponent redox condensation involving o-chloronitrobenzene and benzaldehydes.9Deep eutectic solvents (DESs), categorized as a new generation of ionic liquids, have been known as green solvent systems.10 The combination of hydrogen bond donors and acceptors at a given temperature, which results in a lower melting point, allows DESs to be easily prepared both in the laboratory and large-scale applications.11 Owing to their remarkable properties, DESs have found applications in a wide range of areas, such as alternative solvents for natural compound extraction, potential electrolytes in redox flow batteries and photoelectrochemical cells,12,13 drug delivery systems based on biocompatibility,14 dispersants in the formation of nanoparticles,15 efficient and green catalyst/solvent systems for organic synthesis.16,17 Since the first report on a DES made up of choline chloride (CholineCl) and imidazole (IM) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in 2008, the DES has emerged as a strong basic reagent with low viscosity and high conductivity.18 [CholineCl][Imidazole]2 has been utilized in swelling pretreatment of cellulose,19 starch dissolution and plasticization,20 and pretreatments followed by saccharification of corncob (Scheme 1).18,21
2 in 2008, the DES has emerged as a strong basic reagent with low viscosity and high conductivity.18 [CholineCl][Imidazole]2 has been utilized in swelling pretreatment of cellulose,19 starch dissolution and plasticization,20 and pretreatments followed by saccharification of corncob (Scheme 1).18,21
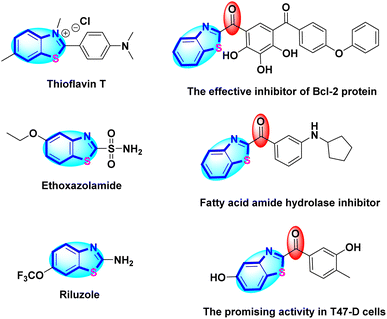
2-Substituted benzothiazoles are classified as benzothiazole scaffolds, which represent important classes of unsaturated heterocycles containing carbon, nitrogen, and sulfur.22 These derivatives have been widely found in biological and medicinally significant structures,23 agrochemicals,24,25 and functionalized materials.26 The benzothiazole moiety is significant in chemistry and is found in various biologically active compounds, including anti-microbial, anti-cancer, anthelmintic, anti-diabetic, anti-tuberculotic, anti-tumor, anti-trypanosomal, anti-viral, antibacterial, antioxidant, anti-glutamate, and anti-parkinsonism agents.27 An uncomplicated and effective transformation using accessible reagents under solvent-free and metal-free circumstances is a pivotal solution to overcome pollution issues arising from large-scale reactions. Numerous protocols have been recently established for synthesizing benzothiazole derivatives using heterogeneous solid acid catalysts,28 ionic liquids,29 microwave irradiation, conventional heating, and mild, solvent-free conditions.30 Researchers considered recent advancements in the synthesis of various benzothiazole derivatives through diverse methodologies, primarily involving the condensation of ortho-amino thiophenols with acids, acid chlorides, aldehydes, esters, nitriles, ketones, and thioesters.31 The condensation between 2-aminothiophenol and aldehydes to form 2-arylbenzothiazoles has been catalyzed by various acidic catalysts under solvent or solvent-free conditions. These catalysts include P2O5,32 Pt/Al2O3,33 Cu(II)–TD@nSiO2,34 TiO2/H2O2,35 ZnCl2/SiO2,36 Cu(II)–Glycerol/MCM-41,37 MIL-101 (Cr),38 ZnO/SiO2–TTIP,39 Amberlite IR-120,40 and Co(MCG)(H2O)3.41 Other approaches were based on the cyclization of substituted thioformanilides under various conditions.42–44 Besides, 2-aroylbenzothiazoles, which contain a ketone (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group, were synthesized through the acylation of benzothiazole with various aryl methyl ketones catalyzed by FeCl3·6H2O/O2,45 CuI/O2 in HBF4/DMSO,46 or I2/KOH.47 Al-Mourabit and co-workers devised a three-component redox condensation using various o-nitrohalobenzenes and acetophenone with elemental sulfur, facilitating a direct, cost-effective, and straightforward synthesis of 2-benzoylbenzothiazoles.48
O) group, were synthesized through the acylation of benzothiazole with various aryl methyl ketones catalyzed by FeCl3·6H2O/O2,45 CuI/O2 in HBF4/DMSO,46 or I2/KOH.47 Al-Mourabit and co-workers devised a three-component redox condensation using various o-nitrohalobenzenes and acetophenone with elemental sulfur, facilitating a direct, cost-effective, and straightforward synthesis of 2-benzoylbenzothiazoles.48
For the synthesis of 2-substituted benzothiazole derivatives using DESs, typical synthetic routes involve condensation reactions between 2-aminobenzenethiol and various substituted aldehydes or ketones. The choice of DESs can influence reaction yield, selectivity, and ease of product isolation.49
With regard to the concerns about environmentally friendly factors, which participate in the synthesis of catalysts and solvents, the drawbacks of using expensive transition metals, environmentally toxic catalysts, large amounts of organic solvents, and inconvenient setups were tackled in this report. A simple procedure was unveiled by employing sulfur as a redox reagent in the condensation of o-chloronitrobenzene and aldehydes or acetophenones under solvent-free conditions. This reaction was catalyzed by [CholineCl][Imidazole]2 under mild conditions. Furthermore, both CholineCl and IM are inexpensive and environment friendly.
2. Experimental section
2.1. Chemicals
CholineCl (98%), IM (assay 99%), 2-fluorobenzaldehyde (assay 97%), 2-chlorobenzaldehyde (assay 99%), 4-fluorobenzaldehyde (assay 98%), 4-bromobenzaldehyde (assay 99%), 4-(dimethylamino)benzaldehyde (assay 99%), methyl 4-formylbenzoate (assay 99%), 4-hydroxy-3-methoxybenzaldehyde (assay 98%), 3,4-dihydroxybenzaldehyde (assay 97%), 2-hydroxy-5-methylbenzaldehyde (assay 98%), furfural (assay 99%), 1H-pyrrole-2-carboxaldehyde (assay 98%), 4-imidazolecarboxaldehyde (assay 98%), 4-pyridinecarboxaldehyde (assay 98%), cyclohexanecarboxaldehyde (assay 97%), acetophenone (assay 99%), 2-hydroxyacetophenone (assay 98%), 3-methylacetophenone (assay 98%), 4-methylacetophenone (assay 95%), 4-methoxyacetophenone (assay 99%), and phenol (assay 99%) were obtained from Sigma-Aldrich. Urea (purity 98%), glycerol (for analysis), zinc chloride (for analysis), benzaldehyde (for synthesis), 4-methoxybenzaldehyde (for synthesis), and TLC silica gel 60 F254 were obtained from Merck. 1-Fluoro-2-nitrobenzene (98%), 1-chloro-2-nitrobenzene (98%), 1-bromo-2-nitrobenzene (98%), and 1-iodo-2-nitrobenzene (98%) were obtained from Across. Ethyl acetate (purity 99.5%) and n-hexane (purity 99.5%) were obtained from Xilong Chemical Co., Ltd (China).2.2. General procedure for the synthesis of DESs from CholineCl and IM
The DES derived from CholineCl and IM was synthesized according to a reported method.50 The DES was prepared by heating a mixture of IM and CholineCl at different molar ratios at 100 °C. The mixture slowly converted into a colorless transparent liquid within an hour. A homogeneous liquid was obtained and characterized using 1H and 13C NMR spectroscopy, FT-IR spectroscopy, and TGA (Scheme 2).2.3. General procedure for the synthesis of DES and 2-substituted benzothiazole derivatives
A mixture of o-chloronitrobenzene (1 mmol), aldehydes (1 mmol) or methyl aryl ketones (1 mmol), and sulfur (2.0 mmol) in the presence of [CholineCl][Imidazole]2 (35 mol%) was heated at 120 °C for 6 h in a round bottom flask. Upon completion of the reaction (monitored using TLC), the reaction mixture was extracted with ethyl acetate (3 × 5 mL) and then concentrated under reduced pressure. The crude product was purified using silica gel chromatography employing a solvent system of ethyl acetate and n-hexane. The products were further analyzed using 1H and 13C-NMR spectroscopy.3. Results and discussion
3.1. Characterization of [CholineCl][Imidazole]2
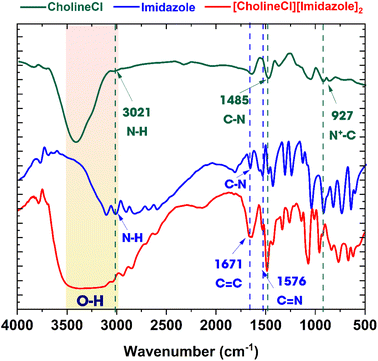
Deep eutectic solvents (DESs) formed through the formation of a hydrogen bond between CholineCl and IM. In this work, the FTIR spectroscopy was used to determine the structure of [CholineCl][Imidazole]2 (Fig. 1). A prominent signal in the range of 3400–3000 cm−1 can be attributed to the O–H stretching vibration of CholineCl, IM, and [CholineCl][Imidazole]2. A peak at 1485 cm−1, attributed to the methyl group of ammonium, and a peak at 927 cm−1, associated with quaternary ammonium absorption, were observed.51 The peaks seen around 1761 cm−1 and 1576 cm−1, indicative of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, respectively, are characteristic signals of IM present in the DES.52 Additionally, the signal at 3021 cm−1 was assigned to the N–H bond, clearly seen in samples of CholineCl, IM, and [CholineCl][Imidazole]2.53 The [CholineCl][Imidazole]2 spectrum exhibited vibrational bands corresponding to both CholineCl and IM. This discovery indicated that the structure of the choline cation remained intact throughout the formation of [CholineCl][Imidazole]2, indicating the formation of hydrogen bonds.
N bonds, respectively, are characteristic signals of IM present in the DES.52 Additionally, the signal at 3021 cm−1 was assigned to the N–H bond, clearly seen in samples of CholineCl, IM, and [CholineCl][Imidazole]2.53 The [CholineCl][Imidazole]2 spectrum exhibited vibrational bands corresponding to both CholineCl and IM. This discovery indicated that the structure of the choline cation remained intact throughout the formation of [CholineCl][Imidazole]2, indicating the formation of hydrogen bonds.
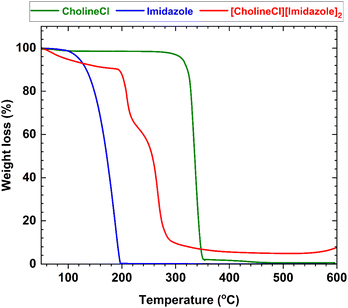
Thermogravimetric analysis (TGA) was used to assess the stability of deep eutectic solvents, namely, CholineCl, IM, and [CholineCl][Imidazole]2, as seen in Fig. 1. The data from TGA indicated that the mass remained relatively stable (approximately 10%) during the initial thermal decomposition step up to 220 °C. However, prior research has demonstrated that increasing the temperature from 200 °C to 300 °C results in the complete disintegration of DESs.54 During the final thermal degradation phase, [CholineCl][Imidazole]2 underwent full decomposition within the temperature range of 300–600 °C. Consequently, at the temperature established in our investigation, the DES remained stable and did not degrade (Fig. 2).
Fig. S4.1† shows the 1H and 13C NMR spectra of the catalyst. As seen in the 1H NMR spectra, signals [δH 7.76 (s) and 7.13 (s)] correspond to the IM moiety. CholineCl shows signals [δH 4.06–4.02 (m), 3.50–3.47 (m), and 3.17 (s)]. The signals in the NMR spectra indicate that the DES is free of impurities and confirm the molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of CholineCl and IM.
2 of CholineCl and IM.
3.2. Optimization of reaction conditions
After the successful synthesis and characterization of [CholineCl][Imidazole]2, a variety of reactions with diverse parameters, including temperature, duration, and catalyst loading, were evaluated to optimize the conditions using the model reaction of o-chloronitrobenzene, benzaldehyde, and sulfur (Scheme 3).We examined the influence of catalysts on the synthesis of 2-phenylbenzo[d]thiazole, and the results are shown in Table 1. The catalytic efficiency was investigated by conducting the reaction with different catalysts, including [CholineCl][ZnCl2]3, [CholineCl][Phenol]2, CholineCl, [CholineCl][Citric acid], [CholineCl][Oxalic acid], [CholineCl][Lactic acid], and [CholineCl][Salicylic acid], and without a catalyst. The conversion efficiency was 31%, 29%, and 17% for [CholineCl][Urea]2, [CholineCl][Glycerol]2, and [CholineCl][Phenol]2, respectively, as a catalyst (Table 1, entries 1–3). Conversely, [CholineCl][Urea]2, [CholineCl][Imidazole]2, and IM, bearing pairs of electrons representing Lewis bases, resulted in higher conversion efficiencies. The combination of CholineCl and IM has paved a new avenue for the synthesis of 2-phenylbenzothiazole by employing sulfur in this redox condensation. Thereafter, optimal conditions were applied to scale up the reaction of o-chloronitrobenzene, benzaldehyde, and sulfur from 1 mmol to 10 mmol without a significant drop in conversion.
| Entry | Catalysts | Yieldb (%) |
|---|---|---|
a Reaction conditions: o-chloronitrobenzene (1 mmol), benzaldehyde (1 mmol), sulfur (2 mmol) at 120 °C for 6 h with 35 mol% of the catalyst.b Isolated yield using chromatography (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 8 EA = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).c No reaction.d 10 mmol scale. 2).c No reaction.d 10 mmol scale. |
||
| 1 | [CholineCl][Urea]2 | 31 |
| 2 | [CholineCl][Glycerol]2 | 29 |
| 3 | [CholineCl][Phenol]2 | 17 |
| 4 | [CholineCl][ZnCl2]3 | NRc |
| 5 | Choline chloride | 5 |
| 6 | Imidazole | 65 |
| 7 | None | NRc |
| 8 | [CholineCl][Imidazole]2 | 78 (78)d |
| 9 | [CholineCl][Oxalic acid] | 35 |
| 10 | [CholineCl][Citric acid] | 32 |
| 11 | [CholineCl][Salicylic acid] | 31 |
| 12 | [CholineCl][Lactic acid] | 25 |
The impact of solvents on the synthesis of 2-phenylbenzo[d]thiazole is illustrated in Table 2. We investigated various solvents, namely, DMSO, DMF, 1,4-dioxane, and sulfolane, and under solvent-free conditions. With DMSO as the solvent, the yield of the reaction was at about 46%, while the yield of 2-phenylbenzo[d]thiazole was 78% under solvent-free conditions. DMSO is a polar solvent that can be used to dissolve organic compounds.55 Additionally, we examined dimethylformamide (DMF) and sulfolane, and the finding showed that yields of about 34% and 35%, respectively, was obtained. Meanwhile, the yield tended to increase when the solvent was changed from DMF to 1,4-dioxane, reaching 52%. In conclusion, solvent-free conditions provided the best reaction outcome in the investigation.
| Entry | Solvent | [CholineCl][Imidazole]2 (mol%) | Ratio of a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ca (mmol) ca (mmol) |
Yieldb (%) |
|---|---|---|---|---|
a o-Chloronitrobenzene (a), benzaldehyde (b), sulfur (c).b Isolated yield using chromatography (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 8 EA = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). |
||||
| 1 | DMSO | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
46 |
| 2 | DMF | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
34 |
| 3 | 1,4-Dioxane | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
52 |
| 4 | Sulfolane | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
35 |
| 5 | No solvent | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
78 |
The reaction time, catalyst loading, and temperature was investigated for the synthesis of 2-arylbenzothiazole (Table 3). The reaction was carried out at various reaction times including 1, 2, 4, 6, and 8 h at 120 °C (Table 3, entries 1–5). The results showed that the yield of the reaction tended to increase with prolonged reaction time. For example, the yield was 15%, 33%, and 64% with increasing the time from 1 to 6 h. The highest yield of about 78% was obtained at 6 h and tended to increase slowly when the reaction time was extended to 8 h. Therefore, a reaction time of 6 h was chosen for the next investigation. We investigated the effect of temperature on the yield of 2-arylbenzothiazaole at 6 h, with temperatures of 80 °C, 100 °C, 120 °C and 140 °C (Table 3, entries 4–8). The reaction yield was 13% at 80 °C and increased to about 30% at 100 °C. Interestingly, the yield dramatically improved to 78% at 120 °C, however, the yield declined with increasing temperature (140 °C), resulting in a yield of 65%. Thus, 120 °C was chosen as the ideal investigation temperature in this work. To choose the optimal reaction conditions for the synthesis of 2-arylbenzothiazole, we examined the effect of catalyst loading on yield (Table 3, entry 4 and entries 9–12). The reaction did not occur in the absence of a catalyst (Table 4, entry 9). The yield was 27% with 0.15 mol% catalyst, while the yield was 47% when catalyst loading was increased from 0.15 mol% to 0.25 mol%. The yield of the reaction improved significantly with 0.35 mol% and 0.5 mol% catalyst loading, resulting in values of 72% and 77%, respectively. Hence, the optimal catalyst loading for the reaction was 0.35 mol%.
| Entry | Temperature (°C) | Time (h) | [CholineCl][Imidazole]2 (mol%) | Ratio of a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ca (mmol) ca (mmol) |
Yieldb (%) |
|---|---|---|---|---|---|
a o-Chloronitrobenzene (a), benzaldehyde (b), sulfur (c).b Isolated yield using chromatography (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 8 EA = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).c NR: no reaction. 2).c NR: no reaction. |
|||||
| 1 | 120 | 1 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
15 |
| 2 | 120 | 2 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
33 |
| 3 | 120 | 4 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
64 |
| 4 | 120 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
78 |
| 5 | 120 | 8 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 |
| 6 | 80 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
13 |
| 7 | 100 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
30 |
| 8 | 140 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
65 |
| 9 | 120 | 6 | No catalyst | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
NRc |
| 10 | 120 | 6 | 0.15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
27 |
| 11 | 120 | 6 | 0.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
49 |
| 12 | 120 | 6 | 0.50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
77 |
| Entry | DES | Time (h) | Catalyst loading (mol%) | Ratio of a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ca (mmol) ca (mmol) |
Yieldb (%) |
|---|---|---|---|---|---|
a o-Chloronitrobenzene (a), benzaldehyde (b), sulfur (c).b Isolated yield using chromatography (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 8 EA = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). |
|||||
| 1 | [CholineCl][Imidazole]2 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 |
| 2 | [CholineCl][Imidazole]2 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
78 |
| 3 | [CholineCl][Imidazole]2 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
78 |
| 4 | [CholineCl][Imidazole]2 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
48 |
| 5 | [CholineCl][Imidazole]2 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
46 |
| 6 | [CholineCl]3[Imidazole] | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
31 |
| 7 | [CholineCl]3[Imidazole]5 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
57 |
| 8 | [CholineCl]3[Imidazole]10 | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
64 |
| 9 | [CholineCl][Imidazole] | 6 | 0.35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
47 |
In order to optimize the yield of the reaction, we evaluated the concentration of sulfur and the ratio of DESs for the synthesis of 2-phenylbenzo[d]thiazole. The results are illustrated in Table 4. The concentration of sulfur was changed from 1 to 5 mmol (Table 4, entries 1–5), using [CholineCl][Imidazole]2 as the catalyst. The yield reached around 50% with 1 mmol of sulfur, but it increased to 78% with 2 mmol of sulfur. The increase in the amount of sulfur from 2 to 3 mmol did not significantly change the yield, which remained at 78%. Interestingly, the yield of 2-phenylbenzo[d]thiazole was 46% when using 4 mmol and 5 mmol of sulfur. In general, increasing sulfur concentration did not enhance the reaction yield. Thus, 2 mmol of sulfur was chosen for further investigation. The ratio of the DES was examined to optimize the reaction yield (Table 4, entries 6–9). The yield of 2-phenylbenzo[d]thiazole was 31% using [CholineCl]3[Imidazole]. The increase in the DES ratio resulted in an elevated reaction yield. For instance, 57% and 64% 2-phenylbenzo[d]thiazole were obtained using [CholineCl]3[Imidazole]5 and [CholineCl]3[Imidazole]10, respectively. The highest yield of 78% was achieved with [CholineCl][Imidazole]2 after 6 h at 120 °C. Nevertheless, the yield decreased sharply when the ratio of CholineCl to IM was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, resulting in only 47%. Following the examination, 2 mmol of sulfur and [CholineCl][Imidazole]2 were used to optimize substrate ratios and the ratio of the DES (Scheme 4).
1, resulting in only 47%. Following the examination, 2 mmol of sulfur and [CholineCl][Imidazole]2 were used to optimize substrate ratios and the ratio of the DES (Scheme 4).
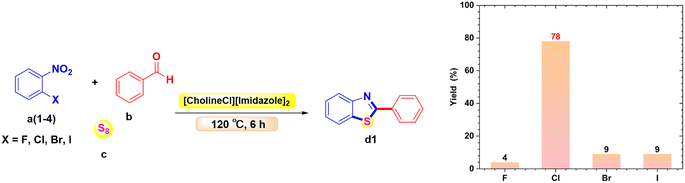
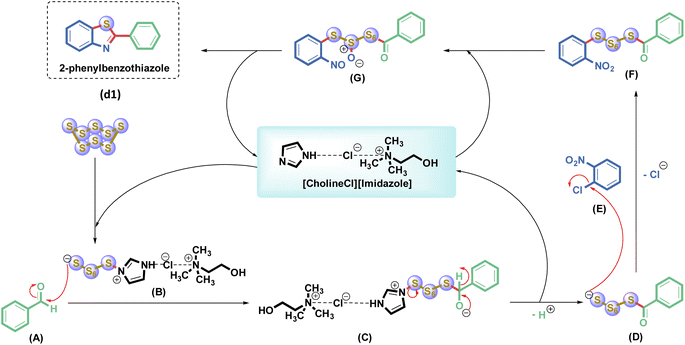
The reactivity of a halogen nitrobenzene substrate was investigated under the same reaction conditions (Scheme 5). The halogen nitrobenzenes investigated included 1-fluoro-2-nitrobenzene, 1-chloro-2-nitrobenzene, 1-bromo-2-nitrobenzene, and 1-iodo-2-nitrobenzene with benzaldehyde and sulfur in the presence of [CholineCl][Imidazole]2 at 120 °C for 6 h. The results showed that the substrate with the –F substituent gave a very low yield of the product (d1), only about 4%. However, when the halogen group was replaced by –Cl, –Br, or –I, the yield of product increased, reaching 78%, 9%, and 9%, respectively. This is because in order to form (d1), a replacement of the halogen group is required. Therefore, the halogen group must be easy to extract, or in other words, the C–X bond must be the least stable to react well. Of the four derivatives (X = –F, –Cl, –Br, and –I), the derivative containing –F is the most difficult to replace because the atomic radius of –F is very small, making the electron density at the bond very high and thus making it difficult to break; thus, the efficiency of the reaction using the 1-fluoro-2-nitrobenzene derivative is poor. In the remaining three derivatives, in terms of radius, the radius of Cl < Br < I leads to –I being the best leaving group. However, owing to the p–π resonance of the halogen group from layer three onwards, this resonance will gradually increase from –Cl to –I because this factor depends on their non-bonding electron pairs. These derivatives will react easily when these electron pairs are far from the nucleus and the element has low electronegativity. This results in the substrate bearing the –Cl substituent giving the best product formation yield.
To elucidate the importance of the catalyst, a leaching test was carried out under optimal reaction conditions (Fig. 3). Chloronitrobenzene (1 mmol), benzaldehyde (1 mmol), and sulfur (2 mmol) in the presence of [CholineCl][Imidazole]2 under standard heating conditions were used. The examination of the response advanced via two separate phases: (i) the preliminary phase of the reaction, which occurred under ideal circumstances for 3 hours. The response was then divided into three segments for additional analysis. The reaction concluded in the first phase, resulting in a yield of 32% after catalytic filtration and crystallization to separate the products. (ii) The reaction persisted under ideal circumstances, achieving an efficiency of roughly 44% after catalyst removal via filtration, consistent with the results of the preceding phase. In the third phase, the reaction was prolonged for 3 hours under standard heating conditions, following filtration and catalyst removal, yielding 78% of 2-phenylbenzo[d]thiazole at completion, which nearly matched the efficiency yield attained by the same reaction condition.
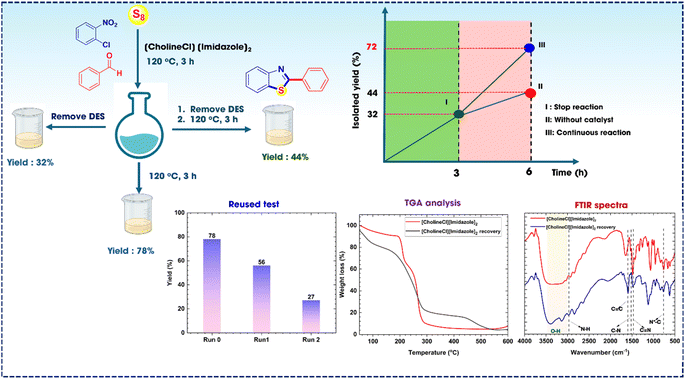 | ||
| Fig. 3 Leaching test procedure: (I) reaction stopped; (II) reaction without [CholineCl][Imidazole]2; (III) continuous reaction with [CholineCl][Imidazole]2. | ||
The [CholineCl][Imidazole]2 catalyst was assessed through reuse testing, thermogravimetric analysis (TGA), and Fourier-transform infrared (FTIR) spectroscopy (Fig. 3). The findings indicated that the catalyst was capable of being utilized twice. The yield was 56% after the first run and decrease to 27% after the second run. Nevertheless, during the two recovery periods, the yield markedly decreased due to the production of HCl during the reaction, which impaired the activity of [CholineCl][Imidazole]2. Our work presents a straightforward, effective, and eco-friendly method for the synthesis of 2-substituted benzothiazole derivatives. Subsequently, TGA analysis was used to assess the stability of the catalyst; upon recovery, the catalyst remained stable within the investigated temperature range. FTIR spectra elucidated the structure of the [CholineCl][Imidazole]2 catalyst, with a significant signal in the region of 3400–3000 cm−1 likely corresponding to the O–H stretching vibration of both the original and recovered catalysts. In comparison to the original catalyst, a distinctive signal was detected; notably, the characteristic bonds, including N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, and N
C, C–N, and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, were present in both the recovered and original spectra. The findings indicated that the catalyst structure remained intact post-recovery.
N, were present in both the recovered and original spectra. The findings indicated that the catalyst structure remained intact post-recovery.
During the reaction, HCl was generated as a by-product, which caused a significant decrease in the activity of [CholineCl][Imidazole]2. Based on the FTIR spectra results of the [CholineCl][Imidazole]2 (recovery) and [CholineCl][Imidazole]2/HCl, a similarity was observed (ESI, Fig. S2.1†), which may indicate that the structure of [CholineCl][Imidazole]2 has changed, resulting in a decrease in the absorption of the signals.
3.3. Assessment of green metrics
An increasing focus has been placed on developing environmentally friendly and sustainable technologies to synthesize organic molecules. Green chemistry is an example of this approach, which offers a framework for evaluating the environmental sustainability of chemical processes. Green chemistry is an example of this method. An illustration of this may be seen in the use of [CholineCl][Imidazole]2 as a catalyst for the synthesis of 2-substituted benzothiazole derivatives (the values that were computed are presented in the ESI†). As per the above results, it was concluded that the reaction has a low environment-factor (E-factor = 0.61), high atom economy (AE = 64.54%), high process mass intensity (PMI = 1.98), and medium reaction mass efficiency (RME = 50.34%), with an acceptable eco-score (71.0%). These values clearly indicate the eco-friendliness of the present synthesis. The exceptional results demonstrated the sustainable and eco-friendly characteristics of using the [CholineCl][Imidazole]2 catalyst in the synthesis of poly-functionalized IM scaffolds, with little environmental impact. This represents significant progress in the quest for developing sustainable and durable methods for synthesizing organic compounds.3.4. Synthesis of 2-substituted benzothiazole derivatives
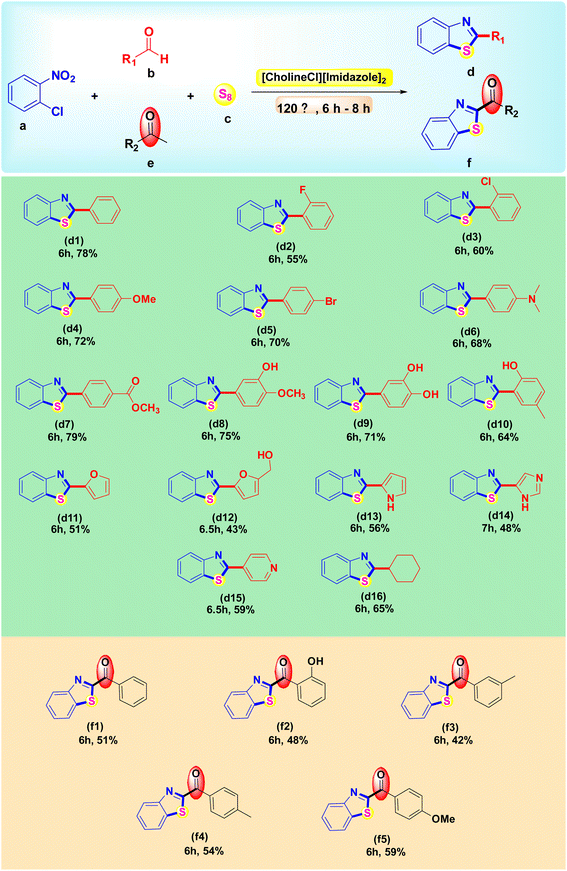
Under optimal conditions, various aldehydes were simultaneously harnessed for the synthesis of 2-substituted benzothiazole derivatives. In this study, we used the derivatives of benzaldehyde and acetophenone to synthesize 2-substitute benzothiazole derivatives. As shown in Scheme 5, most of these substrates reacted smoothly to obtain products in moderate-to-good yields. The impact on yields was seen when the reaction included aromatic aldehydes with substituents at the ortho position, while substituents at the para or in combination with the meta position produced the desired products in greater yields. Moreover, cyclohexanecarboxaldehyde, a less active aldehyde, gave an acceptable yield. On the contrary, when the reaction was performed with five-membered heterocyclic aldehydes such as furan, pyrrole, and IM, the scaffold expressed lower activity in yields than the benzene-ring. The lowest yield belongs to 5-hydroxymethylfurfural possibly owing to the instability of this aldehyde. In addition to aldehydes, aryl methyl ketones were utilized to widen the scope. Some acetophenones containing substituents at the ortho, meta, and para position were chosen to synthesize 2-aroylbenzothiazole derivatives.3.5. Mechanism
The proposed reaction mechanism is illustrated in Scheme 6. The DES catalyst, composed of CholineCl and IM, exhibits strong basicity due to sp2 nitrogen, which facilitates the ring-opening of octasulfur, producing the nucleophile (B). Nucleophile B subsequently engages in nucleophilic substitution with the carbonyl group of benzaldehyde (A), forming an intermediate (D) through dehydrogenation and resulting in a dipolar tetrahedral intermediate (C). Intermediate D undergoes further substitution with 1-nitro-2-chlorobenzene (E), leading to the elimination of Cl− and the formation of intermediate F. The catalyst then promotes the reduction of SO3− while simultaneously eliminating S6 via a carbonyl group displacement and ring closure of intermediate (G), culminating in the formation of the product (d1).3.6. The comparison of this work to previous works
This work investigates a DES formed using CholineCl and IM in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio as a novel, eco-friendly catalyst for synthesizing 2-phenylbenzothiazole and compares its performance to previous research. Earlier studies employing N-methylmorpholine and NH4Cl yielded moderate results but relied on toxic reagents and harsh conditions. Similarly, research utilizing MeSO3H/SiO2 and Cu(OAc)2 achieved high efficiency but raised significant environmental and safety concerns. In contrast, the DES, composed of biodegradable components such as CholineCl and IM, operate under milder conditions (120 °C for 6 hours), effectively reducing energy consumption and eliminating the need for hazardous solvents such as toluene. Although the DES yielded a slightly lower product (78%) compared to previous catalysts, its sustainability, safety, and cost-efficiency advantages are substantial. Moreover, the DES catalyst can be readily removed from the reaction mixture and reutilized in future reactions with minimum activity loss, thus enhancing sustainability by decreasing waste and the need for new catalysts (Table 5).
2 ratio as a novel, eco-friendly catalyst for synthesizing 2-phenylbenzothiazole and compares its performance to previous research. Earlier studies employing N-methylmorpholine and NH4Cl yielded moderate results but relied on toxic reagents and harsh conditions. Similarly, research utilizing MeSO3H/SiO2 and Cu(OAc)2 achieved high efficiency but raised significant environmental and safety concerns. In contrast, the DES, composed of biodegradable components such as CholineCl and IM, operate under milder conditions (120 °C for 6 hours), effectively reducing energy consumption and eliminating the need for hazardous solvents such as toluene. Although the DES yielded a slightly lower product (78%) compared to previous catalysts, its sustainability, safety, and cost-efficiency advantages are substantial. Moreover, the DES catalyst can be readily removed from the reaction mixture and reutilized in future reactions with minimum activity loss, thus enhancing sustainability by decreasing waste and the need for new catalysts (Table 5).
| Entry | Catalyst | Condition | Yield (%) | Reference |
|---|---|---|---|---|
| 1 | N-Methylmorpholine, 4 eq. | 130 °C, 16 h | 80 | 56 |
| 2 | NH4Cl (70% mol), O2 air, CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (15 H2O (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
rt, 30 min | 84 | 57 |
| 3 | MeSO3H/SiO2 (1 mL/0.3 g) | 140 °C, 2 h | 87 | 58 |
| 4 | PBu3, toluene | rt, refluxing, 10 min | 90 | 59 |
| 5 | Cu(OAc)2, Et3N, ethanol | 70 °C, 6 h | 86 | 60 |
| 6 | [CholineCl][Imidazole]2 (35% mol) | 120 °C, 6 h | 78 | This work |
4. Conclusion
In conclusion, we effectively synthesized 2-substituted benzothiazole derivatives via a solvent-free approach with the [CholineCl][Imidazole]2 catalyst. The reaction performance is achieved a maximum of 78% 2-phenylbenzo[d]thiazole, and 21 examples were synthesized using this high-yield and green method. The [CholineCl][Imidazole]2 catalyst was characterized using 1H NMR, 13C NMR, FTIR techniques, and the stability of the DES was evaluated using TGA analysis. Additionally, we have offered a detailed account of a prospective study to clarify the function of the [CholineCl][Imidazole]2 catalyst in the cyclization of chloronitrobenzene, benzaldehyde, and sulfur. The catalyst exhibited reusability and enabled environmentally sustainable techniques using a one-pot, multi-component reaction process. Utilizing solvent-free reactions in organic chemical synthesis effectively addresses issues related to environmentally harmful solvents. Future investigations should focus on various variants of the substrate 1-chloro-2-nitrobenzene in the synthesis of 2-substituted benzothiazole derivatives.Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.Author contributions
Vy Anh Truong, Minh Hai Tran, and Trinh Hao Nguyen: investigation, methodology, resources, formal analysis, validation, data curation, and writing – original draft. Hai Truong Nguyen: methodology, resources, formal analysis, validation, data curation, writing – review and editing, and supervision.Conflicts of interest
There are no conflicts to declare.References
- S. J. Barnes, S. Staude, M. Le Vaillant, R. Piña and P. C. Lightfoot, Ore Geol. Rev., 2018, 101, 629–651 CrossRef.
- J.-G. Wagenfeld, K. Al-Ali, S. Almheiri, A. F. Slavens and N. Calvet, Waste Manage. Res., 2019, 95, 78–89 CrossRef.
- M. Maji, D. Panja, I. Borthakur and S. Kundu, Org. Chem. Front., 2021, 8, 2673–2709 RSC.
- T. B. Nguyen, Adv. Synth. Catal., 2020, 362, 3448–3484 CrossRef CAS.
- M.-H. D. Dang, L. H. T. Nguyen and P. H. Tran, Synthesis, 2020, 52, 1687–1694 CrossRef CAS.
- T. T. Nguyen and P. H. Tran, RSC Adv., 2020, 10, 9663–9671 RSC.
- T. B. Nguyen, Adv. Synth. Catal., 2017, 359, 1066–1130 CrossRef CAS.
- X. Li, W. Ma, H. Li, Q. Zhang and H. Liu, Coord. Chem. Rev., 2020, 408, 213191 CrossRef CAS.
- Q. A. Ngo, P. Retailleau and T. B. Nguyen, Green Chem., 2017, 19, 4289–4293 RSC.
- J. Cao and E. Su, J. Cleaner Prod., 2021, 314, 127965 CrossRef CAS.
- Y. Wang, Y. Hou, W. Wu, D. Liu, Y. Ji and S. Ren, Green Chem., 2016, 18, 3089–3097 RSC.
- M. H. Chakrabarti, F. S. Mjalli, I. M. AlNashef, M. A. Hashim, M. A. Hussain, L. Bahadori and C. T. J. Low, Renewable Sustainable Energy Rev., 2014, 30, 254–270 CrossRef CAS.
- P. T. Nguyen, T.-D. T. Nguyen, V. S. Nguyen, D. T.-X. Dang, H. M. Le, T.-C. Wei and P. H. Tran, J. Mol. Liq., 2019, 277, 157–162 CrossRef CAS.
- P. Pradeepkumar, A. M. Elgorban, A. H. Bahkali and M. Rajan, New J. Chem., 2018, 42, 10366–10375 RSC.
- J. D. Mota-Morales, M. C. Gutiérrez, M. L. Ferrer, R. Jiménez, P. Santiago, I. C. Sanchez, M. Terrones, F. Del Monte and G. Luna-Bárcenas, J. Mater. Chem., 2013, 1, 3970–3976 RSC.
- M. Ruesgas-Ramón, M. C. Figueroa-Espinoza and E. Durand, J. Agric. Food Chem., 2017, 65, 3591–3601 CrossRef.
- A. Kalyniukova, J. Holuša, D. Musiolek, J. Sedlakova-Kadukova, J. Płotka-Wasylka and V. Andruch, Ind. Crops Prod., 2021, 172, 114047 CrossRef CAS.
- Y. Hou, Y. Gu, S. Zhang, F. Yang, H. Ding and Y. Shan, J. Mol. Liq., 2008, 143, 154–159 CrossRef CAS.
- J. A. Sirviö, K. Hyypiö, S. Asaadi, K. Junka and H. Liimatainen, Green Chem., 2020, 22, 1763–1775 RSC.
- M. Zdanowicz, T. Spychaj and H. Mąka, Carbohydr. Polym., 2016, 140, 416–423 CrossRef CAS PubMed.
- A. Procentese, E. Johnson, V. Orr, A. G. Campanile, J. A. Wood, A. Marzocchella and L. Rehmann, Bioresour. Technol., 2015, 192, 31–36 CrossRef CAS PubMed.
- M. C. Henry, V. M. Abbinante and A. Sutherland, Eur. J. Org Chem., 2020, 2020, 2819–2826 CrossRef CAS.
- M. Komiya, S. Asano, N. Koike, E. Koga, J. Igarashi, S. Nakatani and Y. Isobe, Chem. Pharm. Bull., 2013, 61, 1094–1097 CrossRef CAS PubMed.
- A. Spadaro, M. Frotscher and R. W. Hartmann, J. Med. Chem., 2012, 55, 2469–2473 CrossRef CAS.
- Y. Liao, H. Qi, S. Chen, P. Jiang, W. Zhou and G.-J. Deng, Org. Lett., 2012, 14, 6004–6007 CrossRef CAS PubMed.
- D. Wang, J. Albero, H. García and Z. Li, J. Catal., 2017, 349, 156–162 CrossRef CAS.
- K. P. Yadav, M. A. Rahman, S. Nishad, S. K. Maurya, M. Anas and M. Mujahid, Intell. Pharm., 2023, 1, 122–132 Search PubMed.
- M. M. Heravi, E. Hashemi, Y. S. Beheshtiha, K. Kamjou, M. Toolabi and N. Hosseintash, J. Mol. Catal. A:Chem., 2014, 392, 173–180 CrossRef CAS.
- F. G. Zadeh, B. Asadi, I. Mohammadpoor-Baltork, S. Tangestaninejad, V. Mirkhani, M. Moghadam and A. Omidvar, RSC Adv., 2023, 13, 31213–31223 RSC.
- M. N. Bhoi, M. A. Borad, E. A. Pithawala and H. D. Patel, Arabian J. Chem., 2019, 12, 3799–3813 CrossRef CAS.
- L. M. Bouchet, A. A. Heredia, J. E. Argüello and L. C. Schmidt, Org. Lett., 2020, 22, 610–614 CrossRef CAS PubMed.
- S. V. Nalage, S. V. Bhosale, D. S. Bhosale and W. N. Jadhav, Chin. Chem. Lett., 2010, 21, 790–793 CrossRef CAS.
- C. Chaudhari, S. M. A. H. Siddiki and K.-i. Shimizu, Tetrahedron Lett., 2015, 56, 4885–4888 CrossRef CAS.
- M. Zakeri, M. Moghadam, V. Mirkhani, S. Tangestaninejad, I. Mohammadpoor-Baltork and Z. Pahlevanneshan, Appl. Organomet. Chem., 2018, 32, e3937 CrossRef.
- K. Bahrami, M. M. Khodaei and F. Naali, J. Exp. Nanosci., 2016, 11, 148–160 CrossRef CAS.
- H. A. Soliman, M. El-Shahat and A.-G. Soliman, Lett. Org. Chem., 2019, 16, 584–591 CrossRef CAS.
- N. N. Pesyan, H. Batmani and F. Havasi, Polyhedron, 2019, 158, 248–254 CrossRef.
- E. Niknam, F. Panahi, F. Daneshgar, F. Bahrami and A. Khalafi-Nezhad, ACS Omega, 2018, 3, 17135–17144 CrossRef CAS PubMed.
- K. Bahrami and Z. Karami, J. Exp. Nanosci., 2018, 13, 272–283 CrossRef CAS.
- M. Chhabra, S. Sinha, S. Banerjee and P. Paira, Bioorg. Med. Chem. Lett., 2016, 26, 213–217 CrossRef CAS PubMed.
- A. Sethiya, N. Sahiba, P. Teli, J. Soni and S. Agarwal, Mol. Diversity, 2022, 26, 513–553 CrossRef CAS.
- H. Sharghi, S. F. Razavi, M. Aberi, F. Tavakoli and M. Shekouhy, ChemistrySelect, 2020, 5, 2662–2671 CrossRef CAS.
- L. M. Bouchet, A. A. Heredia, J. E. Argüello and L. C. Schmidt, Org. Lett., 2019, 22, 610–614 CrossRef PubMed.
- Y. Cheng, J. Yang, Y. Qu and P. Li, Org. Lett., 2012, 14, 98–101 CrossRef CAS.
- S. Liu, R. Chen, H. Chen and G.-J. Deng, Tetrahedron Lett., 2013, 54, 3838–3841 CrossRef CAS.
- Q. Feng and Q. Song, Adv. Synth. Catal., 2014, 356, 2445–2452 CrossRef CAS.
- Q. Gao, X. Wu, F. Jia, M. Liu, Y. Zhu, Q. Cai and A. Wu, J. Org. Chem., 2013, 78, 2792–2797 CrossRef CAS.
- T. B. Nguyen, K. Pasturaud, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2015, 17, 2562–2565 CrossRef CAS.
- S. Dhadda, A. K. Raigar, K. Saini, Manju and A. Guleria, Sustainable Chem. Pharm., 2021, 24, 100521 CrossRef CAS.
- J. A. Sirviö, K. Hyypiö, S. Asaadi, K. Junka and H. Liimatainen, Green Chem., 2020, 22, 1763–1775 RSC.
- W. J. Yang, T. Cai, K.-G. Neoh, E.-T. Kang, S. L.-M. Teo and D. Rittschof, Biomacromolecules, 2013, 14, 2041–2051 CrossRef CAS PubMed.
- T. H. Nguyen, H. B. Phan, D. D. Le, H. T. T. Nguyen, K. N. Tran, L. B. Nguyen and P. H. Tran, Biomass Bioenergy, 2024, 180, 107004 CrossRef CAS.
- I.-C. Poplăcean, M. Mureşan-Pop, M. Vasilescu, A. Simion and S. Simon, J. Mol. Struct., 2025, 1321, 140094 CrossRef.
- A. Satlewal, R. Agrawal, S. Bhagia, J. Sangoro and A. J. Ragauskas, Biotechnol. Adv., 2018, 36, 2032–2050 CrossRef CAS PubMed.
- N. Keppeler, N. R. S. Vagula, M. A. Komesu, N. I. Malek and O. A. El Seoud, J. Mol. Liq., 2024, 397, 124130 CrossRef CAS.
- L. A. Nguyen, Q. A. Ngo, P. Retailleau and T. B. Nguyen, Green Chem., 2017, 19, 4289–4293 RSC.
- B. Maleki and H. Salehabadi, Eurasian J. Chem., 2010, 1, 377–380 CAS.
- H. Sharghi and O. Asemani, Synth. Commun., 2009, 39, 860–867 CrossRef CAS.
- F. L. Coelho and L. F. Campo, Tetrahedron Lett., 2017, 58, 2330–2333 CrossRef CAS.
- Y. Sun, H. Jiang, W. Wu, W. Zeng and X. Wu, Org. Lett., 2013, 15, 1598–1601 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07400a |
| ‡ The authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |