Open Access Article
Open Access ArticleFine-regulation of gradient gate-opening in nanoporous crystals for sieving separation of ternary C3 hydrocarbons†
Shuang
Liu‡
a,
Yuhang
Huang‡
b,
Jingmeng
Wan
b,
Jia-Jia
Zheng
c,
Rajamani
Krishna
 d,
Yi
Li
b,
Kai
Ge
*b,
Jie
Tang
b and
Jingui
Duan
d,
Yi
Li
b,
Kai
Ge
*b,
Jie
Tang
b and
Jingui
Duan
 *b
*b
aHenan Engineering Research Center for Green Synthesis of Pharmaceuticals, College of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu 476000, China
bState Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: gekai@njtech.edu.cn; duanjingui@njtech.edu.cn
cLaboratory of Theoretical and Computational Nanoscience, National Center for Nanoscience and Technology, Chinese Academy of Sciences, Beijing 100190, China
dVan't Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands
First published on 2nd April 2024
Abstract
The adsorptive separation of ternary propyne (C3H4)/propylene (C3H6)/propane (C3H8) mixtures is of significant importance due to its energy efficiency. However, achieving this process using an adsorbent has not yet been accomplished. To tackle such a challenge, herein, we present a novel approach of fine-regulation of the gradient of gate-opening in soft nanoporous crystals. Through node substitution, an exclusive gate-opening to C3H4 (17.1 kPa) in NTU-65-FeZr has been tailored into a sequential response of C3H4 (1.6 kPa), C3H6 (19.4 kPa), and finally C3H8 (57.2 kPa) in NTU-65-CoTi, of which the gradient framework changes have been validated by in situ powder X-ray diffractions and modeling calculations. Such a significant breakthrough enables NTU-65-CoTi to sieve the ternary mixtures of C3H4/C3H6/C3H8 under ambient conditions, particularly, highly pure C3H8 (99.9%) and C3H6 (99.5%) can be obtained from the vacuum PSA scheme. In addition, the fully reversible structural change ensures no loss in performance during the cycling dynamic separations. Moving forward, regulating gradient gate-opening can be conveniently extended to other families of soft nanoporous crystals, making it a powerful tool to optimize these materials for more complex applications.
Introduction
Efficient separation of the ternary C3 hydrocarbons is crucial for the petrochemical industry as C3H6 is an essential feedstock for valuable commodities.1 Currently, the separation process involves energy-intensive catalytic hydrogenation and cryogenic distillation, which accounts for nearly 3% of all separation energy.1–3 Therefore, there is a need for a more energy-efficient technology for this task. Adsorption separation using porous materials, specifically porous coordination polymers (PCPs), shows great promise due to their finely designed pore chemistry.4–13 In this regard, recent progress has been made in binary separations using strategies such as π-complexation, sieving strategies and kinetic diffusion.14–23 However, the similar molecular sizes and kinetic diameters exacerbate the challenge of C3H4/C3H6/C3H8 separation, particularly by one material.Recent research has shown that the purification of ethylene from a complex mixture of C2 hydrocarbons and carbon dioxide can be achieved using sequentially packed sorbents, where the three impurities were captured by three PCPs.24 Although this strategy requires overcoming the difficulties of preparation of the three materials, and also their rational packing, it enables us to believe that integrating gradient sorbent–sorbate interactions may address the challenge of C3H4/C3H6/C3H8 separation in a single domain, as well as the precise understanding of hierarchical sites or gradient gate-openings.
The concept of hierarchical sites holds certain theoretical feasibility, but its implementation is hindered by the significant limitations in rational assembly of these sites in a confined nanospace. Comparably, the strategy of gradient gate-openings shows promising prospects. Since the early reports of soft crystals,25,26 the soft nature of these materials has been gradually understood, that is, the stronger the interaction, the earlier the gate-opening.27,28 In addition, soft frameworks can exhibit sensitive responses to even extremely small stimuli.29–31 Given these advantages, significant efforts have been dedicated to the construction of soft PCPs;27,29,32–35 however, neither the fine predesign nor function-oriented synthesis of such a material, whose framework can sequentially recognize these similar molecules, has yet been reported.
During our investigation of porous materials,20,36,37 we have reported a soft crystal, NTU-65, [Cu(L)2(SiF6)] (L = 1,4-di(1H-imidazolyl)benzene). The temperature dependent gate-opening allows it to show a sieving separation of C2H4/C2H2/CO2 mixtures.
However, this framework cannot separate the equimolar C3H6/C3H8 due to a small difference in uptake at this ratio caused by the gate-opening effect which either occurs too early or too late (Fig. S2†). Inspired by reticular chemistry38 and NTU-65, herein, we report a fine tuning of the framework softness via node substitution in a series of PCPs (NTU-65-series: NTU-65-FeZr, NTU-65-FeTi, NTU-65-CoZr and NTU-65-CoTi, Scheme 1), as the metal nodes may alter the transformation characteristics of soft structures. As expected, a sole gate-opening toward C3H4 in NTU-65-FeZr evolves into a gradient response to C3H4 and C3H6 in NTU-65-CoTi, yielding an unprecedented breakthrough of sieving separation of C3H4/C3H6/C3H8.
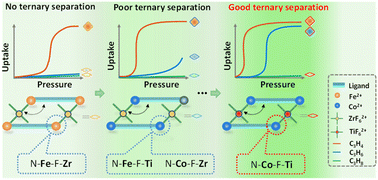 | ||
| Scheme 1 Illustration of fine-regulation of gradient gate-opening in soft NTU-65-seriesvia node substitution for sieving separation of C3 hydrocarbons in a single domain. | ||
Results and discussion
Solvothermal reactions of L and the corresponding metal salt provide NTU-65-series (Fig. S3†). They crystallize in the monoclinic system (Table S1†) and adopt the same coordination configurations. The M1 centres (M1: Fe2+ or Co2+) of NTU-65-series are connected by four imidazole N atoms in a planar configuration from four L and two F atoms in the axial vertex from two M2F62− (M2: Zr4+ or Ti4+) ions. As a result, cross-linked 2D channels are observed in the packed frameworks, although the window apertures undergo minimal changes (Fig. 1a, b and S4–S8†), along with similar accessible volumes (43.5%, 44.8%, 42.4% and 44.8%).39 To reach the adjacent nodes, the dihedral angle between the benzene and imidazole rings in L in NTU-65-series shows significant changes. For example, the angle of the LCis in NTU-65-FeZr is as large as 14.81°, while this angle decreases to 3.45° in NTU-65-FeTi (Fig. 1c). Additionally, the dihedral angle in LTrans in NTU-65-CoTi is up to 22.69°. Therefore, different degrees of softness are expected in the NTU-65-series. Phase purity of the four crystals has been confirmed by X-ray powder diffraction measurement (Fig. S9†). In addition, all samples are thermally stable, up to 350 °C (Fig. S10†).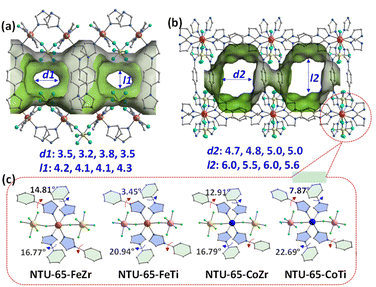 | ||
| Fig. 1 Crystal structure of NTU-65-series along the a-axis (a) and b-axis (b). Comparison of the dihedral angle in L in NTU-65 series (c). | ||
Permanent porosity of the activated NTU-65-series was evaluated using N2 adsorption measurements at 77 K (Fig. S12†). Initially, all samples show minimal N2 uptake until reaching P/P0 = 0.61, 0.26, 0.27 and 0.24. However, a peculiar ‘kink’ phenomenon occurred in all of them. This is due to the rapid increase in adsorption capacity of the crystals after instantaneous gate-opening under this condition, accompanied by a rapid decrease in system pressure, to P/P0 = 0.10, 0.07, 0.05 and 0.04, respectively. Subsequently, N2 uptake increases with increased pressure in NTU-65-FeZr, while type-I adsorption isotherms were observed in NTU-65-FeTi and NTU-65-CoTi. Differently, NTU-65-CoZr exhibits a second adsorption platform before reaching P/P0 = 0.6, followed by pressure-dependent uptake. Despite these differences, all four crystals demonstrated a similar maximum N2 capacity (219.5 to 236.2 cm3 g−1). Evaluated by using these isotherms, the crystals exhibit comparable surface areas (540–610 m2 g−1) and pore volumes (0.34–0.36 cm3 g−1) (Fig. S13, S14 and Table S2†). Additionally, all isotherms display desorption hysteresis, confirming the filling effect of the soft frameworks.40,41
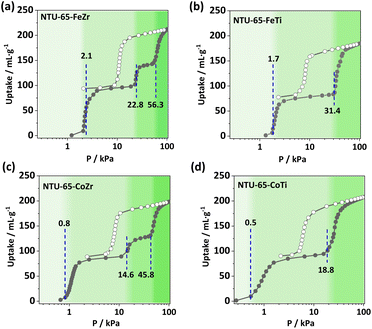
To further investigate structural softness, CO2 isotherms were collected at 195 K, as CO2 may interact differently with the frameworks compared to N2.42,43 Unlike the ‘kink’ phenomenon in the above N2 isotherms, all CO2 uptakes of the NTU-65-series increase with elevated pressure. Meanwhile, gradient adsorption platforms indicating sequential gate-openings were clearly observed in NTU-65-series. For NTU-65-FeZr, the first gate-opening occurs at 2.1 kPa, followed by the second and third gate-opening at 22.8 and 56.3 kPa, respectively (Fig. 2a). However, NTU-65-FeTi shows an earlier first gate-opening pressure (OP) of 1.7 kPa. The second and third gate-openings in NTU-65-FeZr merge into one in NTU-65-FeTi, at 31.4 kPa (Fig. 2b). Notably, three gate-openings appear again in NTU-65-CoZr (starting at 0.8, 14.6 and 45.8 kPa), whose responsive pressures are all lower than that of NTU-65-FeZr, and the first OP is also lower than that of NTU-65-FeTi (Fig. 2c). Remarkably, NTU-65-CoTi exhibits an advancement of the first OP at 0.5 kPa, followed by a second quick uptake at 18.8 kPa (Fig. 2d). Therefore, these isotherms indicate that the four activated crystals possess non-porous characteristics and can be opened by N2 or CO2 once the stimuli exceed the energy barrier controlling the framework closure. Meanwhile, the gradually decreased pressure (2.1 to 0.5 kPa) for the first gate-opening reflects the fact that the four frameworks show different sensitivities to external stimuli. Additionally, the difference in multi-step gate-openings in them validates the effectiveness of the node substitution strategy in regulation of the framework with gradient softness, which may provide a promising platform for sequential recognition of gases with extremely small differences.
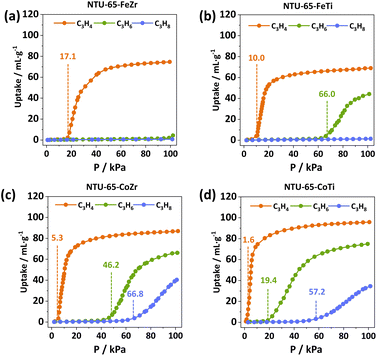
To investigate the function of gradient and sensitive gate-openings, adsorption isotherms of C3H4, C3H6 and C3H8 were collected on NTU-65-series (Fig. 3 and S15–S18†). NTU-65-FeZr exhibits minimal uptake of C3H6 and C3H8 at the threshold pressure, whereas C3H4 adsorption begins at 17.1 kPa with a maximum capacity of 74.7 cm3 g−1 (Fig. 3a). Meanwhile, the starting OP is advanced to 10.0 kPa in NTU-65-FeTi, along with nearly the same C3H4 capacity (69.0 cm3 g−1) (Fig. 3b). Unlike NTU-65-FeZr, C3H6 molecules open the framework of NTU-65-FeTi at 66.0 kPa with a capacity of 44.2 cm3 g−1 at 100 kPa (Fig. 3c). Interestingly, the OP of the three gases (C3H4: 5.3 kPa, C3H6: 46.2 kPa and C3H8: 66.8 kPa) exhibit a forward movement again in NTU-65-CoZr (Fig. 3d). However, the isotherms indicate two crucial issues that may arise during the dynamic separation of such ternary mixtures: (1) insufficient partial pressure for C3H4 adsorption (far larger than 1 kPa); and (2) lower separation performance between C3H6 and C3H8 (small uptake difference of only 7.6 cm3 g−1 at around 50 kPa). Remarkably, these issues may be solved by using NTU-65-CoTi, which exhibits rapid uptake of C3H4 at 1.6 kPa and a significantly improved uptake difference between C3H6 and C3H8, reaching as high as 55.9 cm3 g−1 at 50 kPa. Additionally, the total C3H4 capacity is increased to 95.9 cm3 g−1. Moreover, the uptake ratios of binary mixtures (C3H4/C3H6, C3H6/C3H8 and C3H4/C3H8) were significantly and simultaneously improved compared to that of other benchmark materials (Table 1 and S3†).
| PCPs | C3H4 | C3H6 | C3H8 | Uptake ratios | ||
|---|---|---|---|---|---|---|
| 0.1 bar | 0.5 bar | 0.5 bar | C3H4/C3H6 (0.1/0.5) | C3H6/C3H8 (0.5/0.5) | C3H4/C3H8 (0.1/0.5) | |
| NTU-65-CoTi | 73.93 | 57.44 | 1.55 | 1.28 | 37.05 | 47.70 |
| NTU-65-CoZr | 46.24 | 8.55 | 0.96 | 5.41 | 8.91 | 48.17 |
| NTU-65-FeTi | 3.93 | 1.27 | 0.73 | 3.01 | 1.74 | 5.34 |
| NTU-65-FeZr | 0.59 | 0.74 | 0.51 | 0.80 | 1.45 | 1.16 |
To confirm the systemic structural changes, in situ powder X-ray diffraction patterns/sorption measurements were performed (Fig. 4). The characteristic peaks corresponding to crystal faces of [0 2 0] and [0 0 1] in the as-synthesized phases (at about 6.5 and 7.2°) become weaker and shift to a higher-angle region in the activated crystals, indicative of structural contraction commonly observed in flexible PCPs. However, these two peaks reappear at similar positions to those obtained from their as-synthesized phases after an increase in pressure. Additionally, these open structures transform back into the closed state when the pressure decreases to a very low value, showing reversible sorption and structural changes in NTU-65-series. In addition, gas-loaded crystallographic analysis has also been attempted to explore the detailed structural changes. However, the small crystal size and crack after activation results in extremely weak or no diffraction. To understand the trend in softness of these PCPs, we conducted density functional theory (DFT) calculations taking C3H4 as the probe molecule (Fig. S20†). Previously, we have shown that the gate-opening pressure of PCPs induced by gas adsorption is dependent on the binding energy of the gas molecule with the PCP.44 We therefore calculated the binding energies of C3H4 with the NTU-65-series. The binding energy increases (becomes more negative) in the following order: NTU-65-FeZr (–7.8 kcal mol−1) < NTU-65-FeTi (–8.1 kcal mol−1) < NTU-65-CoZr (–8.5 kcal mol−1) < NTU-65-CoTi (–9.1 kcal mol−1). This is consistent with the order of gate-opening pressure of these PCPs in response to C3H4 adsorption for these PCPs (Fig. 3).
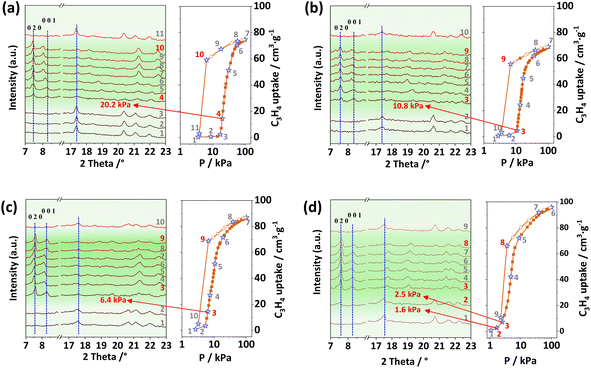 | ||
| Fig. 4 In situ PXRD patterns of NTU-65-FeZr (a), NTU-65-FeTi (b), NTU-65-CoZr (c) and NTU-65-CoTi (d) during C3H4 adsorption/desorption at 273 K. | ||
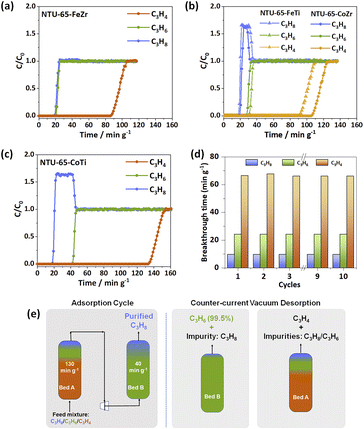
The sequential recognition of C3 hydrocarbons encouraged us to perform dynamic separations. Given the commonly encountered ratio of C3 hydrocarbons in industry, a simulated feed-gas of C3H4/C3H6/C3H8 (0.5/49.75/49.75, v/v/v) was used for breakthrough experiments at 273 K. Consistent with the single-component gas isotherms, C3H6 and C3H8 elute simultaneously from the NTU-65-FeZr sample bed, while C3H4 was captured until 85.8 min g−1, showing impossible ternary separation (Fig. 5a). For NTU-65-CoZr, C3H8 was detected as the first elution at 19.3 min g−1, followed by C3H6 at 27.8 min g−1. Additionally, C3H4 passes through the column at 106.3 min g−1 (Fig. 5b). Considering the OP of C3H6 (46.2 kPa) and the small uptake difference (7.6 cm3 g−1) of C3H6/C3H8 in NTU-65-CoZr, the observable separation time of C3H6/C3H8 should be attributed to the slight pore opening, caused by accumulated C3H6 adsorption. This phenomenon is similar to the selective C3H4 or C2H2 capture in GeFSIX-dps-Zn45 and Co(4-DPDS)2MoO4,46 respectively, where the partial pressures of C3H4 (10 kPa) or C2H2 (1 kPa) in the feed-gas for breakthrough separations are lower than that of the corresponding OPs (∼16 kPa and ∼17 kPa) of the frameworks at the same temperature. In addition, a similar and insufficient ternary separation was also observed in NTU-65-FeTi. Remarkably, NTU-65-CoTi exhibits a significant separation of the ternary mixture of C3H4/C3H6/C3H8, in which highly pure C3H8 elutes out at 17.0 min g−1, while the outflows of C3H6 and C3H4 from the sample bed are around 41.1 min g−1 and 132.9 min g−1 (Fig. 5c). Additionally, the further extended retention time of C3H4 (from 85.8 min g−1 in NTU-65-FeZr to 132.9 min g−1 in NTU-65-CoTi) reflects the increased cumulative effect in NTU-65-series, as structural deformation becomes easier. To the best of our knowledge, the PCPs, including rigid and soft frameworks, have been widely explored for gas separations; however, this is the first example that can separate C3 hydrocarbons in one-step in a single domain. Furthermore, this promising separation was also confirmed at increased velocity (5 and 10 mL min−1) of such ternary mixtures, as well as the binary mixtures (Fig. S21–S27 and Tables S4–S7†). More importantly, due to the reversible structural changes, there is almost no performance loss during the cycling measurments on NTU-65-CoTi (Fig. 5d and S28†), a critical factor in evaluating the feasibility of the adsorbents.
Based on these breakthrough results results, how the NTU-65-CoTi might be possible to procure pure C3H6 and C3H8 are mostly expected. As shown in Fig. 5e, two beds are needed, Bed A and B, both packed with NTU-65-CoTi.47,48 During the adsorption cycle, the two beds A and B operate sequentially. The operation of Bed A and B should last for at least 130 min g−1 and 35 min g−1, respectively. Highly pure C3H8 (99.9%, GC limitation) is recovered from Bed B at the end of the adsorption cycle. After termination of the adsorption cycle, both Bed A and B are subjected to counter-current vacuum desorption. From Bed A, the product C3H4 with improved concentration (from 0.5% to 6.3%) can be obtained, while from Bed B, the product C3H6 can be harvested with a purity approaching 99.5%.
Conclusions
In summary, due to the demand for energy-efficient separation of C3 hydrocarbons, we here report a gradient regulation of framework softness via the node substitution approach. The exclusive response to C3H4 in NTU-65-FeZr has been tailored into a sequential capture of C3H4 and C3H6 in NTU-65-CoTi. This breakthrough enables an unprecedented ability for sieving separation of the ternary C3H4/C3H6/C3H8 mixtures in a single domain. By following designed procedures, highly pure C3H8 (99.9%) and C3H6 (99.5%) can be obtained. Moving forward, given that the conditions for feasible gas separation vary significantly, the precise understanding and ability to tune the gate-openings of soft PCPs are particularly important, potentially indicating a path to other challenging multi-component systems.Data availability
The data supporting the findings of this study are available within the article and/or its ESI.†Author contributions
J. D. conceived the research idea and designed the experiments. S. L. and Y. H. conducted the experiments. S. L., Y. H., J. W., Y. L., and J. T. analysed and discussed the data. J. Z. and K. R. performed the calculations. J. D. and K. G. wrote the paper. All authors offered constructive comments on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful for the financial support of the National Natural Science Foundation of China (22171135), the Innovative Research Team Program by the Ministry of Education of China (IRT-17R54), the National Natural Science Foundation of Jiangsu Province (BK20231269), the State Key Laboratory of Materials-Oriented Chemical Engineering (SKL-MCE-23A18) and the Education Department of Henan Province (22A150050 and 2023GGJS131).Notes and references
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- Y. Wang, N. Y. Huang, X. W. Zhang, H. He, R. K. Huang, Z. M. Ye, Y. Li, D. D. Zhou, P. Q. Liao, X. M. Chen and J. P. Zhang, Angew. Chem., Int. Ed., 2019, 58, 7692–7696 CrossRef CAS PubMed.
- F. A. Da Silva and A. E. Rodrigues, AIChE J., 2001, 47, 341–357 CrossRef CAS.
- B. Y. Li, Z. J. Zhang, Y. Li, K. X. Yao, Y. H. Zhu, Z. Y. Deng, F. Yang, X. J. Zhou, G. H. Li, H. H. Wu, N. Nijem, Y. J. Chabal, Z. P. Lai, Y. Han, Z. Shi, S. H. Feng and J. Li, Angew. Chem., Int. Ed., 2012, 51, 1412–1415 CrossRef CAS PubMed.
- G. K. H. Shimizu, J. M. Taylor and S. Kim, Science, 2013, 341, 354–355 CrossRef CAS PubMed.
- K. S. Walton, Nat. Chem., 2014, 6, 277–278 CrossRef CAS PubMed.
- S. H. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schroder, Nat. Chem., 2015, 7, 121–129 CrossRef CAS PubMed.
- T. M. McDonald, J. A. Mason, X. Q. Kong, E. D. Bloch, D. Gygi, A. Dani, V. Crocella, F. Giordanino, S. O. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L. Dzubak, R. Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. W. F. Wan, D. Prendergast, J. B. Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and J. R. Long, Nature, 2015, 519, 303–308 CrossRef CAS PubMed.
- X. L. Cui, K. J. Chen, H. B. Xing, Q. W. Yang, R. Krishna, Z. B. Bao, H. Wu, W. Zhou, X. L. Dong, Y. Han, B. Li, Q. L. Ren, M. J. Zaworotko and B. L. Chen, Science, 2016, 353, 141–144 CrossRef CAS PubMed.
- S. Krause, V. Bon, I. Senkovska, U. Stoeck, D. Wallacher, D. M. Tobbens, S. Zander, R. S. Pillai, G. Maurin, F. X. Coudert and S. Kaskel, Nature, 2016, 532, 348–352 CrossRef CAS PubMed.
- A. Cadiau, Y. Belmabkhout, K. Adil, P. M. Bhatt, R. S. Pillai, A. Shkurenko, C. Martineau-Corcos, G. Maurin and M. Eddaoudi, Science, 2017, 356, 731–735 CrossRef CAS PubMed.
- P.-Q. Liao, N.-Y. Huang, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Science, 2017, 356, 1193–1196 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444–1230455 CrossRef PubMed.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140 CrossRef CAS PubMed.
- J. E. Bachman, M. T. Kapelewski, D. A. Reed, M. I. Gonzalez and J. R. Long, J. Am. Chem. Soc., 2017, 139, 15363–15370 CrossRef CAS PubMed.
- L. Yang, X. Cui, Q. Yang, S. Qian, H. Wu, Z. Bao, Z. Zhang, Q. Ren, W. Zhou, B. Chen and H. Xing, Adv. Mater., 2018, 30, 1705374 CrossRef PubMed.
- M.-H. Yu, B. Space, D. Franz, W. Zhou, C. He, L. Li, R. Krishna, Z. Chang, W. Li, T.-L. Hu and X.-H. Bu, J. Am. Chem. Soc., 2019, 141, 17703–17712 CrossRef CAS PubMed.
- T. Ke, Q. Wang, J. Shen, J. Zhou, Z. Bao, Q. Yang and Q. Ren, Angew. Chem., Int. Ed., 2020, 59, 12725–12730 CrossRef CAS PubMed.
- H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu and D. Li, Nature, 2021, 595, 542–548 CrossRef CAS PubMed.
- Q. Dong, Y. Huang, J. Wan, Z. Lu, Z. Wang, C. Gu, J. Duan and J. Bai, J. Am. Chem. Soc., 2023, 145, 8043–8051 CrossRef CAS PubMed.
- H. Wang, X. L. Dong, V. Colombo, Q. N. Wang, Y. Y. Liu, W. Liu, X. L. Wang, X. Y. Huang, D. M. Proserpio, A. Sironi, Y. Han and J. Li, Adv. Mater., 2018, 30, 1805088–1805096 CrossRef PubMed.
- D. Antypov, A. Shkurenko, P. M. Bhatt, Y. Belmabkhout, K. Adil, A. Cadiau, M. Suyetin, M. Eddaoudi, M. J. Rosseinsky and M. S. Dyer, Nat. Commun., 2020, 11, 6099 CrossRef CAS PubMed.
- X. Li, J. Liu, K. Zhou, S. Ullah, H. Wang, J. Zou, T. Thonhauser and J. Li, J. Am. Chem. Soc., 2022, 144, 21702–21709 CrossRef CAS PubMed.
- K. J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q. Y. Zhang and M. J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS PubMed.
- R. Kitaura, K. Seki, G. Akiyama and S. Kitagawa, Angew. Chem., Int. Ed., 2003, 42, 428–431 CrossRef CAS PubMed.
- S. Kitagawa and M. Kondo, Bull. Chem. Soc. Jpn., 1998, 71, 1739–1753 CrossRef CAS.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed.
- N. Behera, J. Duan, W. Jin and S. Kitagawa, EnergyChem, 2021, 3, 100067 CrossRef CAS.
- V. I. Nikolayenko, D. C. Castell, D. Sensharma, M. Shivanna, L. Loots, K. A. Forrest, C. J. Solanilla-Salinas, K. I. Otake, S. Kitagawa, L. J. Barbour, B. Space and M. J. Zaworotko, Nat. Chem., 2023, 15, 542–549 CrossRef CAS PubMed.
- D. D. Zhou, P. Chen, C. Wang, S. S. Wang, Y. Du, H. Yan, Z. M. Ye, C. T. He, R. K. Huang, Z. W. Mo, N. Y. Huang and J. P. Zhang, Nat. Mater., 2019, 18, 994–998 CrossRef CAS PubMed.
- L. B. Li, R. B. Lin, R. Krishna, X. Q. Wang, B. Li, H. Wu, J. P. Li, W. Zhou and B. L. Chen, J. Am. Chem. Soc., 2017, 139, 7733–7736 CrossRef CAS PubMed.
- J. A. Mason, J. Oktawiec, M. K. Taylor, M. R. Hudson, J. Rodriguez, J. E. Bachman, M. I. Gonzalez, A. Cervellino, A. Guagliardi, C. M. Brown, P. L. Llewellyn, N. Masciocchi and J. R. Long, Nature, 2015, 527, 357–361 CrossRef CAS PubMed.
- J. H. Lee, S. Jeoung, Y. G. Chung and H. R. Moon, Coord. Chem. Rev., 2019, 389, 161–188 CrossRef CAS.
- M. Bonneau, C. Lavenn, J.-J. Zheng, A. Legrand, T. Ogawa, K. Sugimoto, F.-X. Coudert, R. Reau, S. Sakaki, K.-i. Otake and S. Kitagawa, Nat. Chem., 2022, 14, 816–822 CrossRef CAS PubMed.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- Y. Huang, J. Wan, T. Pan, K. Ge, Y. Guo, J. Duan, J. Bai, W. Jin and S. Kitagawa, J. Am. Chem. Soc., 2023, 145, 24425–24432 CrossRef CAS PubMed.
- J. Wan, H. L. Zhou, K. Hyeon-Deuk, I. Y. Chang, Y. Huang, R. Krishna and J. Duan, Angew. Chem., Int. Ed., 2023, 62, e202316792 CrossRef CAS PubMed.
- M. J. Kalmutzki, N. Hanikel and O. M. Yaghi, Sci. Adv., 2018, 4, eaat9180 CrossRef CAS PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- S. Sen, N. Hosono, J. J. Zheng, S. Kusaka, R. Matsuda, S. Sakaki and S. Kitagawa, J. Am. Chem. Soc., 2017, 139, 18313–18321 CrossRef CAS PubMed.
- C. Gu, N. Hosono, J. J. Zheng, Y. Sato, S. Kusaka, S. Sakaki and S. Kitagawa, Science, 2019, 363, 387–391 CrossRef CAS PubMed.
- Q. Dong, X. Zhang, S. Liu, R. B. Lin, Y. Guo, Y. Ma, A. Yonezu, R. Krishna, G. Liu, J. Duan, R. Matsuda, W. Jin and B. Chen, Angew Chem. Int. Ed. Engl., 2020, 59, 22756–22762 CrossRef CAS PubMed.
- C. Kang, Z. Zhang, S. Kusaka, K. Negita, A. K. Usadi, D. C. Calabro, L. S. Baugh, Y. Wang, X. Zou, Z. Huang, R. Matsuda and D. Zhao, Nat. Mater., 2023, 22, 636–643 CrossRef CAS PubMed.
- J. J. Zheng, S. Kusaka, R. Matsuda, S. Kitagawa and S. Sakaki, J. Am. Chem. Soc., 2018, 140, 13958–13969 CrossRef CAS PubMed.
- T. Ke, Q. J. Wang, J. Shen, J. Y. Zhou, Z. B. Bao, Q. W. Yang and Q. L. Ren, Angew. Chem., Int. Ed., 2020, 59, 12725–12730 CrossRef CAS PubMed.
- F. Zheng, R. Chen, Y. Liu, Q. Yang, Z. Zhang, Y. Yang, Q. Ren and Z. Bao, Adv. Sci., 2023, 10, 2207127 CrossRef CAS PubMed.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed.
- R. Krishna, ACS Omega, 2020, 5, 16987–17004 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2284815–2284818. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc05489f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |