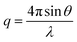Open Access Article
Open Access ArticleStructure–function relationships in pure archaeal bipolar tetraether lipids†
Ahanjit
Bhattacharya
 ab,
Isaac D.
Falk
a,
Frank R.
Moss
III
ab,
Isaac D.
Falk
a,
Frank R.
Moss
III
 c,
Thomas M.
Weiss
d,
Khoi N.
Tran
c,
Thomas M.
Weiss
d,
Khoi N.
Tran
 a,
Noah Z.
Burns
a,
Noah Z.
Burns
 *a and
Steven G.
Boxer
*a and
Steven G.
Boxer
 *a
*a
aDepartment of Chemistry, Stanford University, Stanford, CA 94305, USA. E-mail: sboxer@stanford.edu; nburns@stanford.edu
bStanford Center for Innovation in Global Health, Stanford University, Stanford, CA 94305, USA
cLinac Coherent Light Source, SLAC National Accelerator Laboratory, Menlo Park, CA 94025, USA
dStanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, Menlo Park, CA 94025, USA
First published on 7th August 2024
Abstract
Archaeal bipolar tetraether lipids (BTLs) are among the most unusual lipids occurring in nature because of their presumed ability to span the entire membrane to form a monolayer structure. It is believed that because of their unique structural organization and chemical stability, BTLs offer extraordinary adaptation to archaea to thrive in the most extreme milieus. BTLs have also received considerable attention for development of novel membrane-based materials. Despite their fundamental biological significance and biotechnological interests, prior studies on pure BTLs are limited because of the difficulty to extract them in pure form from natural sources or to synthesize them chemically. Here we have utilized chemical synthesis to enable in-depth biophysical investigations on a series of chemically pure glycerol dialkyl glycerol tetraether (GDGT) lipids. The lipids self-assemble to form membrane-bound vesicles encapsulating polar molecules in aqueous media, and reconstitute a functional integral membrane protein. Structural properties of the membranes were characterized via small-angle X-ray scattering (SAXS) and cryogenic electron microscopy (cryo-EM). SAXS studies on bulk aqueous dispersions of GDGT lipids over 10–90 °C revealed lamellar and non-lamellar phases and their transitions. Next we asked whether vesicles overwhelmingly composed of a single GDGT species can undergo fusion as it is difficult to conceptualize such behavior with the assumption that such membranes have a monolayer structure. Interestingly, we observed that GDGT vesicles undergo fusion with influenza virus with lipid mixing kinetics comparable to that with vesicles composed of monopolar phospholipids. Our results suggest that GDGT membranes may consist of regions with a bilayer structure or form bilayer structures transiently which facilitate fusion and thus offer insight into how archaea may perform important physiological functions that require dynamical membrane behavior.
Introduction
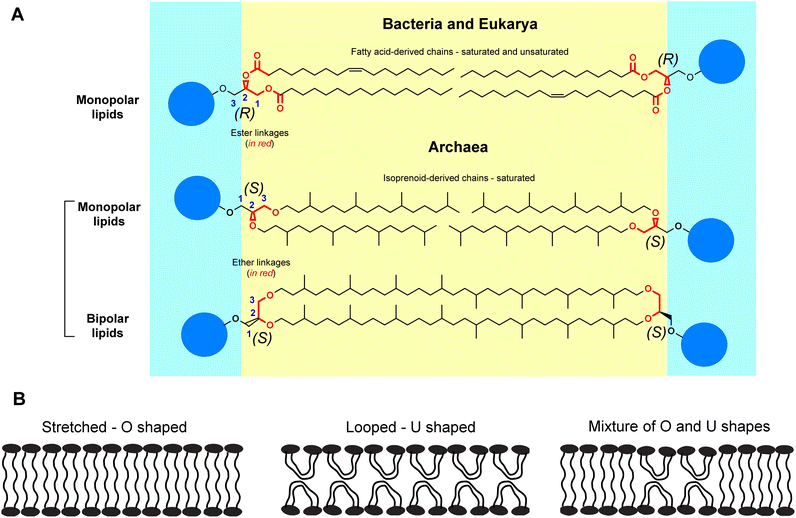
Archaea are an enigmatic group of prokaryotic organisms. Originally thought to be extremophiles, archaea have been found in every realm of the biosphere including the human microbiome.1 Archaea constitute ∼1.3% of the total biomass on the Earth,2 yet they have remained quite underexplored. While archaea resemble bacteria in certain superficial ways, fundamental differences in their biochemistry led to their classification as a separate domain of life. For instance, archaeal lipids differ significantly from the lipids found in bacteria (and eukaryotes) in terms of structural features, such as, absolute stereochemistry of the glycerol moiety, and the nature of the hydrocarbon chains and their linkages to the same.3,4 These distinctions between the lipids of archaea and bacteria (and eukarya) form the basis of a dichotomy known as the “lipid divide” (Fig. 1A).5 In archaeal lipids, the hydrocarbon chains have multiple methyl branches and are of isoprenoid origin – lacking any unsaturation in most cases. Their hydrocarbon chains are connected to the sn-glycerol-1-phosphate backbone via ether linkages. In bacterial lipids, the hydrocarbon chains are fatty acid-based, contain various degrees of unsaturation, and the hydrocarbon chains are connected to the sn-glycerol-3-phosphate backbone through ester linkages. Typical lack of unsaturation and the presence of ether linkages make the archaeal lipids extremely stable to aerial oxidation and hydrolysis respectively. Due to this exceptional chemical stability, archaeal lipids serve as excellent biomarkers for biogeochemical and climate studies.6Remarkably, a major class of archaeal lipids has a bipolar structure – which is analogous to two monopolar lipids covalently joined at the ends of their hydrocarbon tails. Bipolar lipids render archaeal membranes mechanically stable and impermeable even at high temperatures and at highly acidic pH.7,8 Although bipolar lipids are found in some bacteria, those are fatty acid-derived, and usually do not constitute the major lipid species in a membrane.9 Glycerol dialkyl glycerol tetraethers (GDGT) are one of the primary bipolar tetraether lipids (BTLs) found in archaea. Thermoacidophilic archaea possess GDGTs alongside other BTLs while methanogenic archaea possess GDGTs alongside monopolar lipids.10
A wealth of information on bacterial and eukaryotic membrane biology has been obtained from biophysical studies on model phospholipid membranes. Similarly, fundamental understanding of structure–function relationships in GDGTs through biophysical studies can further our understanding of the membrane-based cellular processes of archaea. For example, the bipolar nature of the GDGTs gives rise to the question of how these molecules are organized within a membrane and how such organization influences membrane function? Three types of limiting molecular arrangements can be imagined (Fig. 1B): (1) stretched – the lipid molecules are O-shaped and span the entire width of the membrane to form a monolayer; (2) looped – the lipid molecules are bent (i.e., U-shaped) at the hydrocarbon region so that the two headgroups face the same side of the membrane, and the membrane more closely resembles a bilayer; and (3) mixed – where both O- and U-shaped lipids coexist. There is considerable evidence that suggests that a majority of the lipids occur in a stretched conformation. For instance, in contrast to bilayer membranes, freeze-fracture electron microscopy studies on crude archaeal tetraether lipid vesicles have revealed the absence of a preferential fracture plane, which can be explained by considering that the lipids adopt a stretched conformation.11–13 Although there is a report mentioning exclusive formation of U-shaped bilayers of crude GDGTs on a solid substrate via transfer of Langmuir films,14 evidence of such structures in biological membranes or model vesicles is lacking. The possibility of a small percentage of U-shaped lipids coexisting with O-shaped lipids has been speculated.15,16 Nevertheless, functional evidence of the presence of such structures in compositionally well-defined GDGT systems is lacking.
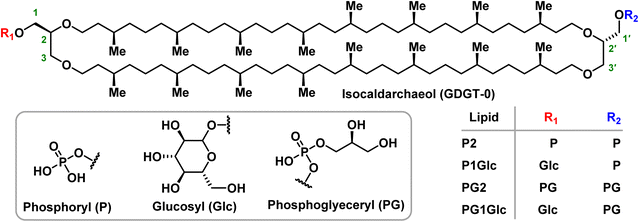
Unlike bacterial or eukaryotic lipids, the inaccessibility of pure archaeal BTLs such as GDGTs poses a roadblock to comprehensive biophysical studies which typically require many milligrams of lipids. Moreover, access to pure archaeal BTL standards will facilitate discovery of archaeal lipids biosynthetic enzymes and their mechanistic studies – an area where fundamental understanding has started to deepen only recently.17,18 Although a few model monopolar archaeal lipids in the form of ester- or ether-linked diphytan(o)yl phospholipids are commercially available, pure GDGT lipids are difficult to access. Also, the presence of multiple stereocentres makes these lipids challenging to synthesize chemically. To date, no GDGT lipids can be purchased from commercial vendors in pure form. Therefore, most biophysical studies until now have been performed on crude BTL extracts19–23 or model bipolar lipids.24–29 There are a few reports of semisynthetic approaches where headgroups were installed onto a heterogeneous mixture of core GDGTs extracted from microbial cultures.30,31 Only a handful of examples exist where pure species of polar GDGT lipids have been accessed via total synthetic approaches.32–34 In addition, a few pure hemi-macrocyclic tetraether lipids were previously reported which differed in molecular architecture as compared to the natural macrocyclic counterparts.24,27 Also, the previous works were primarily focused at advancing the synthetic methodology and not for carrying out comprehensive biophysical studies on those lipids. In this work we took advantage of synthetic chemistry to generate a series of four parallel GDGT-0 lipids in multi-milligram quantities (Fig. 2). Among these, two lipids are symmetric (P2 and PG2) and two are unsymmetric (P1Glc and PG1Glc) in terms of head group composition. The headgroups (phosphate, phosphoglycerol, glucose) that are present in the GDGTs in this study can be found naturally although symmetric combinations of those headgroups on an individual lipid are not yet reported to occur naturally. We studied the self-assembly behaviour of these GDGT lipids in various aqueous buffers and identified conditions in which membrane-bound giant vesicles are formed. We characterized the structural properties of GDGT membranes using small-angle X-ray scattering (SAXS), and cryogenic electron microscopy. We used SAXS also to characterize the transitions between various mesophases under a range of conditions of ionic strengths, divalent cation, and pH over a wide temperature range. Interestingly, we identified non-lamellar phases such as hexagonal and cubic phases under many conditions which inspired us to explore whether they have any functional implications, especially in processes like membrane fusion which are thought to proceed through non-lamellar intermediates. Indeed, we found that membranes composed of GDGT lipids undergo facile acidic pH-induced fusion with influenza-virus. To the best of our knowledge, this is the first demonstration of membrane fusion of compositionally well-defined macrocyclic BTLs. Our results provide mechanistic insight into how archaeal membranes undergo fundamental dynamical processes such as fusion.
Experimental section
Preparation of buffers
The following buffers were used and the osmolalities were measured using an Advanced Instruments Model 3320 osmometer:Tris buffer: 50 mM tris, pH 7.5. Osmolality: 88 mOsmol kg−1.
HB buffer: 20 mM HEPES–Na, 150 mM NaCl, pH 7.2. Osmolality: 316 mOsmol kg−1.
Vesicle buffer: 10 mM Na–phosphate, 90 mM sodium citrate, and 150 mM NaCl (pH 7.4). Osmolality: 545 mOsmol kg−1.
Fusion buffer: 10 mM Na–phosphate, 90 mM sodium citrate, and 150 mM NaCl (pH 5.1). Osmolality: 510 mOsmol kg−1.
Optical microscopy
Fluorescence images were acquired with a Nikon Ti–U microscope using a 100× oil immersion apochromat TIRF objective (NA = 1.49) (Nikon Instruments, Melville, NY). A Spectra-X LED Light Engine (Lumencor, Beaverton, OR) was used for illumination, and an Andor iXon 897 EMCCD camera (Andor Technologies, Belfast, UK) with 16 bit image settings. Images were captured with Metamorph software version 7.10.4 (Molecular Devices, Sunnyvale, CA).Negative-staining transmission electron microscopy (TEM)
Formvar-coated Cu grids (300 mesh, Catalog #FCF300-Cu, Electron Microscopy Sciences) were glow-discharged using a Denton BenchTop™ Turbo setup and used freshly. 5 μL of a vesicle sample was added to formvar-coated Cu grid surface and allowed to sit for 3 min. The grids were washed by touching the surface of 2 drops of Milli-Q placed on a parafilm sheet. 3 drops of 1% uranyl acetate in ultrapure water were then added to the grid and the 3rd drop was allowed to sit on the grid for 1 min. Afterward, the excess staining solution was blotted into a filter paper. The grids were dried in air. Finally, the grids were imaged on a JEOL JEM-1400 microscope equipped with a Gatan OneView (4k × 4k resolution) camera.Expression, purification, and reconstitution of photosynthetic reaction centres (RCs) in GDGT membranes
Rhodobacter spheroides RCs (M252V mutant which does not assemble with QA) were isolated as previously described.35 The protein was stored in 10 mM tris (pH 8.0), containing 0.03% (w/v) LDAO. For reconstitution into vesicles, detergent removal method was used. Briefly, a film was prepared in a glass vial by evaporating solvents from 80 μL of 5 mg mL−1 solution of PG2 (or PG1Glc) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CHCl3
1 CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH. The vial was kept in the desiccator for 12 h. The film was hydrated by gently tapping with 400 μL of a solution consisting of 10 mM tris (pH 8.0), 30 mM octyl glucoside, 0.26 mM LDAO, and 6 μM RC. The protein to GDGT lipid ratio was about 1
MeOH. The vial was kept in the desiccator for 12 h. The film was hydrated by gently tapping with 400 μL of a solution consisting of 10 mM tris (pH 8.0), 30 mM octyl glucoside, 0.26 mM LDAO, and 6 μM RC. The protein to GDGT lipid ratio was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The lipid/detergent/protein mixture was added to 18 mg of pre-cleaned wet BioBeads SM-2 resin (soaked in Milli-Q water) and allowed to tumble for 30 min at RT. Afterwards, the solution was separated from the beads and taken in a 10 kDa MWCO dialysis cassette. Dialysis was carried out against 10 mM tris buffer (pH 8.0) for 48 h with 2 changes of buffer in between and a clear light purple dispersion is collected. No residual detergents were detected by mass spectrometry. transient (fs ps−1) absorption spectroscopy measurements for RCs shown in Fig. S3† were obtained in the laboratory of Professor Dewey Holten and Christine Kirmaier by Dr Kaitlin Faries at Washington University using previously described methods. We are indebted to them for providing this data.
100. The lipid/detergent/protein mixture was added to 18 mg of pre-cleaned wet BioBeads SM-2 resin (soaked in Milli-Q water) and allowed to tumble for 30 min at RT. Afterwards, the solution was separated from the beads and taken in a 10 kDa MWCO dialysis cassette. Dialysis was carried out against 10 mM tris buffer (pH 8.0) for 48 h with 2 changes of buffer in between and a clear light purple dispersion is collected. No residual detergents were detected by mass spectrometry. transient (fs ps−1) absorption spectroscopy measurements for RCs shown in Fig. S3† were obtained in the laboratory of Professor Dewey Holten and Christine Kirmaier by Dr Kaitlin Faries at Washington University using previously described methods. We are indebted to them for providing this data.
Surface pressure-area isotherms
Surface pressure-area isotherms of monomolecular layers were recorded at room temperature (22 °C) using a KSV NIMA KN 2002 (Biolin Scientific) Langmuir trough equipped with a Teflon trough (243 cm2 or 555 cm2) and symmetric delrin barriers. A Wilhelmy plate made of a 30 mm × 14 mm piece of Whatman #1 filter paper was used to monitor the surface pressure. At first, the trough surface was rinsed with methanol. Next, Milli-Q water was used to thoroughly clean the trough. Typically, a solution of the lipids of known concentration in chloroform (or 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform
1 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol) was slowly added dropwise onto the surface of the water using a glass microsyringe. The solvent was allowed to evaporate for at least 20 min. The barriers were then compressed at a rate of 10 mm min−1 and pressure-area isotherms were recorded.
methanol) was slowly added dropwise onto the surface of the water using a glass microsyringe. The solvent was allowed to evaporate for at least 20 min. The barriers were then compressed at a rate of 10 mm min−1 and pressure-area isotherms were recorded.
Small-angle X-ray scattering (SAXS)
SAXS data were collected at the Beamline 4-2 of Stanford Synchrotron Radiation Lightsource. The distance between the sample and the detector (Pilatus3 X 1 M) was 1.2 m and the energy of the X-rays was 11 keV (λ = 1.127 Å). Momentum transfer (q) was measured over a range of 0.01–0.75 Å−1, where q is modulus of the scattering vector defined as follows:where θ = scattering angle, λ = wavelength of the X-rays.
Two kinds of experiments were performed:
(1) Scattering from unilamellar vesicles. Small unilamellar vesicles of P2, PG2, and PG1Glc were prepared by extruding a multilamellar dispersion in tris buffer through a 50 nm polycarbonate membrane filter. In the case of P1Glc, the lipid dispersion contained a significant amount of insoluble aggregates which could not be fully dispersed by bath sonication and those stuck to the membrane filters during extrusion. Therefore, the dispersion was subject to probe ultrasonication which helped to break down the larger aggregates. ∼30 μL volumes of the vesicle dispersions were taken in 0.2 mL microtubes and loaded onto an automated sample loader at the beamline. Samples were exposed for a variable time (1 s or 2 s) and variable number of scans (24–50) were taken depending on the concentration of the lipids. Each sample was measured after storing at room temperature for up to a day and identical scattering profiles were obtained. Details of the SAXS data fitting is provided in the ESI† (“Fitting of SAXS data from unilamellar vesicles” section).
(2) Scattering from bulk dispersions. In these experiments, a thin film of a GDGT lipid was deposited on the walls of a glass vial. The film was hydrated with an aqueous buffer (typically 50 mM tris, pH 7.5) by a combination of vortexing and bath sonication. Additional components such as salt (NaCl), or MgCl2 were included in the hydration buffer. The resulting lipid dispersion was loaded into capillaries (Quartz capillaries from Hampton Research having the following specifications: length – 80 mm, outer diameter – 1.5 mm, wall thickness – 0.01 mm) and placed in a custom designed temperature-controlled capillary holder. Temperature was raised typically in steps of 10 °C over the range 10–90 °C. About 20 min was allowed at each temperature before collection of data. Typically, 20 consecutive 1 s scans were taken and averaged. Lipid phases were identified from the ratios of q-values of the Bragg peaks.
Cryogenic transmission electron microscopy (cryo-TEM)
Unilamellar vesicles were prepared in a manner identical to that for SAXS experiments. Cryo-TEM was carried out at the Stanford Cryo-Electron Microscopy Center (cEMc). 3–5 μL of an SUV dispersion was applied to a Quantifoil holey carbon grid (Quantifoil Micro Tools GmbH) that was previously glow discharged, blotted for 1–2 seconds, and plunge-frozen in liquid ethane using a Vitrobot Mark IV System (Thermo Scientific). The frozen grids were imaged with an FEI Tecnai F20 electron microscope (FEI Company) equipped with a Gatan K2 Summit direct detection device (Gatan Inc.) with a dose rate of 6–10 e− per px per s. A low pass filter of 0.1 and mean shrink of 4 were applied to the raw images for visualization only. The raw images were processed with RELION 3.0. Overlapping regions of the bilayers (particles) were picked with the manual picking tool, extracted, CTF-corrected, and 2D averaged. The 2D classes with good alignment were reclassified into a single 2D class. These particles were randomly divided into 2 subsets and each subset was aligned and averaged independently. The electron scattering profiles of the bilayers were calculated by taking a line profile of the 2D classes through the centre in FIJI. The peak-to-peak distances were calculated by fitting each profile to 2 Gaussian functions and computing the difference between each peak (https://terpconnect.umd.edu/%7Etoh/spectrum/CurveFittingC.html). The uncertainty was calculated as the standard deviation between the peak-to-peak distances of each independent half of each dataset.Fusion of GDGT vesicles with influenza virus
Previously described experimental design and methodology of data analysis for viral membrane fusion was employed here.36,37Results and discussion
Synthesis of GDGT lipids
In earlier work led by the Burns group, an efficient synthetic route to the core structure of parallel GDGT-0 (iso-caldarchaeol) was described.34 In the same work, the synthesis of P2 and P1Glc were reported. In this work, along with P2 and P1Glc, the synthesis of two additional GDGT lipids – PG2 and PG1Glc was achieved (Fig. 2, ESI Schemes S1–S7†). It should be noted that the glucosyl groups in P1Glc and PG1Glc are present as a mixture of anomers. PG1Glc is closest in structure to the naturally occurring archaeal lipid β-L-gulose-GDGT-PG.38Self-assembly of GDGTs in aqueous media
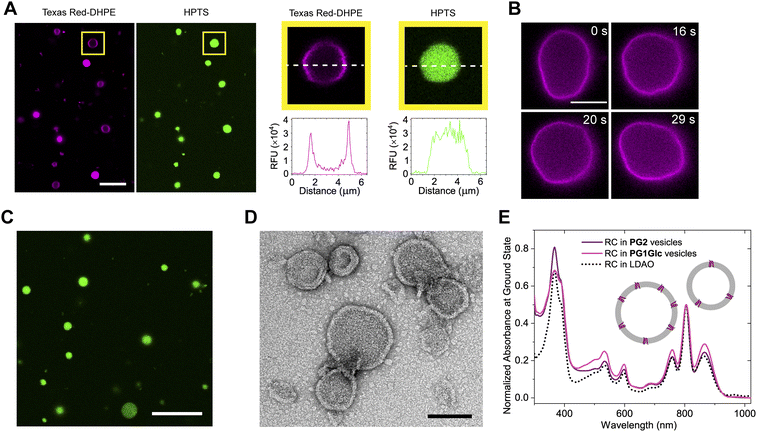
We found that the GDGTs spontaneously self-assembled into giant vesicles (∼1–20 μm diameter) in aqueous media upon hydration of a thin lipid film (typically after incubating at 65 °C) and the membranes were visualized by staining with the lipophilic dye-labelled Texas Red-DHPE (Fig. 3A and S1†). If a water-soluble fluorescent dye such as pyranine (HPTS) was added to the hydration solution, the vesicles encapsulated the dye in the inner aqueous volume (Fig. 3A and S1†). The GDGT vesicle membranes were observed under the microscope to display fluid-like behaviour such as shape fluctuations (Fig. 3B). However, it is noteworthy that unlike typical fluid phase phospholipids (e.g. POPC), the GDGT lipid films required increased mechanical forces (i.e., scratching, vortexing, sonication) to disperse into vesicles, suggesting that the lipids are tightly packed in the thin films. Thin films of the glucose-containing lipids (P1Glc and PG1Glc) were harder to hydrate likely because of extensive intermolecular hydrogen-bonding among the sugar headgroups.10 We empirically observed that the efficiency of hydration of the lipid films depended on the nature and ionic strengths of the buffers. Tris buffer (50 mM, pH 7.5) was found to be the most suitable for hydration of all the lipids studied. Only PG2 films could readily hydrate in several other buffers like HEPES, PBS, and even in pure water to form vesicles. Also, only PG2 could hydrate to form giant vesicles in the presence of proteins such as superfolder green fluorescent protein (sfGFP) and encapsulate the protein (Fig. 3C) while other GDGTs lipids formed aggregates. Next, we tested whether the multilamellar lipid dispersions can be extruded into smaller vesicles via extrusion through membrane filters. Using negative-staining TEM we confirmed the presence of vesicles having diameters less than 200 nm (Fig. S2†).Given the large difference in intermolecular packing as compared to typical phospholipids, it is not obvious whether integral membrane proteins of non-archaeal origin can be reconstituted into GDGT vesicles. There are only a few examples of reconstitution of non-archaeal integral membrane proteins into crude archaeal GDGT lipid membranes. Elferink et al. reconstituted bovine cytochrome c-oxidase in lipid extracts from Sulfolobus acidocaldarius primarily composed of bipolar lipids.11,39 Curiously, archaea are not known to be involved in photosynthesis, which is a heavily membrane-dependent process.5 Therefore, we chose to reconstitute a bacterial photosynthetic reaction centre (RC) protein into GDGT membranes. First, we reconstituted RC in PG2 membranes using detergent removal method. We then performed negative-staining TEM on the protein-lipid dispersion and detected spherical or nearly spherical structures of diameters 40–370 nm (mean: 119 ± 63 nm, n = 108) corresponding to the proteoliposomes (Fig. 3D). To ascertain that the RC is still intact and functional after reconstitution into archaeal lipid, we first recorded the UV-Vis spectra, which confirmed that the protein is still properly assembled, and that the chromophores are still in place (Fig. 3E). We obtained a spectrum with similar features when the RCs were reconstituted into PG1Glc vesicles using similar methodology (Fig. 3E) suggesting that the protein is properly folded in these membranes too. Finally, we performed ultrafast transient absorption spectroscopy to see whether the RC is still able to perform primary electron transfer in this new environment. The results showed that RC can still undergo charge transfer, and the kinetics and mechanism are largely unchanged (Fig. S3A and B†).
Self-assembly of GDGTs at the air–water interface
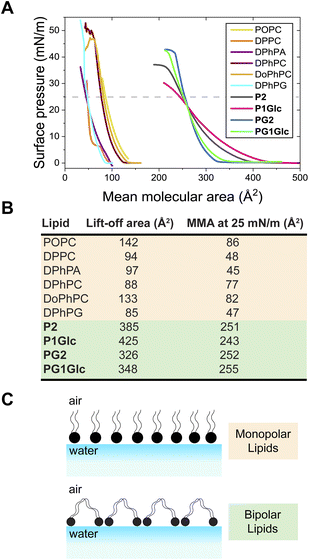
Amphiphilic molecules self-assemble at the air–water interface to form insoluble monomolecular layers with the polar headgroup facing water and the hydrophobic region positioned in air. Since GDGTs have two polar head groups we were curious to test how they self-assemble at the air–water interface and therefore understand how flexible these molecules are conformationally. Using a Langmuir–Blodgett trough, we found that GDGT lipids form stable monomolecular layers at the air–water interface as evident from measurements of surface pressure–area (π–A) isotherms obtained by the Wilhelmy plate method (Fig. 4A and S4†). The isotherms exhibited stable liquid expanded behaviour lacking any first order transitions. In the case of P2, P1Glc, PG2, and PG1Glc, the surface pressure reached a plateau at significantly high values of 36 mN m−1, 30 mN m−1, 42 mN m−1, and 41 mN m−1 respectively, which is indicative of monolayer stability.The results were compared with those obtained with monopolar phospholipids having straight chains (POPC, DPPC) and archaeal-inspired branched-chain phospholipids (DPhPA, DPhPC, DoPhPC, DPhPG) (Fig. 4A and S4†). We found that the GDGTs display significantly higher mean molecular areas (MMA) as compared to monopolar lipids (Fig. 4B). At the lift-off point, the GDGTs display MMA considerably higher than 300 Å2 while at a surface pressure of 25 mN m−1, all GDGTs display MMA of approximately 250 Å2. The corresponding values for monopolar lipids are at least 3–4 times smaller. The high MMA values of the GDGTs can be explained by suggesting that these lipids have the conformational flexibility to loop into a U-shaped conformation at the air–water interface – i.e., with both headgroups facing water and the hydrocarbon chains forming an arch (Fig. 4C).
Measurement of structural properties of GDGT membranes
There are well-established X-ray based methods for elucidating the structure of lipid bilayers.40,41 Here we measured the thicknesses of the GDGT membranes from small-angle X-ray scattering (SAXS) on unilamellar vesicles in solution. In this method, only diffuse scattering is obtained from uncorrelated membranes and therefore no Bragg peaks are observed.42 The observed scattering intensity profiles result from the difference in scattering length densities between the lipids and the buffer.42 In a typical experiment, we obtained scattering intensity profiles from GDGT unilamellar vesicles characterized by three broad lobes (Fig. 5A). The profiles were fitted to a well-established model based on a simulated annealing algorithm that has been previously used to compute asymmetric bilayer electron density profiles (EDP);42–44 all fitting parameters are provided in Table S1.† A typical EDP is characterized by two peaks corresponding to the electron-rich lipid headgroup regions separated by a trough corresponding to the electron-deficient hydrocarbon region (Fig. 5B). Therefore, the head-to-head distances in a lipid membrane can be readily measured from the peak-to-peak distances of the EDPs which ranged between 38–40 Å for the GDGT lipids (Fig. 5B). The measurements are in good agreement with a previous study where lamellar repeat distances (which includes thickness of water layer too) of ∼49–50 Å were measured in crude tetraether lipid dispersions7 or multilamellar vesicles.45 Cryogenic transmission electron microscopy was used to confirm the unilamellar nature of the vesicles and measure the thicknesses of the membranes. Small unilamellar vesicles of GDGTs were vitrified on holey carbon grids using standard techniques and imaged by cryo-EM. The overwhelming majority of the vesicles were unilamellar displaying uni-vesicular or multi-vesicular (or “vesicle-in-vesicle”) architectures (Fig. 5C, S5A and B†). In addition, some sponge-like morphologies comprising of an interconnected network of membranes46 were observed in case of P2 and PG1Glc which were excluded from bilayer profile analysis (Fig. S5C†). We measured the thickness of each membrane by segmenting vesicles into small regions and aligning and averaging the segments to derive 2D class averages (Fig. 5C). The linear profile of each membrane was measured across the centre of the aligned and averaged images to obtain corresponding electron scattering profiles (ESPs) (Fig. 5D). In agreement with the SAXS results, we found that the membrane thicknesses for all GDGT lipids were similar. Congruent with previous work, measured membrane thicknesses were approximately 8 Å thinner than those measured by SAXS (Fig. 5D), likely due to the different physics of X-ray and electron scattering, the different resolutions of the two methods, and the influence of the contrast transfer function (CTF) of the electron microscope on the process of image formation.47 Regarding the latter, the strong Fresnel fringe adjacent to the outer leaflet indicates that the CTF is incompletely corrected, and this trough of negative intensity likely affects the measured peak-to-peak distance.47 We also note that the membrane profiles obtained from cryo-EM have a different shape than those obtained from SAXS because they represent the projection of the spherical density rather than the EDP normal to the plane of the bilayer. Regardless of the absolute membrane thickness, cryo-EM can measure the relative thicknesses of membranes composed of GDGT analogues with sub-Ångström precision. Together, the SAXS and cryo-EM data demonstrate that the thicknesses of GDGT membranes is primarily determined by their hydrocarbon chains rather than their headgroups.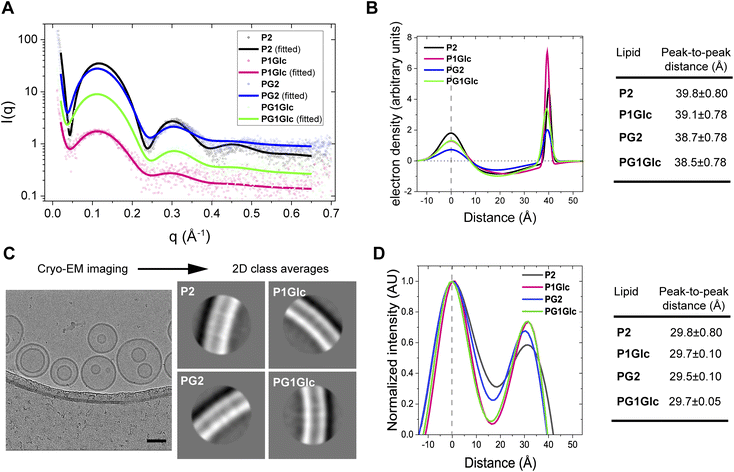 | ||
| Fig. 5 Structural characterization of GDGT membranes. (A) Small-angle X-ray scattering (SAXS) intensity profiles from unilamellar vesicles of GDGTs in tris buffer (50 mM, pH 7.5) at room temperature. The solid lines correspond to the data fitted to the raw data points (open circles). (B) Electron density profiles (EDP) of GDGT membranes calculated from the SAXS intensity profiles. The peak-to-peak distance corresponds to the head-to-head distances of lipids in a membrane. The centres of the 1st peaks of all EDPs were arbitrarily set to ‘0’. All fit parameters are summarized in Table S1.† (C) Representative cryogenic electron microscopy images of unilamellar (and multi-compartment) vesicles of P2 prepared in tris buffer (50 mM, pH 7.5). Scale bar represents 50 nm. The adjoining images show the 2D class averages for each lipid. (D) Electron scattering profiles (ESPs) of GDGT membranes calculated from cryo-EM images. The peak-to-peak distances correspond to the thicknesses of the membranes. The centres of the 1st peaks of all ESPs were arbitrarily set to ‘0’. All error values correspond to fitting error. | ||
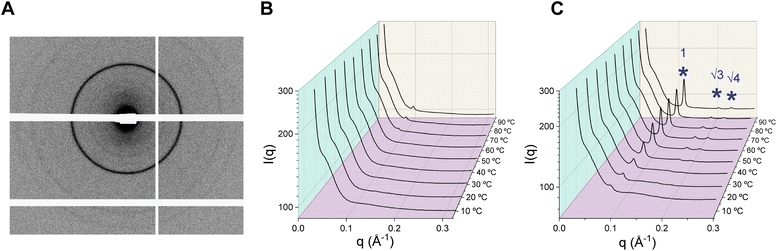
Characterization of mesophases formed by GDGTs
Lipid molecules self-assemble into supramolecular structures called mesophases (e.g. lamellar, hexagonal, cubic) depending on their molecular geometry and external factors. Functional roles of lipids are dictated by their propensity to form specific mesophases. For example, the tendency of phosphoethanolamine lipids to form inverted non-lamellar phases has been associated with their role in facilitating fusion of biological membranes via induction of negative curvature.48 SAXS on bulk lipid dispersions produces characteristic diffraction patterns from which specific mesophases can be ascertained (Fig. 6A).49 We sought to establish a link between the mesophase behaviour of the lipids across a wide temperature range and their probable functional roles in archaeal cell membranes. Differential scanning calorimetry (DSC) studies on bulk GDGT lipid mixtures from extracts typically revealed that the lipids exist in fluid phases at all temperatures and do not exhibit any endothermic lamellar phase transitions (i.e. gel-to-fluid).34 However, it may be challenging to detect transitions between lamellar and non-lamellar phases which are typically characterized by low enthalpy changes.50 Moreover, each DSC measurement requires a significant amount of material that is not always practical to recover. Therefore, we characterized the mesophases formed by aqueous GDGT dispersions using synchrotron SAXS over 10–90 °C under the influence of (i) varying salt concentration; (ii) presence of Mg2+; and (iii) acidic pH. The results are summarized in Table 1.| Conditions | P2 | P1Glc | PG2 | PG1Glc | |
|---|---|---|---|---|---|
| Variable salt concentration | Buffer only (pH 7.5) | Disordered lamellar (10–90 °C) | Disordered lamellar (10–30 °C), hexagonal (40–90 °C) | Disordered lamellar (10–90 °C) | Disordered lamellar (10–90 °C) |
| Buffer + 0.1 M NaCl (pH 7.5) | Disordered hexagonal (10–20 °C) | Disordered lamellar (10–40 °C) | Disordered lamellar (10–90 °C) | Disordered lamellar (10–90 °C) | |
| Hexagonal (30–70 °C) | Hexagonal (50–90 °C) | ||||
| Hexagonal + cubic Pn3m (80–90 °C) | |||||
| Buffer + 0.5 M NaCl (pH 7.5) | Lamellar (10–40 °C) | (Not dispersible) | Disordered lamellar (10–90 °C) | Disordered lamellar (10–20 °C) | |
| Cubic la3d (50 °C) | Hexagonal (30–90 °C) | ||||
| Hexagonal (60–90 °C) | |||||
| Buffer + 1.0 M NaCl (pH 7.5) | Lamellar (10–30 °C) | (Not dispersible) | Lamellar (10–20 °C) | Hexagonal (10–90 °C) | |
| Lamellar + hexagonal (40–60 °C) | Disordered lamellar (30–90 °C) | ||||
| Hexagonal (70–90 °C) | |||||
| Mg2+ | Buffer + 10 mM Mg2+ (pH 7.5) | Lamellar (10–90 °C) | Lamellar phase 1 (10–90 °C) | Disordered lamellar (10–40 °C) | Hexagonal (10–90 °C) |
| Lamellar phase 2 (10–90 °C) | Hexagonal (50–90 °C) | ||||
| Acidic pH | Buffer (pH 5.1) | Disordered lamellar (10–90 °C) | Disordered lamellar (10–90 °C) | Disordered lamellar (10–90 °C) | Disordered lamellar (10–90 °C) |
| Buffer (pH 2.8) | (Not dispersible) | (Not dispersible) | Hexagonal (30–50 °C) | Hexagonal (10–90 °C) |
In a low ionic strength buffer such as 50 mM tris (pH 7.5), the SAXS intensity profiles of P2, PG2, and PG1Glc were mostly featureless (Fig. 6B, S6, S8 and S9†) over the entire temperature range. Lack of features suggest that the lipids mostly formed uncorrelated bilayers in tris buffer due to repulsive forces between the charged headgroups. Repulsive forces may also arise from mutual steric hindrance between undulating membranes stacked in a liquid crystalline multilayer.51P1Glc exhibited a weak hexagonal phase at 30 °C which gradually increased in intensity with temperature (Fig. S7†). Addition of salts is expected to screen the electrostatic repulsion and generate more ordered structures. Therefore, we investigated the effect of 0.1 M, 0.5 M, and 1 M NaCl added to 50 mM tris buffer (pH 7.5). Among all lipids, P2 exhibited the most complex behaviour at varying salt concentrations (Fig. S6†). In the presence of 0.1 M NaCl, somewhat broad and low intensity HII peaks were observed at 10–20 °C which became sharper and more intense over 30–70 °C. Over 80–90 °C, a Pn3m cubic phase coexisted with the HII phase. In 0.5 M NaCl, strong lamellar peaks were seen at 10 °C which fully transitioned to HII peaks at 60 °C and above via an la3d cubic phase at 50 °C. In 1.0 M NaCl, strong lamellar peaks existed over 10–30 °C which coexisted with an HII phase at 40–60 °C and an HII phase only was observed at higher temperatures. P1Glc was difficult to disperse even in the presence of 0.1 M NaCl and a hexagonal phase was detectable at 50 °C and above (Fig. S7†). P1Glc film could not be hydrated at higher salt concentrations. PG2 exhibited disordered phases at all salt concentrations except a small degree of lamellar ordering over 10–30 °C in the presence of 1 M NaCl (Fig. S8†). These observations are in good agreement with microscopy studies where we see facile formation of PG2 vesicles in buffers having a similar range of ionic strengths. PG1Glc displayed disordered phases in 0.1 M NaCl, but a hexagonal phase started to appear above 30 °C in the presence of 0.5 M NaCl (Fig. S9†). In the presence of 1 M NaCl, a small hexagonal phase peak was detectable even at 10 °C which increased in intensity with temperature (Fig. S9†). It is noteworthy that formation of non-lamellar phases (hexagonal and/or cubic) at high temperatures have been observed in crude archaeal bipolar lipid mixtures52 or archaeal-inspired model bipolar lipids27 as well. Our results suggest that such behaviour can be retained in pure individual GDGT species too.
Next, we studied the effect of Mg2+ ions on the phase behaviour of GDGTs (Fig. S10A–D†). Both phosphate-containing lipids P2 and P1Glc exhibited strong lamellar ordering at all temperatures in the presence of MgCl2. P2 exhibited sharp Bragg peaks at all temperatures with lamellar d-spacing of 4.95 nm at 10 °C which monotonically decreased to 3.93 nm at 90 °C. P1Glc exhibited Bragg peaks corresponding to a mixture of two lamellar phases having lamellar d-spacing of 4.67 nm and 4.49 nm at 10 °C likely arising from a mixture of stiff and disordered hydrocarbon chains as previously observed with crude archaeal BTL extracts.52 The spacings monotonically decreased to 3.99 nm and 3.95 nm respectively at 90 °C. On the other hand, the phosphoglycerol-containing lipids PG2 and PG1Glc exhibited hexagonal phases in the presence of MgCl2. In the case of PG2, a weak and broad hexagonal phase peak appeared at 50 °C and it became more prominent at higher temperatures. For PG1Glc, a poorly resolved broad 1st HII peak appeared at 10 °C which increased in prominence with temperature, and the 2nd and 3rd HII peaks could be unambiguously assigned only above 50 °C. We reason that upon binding of Mg2+ to the PG headgroup phosphates, they bridge together. Consequently, the volume of the headgroup region becomes smaller in comparison to the hydrocarbon region and the molecules organize into a hexagonal phase. Interestingly, we also observed Mg2+-induced HII phase formation in the monopolar diphytanoyl archaeal lipid DPhPG (which has a negative spontaneous curvature) at all temperatures (Fig. S11†), which supports the idea that bridging of PG-headgroups by Mg2+ ions is the primary driver of HII phase formation.
Since GDGT lipids occur in thermoacidophilic archaea, we investigated the effect of acidic pH on their mesophase behaviour. The phosphate containing lipids P2 and P1Glc could not be dispersed in 50 mM tris (pH 2.8) likely because of partial protonation of the phosphate groups. Interestingly, both the phosphoglycerol containing lipids PG2 and PG1Glc were found to form hexagonal phases at pH 2.8 over the temperature range (30–90 °C) surveyed (Fig. S10E and F†). For PG1Glc, the HII peaks were sharp and three HII peaks (ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √3
√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √4) were detected at all temperatures. We reason that due to protonation of the PG groups (pKa ∼ 3)53 they become less hydrated and therefore decrease in volume. Notably, we did not observe hexagonal phase in PG2 and PG1Glc at pH 5.1, where the PG headgroups are expected to be fully deprotonated.
√4) were detected at all temperatures. We reason that due to protonation of the PG groups (pKa ∼ 3)53 they become less hydrated and therefore decrease in volume. Notably, we did not observe hexagonal phase in PG2 and PG1Glc at pH 5.1, where the PG headgroups are expected to be fully deprotonated.
Fusion behaviour of membranes composed of GDGTs
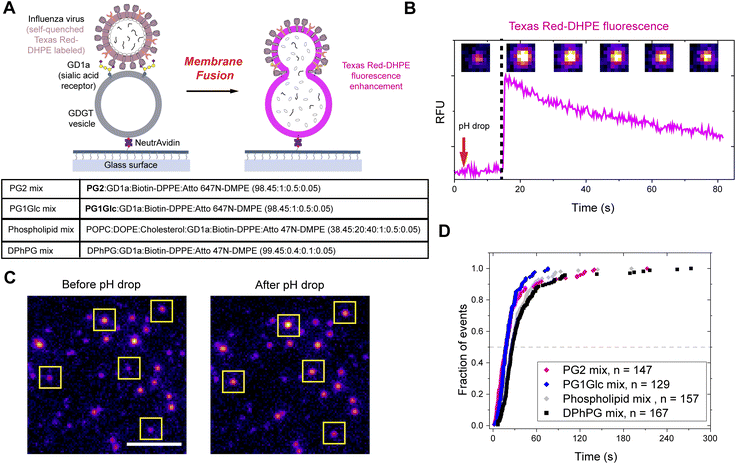
Like any organism, archaea must perform dynamical membrane processes such as fission and fusion during cell division, membrane vesicle budding, or viral entry/egress. However, there is a lack of clarity regarding how archaeal membranes primarily composed of bipolar lipids can undergo fusion (or fission). In a canonical model of bilayer membrane fusion, the outer leaflets of apposing membranes first merge to form a stalk-like hemifusion intermediate.48 This step is triggered by fusogenic agents like divalent cations, acidic pH, polyethylene glycol, or fusion peptides which help overcome the energetic barriers associated with various intermediate steps along the fusion pathway. In the next step, the inner leaflets merge via transient non-lamellar intermediates to open a fusion pore and the two membranes completely fuse along with mixing of inner aqueous contents. In the case where GDGT lipids form a monolayer membrane with all lipids in membrane-spanning conformation, there would likely be high energy barriers and geometrical constraints to forming various fusion intermediates as compared to bilayer membranes. Therefore, we hypothesize that at least some regions of the GDGT membrane should have a bilayer structure which is only possible if some lipids adopt a looping or U-shaped conformation (Fig. 1B). Whether the bipolar lipid membranes can undergo fusion or not and how such behaviour is related to the conformations of lipids (O vs. U) has been a subject of intense investigation. A theoretical study predicted that membrane fusion of bipolar lipid membranes take place through regions consisting of U-shaped lipids.54 Leriche and coworkers demonstrated that vesicles composed purely of an acyclic bipolar lipid can undergo Ca2+-induced fusion without loss of contents.55 They further probed lipid ordering using 2H-NMR and concluded that the bipolar lipids adopt both O- and U-shapes in membranes although precise percentages were not determined. 2H-NMR experiments on model acyclic bipolar lipid membranes suggest that 90% of the lipids exist in O-shape and 10% in U-shape.29 In principle, atomic force microscopy (AFM) may be used to visualize O- and U- shaped regions since they will be expected to have different thicknesses;56 however, such studies will ideally need to be performed on planar supported membranes or GUV patches. Given that the GDGTs in our study are anionic, planar supported membranes or membrane patches could not be generated on clean glass surface (which is negatively charged) via vesicle fusion or rupture of GUVs. Many studies on archaeal lipid mixtures attempted to establish a rational link between the lipid structures and membrane fusion (or the lack thereof) induced by Ca2+ or PEG.13,57–59 For example, Relini et al. noted that vesicles formed from archaeal lipid extracts which displayed transformations between lamellar and non-lamellar phases underwent fusion implying that lipids that can form non-lamellar phases are necessary to induce membrane curvature during fusion.13In previous sections, we showed that under the influence of temperature, salt concentration, pH, or divalent cations, GDGTs show diverse mesophases, especially the formation of inverted non-lamellar phases. Although those lamellar to non-lamellar phase transitions were observed in bulk lipid dispersions, the presence of non-lamellar phase lipids in membranes likely increase the propensity to form local transient non-lamellar structures essential for fusion to take place. Therefore, we asked whether the tendency of GDGT lipids to form non-lamellar phases is related to membrane fusion. To test the fusion behaviour of GDGT vesicles, we devised a novel assay where we chose influenza virus as a fusogenic agent as the latter possesses an efficient fusion machinery embedded in a bilayer lipid membrane that triggers a fusion pathway representative of many biological fusion processes.60
We followed a widely utilized fluorescence microscopy assay to observe fusion of viruses with lipid vesicles on a single particle level.36,37 In this method, the vesicles are allowed to undergo pH-induced fusion with bound influenza virus labelled with self-quenching concentrations of Texas Red-DHPE (Fig. 7A). 100 nm vesicles composed overwhelmingly of GDGT lipids (PG2 mix – PG2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GD1a
GD1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Biotin-DPPE
Biotin-DPPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Atto 647N-DMPE 98.45
Atto 647N-DMPE 98.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05; PG1Glc mix – PG1Glc
0.05; PG1Glc mix – PG1Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GD1a
GD1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Biotin-DPPE
Biotin-DPPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Atto 647N-DMPE 98.45
Atto 647N-DMPE 98.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 by molar ratio) were prepared in a pH 7.4 buffer. The vesicles were tethered to a functionalized glass slide through interaction of neutravidin with Biotin-DPPE (Fig. 7A). Next, influenza virus particles labelled with a self-quenching concentration of Texas Red-DHPE were added. The α2,3-silaic acid containing sialoglycolipid GD1a served as the receptor for influenza hemagglutinin binding. The pH was lowered by flowing in an acidic buffer (pH 5.1). Upon hemifusion, the outer leaflets of viral and vesicle membranes merge and the Texas Red-DHPE gets diluted. Consequently, its fluorescence quenching is relieved as evident from a sudden spike in fluorescence intensity (Fig. 7B).
0.05 by molar ratio) were prepared in a pH 7.4 buffer. The vesicles were tethered to a functionalized glass slide through interaction of neutravidin with Biotin-DPPE (Fig. 7A). Next, influenza virus particles labelled with a self-quenching concentration of Texas Red-DHPE were added. The α2,3-silaic acid containing sialoglycolipid GD1a served as the receptor for influenza hemagglutinin binding. The pH was lowered by flowing in an acidic buffer (pH 5.1). Upon hemifusion, the outer leaflets of viral and vesicle membranes merge and the Texas Red-DHPE gets diluted. Consequently, its fluorescence quenching is relieved as evident from a sudden spike in fluorescence intensity (Fig. 7B).
Interestingly, we observed on a single particle level that many dim fluorescent spots (corresponding to a virus particle labelled with self-quenched TR-DHPE) turned brighter (Fig. 7C). We plotted the time to the onset of lipid mixing into cumulative distribution function for many vesicle populations and found that the fusion of GDGT vesicles follow a kinetics comparable to that with vesicles composed of monopolar phospholipids and cholesterol (“phospholipid mix”)33 or vesicles composed primarily of monopolar archaeal-type lipids (“DPhPG mix”) (Fig. 7D). Next, we tested whether the small percentage (1.55 mol%) of monopolar lipids (i.e. bilayer forming) added to the GDGT vesicles in the previous experiments necessary for viral attachment and surface tethering have any significant influence over the outcome of membrane fusion. We prepared PG2 vesicles containing even smaller percentages (1 mol% and 0.5 mol%) of monopolar lipids and still observed many lipid mixing events (Fig. S12†). The kinetics of those events were nearly identical to that observed with “DPhPG mix” (having 100 mol% of monopolar lipids) vesicles (Fig. S12†). Therefore, we reason that the tiny percentages of monopolar lipids necessary to functionalize the GDGT vesicles do not have any significant influence on the outcome of the membrane fusion process and the fusion of GDGT vesicles is likely mediated by bilayer regions formed from U-shaped lipids.
Next, we asked whether the fusion events led to full fusion or whether they were arrested at the hemifusion stage. To address this question, we prepared “PG2 mix” vesicles encapsulating self-quenching concentration of the water-soluble dye sulforhodamine B (SRB) (Fig. S13A†). In the event of a full fusion, the interiors of the virus and vesicle compartments become one and the fluorescence of SRB is enhanced upon dilution.61 We allowed unlabelled influenza virus particles to bind to tethered GDGT vesicles and then lowered the pH to 5.1. We observed many events where a dim fluorescent spot turned brighter indicating transfer of SRB from vesicle to virus through the fusion pore with nearly identical kinetics as compared to vesicles composed of monopolar phospholipids (“phospholipid mix”) (Fig. S13B and C†).
Conclusions
We have described the synthesis of a series of chemically pure GDGT-0 lipids which enabled in-depth biophysical investigations on those. Although symmetric combinations of headgroups (such as in P2 and PG2) are not yet reported to occur naturally, overall, we found that their self-assembly and structural properties share many parallels with those of their unsymmetric counterparts (i.e.P1Glc and PG1Glc respectively). Therefore, for many bottom-up biophysical studies, symmetric GDGTs, which are easier to synthesize, should serve as reasonably good model lipids. Symmetric GDGT lipids are also of interest to computational researchers likely because of the ease of theoretical modelling.62,63 Therefore, availability of experimental data on such lipids will be beneficial for future computational studies. Archaeal BTLs have long been considered as excellent building blocks of liposomal drug delivery systems, lipid nanoparticles for mRNA delivery, synthetic cell membranes, or as matrix for membrane protein reconstitution with exceptional long-term structural and thermal stabilities.10,64–66 It is notable that the lipid PG2 (followed by PG1Glc) offered the most optimal properties (such as ease of dispersion and tolerance to many buffers and macromolecules) for broad applicability akin to typical glycerophospholipids, and therefore may find many applications as a model GDGT such as mentioned above. Although P2 and P1Glc are not ideal for forming vesicles on their own, they can nevertheless be used as minor components in lipid mixtures.In this work, we also addressed the question of what conformations GDGT lipids adopt in a membrane and how those impact the functional behaviour of the membranes. While it is generally considered that archaeal BTLs adopt a membrane-spanning conformation (O-shape), we have provided structural and functional evidence that GDGT-0 lipids possess the conformational flexibility to fold into U-shapes in a membrane. First, we showed that the GDGT-0 lipids adopt U-shapes in monomolecular films at the air–water interface as evident from the significantly higher mean molecular area occupied by these lipids in comparison to monopolar lipids. Conformational flexibility is further evident from the rich mesophase behaviour for each GDGT lipid which were found to transform between lamellar and non-lamellar phases at various combinations of buffer and temperature conditions. Next, we developed a novel assay where we showed that vesicles primarily composed of a single GDGT lipid species can undergo pH-induced fusion with influenza virus particles at kinetic rates comparable to that measured with conventional monopolar lipid vesicles. Such behaviour can be explained by considering that the GDGT membranes at least partially consist of bilayer regions composed of U-shaped lipids that act as sites of fusion. Whether the influenza fusion protein (hemagglutinin) has any direct role in inducing local bilayer structures that allow membrane hemifusion will require further investigation. Previous reports suggest that viral fusion peptides can induce inverted non-lamellar phases in phosphoethanolamine lipids which have a tendency to form hexagonal phases at high temperatures.67,68 Similarly, the tendency of GDGT-0 lipids to form inverted non-lamellar phases possibly allows stabilization of fusion intermediates upon interaction with influenza viral fusion peptide.
Examples from archaeal biology indeed support our idea that GDGT-0 lipids have a greater tendency to adopt U-shapes and play a pivotal role in membrane dynamics. For instance, it has been observed that enveloped archaeal viruses such as Sulfolobus Spindle-Shaped Virus 1 (SSV1) consist of a disproportionately larger fraction (68.1%) of GDGT-0 lipids in their envelope as compared to its host Sulfolobus solfataricus (0.8%).69 The presence of high fraction of GDGT-0 lipids in the viral membranes potentially facilitates their fusion with the host membranes both during entry and budding. Another matter that will be interesting to investigate in the future is the impact of the cycloalkane rings in the hydrocarbon region of the higher order GDGT-n (n ≥ 1) lipids. GDGTs containing n cyclopentane rings are expected to be conformationally rigid and less likely to loop into a U-shape. Future studies involving fusion of archaeal enveloped viruses with model membranes consisting of GDGTs with variable number of rings (n ≥ 0) will indeed provide deeper insights into fusion mechanisms of such viruses.
Finally, the observation that GDGT vesicles can fuse with influenza virus also opens the possibility of their application as novel chemically stable components of optical sensing devices for cell-free detection of infectious virus particles, and such efforts are currently underway.
Data availability
Data presented in this article are available in the main manuscript and the ESI.† Additional data can be available upon reasonable request made to the corresponding authors.Author contributions
AB performed the biophysical experiments under the guidance of SGB. IDF carried out the synthesis of GDGT lipids under the guidance of NZB. FRM and AB performed the cryo-EM experiments. TMW collaborated on the SAXS experimental setup and data analysis software. KNT purified photosynthetic reaction centre. AB, FRM, NZB and SGB wrote the paper with inputs from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was carried out with support from the NSF (MCB-1915727) and NIH (R35GM118044) grants awarded to SGB and from the NSF (CAREER CHE-1846512) grant awarded to NZB. AB acknowledges an Education & Outreach Grant offered by nano@stanford (which is supported by the National Science Foundation as part of the National Nanotechnology Coordinated Infrastructure under award ECCS-2026822) for partial financial support. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF), supported by the National Science Foundation under award ECCS-2026822. Electron microscopy imaging carried out at the Cell Sciences Imaging Facility (CSIF) at Stanford University was supported, in part, by ARRA Award Number 1S10RR026780-01 from the National Center for Research Resources (NCRR). Use of the Stanford Synchrotron Radiation Lightsource (SSRL), SLAC National Accelerator Laboratory, is supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under Contract DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (P30GM133894). The Pilatus detector at beamline 4-2 at SSRL was funded under National Institutes of Health Grant S10OD021512. We thank Profs. Dewey Holten and Christine Kirmaier, and Dr Kaitlin Faries of Washington University for providing the ultrafast spectroscopic measurements on photosynthetic reaction centre shown in Fig. S3.† We thank Sawan Kumar Jha (Red Horse Lab, Stanford) and Suraj Borkar (Gerald Fuller Lab, Stanford) for collaborating with for collaborating with confocal microscopy and Langmuir trough experiments respectively. We thank Srijit Mukherjee (Boxer Lab) for insightful suggestions regarding the manuscript.Notes and references
- N. O. Medina-Chávez and M. Travisano, Front. Genet., 2022, 12, 693193 CrossRef PubMed.
- Y. M. Bar-On, R. Phillips and R. Milo, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6506–6511 CrossRef PubMed.
- A. Caforio and A. J. M. Driessen, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2017, 1862, 1325–1339 CrossRef PubMed.
- A. Bhattacharya, Emerging Top. Life Sci., 2022, 6, 571–582 CrossRef PubMed.
- V. Sojo, BioEssays, 2019, 41, 1800256 CrossRef PubMed.
- S. Schouten, E. C. Hopmans and J. S. Sinninghe Damsté, Org. Geochem., 2013, 54, 19–61 CrossRef.
- P. L. G. Chong, Chem. Phys. Lipids, 2010, 163, 253–265 CrossRef.
- A. Bonanno, R. C. Blake and P. L. G. Chong, Int. J. Mol. Sci., 2019, 20, 5308 CrossRef CAS PubMed.
- J. S. Sinninghe Damsté, W. I. C. Rijpstra, E. C. Hopmans, J. W. H. Weijers, B. U. Foesel, J. Overmann and S. N. Dedysh, Appl. Environ. Microbiol., 2011, 77, 4147–4154 CrossRef PubMed.
- G. D. Sprott, Archaeal Membrane Lipids and Applications, eLS, 2011, DOI:10.1002/9780470015902.a0000385.pub3.
- M. G. L. Elferink, J. G. De Wit, R. Demel, A. J. M. Driessen and W. N. Konings, J. Biol. Chem., 1992, 267, 1375–1381 CrossRef CAS.
- T. J. Beveridge, C. G. Choquet, G. B. Patel and G. D. Sprott, J. Bacteriol., 1993, 175, 1191–1197 CrossRef CAS PubMed.
- A. Relini, D. Cassinadri, Q. Fan, A. Gulik, Z. Mirghani, M. De Rosa and A. Gliozzi, Biophys. J., 1996, 71, 1789–1795 CrossRef CAS.
- S. Dante and M. De Rosa, et al. , Thin Solid Films, 1996, 285, 459–463 CrossRef.
- A. Gliozzi, R. Rolandi, M. De Rosa and A. Gambacorta, J. Membr. Biol., 1983, 75, 45–56 CrossRef CAS PubMed.
- M. De Rosa, A. Gambacorta and B. Nicolaus, J. Memb. Sci., 1983, 16, 287–294 CrossRef CAS.
- C. T. Lloyd, D. F. Iwig, B. Wang, M. Cossu, W. W. Metcalf, A. K. Boal and S. J. Booker, Nature, 609, 197–203 CrossRef CAS PubMed.
- Z. Zeng, H. Chen, H. Yang, Y. Chen, W. Yang and P. V Welander, Nat. Commun., 2022, 13, 1545 CrossRef CAS.
- S. Dante, F. Rustichelli, B. Yang, M. De Rosa, A. Morana, E. Maccioni, C. Nicolini and V. I. Troitsky, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1995, 262, 191–207 CrossRef.
- C. Dietrich, L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson and E. Gratton, Biophys. J., 2001, 80, 1417–1428 CrossRef CAS.
- P. L. G. Chong, M. Sulc and R. Winter, Biophys. J., 2010, 99, 3319–3326 CrossRef CAS.
- H. Shimada and A. Yamagishi, Biochemistry, 2011, 50, 4114–4120 CrossRef CAS PubMed.
- M. L. Bode, S. R. Buddoo, S. H. Minnaar and C. A. du Plessis, Chem. Phys. Lipids, 2008, 154, 94–104 CrossRef CAS PubMed.
- G. Lecollinet, J. W. G. Annette Gulik, G. Mackenzie, T. Benvegnu and D. Plusquellec, Chem.–Eur. J., 2002, 8, 585–593 CrossRef CAS.
- T. Benvegnu, G. Lecollinet, J. Guilbot, M. Roussel, M. Brard and D. Plusquellec, Polym. Int., 2003, 52, 500–506 CrossRef CAS.
- M. Brard, W. Richter, T. Benvegnu and D. Plusquellec, J. Am. Chem. Soc., 2004, 126, 10003–10012 CrossRef CAS PubMed.
- A. Jacquemet, C. Mériadec, L. Lemiègre, F. Artzner and T. Benvegnu, Langmuir, 2012, 28, 7591–7597 CrossRef CAS.
- Y. H. Kim, G. Leriche, K. Diraviyam, T. Koyanagi, K. Gao, D. Onofrei, J. Patterson, A. Guha, N. Gianneschi, G. P. Holland, M. K. Gilson, M. Mayer, D. Sept and J. Yang, Sci. Adv., 2019, 5, eaaw4783 CrossRef PubMed.
- D. P. Brownholland, G. S. Longo, A. V. Struts, M. J. Justice, I. Szleifer, H. I. Petrache, M. F. Brown and D. H. Thompson, Biophys. J., 2009, 97, 2700–2709 CrossRef CAS.
- M. De Rosa, Thin Solid Films, 1996, 284–285, 13–17 CrossRef.
- G. Schwarzmann, B. Breiden and K. Sandhoff, J. Lipid Res., 2015, 56, 1861–1879 CrossRef CAS.
- T. Eguchi, K. Arakawa, K. Kakinuma, G. Rapp, S. Ghosh, Y. Nakatani and G. Ourisson, Chem.–Eur. J., 2000, 6, 3351–3358 CrossRef CAS.
- K. Arakawa, T. Eguchi and K. Kakinuma, Chem. Lett., 2001, 30, 440–441 CrossRef.
- I. D. Falk, B. Gál, A. Bhattacharya, J. H. Wei, P. V. Welander, S. G. Boxer and N. Z. Burns, Angew. Chem., Int. Ed., 2021, 60, 17491–17496 CrossRef CAS.
- J. B. Weaver, C. Y. Lin, K. M. Faries, I. I. Mathews, S. Russi, D. Holten, C. Kirmaier and S. G. Boxer, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2116439118 CrossRef CAS PubMed.
- R. J. Rawle, S. G. Boxer and P. M. Kasson, Biophys. J., 2016, 111, 123–131 CrossRef CAS PubMed.
- K. N. Liu and S. G. Boxer, Biophys. J., 2020, 118, 2426–2433 CrossRef CAS PubMed.
- M. Swain, J. R. Brisson, G. D. Sprott, F. P. Cooper and G. B. Patel, Biochim. Biophys. Acta, Lipids Lipid Metab., 1997, 1345, 56–64 CrossRef CAS PubMed.
- M. G. L. Elferink, J. G. de Wit, A. J. M. Driessen and W. N. Konings, Eur. J. Biochem., 1993, 214, 917–925 CrossRef CAS.
- J. A. Bouwstra, G. S. Gooris, W. Bras and H. Talsma, Chem. Phys. Lipids, 1993, 64, 83–98 CrossRef CAS.
- J. F. Nagle and S. Tristram-Nagle, Biochim. Biophys. Acta, Biomembr., 2000, 1469, 159–195 CrossRef CAS.
- M. R. Brzustowicz and A. T. Brunger, J. Appl. Crystallogr., 2005, 38, 126–131 CrossRef CAS.
- F. R. Moss, S. R. Shuken, J. A. M. Mercer, C. M. Cohen, T. M. Weiss, S. G. Boxer and N. Z. Burns, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9098–9103 CrossRef CAS.
- F. R. Moss, G. E. Cabrera, G. M. McKenna, G. J. Salerno, S. R. Shuken, M. L. Landry, T. M. Weiss, N. Z. Burns and S. G. Boxer, ACS Chem. Biol., 2020, 15, 2986–2995 CrossRef.
- P. L. G. Chong, M. Zein, T. K. Khan and R. Winter, J. Phys. Chem. B, 2003, 107, 8694–8700 CrossRef CAS.
- A. Bhattacharya, H. Niederholtmeyer, K. A. Podolsky, R. Bhattacharya, J.-J. Song, R. J. Brea, C.-H. Tsai, S. K. Sinha and N. K. Devaraj, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 18206–18215 CrossRef CAS PubMed.
- F. A. Heberle, M. Doktorova, H. L. Scott, A. D. Skinkle, M. N. Waxham and I. Levental, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 19943–19952 CrossRef CAS.
- A. Joardar, G. P. Pattnaik and H. Chakraborty, J. Membr. Biol., 2022, 255, 211–224 CrossRef CAS PubMed.
- C. V. Kulkarni, W. Wachter, G. Iglesias-Salto, S. Engelskirchen and S. Ahualli, Phys. Chem. Chem. Phys., 2011, 13, 3004–3021 RSC.
- J. M. Seddon, G. Cevc and D. Marsh, Biochemistry, 1983, 22, 1280–1289 CrossRef CAS PubMed.
- W. Helfrich, Z. Naturforsch., 1978, 33, 305–315 CrossRef.
- A. Gulik, V. Luzzati, M. De Rosa and A. Gambacorta, J. Mol. Biol., 1985, 182, 131–149 CrossRef CAS PubMed.
- D. Marsh, Handbook of Lipid Bilayers, CRC Press, 2nd edn, 2013 Search PubMed.
- T. R. Galimzyanov, P. I. Kuzmin, P. Pohl and S. A. Akimov, Soft Matter, 2016, 12, 2357–2364 RSC.
- G. Leriche, D. Stengel, D. Onofrei, T. Koyanagi, G. P. Holland and J. Yang, Sci. Rep., 2019, 9, 19359 CrossRef CAS.
- S. Vidawati, J. Sitterberg, U. Bakowsky and U. Rothe, Colloids Surf., B, 2010, 78, 303–309 CrossRef CAS PubMed.
- A. Relini, D. Cassinadri, Z. Mirghani, O. Brandt, A. Gambacorta, A. Trincone, M. De Rosa and A. Gliozzi, Biochim. Biophys. Acta, Biomembr., 1994, 1194, 17–24 CrossRef CAS.
- M. G. L. Elferink, J. Van Breemen, W. N. Konings, A. J. M. Driessen and J. Wilschut, Chem. Phys. Lipids, 1997, 88, 37–43 CrossRef CAS PubMed.
- R. Kanichay, L. T. Boni, P. H. Cooke, T. K. Khan and P. L. G. Chong, Archaea, 2003, 1, 175–183 CrossRef CAS PubMed.
- M. Kielian, Annu. Rev. Virol., 2014, 1, 171–189 CrossRef.
- K. N. Liu and S. G. Boxer, Biophys. J., 2021, 120, 4832–4841 CrossRef CAS.
- W. Shinoda, K. Shinoda, T. Baba and M. Mikami, Biophys. J., 2005, 89, 3195–3202 CrossRef CAS.
- A. O. Chugunov, P. E. Volynsky, N. A. Krylov, I. A. Boldyrev and R. G. Efremov, Sci. Rep., 2014, 4, 7462 CrossRef CAS.
- A. C. Jacobsen, S. M. Jensen, G. Fricker, M. Brandl and A. H. Treusch, Eur. J. Pharm. Sci., 2017, 108, 101–110 CrossRef CAS PubMed.
- V. L. Sedlmayr, S. Schobesberger, S. Spitz, P. Ertl, D. J. Wurm, J. Quehenberger and O. Spadiut, Eur. J. Pharm. Biopharm., 2024, 197, 114213 CrossRef CAS PubMed.
- H. P. Rahn, J. Sun, Z. Li, R. M. Waymouth, R. Levy and P. A. Wender, Biomacromolecules, 2024, 25, 4305–4316 CrossRef CAS PubMed.
- B. G. Tenchov, R. C. Macdonald and B. R. Lentz, Biophys. J., 2013, 104, 1029–1037 CrossRef CAS PubMed.
- S. T. Smrt, A. W. Draney and J. L. Lorieau, J. Biol. Chem., 2015, 290, 228–238 CrossRef CAS.
- E. R. J. Quemin, M. K. Pietilä, H. M. Oksanen, P. Forterre, W. I. C. Rijpstra, S. Schouten, D. H. Bamford, D. Prangishvili and M. Krupovic, J. Virol., 2015, 89, 11681–11691 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc03788j |
| This journal is © The Royal Society of Chemistry 2024 |