Open Access Article
Open Access ArticleEnabling amidation in water: micellar catalysis approach for sustainable synthesis of iopamidol†
Anita Zucchi a,
Sara Mattiello
a,
Sara Mattiello a,
Rachele Maschio
a,
Rachele Maschio b,
Valentina Bellottia,
Giovanni B. Giovenzana
b,
Valentina Bellottia,
Giovanni B. Giovenzana b,
Luciano Lattuada
b,
Luciano Lattuada *c and
Luca Beverina
*c and
Luca Beverina *a
*a
aDepartment of Materials Science, University of Milano Bicocca, Via R.Cozzi 55, Milano, I-20125, Italy. E-mail: luca.beverina@unimib.it
bUniversità del Piemonte Orientale, Dipartimento di Scienze del Farmaco, Largo Donegani 2/3, Novara 28100 (NO), Italy
cBracco Imaging S.p.A, Via Egidio Folli 50, Milano, I-20134, Italy
First published on 18th December 2024
Abstract
Micellar catalysis is becoming an increasingly versatile tool to carry out a wide range of organic transformations using water as the reaction medium. The approach was recently found to be effective also in the case of water sensitive organics such as acyl chlorides. This finding is of great relevance for the manufacturing of challenging substrates such as the known iodinated contrast agent iopamidol, requiring the use of aprotic dipolar solvents (DMF, NMP, DMAc) in the key amidation step of an acyl dichloride intermediate with serinol. These solvents are subjected to an increasing regulatory pressure due to safety and environmental concerns. We show that the amidation step can be straightforwardly performed in water containing the industrial surfactant Triton X-100, provided that the employed amine is not water soluble. Accordingly, we developed suitable lipophilic serinol derivatives that, after amidation and hydrolysis, directly gave iopamidol in a one-pot process.
1 Introduction
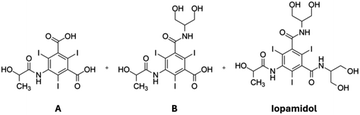
Iodinated contrast agents (ICAs) are non-ionic, water soluble molecules containing iodine atoms which are currently employed worldwide in X-ray diagnostic imaging.1,2These extracellular injectable drugs are particularly valuable in these procedures because they absorb X-rays and appear opaque on radiographic images, by attenuation of the applied ionizing radiation, aiding healthcare professionals in diagnosing and monitoring a wide range of medical conditions.3
Their excellent safety profile, effectiveness, and versatility have made ICAs a crucial tool in modern medicine for accurate diagnosis and treatment planning. Iodinated contrast agents have been on the market for more than 40 years since the launch of iopamidol, the pioneering compound of this category, and they are manufactured on a commodity scale of thousands of tons annually.4–8
Iopamidol9,10 is produced through a sequential synthesis which involves, in the final step, a classical amidation reaction11 between S-5-[[2-(acetyloxy)-1-oxopropyl]amino]-2,4,6-triiodo-1,3-benzenedicarboxylic acid dichloride 1 and an excess of 2-amino-1,3-propandiol (serinol) 2,12 in N,N-dimethylacetamide (DMAc), a common dipolar aprotic solvent, followed by alkaline hydrolysis (Scheme 1).13
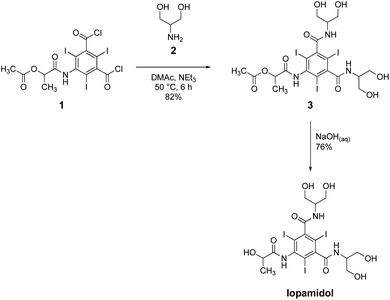 | ||
| Scheme 1 Industrial synthesis of iopamidol.13 | ||
The European Union's Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) legislation has identified DMF, DMAc and NMP as Substances of Very High Concern (SVHC), encouraging the use of safer and more environmentally friendly alternatives.14,15 While, N-butylpyrrolidone (NBP)16–18 and dihydrolevoglucosenone (Cyrene™)19,20 have been proposed as viable and safer alternatives to the currently used dipolar aprotic solvents, their higher boiling points (respectively 241 °C and 227 °C) may pose a problem in the recovery and recycle.
Water as the ultimate green solvent,21 offers multiple advantages: it is abundant, non-toxic, non-flammable, it has a large thermal capacity and a relatively low boiling point. The limited solubility in water of most organics is no longer considered a limit for the development of water-based protocols. It has been demonstrated that reactions can be carried out “on water” in several cases, but the method lacks generality and so the use of water solution of surfactants has become a more reliable and general tool.22,23 Surfactants self-assemble in water forming association colloids featuring lipophilic pockets where organics can accumulate at high effective concentration (lipophilic effect) and react. The method goes by the name of micellar catalysis as reaction becomes more efficient, faster, and compatible with milder conditions due to preferential accumulation. Recently, it was demonstrated that micellar catalysis is also compatible with water sensitive reagents such as acyl chlorides, swiftly and efficiently amidated in water solution of the designer surfactant DL-α-Tocopherol methoxypolyethylene glycol succinate (TPGS-750-M, Scheme 2) with a number of lipophilic amines.24 This water based method complements existing literature where amides are formed in water by condensation of carboxylic acids and amines in the presence of suitable coupling agents like COMU.25,26
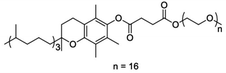 | ||
| Scheme 2 Structure of the designer surfactant TPGS-150-M.29 | ||
TPGS-750-M is certainly efficient but still relatively expensive and thus unsuitable for the production of a commodity chemical such as iopamidol. Efforts are under way to scale up such surfactant and characterize its fate in the waste stream.27 As such, even if not yet competitive with established industrial surfactants, TPGS-750-M might to play an increasingly relevant role in process chemistry. For the purpose of the present study, we relied on industrial surfactants.
The exact details on where the reactions happen (inside the micelle vs. the interphase between polar and nonpolar districts or even completely in the water swelled portion) are reaction and substrate dependent. We however demonstrated that for the Suzuki–Miyaura coupling, also involving an apolar (the halogen or pseudo halogen) and a polar species (the boronic acid or ester), chemicals localize right at the interphase between glycol and alkyl chains, thus further improving on the hydrophobic effect.28
Recovery of the product and purification from the surfactant is possible through extraction with a benign solvent or simple filtration in the case of solid products.
The amidation of 1 with serinol is particularly challenging because of two specific features, so far not addressed in the micellar catalysis literature: (1) serinol is miscible with water in all proportions and features a markedly negative partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = −1.95), and (2) iopamidol is very water soluble and cannot be isolated by extraction with organic solvents. Compound 1 also offers challenges of its own, being rather lipophilic (log
P = −1.95), and (2) iopamidol is very water soluble and cannot be isolated by extraction with organic solvents. Compound 1 also offers challenges of its own, being rather lipophilic (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 4.30) but essentially soluble only in aprotic dipolar solvents and thus intrinsically difficult to disperse even in the presence of performing surfactants. We here show that the direct reaction of 1 and serinol in water solution of surfactants is only possible at very high surfactant concentration, making isolation and purification very challenging. Conversely, the reaction becomes smooth and efficient provided that serinol is converted in suitably lipophilic derivatives via introduction of a variety of protecting groups such as esters, silyl ethers, benzyl and tertbutyl ether. In the case of the particularly performing silyl ether derivatives, amidation is nearly quantitative and cleavage can be performed directly in the reaction mixture, simply by changing the pH. Iopamidol can be isolated from the reaction mixture via elution through ion exchange resins and a crosslinked adsorbent resin. The overall process does not require the use of organic solvents, it is efficient and do not include purification steps not already employed in the industrial manufacturing of iopamidol.
P = 4.30) but essentially soluble only in aprotic dipolar solvents and thus intrinsically difficult to disperse even in the presence of performing surfactants. We here show that the direct reaction of 1 and serinol in water solution of surfactants is only possible at very high surfactant concentration, making isolation and purification very challenging. Conversely, the reaction becomes smooth and efficient provided that serinol is converted in suitably lipophilic derivatives via introduction of a variety of protecting groups such as esters, silyl ethers, benzyl and tertbutyl ether. In the case of the particularly performing silyl ether derivatives, amidation is nearly quantitative and cleavage can be performed directly in the reaction mixture, simply by changing the pH. Iopamidol can be isolated from the reaction mixture via elution through ion exchange resins and a crosslinked adsorbent resin. The overall process does not require the use of organic solvents, it is efficient and do not include purification steps not already employed in the industrial manufacturing of iopamidol.
2 Results and discussion
2.1 Direct amidation of chloride 1 with serinol
We carried out a series of test reactions to identify the most suitable surfactant for our scope as well as to test the reactivity of 1 under standard micellar amidation conditions involving lipophilic amines. We focused our attention on industrial surfactants, three neutral (Kolliphor EL, Triton X-100, Brij L23) and one anionic (Sodium Dodecyl Sulphate, SDS). We also compared the results with surfactant free “on water” conditions.30,31The details of such investigation are discussed in the ESI† and summarized in Table 1. None of the conditions we tested can be considered a viable method to produce iopamidol. Only when working with TX-100 at concentrations too high to be practical we could observe sizeable iopamidol formation. We attributed the result to the particularly good dispersing performances displayed by such surfactant over the very poorly soluble chloride 1 (Fig. 1). We thus focused on TX-100 any further evaluation, also because its biodegradation under aerobic and anaerobic conditions in municipal wastewater sludge is documented.32
 | ||
| Fig. 1 Dispersing capabilities of TX-100 on chloride 1. Left: granules of derivative 1 in plain water. Right: chloride 1 in TX-100 10 wt% after 2 h stirring at r.t. | ||
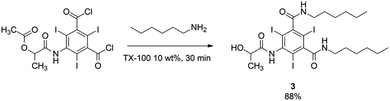
The behaviour of the direct amidation reaction is mostly due to the high hydrophilicity of serinol. In fact, performing the amidation reaction under entry 5 conditions, but using the water insoluble 1-hexylamine instead of serinol leads to the formation of the expected amidation product in 88% yield after 30 min, isolated by filtration of the reaction mixture and washing of the precipitated with water directly on the filter. See Scheme 3.
2.2 Amidation of chloride 1 with protected serinol derivatives
We thus devised a series of serinol protected derivatives enabling (a) more efficient reaction promoting preferential partitioning in the lipophilic districts and (b) direct isolation by filtration from the reaction mixture, due to the hydrophobicity of the amidation product as well.Fig. 2 shows the structure of the ten protected serinol derivatives we synthesized, focusing on improving lipophilicity by means of elaboration of the alcohol functionalities.
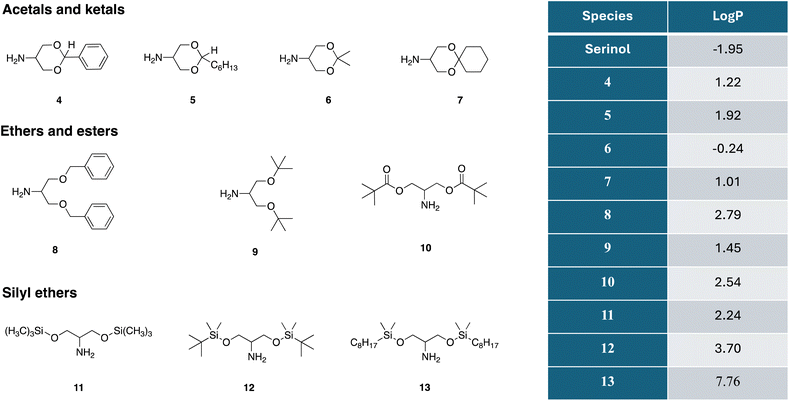 | ||
Fig. 2 Chemical structure and calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for protected serinols 4–13. Log P values for protected serinols 4–13. Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P calculated with consensus model using ChemAxon method Klopman model and the PHYSPROP© database.33,34 P calculated with consensus model using ChemAxon method Klopman model and the PHYSPROP© database.33,34 | ||
These compounds were categorized into three distinct groups based on the specific protection strategies employed. The detailed syntheses of the new compounds are reported in the ESI† section.
Acetals and ketals are popular and versatile protecting groups for 1,3-diols such as serinol. The partition coefficient of the protected derivative can be controlled selecting the suitable carbonyl counterpart. A further attractive feature of this protection scheme is the recyclability of the carbonyl derivative. Deprotection after amidation in fact would lead back to the starting protecting reagent that could be recycled instead of contributing to the production of wastes. We synthetized and tested in the amidation reaction four distinct derivatives: benzylidene acetal (4), heptanal acetal (5), acetonide (6) and cyclohexanone ketal (7). The second group of protected derivatives contains three classical protections of the –OH group: dibenzyl ether (Bn) (8), di-tert-butyl ether (t-Bu) (9) and pivalic diester (10). The aforementioned protective assemblies can be removed as follows: benzyl ether by catalytic hydrogenation, di-tert-butyl ether by acid catalyzed decomposition and the pivalic diester both via acid or basic hydrolysis. Recycling of the protecting group is only possible in the case of the pivalate 10.
The third group contains three different silyl ethers: trimethylsilyl (11), tert-butyldimethyl silyl (12) and dimethyloctyl (13). The silyl group can be removed under mild conditions by using a fluoride source like tetrabutylammonium fluoride (TBAF) or ammonium fluoride (NH4F), to regenerate the original functional group, or by treatment with diluted HCl aqueous solution (around 5 wt%) at room temperature.
The second cleavage strategy is preferable on industrial scale due to the cost and environmental concerns connected with the use of fluorides. All such derivatives were tested in the amidation reaction according to the same general protocol, with minor variations consisting in the mechanical milling of 1 (otherwise employed as pellets) prior to its introduction in the reaction mixture and the nature of the base. We preferentially employed triethylamine, but also in some cases tested diisopropylethylamine, according to recent literature suggesting better performances in the latter case.35
Table 2 shows the results of the amidation reactions. As shown in entries A–D, none of the acetals and ketals derivatives gave satisfactory results. The mass recovery – calculated as the weight ratio between the water insoluble precipitate at the end of the reaction and the combined weight of 1 and the employed serinol derivative – was also particularly poor hinting at sizeable hydrolysis of 1. The issue with such derivatives in a micellar amidation reaction is not the partitioning of the species. We did observe the formation of a water insoluble precipitate, mostly constituted by the monoamidation products. Such behaviour is probably related to the limited stability of acetals and ketals at basic pH and in water, making the cleavage reaction faster than the amidation. Indeed, we investigated the hydrolysis kinetics by GC-MS (ESI† section) and we verified that under identical reaction conditions but without compound 1, the cleavage is complete in 30 min, whereas in the best scenario the amidation reaction takes at least 2 h. Therefore, serinol is formed contributing to further raising the pH of the water phase, eventually leading to the hydrolysis of 1. The only reaction that gave moderately good results is described in entry B2 where a little amount of toluene was added as a mixing aid. Recurrence to small amount of tolerable solvents is a common and resourceful strategy in micellar catalysis, in this particular case improving but not decisively solving the issues encountered with such kind of protection.36,37
| Test | Amine | Reaction conditions | Mass recovery [wt%] | Composition of the water insoluble ppt | Yield of bisamidation product |
|---|---|---|---|---|---|
| A | 4 | TX-100 10 wt%, 3 eq. Et3N, rt | 0 | Complex mixture hydrolysis product | 0 |
| B1 | 5 | TX-100 10 wt%, 3 eq. Et3N, rt, 3 h | 33 | Mono-amidation product, bis-amidation product in traces | 0 |
| B2 | 5 | TX-100 10 wt%, toluene 10 v%, 3 eq. Et3N, rt, 3 h | 55 | Bis-amidation product mainly. Mono amidation product in traces | 55% |
| C1 | 6 | TX-100 10 wt%, 3 eq. Et3N, rt, 3 h | 0 | In solution iopamidol & unprotected monoamidation product in traces | 0 |
| C2 | 6 | TX-100 2 wt%, 2.2 eq. DIPEA, rt, 5 h | 15 | Unreacted 1 | 0 |
| D1 | 7 | TX-100 10 wt%, 3 eq. Et3N, rt, 3 h | 11 | Unreacted 1. Iopamidol detected in solution | 0 |
| E1 | 8 | TX-100 10 wt%, 3 eq. Et3N, rt | 64 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol mixture of mono 1 mol mixture of mono![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bis-amidated product bis-amidated product |
0 |
| F1 | 9 | TX-100 10 wt%, 2.2 eq. DIPEA, rt | 56 | Bis-amidation product | 56 |
| F2 | 9 | TX-100 2 wt%, 2.2 eq. DIPEA, rt, 5 h | 70 | Bis-amidation product | 70 |
| G1 | 10 | TX-100 10 wt%, 3 eq. Et3N, rt, 5h | 32 | Bis-amidation product (partial deacetylation) | 32 |
| G2 | 10 | TX-100 2 wt%, 2.2 eq. DIPEA, rt, 24 h | 23 | Bis-amidation product | 23 |
| H1 | 11 | TX-100 10 wt%, 3 eq. Et3N, rt, 2 h | 70 | Bis-amidation and partial hydrolysis products | 70 |
| H2 | 11 | TX-100, 2 wt%, 3 eq. DIPEA | 68 | Bis-amidation product | 68 |
| J1 | 12 | TX-100 2 wt%, 3 eq. Et3N, rt | 91 | Bis-amidation product | 91 |
| K1 | 13 | TX-100 2 wt%, 3 eq. Et3N, rt | 78 | Bis-amidation product | 78 |
| H3 | 11 | TX-100 2 wt%, 3 eq. Et3N, rt | Not isolated | One pot reaction. Iopamidol isolated in 60% yield | — |
| K2 | 13 | TX-100 2 wt%, 3 eq. Et3N, rt | Not isolated | One pot reaction. Iopamidol isolated in 70% yield | — |
Esters and ethers protected derivatives gave sizably improved results, while also showing some peculiar features of micellar reactions highlighting the importance of achieving a suitable formulation state at all steps. Derivatives 8 and 10 are waxy solids that can be solubilized in the amine only by employing mild heating at 30–50 °C. Derivative 9 is an oil, with a higher solubility in the base. The somewhat limited solubility of 8 and 10 leads to an unwanted formulative evolution of the reaction mixture. As soon as the first amidation takes place, the formation of the insoluble and amorphous intermediate leads to the massive phase segregation of a sticky paste, strongly limiting the mass transport of reagents necessary to achieve full conversion.
As a result, hydrolysis of both 1 and monoamidated intermediate become dominant leading to water soluble products and thus limiting the mass recovery. Conversely, in the case of the tertbutyl ether 9 having both higher solubility and lower melting point, the formulative state of the reaction remains homogeneous at all steps, improving the distribution of the species and thus mass recovery and yield. Silyl ether derivatives further leverage on this effect. Silyl ethers (and silicon) have a remarkably low surface tension and are amongst the best lubricants and antifoaming agents. Asides from being highly soluble in the tertiary amine, they also improve the overall formulative state of the reactions ensuring the formation of a homogeneous dispersion of reagents with a relatively low viscosity at all steps. As shown by entry J1, these characteristic features have a staggering effect on the overall behaviour of the reaction. Not only the mass recovery is essentially quantitative, but the yield is also very high, after a simple silica filtration work up. The use of the TMS and dimetyloctyl derivatives 11 and 13 gave essentially the same results.
2.3 One pot amidation and deprotection direct iopamidol synthesis
The efficiency of the amidation when using silylated serinols was so satisfactory that we devised a one pot protocol for the direct synthesis of iopamidol, without isolating the silylated intermediate. Thus, we repeated reaction H2 and K1 and once essentially only the target deamidation product was visible by TLC inspection of the reaction mixture, we added 5 wt% HCl until acid pH. In the case of protected serinol 13, after 30 min, no silylated material was detected in the reaction mixture. Trimethylsilyl derivative 11 required 12 hours for complete cleavage. In both cases, we proceeded to a simple work up, involving a phase separation of the insoluble silicon phase followed by elution through two ion exchange columns (acidic Amberlite IR-120H, basic Amberstep 900 OH) to remove all electrolytes and pH sensitive compounds and a crosslinked polystyrene one (Amberlite XAD1600) to remove the surfactant and apolar byproducts. Evaporation of the eluted water solution gave pure iopamidol in 70% yield in the case of serinol 13 and of 60% for serinol 11. The different yield in the two cases is in line with the lower amidation yield we observed for 11 over 13.Even if the use of protected serinol 11 leads to a lower yield of iopamidol with respect to both the use of 13 and the patented procedure in DMAC, it still represents by far the preferable route. This is in accord with a lower E-factor of 8, to be compared with 23 for the patented protocol and 420 for 13 based protocol (the two columns required for the synthesis of 13 getting the lion's share in terms of wastes). Moreover, as it is shown in Fig. 3, the use of 11 does not involve any reprotoxic chemical thus representing an overall advantage for the manufacturing of iopamidol.
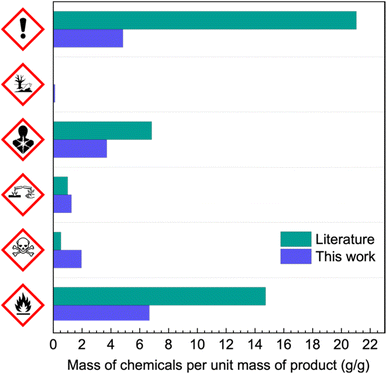 | ||
| Fig. 3 Mass of raw materials per unit mass of product as a function of the corresponding hazard class pictograms for the literature and new syntheses of iopamidol. | ||
3 Conclusions
We demonstrated that by employing lipophilic derivatives of serinol it is possible to enable the preferential amidation of 1 in aqueous media, rather than the hydrolysis of the acyl chlorides functionalities. Furthermore, we highlighted how details in the formulative state of the reaction can have a strong impact on the outcome, unregarding to the partitioning of the species involved. The best class of protecting groups we identified is that of the silyl ethers, not only being stable under the amidation conditions but also strongly and positively impacting on the formulative state of the mixture thanks to the lubricant and antifoaming action of silicones. We realize that the introduction of such protecting groups requires the use of small amounts of toxic materials (see Fig. 3) and that after protection the silicon containing wastes cannot be recycled. We are actively looking for alternatives having a similar impact on both localization and formulative state of the microheterogeneous reactions.Remarkably, in the case of the best performing amidation reaction, we devised a simple work up directly leading to the formation of the target iopamidol contrast agent without employing any organic solvent at all steps.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Anita Zucchi, Valentina Bellotti and Rachele Maschio – methodology and investigation Sara Mattiello – data curation Giovanni Giovenzana and Luciano Lattuada – writing review and editing and conceptualization. Luca Beverina – conceptualization and writing of the original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Bracco Imaging is gratefully acknowledged for financial support. L. B. acknowledges the MUSA – Multilayered Urban Sustainability Action – project, funded by the – NextGenerationEU, under the National Recovery and Resilience Plan (NRRP) Mission 4 Component 2 Investment Line 1.5: strengthening of research structures and creation of R&D “innovation ecosystems”, set up of “territorial leaders in R&D′′Notes and references
- W. A. Kalender, Phys. Med. Biol., 2006, 51, R29–R43 CrossRef PubMed.
- O. W. Linton, Beam Line, 1995, 2, 25–34 Search PubMed.
- J. A. Seibert and J. M. Boone, J. Nucl. Med. Technol., 2005, 33, 3–18 Search PubMed.
- U. Speck, X-Ray Contrast Media, Springer, Berlin, 2018, pp. 20–33 Search PubMed.
- T. Frenzel, R. Lawaczeck, M. Taupitz, G. Jost, J. Lohrke, M. A. Sieber and H. Pietsch, Invest. Radiol., 2015, 50, 671–678 Search PubMed.
- H. Lusic and M. W. Grinstaff, Chem. Rev., 2013, 113, 1641–1666 Search PubMed.
- A. Rutten and M. Prokop, Adv. Anticancer Agents Med. Chem., 2007, 7, 307–316 Search PubMed.
- W. Krause and P. W. Schneider, Top. Curr. Chem., 2002, 222, 107–150 CrossRef CAS.
- The Merck Index, RSC Publishing, 15th edn, 2013, pp. 940–941 Search PubMed.
- D. Pitrè and E. Felder, Invest. Radiol., 1980, 15, S301–S309 CrossRef PubMed.
- J. Magano, Org. Process Res. Dev., 2022, 26, 1562–1689 Search PubMed.
- B. Andreeßen and A. Steinbüchel, AMB Express, 2011, 1, 1–6 Search PubMed.
- P. L. Anelli, M. Brocchetta, L. Lattuada and F. Uggeri, Pure Appl. Chem., 2012, 84, 485–491 CAS.
- A. Jordan, C. G. J. Hall, L. R. Thorp and H. F. Sneddon, Chem. Rev., 2022, 122, 6749–6794 Search PubMed.
- C. J. Clarke, W. C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 Search PubMed.
- J. Sherwood, H. L. Parker, K. Moonen, T. J. Farmer and A. J. Hunt, Green Chem., 2016, 18, 3990–3996 RSC.
- F. De Schouwer, S. Adriaansen, L. Claes and D. E. De Vos, Green Chem., 2017, 19, 4919–4929 RSC.
- J. Lopez, S. Pletscher, A. Aemissegger, C. Bucher and F. Gallou, Org. Process Res. Dev., 2018, 22, 494–503 Search PubMed.
- J. Sherwood, M. De bruyn, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC.
- T. W. Bousfield, K. P. R. Pearce, S. B. Nyamini, A. Angelis-Dimakis and J. E. Camp, Green Chem., 2019, 21, 3675–3681 RSC.
- M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237–4266 RSC.
- B. H. Lipshutz, Green Chem., 2024, 26, 739–752 RSC.
- B. H. Lipshutz, S. Ghorai and M. Cortes-Clerget, Eur. J. Chem., 2018, 24, 6672–6695 Search PubMed.
- M. Shi, N. Ye, W. Chen, H. Wang, C. Cheung, M. Parmentier, F. Gallou and B. Wu, Org. Process Res. Dev., 2020, 24, 1543–1548 CrossRef CAS.
- S. Hazra, F. Gallou and S. Handa, ACS Sustainable Chem. Eng., 2022, 10, 5299–5306 Search PubMed.
- S. Sharma, G. Kaur and S. Handa, Org. Process Res. Dev., 2021, 25, 1960–1965 CrossRef CAS.
- C. Krell, R. Schreiber, L. Hueber, L. Sciascera, X. Zheng, A. Clarke, R. Haenggi, M. Parmentier, H. Baguia, S. Rodde and F. Gallou, Org. Process Res. Dev., 2021, 25, 900–915 Search PubMed.
- A. Ranaudo, C. Greco, G. Moro, A. Zucchi, S. Mattiello, L. Beverina and U. Cosentino, J. Phys. Chem. B, 2022, 126, 9408–9416 CrossRef CAS PubMed.
- B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais, A. Krasovskiy, R. D. Gaston and R. C. Gadwood, J. Org. Chem., 2011, 76, 4379–4391 CrossRef CAS PubMed.
- R. N. Butler and A. G. Coyne, Org. Biomol. Chem., 2016, 14, 9945–9960 RSC.
- S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS PubMed.
- L. Abu-Ghunmi, M. Badawi and M. Fayyad, J. Surfactants Deterg., 2014, 17, 833–838 CrossRef CAS.
- G. Klopman, J. Y. Li, S. Wang, M. Dimayuga, S. Wang and M. Dimayuga, J. Chem. Inf. Comput. Sci., 1994, 34, 752–781 CrossRef CAS.
- F. Csizmadia, A. Tsantili-Kakoulidou, I. Panderi and F. Darvas, J. Pharm. Sci., 1997, 86, 865–871 CrossRef CAS PubMed.
- M. Shi, N. Ye, W. Chen, H. Wang, C. Cheung, M. Parmentier, F. Gallou and B. Wu, Org. Process Res. Dev., 2020, 24, 1543–1548 CrossRef CAS.
- C. M. Gabriel, N. R. Lee, F. Bigorne, P. Klumphu, M. Parmentier, F. Gallou and B. H. Lipshutz, Org. Lett., 2016, 19, 194–197 CrossRef PubMed.
- F. Gallou, P. Guo, M. Parmentier and J. Zhou, Org. Process Res. Dev., 2016, 20, 1388–1391 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details on preliminary amidation tests, synthesis of serinol derivatives, characterizations, stability tests, details on green chemistry metrics. See DOI: https://doi.org/10.1039/d4ra08529a |
| This journal is © The Royal Society of Chemistry 2024 |