Open Access Article
Open Access ArticleTether-entangled conjugated helices†
Ke
Jin
ab,
Zuo
Xiao
 *ab,
Huidong
Xie
ab,
Xingxing
Shen
*c,
Jizheng
Wang
*ab,
Huidong
Xie
ab,
Xingxing
Shen
*c,
Jizheng
Wang
 d,
Xiangyu
Chen
d,
Xiangyu
Chen
 e,
Zhijie
Wang
e,
Zhijie
Wang
 f,
Zujin
Zhao
f,
Zujin
Zhao
 g,
Keyou
Yan
g,
Keyou
Yan
 h,
Yong
Ding
h,
Yong
Ding
 i and
Liming
Ding
i and
Liming
Ding
 *ab
*ab
aCenter for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: xiaoz@nanoctr.cn; ding@nanoctr.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cCollege of Chemical Engineering, Hebei Normal University of Science and Technology, Qinhuangdao 066004, China. E-mail: sxx3694@hevttc.edu.cn
dInstitute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
eBeijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences, Beijing 101400, China
fInstitute of Semiconductors, Chinese Academy of Sciencess, Beijing 100083, China
gState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, China
hSchool of Environment and Energy, South China University of Technology, Guangzhou 510006, China
iBeijing Key Laboratory of Novel Thin-Film Solar Cells, North China Electric Power University, Beijing 102206, China
First published on 24th September 2024
Abstract
A new design concept, tether-entangled conjugated helices (TECHs), is introduced for helical polyaromatic molecules. TECHs consist of a linear polyaromatic ladder backbone and periodically entangling tethers with the same planar chirality. By limiting the length of tether, all tethers synchronously bend and twist the backbone with the same manner, and change it into a helical ribbon with a determinate helical chirality. The 3D helical features are customizable via modular synthesis by using two types of synthons, the planar chiral tethering unit (C2 symmetry) and the docking unit (C2h symmetry), and no post chiral resolution is needed. Moreover, TECHs possess persistent chiral properties due to the covalent locking of helical configuration by tethers. Concave-type and convex-type oligomeric TECHs are prepared as a proof-of-concept. Unconventional double-helix π-dimers are observed in the single crystals of concave-type TECHs. Theoretical studies indicate the smaller binding energies in double-helix π-dimers than conventional planar π-dimers. A concentration-depend emission is found for concave-type TECHs, probably due to the formation of double-helix π-dimers in the excited state. All TECHs show strong circularly polarized luminescence (CPL) with dissymmetric factors (|glum|) generally over 10−3. Among them, the (P)-T4-tBu shows the highest |glum| of 1.0 × 10−2 and a high CPL brightness of 316 M−1 cm−1.
Introduction
Helical polyaromatic molecules have attracted significant interest in recent years due to their aesthetically pleasing molecular structures, unique chemical and physical properties, and diverse applications.1–17 Helicenes and twistacenes are two archetypes that have been extensively investigated (Fig. 1a).18,19 Helicenes are formed of consecutively ortho-fused aromatic or heteroaromatic rings.20–24 The steric repulsion between the spatially adjacent rings or groups endows helicenes with a spiral-staircase-shaped backbone and a helical conjugated system. The helical axis is near orthogonal to the π-surface of aromatic rings. Various helicenes and helicenoids like carbohelicenes,25–28 heterohelicenes,29–39 metallahelicenes,40–45 multiple helicenes,46–54 and expanded helicenes55–64 have been developed. Twistacenes are twisted acenes or benzannulated acenes with steric crowdedness around the periphery.65–83 The crowdedness causes conformational distortion and induces the backbone to adopt a helical conformation, where the helical axis is near parallel to the π-surface of aromatic rings of acenes. Despite structural differences, helicenes and twistacenes share in common that their helical geometry is caused by minimizing the unfavored steric interactions in the planar conformation against the energy cost of distortion.65,84 Thus, the steric effect plays a vital role in deciding the helical structure and configurational stability for helicenes and twistacenes. Just for this reason, challenges like the difficulty in synthesis and chiral resolution, dynamic variation of the helical structures, multiple coexisting conformers, and easily happening racemization (especially for the shorter ones) have accompanied helicenes and twistacenes.47,48,63,85–96Herein, we report a new design of helical polyaromatic molecules, the tether-entangled conjugated helices (TECHs) (Fig. 1a). TECHs contain two parts of structural elements, the linear ladder-type polyaromatic backbone97–103 and the periodically entangling tethers with the same planar chirality. All tethers stretch across the backbone and locate in one side of the molecular plane. By limiting the length of tether, all tethers can synchronously introduce chiral strain to the backbone, and bend and twist the backbone with the same manner. As a result, the backbone is coiled up into a spiral aromatic ribbon with a determinate helical chirality. Different from helicenes and twistacenes, TECHs have following features: (1) the helical geometry of TECHs is no longer decided by the steric effect but by the chiral strain induced from the entangling tethers; (2) the helical chirality of TECHs is exclusive and only decided by the planar chirality of tether; (3) TECHs are static helices with constrained conformational variation; (4) because of the covalent locking of the helical conformation by tethers, TECHs have high configurational stability and persistent chiral properties; (5) the helical structures and chirality of TECHs are customizable by modular synthesis, and no post chiral resolution is needed. In this paper, we introduce the design concept and synthesis analysis for TECHs. Two kinds of TECHs, the concave-type and convex-type oligomeric TECHs, were successfully prepared as a proof-of-concept. Moreover, the concave-type TECH molecules have shown interesting aggregation and chiroptical properties. These molecules can form double-helix dimers via an unconventional embracement-like π–π interaction and exhibit strong circularly polarized luminescence (CPL)104–106 capability.
Results and discussion
Design concept and synthesis analysis
To better describe the design concept of TECHs, two typical ladder-type polyaromatic skeletons, the ladder aromatic lactams107–113 and the ladder oligo(p-aniline)s,114–119 are used as the backbones and the 8-carbons alkyl chains are used as the entangling tethers for building the models of TECHs (Fig. 1b). As shown in Fig. 1b, when using five R-planar-chirality (Rp) tethers to entangle the 21-fused-rings ladder aromatic lactams and the 21-fused-rings ladder oligo(p-aniline)s backbones, all tethers locate in front of the molecular plane, connecting two adjacent bridge nitrogen atoms from top left to bottom right. Due to the geometrical mismatch between backbones and tethers, strain is generated. All tethers synchronously curl the backbones into helical ribbons with the M chirality. The major difference for the two helical ribbons is the spatial location of the entangling tethers. In the case of helical ladder aromatic lactams, all tethers locate in the outer space of the helical structure, covering the convex surface and leaving the concave surface being exposed, whereas in the case of helical ladder oligo(p-aniline)s, all tethers locate in the inner space of the helical structure, covering the concave surface and leaving the convex surface being exposed. We define the former as the concave-type TECHs and the latter as the convex-type TECHs. The cause of this structural difference is due to the different bending direction of the backbones by the tethers. For concave-type TECHs, the bending is toward the opposite side of tethers, whereas for convex-type TECHs, the bending is toward the tethers' side. When using five S-planar-chirality (Sp) tethers to entangle the ladder aromatic lactams and ladder oligo(p-aniline)s, all tethers locate in back of the molecular plane. This time, both helical ribbons show the P chirality and mirror-imaged structures as compared to the M-helices. Therefore, the helical chirality of TECHs is only decided by the planar chirality of tether. The tether length has significant influence to the helical structures of TECHs. As shown in Fig. S1,† longer tethers like C10 or C12 alkyl chains induce little or neglectable chiral strain to the aromatic backbones, leading to less obvious or no helical characteristics. In sharp contrast, shorter tethers like C6 alkyl chains bring larger chiral strain and a higher degree of coiling to the backbones. From the above, it can be seen that the linear polyaromatic ladder backbone and the periodic homochiral entangling tethers in a limited length are the key factors for TECHs. Together they decide the helical shape of TECHs.The synthesis analysis of TECHs is shown in Fig. 2. It is important to note that some helical ladder polymers and oligomers (conjugated or partially conjugated) have been synthesized by developing defect-free annulation reactions120–123 and the use of axial,124–126 planar127,128 and helical129–131 chiral building units. Inspired by these works and also the synthesis of ladder-type conjugated polymers and oligomers,97,103,132–138 we considered that TECHs can be constructed via modular synthesis by using two types of synthons. The first one we call the chiral tethering unit, which has a planar chiral macrocyclic structure with the C2 symmetry and four potential reactive sites (two a1 sites and two b1 sites). The second one we call the docking unit, which has the C2h symmetry and four potential reactive sites (two a2 sites and two b2 sites). If a1 site can selectively bond with a2 site, b1 site can selectively bond with b2 site and no racemization happens for the chiral tethering unit during reaction, we can alternately dock the two building units and generate polymeric TECHs (Fig. 2b). To synthesize oligomeric TECHs, additional terminal units are required. The terminal units are similar to the docking unit but with only one side of reactive sites. Taking the oligomeric TECHs in Fig. 1b for examples, their synthesis can be achieved by using five chiral tethering units, four docking units and two terminal units (Fig. 2c). From the above, it can be concluded that three conditions are required for the synthesis of TECHs: (1) the prepared C2-symmetric chiral tethering units and C2h-symmetric docking units; (2) the selective bonding (a1 to a2, b1 to b2) between the chiral tethering unit and docking unit; (3) no racemization for the chiral tethering units during the reaction. If all the three conditions are satisfied, various TECHs with exclusive 3D geometrical characteristics (e.g. helical diameters and pitches) and chirality are customizable through the above modular synthesis. Fig. S2† shows some designed TECH molecules and their predicted helical structures by PM7.139 It can be seen that even small structural modifications on the chiral tethering units or docking units can lead to dramatical change of the 3D helical geometries.
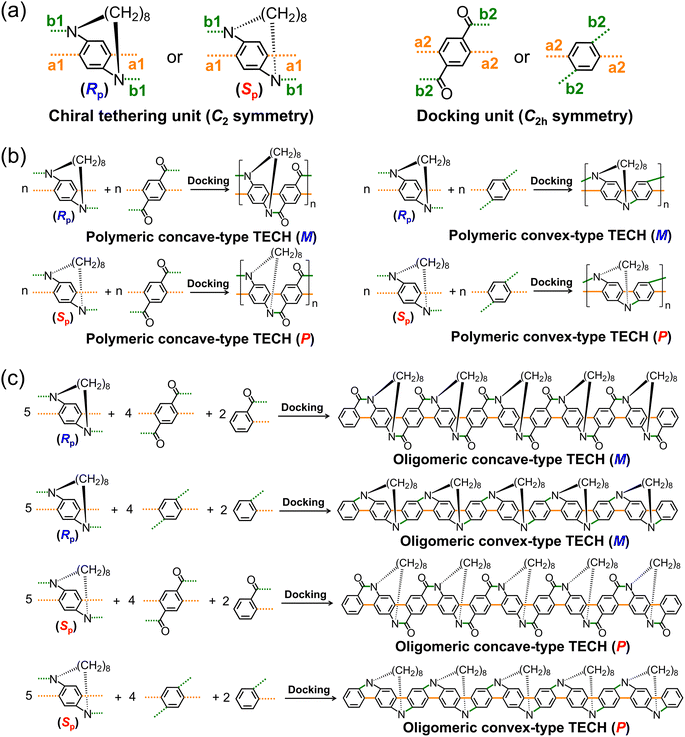 | ||
| Fig. 2 The synthesis analysis of TECHs: (a) the chiral tethering unit and the docking unit; (b) modular synthesis of polymeric TECHs; (c) modular synthesis of oligomeric TECHs. | ||
Synthesis of oligomeric TECHs
Guided by the design concept of TECHs, we start the preparation of oligomeric TECHs. First, we accomplished the synthesis of chiral tethering units (Sp)-3 and (Rp)-3 (Scheme 1). Treating 2,5-dibromoterephthalic acid with diphenylphosphoryl azide, triethylamine and the chiral auxiliary (S)-1-phenylethanol gave (S,S)-1 in 80% yield. Treating (S,S)-1 with 1,8-dibromooctane in diluted solution gave a pair of separable macrocyclic diastereomers (S,Sp,S)-2 and (S,Rp,S)-2 in 26% and 13% yield, respectively. The structures and the absolute configurations for (S,Sp,S)-2 and (S,Rp,S)-2 were unambiguously confirmed by single crystal X-ray diffraction (SCXRD). Treating (S,Sp,S)-2 or (S,Rp,S)-2 with trifluoroacetic acid completely removed the chiral auxiliaries and afforded the planar chiral compounds (Sp)-3 or (Rp)-3 in 94% or 95% yield, respectively. The enantiomeric excess (ee) values for (Sp)-3 and (Rp)-3 are 98.4% and 99.7%, respectively. (Sp)-3 and (Rp)-3 are C2-symmetric molecules with four potential reactive sites, two aryl bromides (a1 site) and two amines (b1 site), satisfying the requirements as chiral tethering units. Bearing in mind that the chiral tethering units should not undergo racemization during TECH synthesis, we tested the configurational stability of (Sp)-3 and (Rp)-3 by heating them at 130 °C for 48 hours. It was found that neither (Sp)-3 nor (Rp)-3 underwent racemization after the thermal treatment (Fig. S3†), suggesting the sufficiently high racemization barrier. This result indicates that (Sp)-3 and (Rp)-3 are suitable chiral tethering units for the synthesis of TECHs. We also tried to synthesis chiral tethering units with shorter tethers like C7 or C6 alkyl chains. However, no isolable product was obtained in the macrocyclization reaction. This could be due to the too high internal strain in these molecules, making them difficult to generate and stabilize.Next, we started to synthesize the P and M oligomeric concave-type TECHs from (Sp)-3 and (Rp)-3, respectively (Scheme 2). For synthesizing shorter TECHs like (P)-T1, (P)-T1-tBu, (P)-T2-tBu and (P)-T3-tBu, the basic strategy is constructing the amide oligomers first (accomplishing the b1 to b2 bonding) and then conducting the Pd-catalyzed consecutive intramolecular C–H arylations140–145 (accomplishing the a1 to a2 bonding, i.e. linear ladderization) at one time to give the target molecules. The synthesis of (P)-T3-tBu is used as an example for a detailed description. Amidation of (Sp)-3 with methyl 4-(chlorocarbonyl)benzoate afforded the bis-amide (Sp)-4-CO2Me in 88% yield. Hydrolysis of (Sp)-4-CO2Me with KOH gave (Sp)-4-CO2H in 97% yield. Then, (Sp)-4-CO2H was converted to acyl chloride and reacted with 2 equiv. of (Sp)-3 and afforded the tetrakis-amide (Sp,Sp,Sp)-7 in 95% yield. Amidation of (Sp,Sp,Sp)-7 with 4-tert-butylbenzoyl chloride afforded the hexakis-amide (Sp,Sp,Sp)-8-tBu in 96% yield. Finally, (P)-T3-tBu was obtained in 61% yield through the Pd-catalyzed linear ladderization (six new C–C bonds formed). It can be seen that the yields for the last ladderization step continuously drop from ∼90% for (P)-T1 and (P)-T1-tBu to 61% for (P)-T3-tBu. This is caused by two reasons. The first one is the accompanied debromination side reaction in the Pd-catalyzed C–H arylation.146 When the number of bromine atoms in the substrate increases, the debromination become more severe. The second one is the generation of curved ladderization isomers. As shown in Fig. S4 and S5,† it is possible to generate two and three isomers in the last step of (P)-T2-tBu and (P)-T3-tBu, respectively. For both cases, the target linear ladderization isomers are always the most thermodynamically favored products, whereas the curved isomers are less favored due to the steric crowdness on backbone. The DFT-optimized structures indicate helicene-like segments in these curved isomers. Despite being energetically unfavorable, small amount of (P)-T2-tBu curved isomer (7% yield), and (P)-T3-tBu curved-1 isomer (15% yield) and curved-2 isomer (7% yield) were observed and isolated from the low-polarity side products of (P)-T2-tBu and (P)-T3-tBu, respectively (see ESI†). Characterization data confirmed the structures shown in Fig. S4 and S5.† This generation of curved isomers highlights the need for strict control of the symmetry of docking unit in TECH synthesis, that is, the docking unit must keep the C2h symmetry to ensure that all tethers can be located on the same side of the molecular plane. In curved isomers, the symmetry of docking unit changed from C2h to C2v, thus leading to tethers on both sides of the molecular plane. This deviates the design target of TECHs.
Due to the increasing debromination side products and the curved ladderization isomers, the one-step ladderization strategy is not applicable for longer TECHs like (P)-T4-tBu and (P)-T5-tBu. Therefore, we adopted a stepwise ladderization strategy for the synthesis of (P)-T4-tBu and (P)-T5-tBu. For (P)-T4-tBu, we conducted the first-step ladderization to build the core structure (P)-T2-CO2H. The synthesis is similar to (P)-T2-tBu (Scheme S1†). Starting from (P)-T2-CO2H, the amide precursor (Sp,P,Sp)-10-tBu was prepared through two amidation processes. Finally, (P)-T4-tBu was obtained in 43% yield from (Sp,P,Sp)-10-tBu through the second-step ladderization. For (P)-T5-tBu, the first-step ladderization afforded the core structure (P)-T3-CO2H. The synthesis is similar to (P)-T3-tBu (Scheme S2†). Then, the amide precursor (Sp,P,Sp)-12-tBu was prepared, and (P)-T5-tBu was obtained in 23% yield from (Sp,P,Sp)-12-tBu through the second-step ladderization. For all M concave-type oligomers ((M)-T1-tBu, (M)-T2-tBu, (M)-T3-tBu, (M)-T4-tBu, (M)-T5-tBu), their syntheses are identical to their P-counterparts but using (Rp)-3 as the starting material (Scheme 2b). The detailed synthetic routes are given in the Scheme S3.†
Next, we synthesized the convex-type TECHs, (P)-T1-convex, (P)-T2-convex, (M)-T1-convex, and (M)-T2-convex (Scheme 3). First, 1 equiv. of (Sp)-3 coupled with 2 equiv of 2-(2,5-difluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane via the Suzuki reaction and gave (Sp)-13 in 85% yield. Then, tBuOK-promoted intramolecular nucleophilic ring-closure reaction119 transformed (Sp)-13 to (P)-T1-convex in 84% yield. For (P)-T2-convex, 1 equiv. of (Sp)-3 first coupled with 1 equiv. of 2-(2,5-difluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane to give (Sp)-14 in 55% yield. (Sp)-14 then coupled with 2,5-difluorobenzene-1,4-diboronicacidbis(pinacol)ester and gave (Sp)-15 in 52% yield. Finally, tBuOK-promoted ring-closure reaction gave (P)-T2-convex in 17% yield. Considering the quite low yield of the final step of (P)-T2-convex, we didn't try to synthesis longer oligomeric convex-type TECHs. For (M)-T1-convex and (M)-T2-convex, they were synthesized similarly as their P-counterparts but with (Rp)-3 as the starting material.
All TECH molecules as well as their intermediates were characterized by nuclear magnetic resonance (NMR) and high-resolution mass (HRMS) spectroscopic methods (see ESI†). For some compounds, (P)-T1, (P)-T2-tBu, (Sp,P,Sp)-11, (Rp,M,Rp)-11, and (P)-T2-convex, their structures and absolute configurations were further determined by SCXRD (vide infra). For concave-type TECHs, the intermediates containing amide moieties generally show broadened NMR spectra due to the slow rotation of the amide C–N bond at room temperature.147–150 However, the NMR spectra of the final TECH molecules show sharp signals, suggesting the enhanced molecular rigidity after ladderization. For convex-type TECHs, (P)-T1-convex and (M)-T1-convex show sharp signals on NMR, whereas (P)-T2-convex and (M)-T2-convex show broadened NMR spectra. This could be due to the lower solubility of (P)-T2-convex and (M)-T2-convex in chloroform and the formation of intermolecular aggregates. Both 1H and 13C NMR spectra indicate the C2 symmetry for all TECH molecules. To further identify the characteristic protons of TECH molecules, 2D-NMR techniques including COSY, NOESY, HSQC, HMBC were employed for (P)-T1-tBu, (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, (P)-T5-tBu, and (P)-T1-convex (Fig. S127–S149†). The key correlations and assigned chemical shifts for these compounds are summarized in Fig. S150–S152.† All aromatic protons and partial aliphatic protons were identified. It is noteworthy that all TECH molecules show signals with negative chemical shift on 1H NMR spectra. These signals belong to the central tether protons pointing downward to the benzene ring of the chiral tethering unit. Taking (P)-T1-tBu and (P)-T1-convex for examples, the chemical shifts for the protons are −0.19 and −1.89 ppm, respectively. Since these protons are very close to the benzene rings, the negative chemical shifts could result from the shielding effect of the central aromatic benzene ring. We carried out DFT calculations to verify this hypothesis (Fig. S153†). First, the calculated chemical shifts for all protons of (P)-T1-tBu and (P)-T1-convex are consistent with the experimental ones. The calculated chemical shifts for the tether protons closest to benzene ring are −0.53 and −2.11 ppm for (P)-T1-tBu and (P)-T1-convex, respectively. Second, the nucleus-independent chemical shift (NICS)151–154 values (e.g. NICS(0), NICS(1), and NICS(−1)) indicate the higher aromaticity of the central benzene ring of (P)-T1-convex than that of (P)-T1-tBu (Fig. S153†). The NICS(0), NICS(1), and NICS(−1) values are −7.28, −8.44, and −7.66 ppm for (P)-T1-tBu, and −9.78, −11.25, and −8.92 ppm for (P)-T1-convex, respectively. Therefore, the tether protons above the central benzene of (P)-T1-convex are more shielded, thus showing a more negative chemical shift. Beside NMR characterization, the HRMS spectra show distinct and expected molecular ion peaks for all TECH molecules (Fig. S154–S170†).
To quantify the chiral strain introduced from tethers, we calculated the strain energy (SE) for all P-TECHs by using the homodesmotic reaction method155–158 (Scheme S4,† Table S1†). The total SE increases along with the number of tethers in either concave-type or convex-type TECHs. The total SE increases from 10.0 kcal mol−1 for (P)-T1 to 47.7 kcal mol−1 for (P)-T5, and from 6.5 kcal mol−1 for (P)-T1-convex to 27.9 kcal mol−1 for (P)-T5-convex. On the other hand, the average SE caused by individual tether shows insignificant change for either concave-type or convex-type TECHs. For concave-type TECHs, the SE per tether ranged from 9.5 kcal mol−1 to 10.0 kcal mol−1, and for convex-type TECHs, the SE per tether ranged from 5.6 kcal mol−1 to 6.5 kcal mol−1. The above results indicate that in a TECH molecule all tethers bend and twist the aromatic backbone with a similar amplitude.
Single crystal structures
Single crystal structures for (P)-T1, (P)-T2-tBu, (Sp,P,Sp)-11, (Rp,M,Rp)-11, and (P)-T2-convex are shown in Fig. 3. These structures provide a solid support for the design concept of TECHs. For (P)-T1, (P)-T2-tBu, and (Sp,P,Sp)-11 that contain one, two, and three Sp entangling tethers in the ladder aromatic lactam backbone, respectively, all tethers were found in the back of the molecular plane, connecting two adjacent bridge nitrogen atoms from top left to bottom right. The chiral strain induced by these tethers coiled the backbones up into helical ribbons. Viewing along the helical axis, all backbones show the clockwise twist (P chirality) and all tethers locate in the outer space of the helical structure. Such concave-type TECH configurations are exactly as what we forecasted in Fig. 1. It was also found that the average bending of the backbone caused by one tether varies in the three molecules. The angles of total bending are ∼30°, ∼107°, and ∼118° for (P)-T1, (P)-T2-tBu and (Sp,P,Sp)-11, respectively, corresponding to ∼30°, ∼54°, and ∼39° average bending per tether. This result suggests that although the TECHs are rigid static helical molecules, there is still freedom of motion in bending and twisting, thus rendering them a certain degree of flexibility. For (Rp,M,Rp)-11 that with the Rp entangling tethers, it shows a mirror-imaged structure of (Sp,P,Sp)-11 and a helical backbone with the anti-clockwise twist (M chirality), confirming that the helical chirality of TECHs is only decided by the planar chirality of tethers. Finally, the single crystal structure of (P)-T2-convex confirmed our prediction about the convex-type TECHs, where the entangling tethers locate in the inner space of the helical structure. The backbone of (P)-T2-convex is bent by ∼56°, corresponding to ∼28° average bending per tether. With the Sp entangling tethers, the backbone also shows a P chirality.A more interesting finding is the formation of double-helix dimer in the crystals of concave-type TECHs (P)-T2-tBu, (Sp,P,Sp)-11, and (Rp,M,Rp)-11. As shown in Fig. 4, two molecules of (P)-T2-tBu (or (Sp,P,Sp)-11, (Rp,M,Rp)-11) embrace each other and form a dimer complex via the concave face of helical backbone.159–161 Short center-to-center contacts (e.g. 3.42–3.77 Å in (P)-T2-tBu dimer, 3.50–3.62 Å in (Sp,P,Sp)-11 dimer, 3.57–3.63 Å in (Rp,M,Rp)-11 dimer) between the spatially close aromatic rings of two backbones were found for the three dimers. This result suggests that π–π interaction may play an important role in the dimer formation. However, π-dimerization usually takes place between planar conjugated molecules. To verify the unconventional π–π interaction in the double-helix dimers, we carried out DFT calculations for (P)-T2-tBu and (Sp,P,Sp)-11 dimers. Considering that the terminal groups have little influence to the dimer interactions, we omitted the terminal groups of (P)-T2-tBu and (Sp,P,Sp)-11 and built up two simplified models named as (P)-T2 dimer and (P)-T3 dimer, respectively. The structural optimization was done at the M06-2X(D3)/6-31G(d,p) level with crystal structure as initial guess. The optimized structures of dimers are shown in Fig. 4d. We conducted independent gradient model based on Hirshfeld partition (IGMH) analysis162–165 to visualize the real-space noncovalent interactions. It was found that in both dimers a large area of interaction isosurface spreads between the concave π-surfaces of the two monomer fragments, in accordance with the feature of π–π interaction. Moreover, the sandwiched isosurface continuously twists along with the twisted molecular planes. The interaction region indicator (IRI) analysis166 indicates that the interaction isosurface sandwiched by monomers mainly represents van der Waals attraction (Fig. S171†). The above results verified that considerable π–π interactions exist in the wrapped space of the double-helix dimers and dominate noncovalent interactions. Therefore, they probably provide a driving force for the dimer formation. To get a general understanding of the strength of such unconventional π-dimerization, we investigated the interaction energies (ΔEint) and binding energies (ΔEb) in (P)-T2 dimer and (P)-T3 dimer. For comparison, we also built up two supposititious planar π-dimer models (planar-T2 dimer and planar-T3 dimer) and calculated ΔEint and ΔEb in conventional π-dimers (Fig. S172 and S173†). In the case of T2 dimers, the double-helix (P)-T2 dimer and the planar-T2 dimer show comparable ΔEint of −54.4 and −53.6 kcal mol−1, respectively. However, the ΔEb for (P)-T2 dimer is apparently smaller than that of planar-T2 dimer (−44.7 vs. −50.9 kcal mol−1). This is due to the fact that much higher deformation energy (ΔEd) of 9.7 kcal mol−1 should be overcome for the double-helix dimer formation, whereas the ΔEd for the planar dimer formation is only 2.7 kcal mol−1. The larger ΔEd for double-helix dimer than planar dimer can be directly visualized from the more obvious structural change of the (P)-T2 monomers before and after dimerization (Fig. S172†). In the case of T3 dimers (Fig. S173†), the double-helix (P)-T3 dimer not only shows higher ΔEd than planar-T3 dimer (9.5 vs. 3.9 kcal mol−1), but also shows smaller ΔEint (−64.5 vs. −79.3 kcal mol−1). Thus, the binding energy for (P)-T3 dimer is much smaller than that of planar-T3 dimer (−55.0 vs. −75.4 kcal mol−1). From the above, it can be concluded that the tendency for the double-helix π-dimerization is considerably strong, but is not as strong as the conventional planar π-dimerization, because the monomers have to overcome a larger deformation energy to find a better π–π interaction geometry.
It is worth to note that no double-helix π-dimer is observed in the crystals of (P)-T1 and (P)-T2-convex. For (P)-T1, although it belongs to concave-type TECHs, the conjugated backbone is short. Thus, the π–π interaction for (P)-T1 could be relatively weak and may not play a dominate role in molecular aggregation. For (P)-T2-convex, its concave π-surface is shielded by the entangling tethers. Therefore, the tethers will impede the embracement-like π–π interactions and prevent the formation of dimers. In this regard, only the concave-type TECHs with sufficient backbone length could possess the capability of forming double-helix π-dimers. We envisioned that this capability may be even stronger for longer concave-type TECHs, such as (P)-T4-tBu and (P)-T5-tBu. Because the crystals of (P)-T4-tBu and (P)-T5-tBu were not obtained, we investigated their dimer complexes by theoretical calculation. Considering the systems of (P)-T4 dimer (392 atoms) and (P)-T5 dimer (484 atoms) are too large, we optimized their structures by using the quantum chemistry program ORCA 5.0.4 at the r2SCAN-3c level.167–169 The optimized structures of (P)-T4 dimer and (P)-T5 dimer as well as the IGMH isosurfaces are shown in Fig. S174.† As expected, both dimers show double-helix geometries and embracement-like π–π interactions. Large ΔEint of −91.5 and −113.7 kcal mol−1 were found for (P)-T4 dimer and (P)-T5 dimer, respectively.
Optical and electrochemical properties
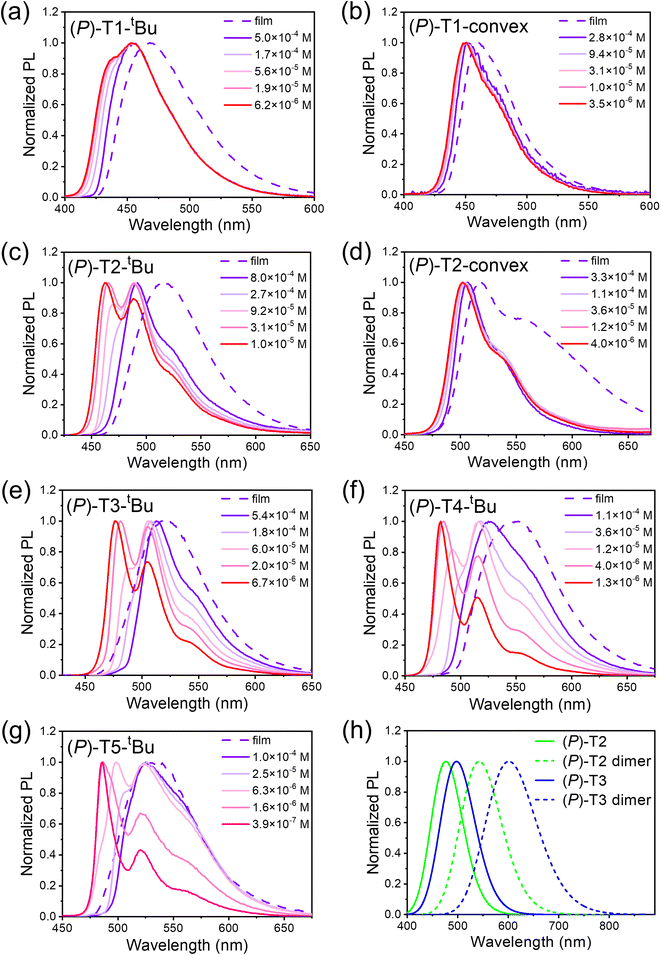
The UV-Vis spectra for concave-type and convex-type TECHs are shown in Fig. 5a and b, respectively. Since the M-helix TECHs gave identical absorption spectra as their P-helix counterparts, only the spectra of P-helix TECHs are presented. For concave-type TECHs, all molecules show two major absorption bands. TD-DFT calculations indicate that the low energy band originates from the S0 → S1 electronic transition, whereas the high energy band comes from other higher energy transitions (Fig. S175†). From (P)-T1-tBu to (P)-T5-tBu, the absorption coefficient of the low energy band increased remarkably due to the significantly enhanced oscillator strength of the S0 → S1 transition. (P)-T5-tBu shows the highest molar absorption coefficient of 1.50 × 105 M−1 cm−1 at 482 nm. For convex-type TECHs, (P)-T1-convex and (P)-T2-convex also show two absorption bands. However, due to the low oscillator strength, the S0 → S1 transition bands for (P)-T1-convex and (P)-T2-convex are weak and broad. For either concave-type or convex-type TECHs, their absorption spectra show distinct bathochromic shifts as the molecular length increases, indicating that the band gap narrows as the conjugated system elongates. This is similar to the planar ladder-type conjugated molecules. The optical band gaps (Eoptg) were estimated from the absorption onsets. (P)-T1-tBu, (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, (P)-T5-tBu, (P)-T1-convex and (P)-T2-convex show Eoptg of 2.86, 2.67, 2.57, 2.51, 2.48, 2.76, and 2.46 eV, respectively.The photoluminescence (PL) spectra of TECH molecules are shown in Fig. 6. It was interesting to find that the concave-type TECHs (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, and (P)-T5-tBu show distinct concentration-dependent emission behavior, whereas the (P)-T1-tBu and the convex-type TECHs (P)-T1-convex and (P)-T2-convex do not. At low concentration, the PL spectra of (P)-T2-tBu to (P)-T5-tBu show clear vibronic progression (e.g. the 0-0 and 0-1 transition peaks) and the profiles are mirror images of the S0 → S1 transition band in absorption spectra, indicating that the emission is from the excited single molecules. When concentration increases, the PL maximum shows large bathochromic shifts (29–44 nm) and the vibronic feature disappears. This phenomenon suggests that excimer-like species may form during the luminescence process.170–173 Reminding that the concave-type TECHs except (P)-T1-tBu possess the capability of forming double-helix π-dimers, we considered that the PL at higher concentration is probably from the excited dimers. This is supported by the fact that the PL spectra are similar for the film and high-concentration solution of (P)-T2-tBu to (P)-T5-tBu.174–178 We also carried out theoretical calculation to investigate the emission property of monomers and dimers. The calculated fluorescence spectra for (P)-T2, (P)-T2 dimer, (P)-T3, and (P)-T3 dimer are shown in Fig. 6h. It can be seen that the excited (P)-T2 dimer and (P)-T3 dimer show much red-shifted emission than their monomers, supporting the hypothesis that the PL at higher concentration could originate from the excited dimers. The measured PL quantum yields for the dilute solution of TECH molecules are 29%, 43%, 57%, 71%, 46%, 27%, and 21% for (P)-T1-tBu, (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, (P)-T5-tBu, (P)-T1-convex, and (P)-T2-convex, respectively. The generally higher emission efficiency of concave-type TECHs than convex-type TECHs can be attributed to their stronger oscillator strength of the S1 → S0 transition (Table S2†). For example, the calculated S1 → S0 oscillator strength are 0.18 and 0.70 for (P)-T1 and (P)-T2, respectively, which are higher than that of their convex counterparts (P)-T1-convex (0.04) and (P)-T2-convex (0.04). In high concentration solution, the PL quantum yields drop for all compounds except (P)-T5-tBu. The values are 22%, 36%, 41%, 49%, 50%, 19%, and 12% for (P)-T1-tBu, (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, (P)-T5-tBu, (P)-T1-convex, and (P)-T2-convex, respectively. The decrease of PL quantum yields in high-concentration solution could be due to the aggregation-caused quenching effect. We further conducted time-resolved fluorescence decay measurements for all P-TECHs solutions at low or high concentrations (Fig. S176†). It was found that for (P)-T1-tBu, (P)-T1-convex, and (P)-T2-convex that do not show concentration-dependent emission behavior, only the single-exponential decay was observed, and their low-concentration and high-concentration solutions show similar fluorescence lifetimes. In contrast, (P)-T2-tBu to (P)-T5-tBu all show bi-exponential decay in the high-concentration solutions, and the ratio of the slow decay component dramatically increased along with concentration. This result suggests that the fast and slow decay components in (P)-T2-tBu to (P)-T5-tBu could come from the excited monomers and dimers, respectively.
The electrochemical property of TECH molecules was studied by cyclic voltammetry (CV) measurements (Fig. S177†). For all molecules, only oxidation peaks were observed. The concave-type TECHs show higher oxidation potentials than the convex-type TECHs. The HOMO levels calculated from the onset oxidation potentials of (P)-T1-tBu, (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, (P)-T5-tBu, (P)-T1-convex, and (P)-T2-convex are −5.61, −5.59, −5.58, −5.56, −5.55, −5.09, and −4.79 eV, respectively. The deeper HOMO levels of the concave-type TECHs can be attributed to the more electron deficient lactam moieties, which pull down energy levels. The LUMO level was calculated by using HOMO level plus Eoptg. The values are −2.75, −2.92, −3.01, −3.05, −3.07, −2.33, and −2.33 eV, respectively. From (P)-T1-tBu to (P)-T5-tBu, the elongation of conjugated system of concave-type TECHs leads to slight increase of HOMO level but dramatic decrease of LUMO level. However, in the case of (P)-T1-convex and (P)-T2-convex, the elongation of conjugated system majorly lifts up HOMO level and has less influence on LUMO level. The DFT-calculated frontier orbitals and energy levels are shown in Fig. S178.† The theoretically predicted variation trends in HOMO and LUMO levels are consistent with the experimental observations.
Chiroptical properties
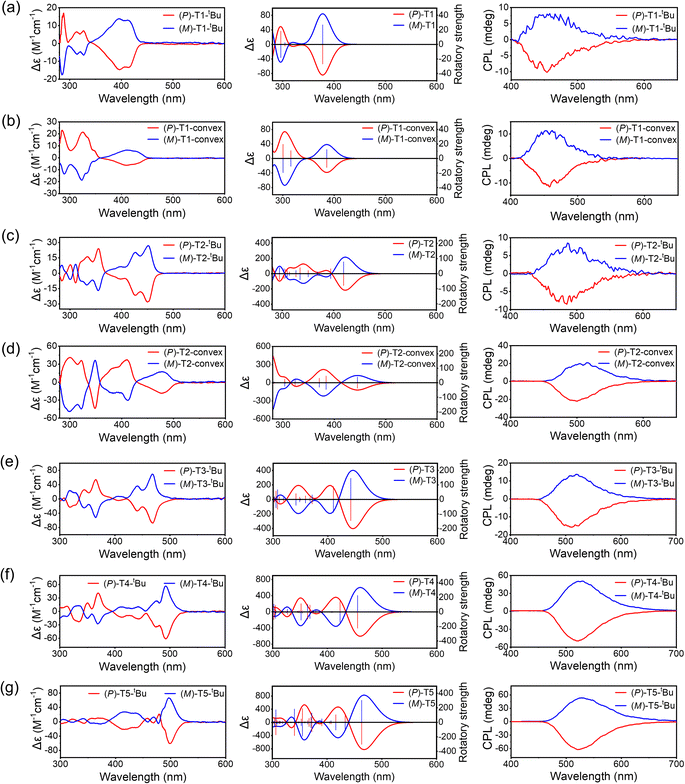
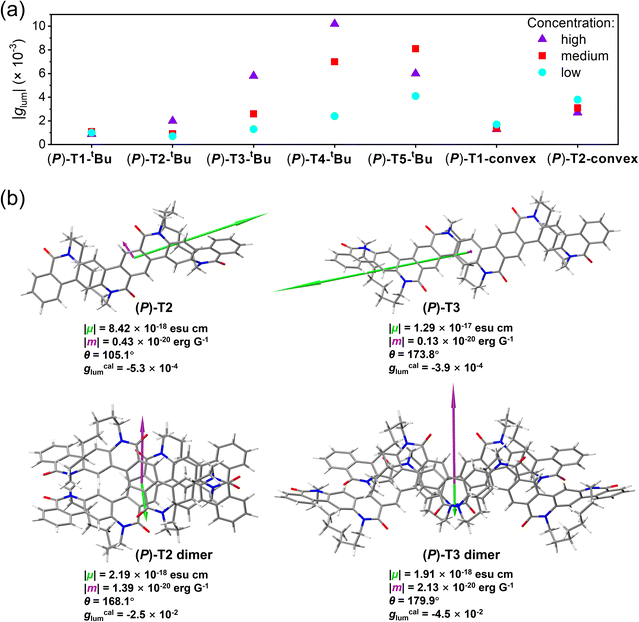
Electronic circular dichroism (CD) and CPL tests were carried out for P- and M-TECHs to investigate their chiral absorption and emission properties. The experimental CD and CPL spectra as well as the theoretical CD spectra for TECHs are shown in Fig. 7. To obtain a good signal to noise ratio on CD spectra, we have to adopt a relatively medium concentration for the test solutions, although there could be co-existed monomers and dimers for the concave-type TECHs. As indicated in Fig. 7, the experimental and theoretical CD spectra have shown good consistency for most of the signals. For all P-TECHs, the CD signals close to band gap show negative Cotton effects, which is in good agreement with the theoretical prediction. All M-TECHs show mirror-imaged CD spectra as their P-counterparts and opposite Cotton effects. For either concave-type or convex-type TECHs, there is an increase tendency in molar circular dichroism (|Δε|) of the near-band gap transitions as the conjugated system elongates. From T1 to T5 concave-type TECHs, the |Δε| increased from cal. 14 M−1 cm−1 to cal. 66 M−1 cm−1. From T1-convex to T2-convex TECHs, the |Δε| increases from cal. 6 M−1 cm−1 to cal. 18 M−1 cm−1. On the other hand, the absorption dissymmetry factors (gabs) for the near-band-gap transitions did not show a regular change. For instance, the gabs for (P)-T1-tBu, (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, (P)-T5-tBu, (P)-T1-convex, and (P)-T2-convex are −7.5 × 10−4, −6.0 × 10−4, −5.3 × 10−4, −8.4 × 10−4, −3.0 × 10−4, −4.7 × 10−4, and −5.0 × 10−4, respectively.All TECH molecules have exhibited prominent CPL-emission capability. As shown in Fig. 7, P- and M-TECHs present mirror-imaged CPL spectra, and the signals have the same sign with the corresponding CD signals near the band gap. The CPL shows an increase tendency along with the elongation of conjugated system. The signals increased from cal. 9 mdeg to cal. 58 mdeg for T1 to T5 concave-type TECHs, and from cal. 11 mdeg to cal. 21 mdeg for T1-convex to T2-convex TECHs, respectively. The luminescence dissymmetric factors (glum) for (P)-T1-tBu, (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, (P)-T5-tBu, (P)-T1-convex, and (P)-T2-convex are −1.1 × 10−3, −9.2 × 10−4, −2.6 × 10−3, −7.0 × 10−3, −8.1 × 10−3, −1.4 × 10−3, and −3.1 × 10−3, respectively. It is surprising that the g-factors are significantly amplified for (P)-T4-tBu and (P)-T5-tBu during the emission process. The |glum| are ∼8 and ∼27 folds greater than the |gabs| for (P)-T4-tBu and (P)-T5-tBu, respectively. For most small molecule chiral emitters, glum corresponds closely to the gabs near the band gap and usually shows the same magnitude.179,180 The deviation of |glum| from |gabs| mainly comes from two possible scenarios: (1) the severe structural change in the excited state via vibrational relaxation; (2) the aggregate formation.180 For rigid aromatic molecules, however, the first circumstance is less possible. We compared the optimized geometries of the ground state and excited state for all P-TECHs in Fig. S179.† It was found that the structural change between the two states is insignificant for all compounds. Moreover, the calculated dissymmetric factors gcalabs and gcallum are close to each other in all cases. Therefore, the relaxation-caused amplification of the dissymmetric factor can be ruled out. Reminding that the concave-type TECHs (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, and (P)-T5-tBu exhibited excimer-like emission in high-concentration solutions, we considered that the amplified glum could be possibly related to aggregation. We investigated the CPL and glum of all P-TECHs at low, medium, and high concentrations (Fig. S180†). The variation of |glum| along with concentration is summarized in Fig. 8a. It can be seen that (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, and (P)-T5-tBu exhibited more pronounced fluctuation of |glum| along with concentration than the other three TECH molecules. The ratios between the highest and lowest |glum| for (P)-T2-tBu, (P)-T3-tBu, (P)-T4-tBu, and (P)-T5-tBu are 2.8, 4.5, 4.3, and 2.0, respectively, whereas the ratios for (P)-T1-tBu, (P)-T1-convex, and (P)-T2-convex are close to unity. This suggests aggregation may exist in the concentrated solutions of (P)-T2-tBu to (P)-T5-tBu. However, the dynamic light scattering (DLS) measurements indicate that they are all in the molecularly dispersed state with no large aggregates (Fig. S182†). The almost unchanged absorption spectra along with concentration also suggest no obvious aggregation (Fig. S183†). These results support the classic excimer mechanism in the concentrated solutions of (P)-T2-tBu to (P)-T5-tBu. Thus, in ground state they mainly exist in the form of monomers, but in the excited state they undergo dimerization to form excimers. The |glum| increment at higher concentrations for (P)-T2-tBu to (P)-T5-tBu could be caused by the formation of double-helix π-dimers in the excited state. To test the hypothesis, we optimized the geometries of S1 state of (P)-T2, (P)-T3 and their dimers (P)-T2 dimer and (P)-T3 dimer, and investigated the S1 → S0 transition characteristics (Fig. 8b). In both cases, the S1 → S0 transition characteristics of monomer and dimer are dramatically different. From monomer to dimer, the electric transition dipole moment (μ) decreases by a factor of 4 and 7 for (P)-T2 and (P)-T3, respectively, whereas the magnetic transition dipole moment (m) increases by a factor of 3 and 17 for (P)-T2 and (P)-T3, respectively, and the θ between μ and m becomes closer to 180°, thus leading to a great enhancement in the g-factors.181–184 The gcallum values for (P)-T2 dimer and (P)-T3 dimer are ∼45 and ∼115 folds higher than those of (P)-T2 and (P)-T3, respectively. The above theoretical studies support the hypothesis that the double-helix π-dimers in the excited state could make an important contribution in the increased |glum| for concentrated solutions. It also implies the reason why |glum| did not change much along with concentration in the case of (P)-T1-tBu, (P)-T1-convex, and (P)-T2-convex is that these molecules do not have the capability of forming double-helix π-dimers. It is worth to note that the |glum| of (P)-T4-tBu in high-concentration solution reached 1.0 × 10−2, which is a considerably high value for organic chiral emitters in solution. The |glum| values for ordinary small molecule chiral emitters are usually around 10−4 to 10−3.185–189 Reminding that (P)-T4-tBu also shows a relatively high PL quantum yield (71% in dilute solution, 49% in high-concentration solution) and a high molar absorption coefficient (1.29 × 105 M−1 cm−1 at 480 nm), (P)-T4-tBu possesses a high CPL brightness179 of 316 M−1 cm−1 (110 M−1 cm−1 in dilute solution), which is among the best values for organic chiral emitters to date.190–194
At last, we investigated the chiroptical stability of TECHs. Traditional optically pure helical polyaromatic molecules like helicenes and twistacenes can lose their optical activity due to the surmountable racemization barriers. However, the racemization for TECHs is almost impossible because the helical configuration is covalently locked by the entangling tethers.159,195–207 Therefore, the TECH molecules will never lose their chiroptical properties unless the entangling tethers are cleaved. After heating all P- and M-TECHs in toluene at 100 °C for 24 h, we found that the CD spectra did not show any decay of signals for all compounds, thus demonstrating the excellent configurational and chiroptical stability of TECHs (Fig. S184†).
Conclusions
In summary, we have introduced a new TECH concept for helical polyaromatic molecules. Different from traditional helicenes and twistacenes, the helical structures of TECHs are no longer decided by the steric effect but by the chiral strain induced from the entangling tethers. TECHs can be directly constructed in enantiopure form via modular synthesis to have unique 3D features. Moreover, the covalent locking of helical configuration by tethers renders them stable and persistent chiral properties. Guided by this concept, we successfully prepared the concave-type and convex-type oligomeric TECHs. The crystal structures of these molecules not only proved the validity and feasibility of the TECH principle, but also unveiled an interesting double-helix π-dimerization behavior of the concave-type TECHs. Prominent and persistent chiroptical properties were observed for all TECH molecules. The (P)-T4-tBu demonstrated the highest |glum| of 1.0 × 10−2 and an impressive CPL brightness of 316 M−1 cm−1. It is expected that by further modifying the chiral tethering units and docking units, more TECH molecules with diverse 3D helical geometries (e.g. different lengths, diameters, and pitches) can be created. With novel and tunable helical architectures and robust chiral properties, TECHs may find interesting applications in the future. Efforts on the synthesis of polymeric TECHs and the exploration of applications are currently under way.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
K. J. and Z. X. conceptualized the work. K. J. and H. X. synthesized the compounds. K. J. performed the characterization and property tests of the compounds. K. J. and Z. X. conducted data analysis. X. S. performed theoretical calculations. J. W., X. C., Z. W., Z. Z., K. Y. and Y. D. directed the experiments and discussed the results. Z. X. and L. D. directed the project and wrote the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors thank the National Key Research and Development Program of China (2022YFB3803300, 2023YFE0116800), Beijing Natural Science Foundation (IS23037) and the Strategic Priority Research Program of Chinese Academy of Sciences (XDB36000000) for financial support. This work is supported by High Performance Computing Center of NCNST, China.References
- Y. Shen and C. F. Chen, Helicenes: synthesis and applications, Chem. Rev., 2012, 112, 1463–1535 Search PubMed.
- D. W. Zhang, M. Li and C. F. Chen, Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes, Chem. Soc. Rev., 2020, 49, 1331–1343 Search PubMed.
- H. V. Anderson, N. D. Gois and W. A. Chalifoux, New advances in chiral nanographene chemistry, Org. Chem. Front., 2023, 10, 4167–4197 Search PubMed.
- Y. Zhu and J. Wang, Helical Synthetic Nanographenes with Atomic Precision, Acc. Chem. Res., 2023, 56, 363–373 Search PubMed.
- Y. Yang, R. C. da Costa, M. J. Fuchter and A. J. Campbell, Circularly polarized light detection by a chiral organic semiconductor transistor, Nat. Photonics, 2013, 7, 634–638 Search PubMed.
- J. Schummer, Molecular Aesthetics: Blind Alleys and Fertile Soils, In Molecular Aesthetics, ed. P. Weibel and Lj. Fruk, MIT Press, Cambridge, MA, 2013, pp. 155–161 Search PubMed.
- L. Zhang, I. Song, J. Ahn, M. Han, M. Linares, M. Surin, H. J. Zhang, J. H. Oh and J. Lin, π-Extended perylene diimide double-heterohelicenes as ambipolar organic semiconductors for broadband circularly polarized light detection, Nat. Commun., 2021, 12, 142 Search PubMed.
- D. Zhu, W. Jiang, Z. Ma, J. Feng, X. Zhan, C. Lu, J. Liu, J. Liu, Y. Hu, D. Wang, Y. S. Zhao, J. Wang, Z. Wang and L. Jiang, Organic donor-acceptor heterojunctions for high performance circularly polarized light detection, Nat. Commun., 2022, 13, 3454 Search PubMed.
- D. Meng, H. Fu, C. Xiao, X. Meng, T. Winands, W. Ma, W. Wei, B. Fan, L. Huo, N. L. Doltsinis, Y. Li, Y. Sun and Z. Wang, Three-Bladed Rylene Propellers with Three-Dimensional Network Assembly for Organic Electronics, J. Am. Chem. Soc., 2016, 138, 10184–10190 Search PubMed.
- S. Rasool, J. Yeop, H. W. Cho, W. Lee, J. W. Kim, D. Yuk and J. Y. Kim, Path to the fabrication of efficient, stable and commercially viable large-area organic solar cells, Mater. Futures, 2023, 2, 032102 Search PubMed.
- C. Gedeon, N. Del Rio, F. Furlan, A. Taddeucci, N. Vanthuyne, V. G. Gregoriou, M. J. Fuchter, G. Siligardi, N. Gasparini, J. Crassous and C. L. Chochos, Rational Design of New Conjugated Polymers with Main Chain Chirality for Efficient Optoelectronic Devices: Carbo[6]Helicene and Indacenodithiophene Copolymers as Model Compounds, Adv. Mater., 2024, 36, e2314337 Search PubMed.
- V. Kiran, S. P. Mathew, S. R. Cohen, I. Hernández Delgado, J. Lacour and R. Naaman, Helicenes—A New Class of Organic Spin Filter, Adv. Mater., 2016, 28, 1957–1962 Search PubMed.
- M. Rickhaus, M. Mayor and M. Juríček, Strain-induced helical chirality in polyaromatic systems, Chem. Soc. Rev., 2016, 45, 1542–1556 Search PubMed.
- R. Rodríguez, C. Naranjo, A. Kumar, P. Matozzo, T. K. Das, Q. Zhu, N. Vanthuyne, R. Gomez, R. Naaman, L. Sánchez and J. Crassous, Mutual Monomer Orientation To Bias the Supramolecular Polymerization of [6]Helicenes and the Resulting Circularly Polarized Light and Spin Filtering Properties, J. Am. Chem. Soc., 2022, 144, 7709–7719 Search PubMed.
- Q. Yan, S. Tao, R. Liu, Y. Zhi and D. Jiang, Crystalline, Porous Helicene Covalent Organic Frameworks, Angew Chem. Int. Ed. Engl., 2024, 63, e202316092 Search PubMed.
- W. C. Guo, W. L. Zhao, K. K. Tan, M. Li and C. F. Chen, B,N-Embedded Hetero[9]helicene Toward Highly Efficient Circularly Polarized Electroluminescence, Angew Chem. Int. Ed. Engl., 2024, 63, e202401835 Search PubMed.
- J. Wade, F. Salerno, R. C. Kilbride, D. K. Kim, J. A. Schmidt, J. A. Smith, L. M. LeBlanc, E. H. Wolpert, A. A. Adeleke, E. R. Johnson, J. Nelson, T. Mori, K. E. Jelfs, S. Heutz and M. J. Fuchter, Controlling anisotropic properties by manipulating the orientation of chiral small molecules, Nat. Chem., 2022, 14, 1383–1389 Search PubMed.
- C. F. Chen and Y. Shen, In Helicene Chemistry: From Synthesis to Applications, Springer, Berlin, Heidelberg, 2017, pp. 1–272 Search PubMed.
- K. K. Wang, Twisted Arenes, Top. Curr. Chem., 2012, 349, 31–61 Search PubMed.
- W. H. Laarhoven and W. J. C. Prinsen, Carbohelicenes and heterohelicenes, Top. Curr. Chem., 1984, 125, 63–130 Search PubMed.
- K. P. Meurer and F. Vögtle, Helical Molecules in Organic Chemistry, Top. Curr. Chem., 1985, 127, 1–76 Search PubMed.
- R. Weitzenböck and A. Klingler, A. Synthese der isomeren Kohlenwasserstoffe 1, 2–5, 6-Dibenzanthracen und 3, 4–5, 6-Dibenzphenanthren, Monatsh. Chem., 1918, 39, 315–323 Search PubMed.
- M. S. Newman and D. Lednicer, The Synthesis and Resolution of Hexahelicene, J. Am. Chem. Soc., 1956, 78, 4765–4770 Search PubMed.
- M. Flammand-Barbieux, J. Nasielski and R. H. Martin, Synthesis of heptahelicene (1) benzo [c] phenanthro [4,3-g]phenanthrene, Tetrahedron Lett., 1967, 7, 743–744 Search PubMed.
- M. Gingras, One hundred years of helicene chemistry. Part 1: non-stereoselective syntheses of carbohelicenes, Chem. Soc. Rev., 2013, 42, 968–1006 Search PubMed.
- M. Gingras, G. Felix and R. Peresutti, One hundred years of helicene chemistry. Part 2: stereoselective syntheses and chiral separations of carbohelicenes, Chem. Soc. Rev., 2013, 42, 1007–1050 Search PubMed.
- M. Gingras, One hundred years of helicene chemistry. Part 3: applications and properties of carbohelicenes, Chem. Soc. Rev., 2013, 42, 1051–1095 Search PubMed.
- L. J. Peng, X. Y. Wang, Z. A. Li and H. Y. Gong, All Carbon Helicenes and π-Extended Helicene Derivatives, Asian J. Org. Chem., 2023, 12, e202300543 Search PubMed.
- K. Dhbaibi, L. Favereau and J. Crassous, Enantioenriched Helicenes and Helicenoids Containing Main-Group Elements (B, Si, N, P), Chem. Rev., 2019, 119, 8846–8953 Search PubMed.
- W. W. Yang and J. J. Shen, Multiple Heterohelicenes: Synthesis, Properties and Applications, Chem.–Eur. J., 2022, 28, e202202069 Search PubMed.
- A. Rajca, M. Miyasaka, M. Pink, H. Wang and S. Rajca, Helically Annelated and Cross-Conjugated Oligothiophenes: Asymmetric Synthesis, Resolution, and Characterization of a Carbon-Sulfur [7]Helicene, J. Am. Chem. Soc., 2004, 126, 15211–15222 Search PubMed.
- K. Yavari, S. Moussa, B. Ben Hassine, P. Retailleau, A. Voituriez and A. Marinetti, 1H-phosphindoles as structural units in the synthesis of chiral helicenes, Angew Chem. Int. Ed. Engl., 2012, 51, 6748–6752 Search PubMed.
- K. Nakano, H. Oyama, Y. Nishimura, S. Nakasako and K. Nozaki, λ5-Phospha[7]helicenes: synthesis, properties, and columnar aggregation with one-way chirality, Angew Chem. Int. Ed. Engl., 2012, 51, 695–699 Search PubMed.
- T. Shibata, T. Uchiyama, Y. Yoshinami, S. Takayasu, K. Tsuchikama and K. Endo, Highly enantioselective synthesis of silahelicenes using Ir-catalyzed [2+2+2] cycloaddition, Chem. Commun., 2012, 48, 1311–1313 Search PubMed.
- H. Oyama, K. Nakano, T. Harada, R. Kuroda, M. Naito, K. Nobusawa and K. Nozaki, Facile Synthetic Route to Highly Luminescent Sila[7]helicene, Org. Lett., 2013, 15, 2104–2107 Search PubMed.
- S. Oda, B. Kawakami, Y. Yamasaki, R. Matsumoto, M. Yoshioka, D. Fukushima, S. Nakatsuka and T. Hatakeyama, One-Shot Synthesis of Expanded Heterohelicene Exhibiting Narrowband Thermally Activated Delayed Fluorescence, J. Am. Chem. Soc., 2022, 144, 106–112 Search PubMed.
- J. K. Li, X. Y. Chen, Y. L. Guo, X. C. Wang, A. C. Sue, X. Y. Cao and X. Y. Wang, B,N-Embedded Double Hetero[7]helicenes with Strong Chiroptical Responses in the Visible Light Region, J. Am. Chem. Soc., 2021, 143, 17958–17963 Search PubMed.
- C. Qu, Y. Xu, Y. Wang, Y. Nie, K. Ye, H. Zhang and Z. Zhang, Bridging of Cove Regions: A Strategy for Realizing Persistently Chiral Double Heterohelicenes with Attractive Luminescent Properties, Angew Chem. Int. Ed. Engl., 2024, 63, e202400661 Search PubMed.
- Z. Sun, W. Xu, S. Qiu, Z. Ma, C. Li, S. Zhang and H. Wang, Thia[n]helicenes with long persistent phosphorescence, Chem. Sci., 2024, 15, 1077–1087 Search PubMed.
- N. Saleh, C. Shen and J. Crassous, Helicene-based transition metal complexes: synthesis, properties and applications, Chem. Sci., 2014, 5, 3680–3694 Search PubMed.
- E. S. Gauthier, R. Rodríguez and J. Crassous, Metal-Based Multihelicenic Architectures, Angew Chem. Int. Ed. Engl., 2020, 59, 22840–22856 Search PubMed.
- L. Norel, M. Rudolph, N. Vanthuyne, J. A. Williams, C. Lescop, C. Roussel, J. Autschbach, J. Crassous and R. Reau, Metallahelicenes: easily accessible helicene derivatives with large and tunable chiroptical properties, Angew Chem. Int. Ed. Engl., 2010, 49, 99–102 Search PubMed.
- S. Graule, M. Rudolph, W. Shen, J. A. Williams, C. Lescop, J. Autschbach, J. Crassous and R. Reau, Assembly of π-conjugated phosphole azahelicene derivatives into chiral coordination complexes: an experimental and theoretical study, Chem.–Eur. J., 2010, 16, 5976–6005 Search PubMed.
- V. Vreshch, M. El Sayed Moussa, B. Nohra, M. Srebro, N. Vanthuyne, C. Roussel, J. Autschbach, J. Crassous, C. Lescop and R. Reau, Assembly of helicene-capped N,P,N,P,N-helicands within Cu(I) helicates: impacting chiroptical properties by ligand-ligand charge transfer, Angew Chem. Int. Ed. Engl., 2013, 52, 1968–1972 Search PubMed.
- J. R. Brandt, X. Wang, Y. Yang, A. J. Campbell and M. J. Fuchter, Circularly Polarized Phosphorescent Electroluminescence with a High Dissymmetry Factor from PHOLEDs Based on a Platinahelicene, J. Am. Chem. Soc., 2016, 138, 9743–9746 Search PubMed.
- W.-B. Lin, M. Li, L. Fang and C.-F. Chen, Recent progress on multidimensional construction of helicenes, Chin. Chem. Lett., 2018, 29, 40–46 Search PubMed.
- K. Kato, Y. Segawa and K. Itami, Symmetric Multiple Carbohelicenes, Synlett, 2018, 30, 370–377 Search PubMed.
- Y.-F. Wu, L. Zhang, Q. Zhang, S.-Y. Xie and L.-S. Zheng, Multiple [n]helicenes with various aromatic cores, Org. Chem. Front., 2022, 9, 4726–4743 Search PubMed.
- T. Mori, Chiroptical Properties of Symmetric Double, Triple, and Multiple Helicenes, Chem. Rev., 2021, 121, 2373–2412 Search PubMed.
- T. Fujikawa, Y. Segawa and K. Itami, Synthesis and Structural Features of Quadruple Helicenes: Highly Distorted pi Systems Enabled by Accumulation of Helical Repulsions, J. Am. Chem. Soc., 2016, 138, 3587–3595 Search PubMed.
- T. Hosokawa, Y. Takahashi, T. Matsushima, S. Watanabe, S. Kikkawa, I. Azumaya, A. Tsurusaki and K. Kamikawa, Synthesis, Structures, and Properties of Hexapole Helicenes: Assembling Six [5]Helicene Substructures into Highly Twisted Aromatic Systems, J. Am. Chem. Soc., 2017, 139, 18512–18521 Search PubMed.
- B. Liu, M. Bockmann, W. Jiang, N. L. Doltsinis and Z. Wang, Perylene Diimide-Embedded Double [8]Helicenes, J. Am. Chem. Soc., 2020, 142, 7092–7099 Search PubMed.
- Y. Chen, C. Lin, Z. Luo, Z. Yin, H. Shi, Y. Zhu and J. Wang, Double π-Extended Undecabenzo[7]helicene, Angew Chem. Int. Ed. Engl., 2021, 60, 7796–7801 Search PubMed.
- W. Niu, Y. Fu, Q. Deng, Z. L. Qiu, F. Liu, A. A. Popov, H. Komber, J. Ma and X. Feng, Enhancing Chiroptical Responses in Helical Nanographenes via Geometric Engineering of Double [7]Helicenes, Angew Chem. Int. Ed. Engl., 2024, 63, e202319874 Search PubMed.
- P. J. Evans, J. Ouyang, L. Favereau, J. Crassous, I. Fernández, J. Perles and N. Martin, Synthesis of a Helical Bilayer Nanographene, Angew Chem. Int. Ed. Engl., 2018, 57, 6774–6779 Search PubMed.
- Y. Nakakuki, T. Hirose, H. Sotome, H. Miyasaka and K. Matsuda, Hexa- peri-hexabenzo[7]helicene: Homogeneously π-Extended Helicene as a Primary Substructure of Helically Twisted Chiral Graphenes, J. Am. Chem. Soc., 2018, 140, 4317–4326 Search PubMed.
- J. Ma, Y. Fu, E. Dmitrieva, F. Liu, H. Komber, F. Hennersdorf, A. A. Popov, J. J. Weigand, J. Liu and X. Feng, Helical Nanographenes Containing an Azulene Unit: Synthesis, Crystal Structures, and Properties, Angew Chem. Int. Ed. Engl., 2020, 59, 5637–5642 Search PubMed.
- X. Xiao, S. K. Pedersen, D. Aranda, J. Yang, R. A. Wiscons, M. Pittelkow, M. L. Steigerwald, F. Santoro, N. J. Schuster and C. Nuckolls, Chirality Amplified: Long, Discrete Helicene Nanoribbons, J. Am. Chem. Soc., 2021, 143, 983–991 Search PubMed.
- Y. Nakakuki, T. Hirose, H. Sotome, M. Gao, D. Shimizu, R. Li, J. Y. Hasegawa, H. Miyasaka and K. Matsuda, Doubly linked chiral phenanthrene oligomers for homogeneously π-extended helicenes with large effective conjugation length, Nat. Commun., 2022, 13, 1475 Search PubMed.
- G. R. Kiel, H. M. Bergman, A. E. Samkian, N. J. Schuster, R. C. Handford, A. J. Rothenberger, R. Gomez-Bombarelli, C. Nuckolls and T. D. Tilley, Expanded [23]-Helicene with Exceptional Chiroptical Properties via an Iterative Ring-Fusion Strategy, J. Am. Chem. Soc., 2022, 144, 23421–23427 Search PubMed.
- J. K. Li, X. Y. Chen, W. L. Zhao, Y. L. Guo, Y. Zhang, X. C. Wang, A. C. Sue, X. Y. Cao, M. Li, C. F. Chen and X. Y. Wang, Synthesis of Highly Luminescent Chiral Nanographene, Angew Chem. Int. Ed. Engl., 2023, 62, e202215367 Search PubMed.
- X. Tian, K. Shoyama, B. Mahlmeister, F. Brust, M. Stolte and F. Würthner, Naphthalimide-Annulated [n]Helicenes: Red Circularly Polarized Light Emitters, J. Am. Chem. Soc., 2023, 145, 9886–9894 Search PubMed.
- G. F. Huo, T. M. Fukunaga, X. Hou, Y. Han, W. Fan, S. Wu, H. Isobe and J. Wu, Facile Synthesis and Chiral Resolution of Expanded Helicenes with up to 35 cata-Fused Benzene Rings, Angew Chem. Int. Ed. Engl., 2023, 62, e202218090 Search PubMed.
- F. Morita, Y. Kishida, Y. Sato, H. Sugiyama, M. Abekura, J. Nogami, N. Toriumi, Y. Nagashima, T. Kinoshita, G. Fukuhara, M. Uchiyama, H. Uekusa and K. Tanaka, Design and enantioselective synthesis of 3D π-extended carbohelicenes for circularly polarized luminescence, Nat. Synth., 2024, 3, 774–786 Search PubMed.
- R. A. Pascal, Twisted acenes, Chem. Rev., 2006, 106, 4809–4819 Search PubMed.
- R. A. Pascal, W. D. McMillan, D. Van Engen and R. G. Eason, Synthesis and structure of longitudinally twisted polycyclic aromatic hydrocarbons, J. Am. Chem. Soc., 1987, 109, 4660–4665 Search PubMed.
- N. Smyth, D. Van Engen and R. A. Pascal, Synthesis of longitudinally twisted polycyclic aromatic hydrocarbons via a highly substituted aryne, J. Org. Chem., 1990, 55, 1937–1940 Search PubMed.
- X. Qiao, D. M. Ho and R. A. Pascal, An Extraordinarily Twisted Polycyclic Aromatic Hydrocarbon, Angew Chem. Int. Ed. Engl., 2003, 36, 1531–1532 Search PubMed.
- J. Lu, D. M. Ho, N. J. Vogelaar, C. M. Kraml and R. A. Pascal, A Pentacene with a 144° Twist, J. Am. Chem. Soc., 2004, 126, 11168–11169 Search PubMed.
- J. Lu, D. M. Ho, N. J. Vogelaar, C. M. Kraml, S. Bernhard, N. Byrne, L. R. Kim and R. A. Pascal, Synthesis, Structure, and Resolution of Exceptionally Twisted Pentacenes, J. Am. Chem. Soc., 2006, 128, 17043–17050 Search PubMed.
- R. S. Walters, C. M. Kraml, N. Ho, D. M. Byrne, Q. Qin, F. J. Coughlin, S. Bernhard and R. A. Pascal, Configurationally Stable Longitudinally Twisted Polycyclic Aromatic Compounds, J. Am. Chem. Soc., 2008, 130, 16435–16441 Search PubMed.
- J. Xiao, H. M. Duong, Y. Liu, W. Shi, L. Ji, G. Li, S. Li, X. W. Liu, J. Ma, F. Wudl and Q. Zhang, Synthesis and structure characterization of a stable nonatwistacene, Angew Chem. Int. Ed. Engl., 2012, 51, 6094–6098 Search PubMed.
- Y. Zhong, B. Kumar, S. Oh, M. T. Trinh, Y. Wu, K. Elbert, P. Li, X. Zhu, S. Xiao, F. Ng, M. L. Steigerwald and C. Nuckolls, Helical ribbons for molecular electronics, J. Am. Chem. Soc., 2014, 136, 8122–8130 Search PubMed.
- W. Fan, T. Winands, N. L. Doltsinis, Y. Li and Z. Wang, A Decatwistacene with an Overall 170 degrees Torsion, Angew Chem. Int. Ed. Engl., 2017, 56, 15373–15377 Search PubMed.
- W. Chen, X. Li, G. Long, Y. Li, R. Ganguly, M. Zhang, N. Aratani, H. Yamada, M. Liu and Q. Zhang, Pyrene-Containing Twistarene: Twelve Benzene Rings Fused in a Row, Angew Chem. Int. Ed. Engl., 2018, 57, 13555–13559 Search PubMed.
- G. Liu, C. Xiao, F. Negri, Y. Li and Z. Wang, Dodecatwistarene Imides with Zigzag-Twisted Conformation for Organic Electronics, Angew Chem. Int. Ed. Engl., 2020, 59, 2008–2012 Search PubMed.
- S. Castro-Fernández, C. M. Cruz, I. F. A. Mariz, I. R. Márquez, V. G. Jimenez, L. Palomino-Ruiz, J. M. Cuerva, E. Maçôas and A. G. Campaña, Two-Photon Absorption Enhancement by the Inclusion of a Tropone Ring in Distorted Nanographene Ribbons, Angew Chem. Int. Ed. Engl., 2020, 59, 7139–7145 Search PubMed.
- S. Ma, J. Gu, C. Lin, Z. Luo, Y. Zhu and J. Wang, Supertwistacene: A Helical Graphene Nanoribbon, J. Am. Chem. Soc., 2020, 142, 16887–16893 Search PubMed.
- X. Yao, W. Zheng, S. Osella, Z. Qiu, S. Fu, D. Schollmeyer, B. Muller, D. Beljonne, M. Bonn, H. I. Wang, K. Müller and A. Narita, Synthesis of Nonplanar Graphene Nanoribbon with Fjord Edges, J. Am. Chem. Soc., 2021, 143, 5654–5658 Search PubMed.
- P. Izquierdo-García, J. M. Fernández-García, I. Fernández, J. Perles and N. Martín, Helically Arranged Chiral Molecular Nanographenes, J. Am. Chem. Soc., 2021, 143, 11864–11870 Search PubMed.
- S. T. Bao, H. Jiang, C. Schaack, S. Louie, M. L. Steigerwald, C. Nuckolls and Z. Jin, Remote Control of Dynamic Twistacene Chirality, J. Am. Chem. Soc., 2022, 144, 18772–18777 Search PubMed.
- R. K. Dubey, M. Melle-Franco and A. Mateo-Alonso, Inducing Single-Handed Helicity in a Twisted Molecular Nanoribbon, J. Am. Chem. Soc., 2022, 144, 2765–2774 Search PubMed.
- Z. Sun, W. Fan, Y. Han, W. Yuan, Y. Ni, J. Wang, H. Wei, Y. Zhao, Z. Sun and J. Wu, Helical fused 1,2:8,9-dibenzozethrene oligomers with up to 201 degrees end-to-end twist: "one-pot" synthesis and chiral resolution, Chem. Sci., 2023, 14, 7922–7927 Search PubMed.
- W. Yang, G. Longhi, S. Abbate, A. Lucotti, M. Tommasini, C. Villani, V. J. Catalano, A. O. Lykhin, S. A. Varganov and W. A. Chalifoux, Chiral Peropyrene: Synthesis, Structure, and Properties, J. Am. Chem. Soc., 2017, 139, 13102–13109 Search PubMed.
- A. Link and C. Sparr, Stereoselective arene formation, Chem. Soc. Rev., 2018, 47, 3804–3815 Search PubMed.
- W. Liu, T. Qin, W. Xie and X. Yang, Catalytic Enantioselective Synthesis of Helicenes, Chem.–Eur. J., 2022, 28, e202202369 Search PubMed.
- T. Hartung, R. Machleid, M. Simon, C. Golz and M. Alcarazo, Enantioselective Synthesis of 1,12-Disubstituted [4]Helicenes, Angew Chem. Int. Ed. Engl., 2020, 59, 5660–5664 Search PubMed.
- F. Wang, F. Gan, C. Shen and H. Qiu, Amplifiable Symmetry Breaking in Aggregates of Vibrating Helical Molecules, J. Am. Chem. Soc., 2020, 142, 16167–16172 Search PubMed.
- G. Li, T. Matsuno, Y. Han, S. Wu, Y. Zou, Q. Jiang, H. Isobe and J. Wu, Fused Quinoidal Dithiophene-Based Helicenes: Synthesis by Intramolecular Radical-Radical Coupling Reactions and Dynamics of Interconversion of Enantiomers, Angew Chem. Int. Ed. Engl., 2021, 60, 10326–10333 Search PubMed.
- M. S. H. Salem, A. Sabri, M. I. Khalid, H. Sasai and S. Takizawa, Two-Step Synthesis, Structure, and Optical Features of a Double Hetero[7]helicene, Molecules, 2022, 27, 9068 Search PubMed.
- T. Ikai, K. Oki, S. Yamakawa and E. Yashima, Stereospecific Synthesis of Enantiopure [6]Helicene Containing a Seven-Membered Ring and [7]Helicene by Acid-Promoted Stepwise Alkyne Annulations of Doubly Axial-Chiral Precursors, Angew Chem. Int. Ed. Engl., 2023, 62, e202301836 Search PubMed.
- V. M. Freixas, N. Oldani, S. Tretiak and S. Fernandez-Alberti, Twisting Aromaticity and Photoinduced Dynamics in Hexapole Helicenes, J. Phys. Chem. Lett., 2023, 14, 10145–10150 Search PubMed.
- S. M. Guo, S. Huh, M. Coehlo, L. Shen, G. Pieters and O. Baudoin, A C-H activation-based enantioselective synthesis of lower carbo[n]helicenes, Nat. Chem., 2023, 15, 872–880 Search PubMed.
- W. L. Xu, R. X. Zhang, H. Wang, J. Chen and L. Zhou, Helicoselective Synthesis of Indolohelicenoids through Organocatalytic Central-to-Helical Chirality Conversion, Angew Chem. Int. Ed. Engl., 2024, 63, e202318021 Search PubMed.
- F. Gan, G. Zhang, J. Liang, C. Shen and H. Qiu, π-Extended Diaza[7]helicenes with Dual Negatively Curved Heptagons: Extensive Synthesis and Spontaneous Resolution into Strippable Homochiral Lamellae with Helical Symmetry, Angew Chem. Int. Ed. Engl., 2024, 63, e202320076 Search PubMed.
- P. Jiang, A. S. Mikherdov, H. Ito and M. Jin, Crystallization-Induced Chirality Transfer in Conformationally Flexible Azahelicene Au(I) Complexes with Circularly Polarized Luminescence Activation, J. Am. Chem. Soc., 2024, 146, 12463–12472 Search PubMed.
- J. S. Wu, S. W. Cheng, Y. J. Cheng and C. S. Hsu, Donor-acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells, Chem. Soc. Rev., 2015, 44, 1113–1154 Search PubMed.
- A. Fukazawa and S. Yamaguchi, Ladder π-conjugated materials containing main-group elements, Chem.–Asian J., 2009, 4, 1386–1400 Search PubMed.
- J. Chen, K. Yang, X. Zhou and X. Guo, Ladder-Type Heteroarene-Based Organic Semiconductors, Chem.–Asian J., 2018, 13, 2587–2600 Search PubMed.
- C. Zhu, A. J. Kalin and L. Fang, Covalent and Noncovalent Approaches to Rigid Coplanar π-Conjugated Molecules and Macromolecules, Acc. Chem. Res., 2019, 52, 1089–1100 Search PubMed.
- Z. Xiao, S. Yang, Z. Yang, J. Yang, H. L. Yip, F. Zhang, F. He, T. Wang, J. Wang, Y. Yuan, H. Yang, M. Wang and L. Ding, Carbon-Oxygen-Bridged Ladder-Type Building Blocks for Highly Efficient Nonfullerene Acceptors, Adv. Mater., 2019, 31, e1804790 Search PubMed.
- S. Che and L. Fang, Porous Ladder Polymer Networks, Chem, 2020, 6, 2558–2590 Search PubMed.
- J. Lee, Recent Progress in Synthesis of Conjugated Ladder Polymers, Asian J. Org. Chem., 2023, 12, e202300104 Search PubMed.
- E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Circularly Polarized Luminescence from Simple Organic Molecules, Chem.–Eur. J., 2015, 21, 13488–13500 Search PubMed.
- Z.-L. Gong, X. Zhu, Z. Zhou, S.-W. Zhang, D. Yang, B. Zhao, Y.-P. Zhang, J. Deng, Y. Cheng, Y.-X. Zheng, S.-Q. Zang, H. Kuang, P. Duan, M. Yuan, C.-F. Chen, Y. S. Zhao, Y.-W. Zhong, B. Z. Tang and M. Liu, Frontiers in circularly polarized luminescence: molecular design, self-assembly, nanomaterials, and applications, Sci. China: Chem., 2021, 64, 2060–2104 Search PubMed.
- X. Yang, X. Gao, Y.-X. Zheng, H. Kuang, C.-F. Chen, M. Liu, P. Duan and Z. Tang, Recent Progress of Circularly Polarized Luminescence Materials from Chinese Perspectives, CCS Chem., 2023, 5, 2760–2789 Search PubMed.
- Y. Wang, Y. Yu, H. Liao, Y. Zhou, I. McCulloch and W. Yue, The Chemistry and Applications of Heteroisoindigo Units as Enabling Links for Semiconducting Materials, Acc. Chem. Res., 2020, 53, 2855–2868 Search PubMed.
- Y. Zhou, W. Zhang and G. Yu, Recent structural evolution of lactam- and imide-functionalized polymers applied in organic field-effect transistors and organic solar cells, Chem. Sci., 2021, 12, 6844–6878 Search PubMed.
- J. Cao, Q. Liao, X. Du, J. Chen, Z. Xiao, Q. Zuo and L. Ding, A pentacyclic aromatic lactam building block for efficient polymer solar cells, Energy Environ. Sci., 2013, 6, 3224–3228 Search PubMed.
- J. Cao, C. Zuo, B. Du, X. Qiu and L. Ding, Hexacyclic lactam building blocks for highly efficient polymer solar cells, Chem. Commun., 2015, 51, 12122–12125 Search PubMed.
- W. Hu, Z. Zhang, Y. Xu, J. Cao, S. Liu, Z. Tang, B. Zhao and J. Zhang, An axisymmetric heptacyclic lactam unit for efficient polymer solar cells, J. Mater. Chem. C, 2018, 6, 6911–6915 Search PubMed.
- A. Onwubiko, W. Yue, C. Jellett, M. Xiao, H. Y. Chen, M. K. Ravva, D. A. Hanifi, A. C. Knall, B. Purushothaman, M. Nikolka, J. C. Flores, A. Salleo, J. L. Bredas, H. Sirringhaus, P. Hayoz and I. McCulloch, Fused electron deficient semiconducting polymers for air stable electron transport, Nat. Commun., 2018, 9, 416 Search PubMed.
- Y. Lu, Z. D. Yu, R. Z. Zhang, Z. F. Yao, H. Y. You, L. Jiang, H. I. Un, B. W. Dong, M. Xiong, J. Y. Wang and J. Pei, Rigid Coplanar Polymers for Stable n-Type Polymer Thermoelectrics, Angew Chem. Int. Ed. Engl., 2019, 58, 11390–11394 Search PubMed.
- N. Blouin, A. Michaud, S. Wakim, P. L. T. Boudreault, M. Leclerc, B. Vercelli, S. Zecchin and G. Zotti, Optical, Electrochemical, Magnetic, and Conductive Properties of New Polyindolocarbazoles and Polydiindolocarbazoles, Macromol. Chem. Phys., 2006, 207, 166–174 Search PubMed.
- T. Janosik, N. Wahlström and J. Bergman, Recent progress in the chemistry and applications of indolocarbazoles, Tetrahedron, 2008, 64, 9159–9180 Search PubMed.
- J. Li and A. C. Grimsdale, Carbazole-based polymers for organic photovoltaic devices, Chem. Soc. Rev., 2010, 39, 2399–2410 Search PubMed.
- T. Janosik, A. Rannug, U. Rannug, N. Wahlström, J. Slätt and J. Bergman, Chemistry and Properties of Indolocarbazoles, Chem. Rev., 2018, 118, 9058–9128 Search PubMed.
- H. Jiang, S. Zhu, Z. Cui, Z. Li, Y. Liang, J. Zhu, P. Hu, H. L. Zhang and W. Hu, High-performance five-ring-fused organic semiconductors for field-effect transistors, Chem. Soc. Rev., 2022, 51, 3071–3122 Search PubMed.
- H. Wang, H. Zhao, S. Chen, L. Bai, Z. Su and Y. Wu, Effective Synthesis of Ladder-type Oligo(p-aniline)s and Poly(p-aniline)s via Intramolecular SNAr Reaction, Org. Lett., 2021, 23, 2217–2221 Search PubMed.
- T. Ikai, T. Yoshida, K. Shinohara, T. Taniguchi, Y. Wada and T. M. Swager, Triptycene-Based Ladder Polymers with One-Handed Helical Geometry, J. Am. Chem. Soc., 2019, 141, 4696–4703 Search PubMed.
- W. Zheng, T. Ikai and E. Yashima, Synthesis of Single-Handed Helical Spiro-Conjugated Ladder Polymers through Quantitative and Chemoselective Cyclizations, Angew. Chem., Int. Ed., 2021, 60, 11294–11299 Search PubMed.
- T. Ikai, S. Miyoshi, K. Oki, R. Saha, Y. Hijikata and E. Yashima, Defect-Free Synthesis of a Fully π-Conjugated Helical Ladder Polymer and Resolution into a Pair of Enantiomeric Helical Ladders, Angew. Chem., Int. Ed., 2023, 62, e202301962 Search PubMed.
- T. Iwasaki, Y. Kohinata and H. Nishide, Poly(thiaheterohelicene): A Stiff Conjugated Helical Polymer Comprised of Fused Benzothiophene Rings, Org. Lett., 2005, 7, 755–758 Search PubMed.
- K. Takaishi, S. Hinoide, T. Matsumoto and T. Ema, Axially Chiral peri-Xanthenoxanthenes as a Circularly Polarized Luminophore, J. Am. Chem. Soc., 2019, 141, 11852–11857 Search PubMed.
- T. Ikai, N. Mishima, T. Matsumoto, S. Miyoshi, K. Oki and E. Yashima, 2,2’-Tethered Binaphthyl-Embedded One-Handed Helical Ladder Polymers: Impact of the Tether Length on Helical Geometry and Chiroptical Property, Angew. Chem., Int. Ed., 2024, 63, e202318712 Search PubMed.
- H.-C. Zhang and L. Pu, Synthesis of Helical Polybinaphthyls, Macromolecules, 2004, 37, 2695–2702 Search PubMed.
- R. Ammenhäuser, J. M. Lupton and U. Scherf, Chain-Length Dependence of the Optical Activity of Helical Triptycene-Based π-Conjugated Ladder Polymers, Adv. Opt. Mater., 2024, 12, 2301857 Search PubMed.
- R. Fiesel, J. Huber and U. Scherf, Synthesis of an Optically Active Poly(para-phenylene) Ladder Polymer, Angew. Chem., Int. Ed., 1996, 35, 2111–2113 Search PubMed.
- W. Zheng, K. Oki, R. Saha, Y. Hijikata, E. Yashima and T. Ikai, One-Handed Helical Tubular Ladder Polymers for Chromatographic Enantioseparation, Angew. Chem., Int. Ed., 2023, 62, e202218297 Search PubMed.
- R. Ammenhäuser, P. Klein, E. Schmid, S. Streicher, J. Vogelsang, C. W. Lehmann, J. M. Lupton, S. C. J. Meskers and U. Scherf, Circularly Polarized Light Probes Excited-State Delocalization in Rectangular Ladder-type Pentaphenyl Helices, Angew. Chem., Int. Ed., 2023, 62, e202211946 Search PubMed.
- X. Xiao, Q. Cheng, S. Bao, Z. Jin, S. Sun, H. Jiang, M. L. Steigerwald and C. Nuckolls, Single-Handed Helicene Nanoribbons via Transfer of Chiral Information, J. Am. Chem. Soc., 2022, 144, 20214–20220 Search PubMed.
- A. C. Grimsdale and K. Müllen, Oligomers and Polymers Based on Bridged Phenylenes as Electronic Materials, Macromol. Rapid Commun., 2007, 28, 1676–1702 Search PubMed.
- J. Lee, B. B. Rajeeva, T. Yuan, Z. H. Guo, Y. H. Lin, M. Al-Hashimi, Y. Zheng and L. Fang, Thermodynamic synthesis of solution processable ladder polymers, Chem. Sci., 2016, 7, 881–889 Search PubMed.
- J. Lee, A. J. Kalin, T. Yuan, M. Al-Hashimi and L. Fang, Fully conjugated ladder polymers, Chem. Sci., 2017, 8, 2503–2521 Search PubMed.
- L. Fang, A. Kalin and J. Lee, Annulation Reactions for Conjugated Ladder-Type Oligomers, Synlett, 2018, 29, 993–998 Search PubMed.
- Z. D. Yu, Y. Lu, J. Y. Wang and J. Pei, Conformation Control of Conjugated Polymers, Chem.–Eur. J., 2020, 26, 16194–16205 Search PubMed.
- Y. Dong, J. Ma, S. Yang and H. Yang, Polyimides and Their Diverse Applications in Multiple Optoelectronic Devices, Adv. Devices Instrum., 2023, 4, 0011 Search PubMed.
- H. Fan, H. Zhao and Y. Wu, Progress in conjugated ladder polymers, Sci. Sin.: Chim., 2021, 51, 1150–1167 Search PubMed.
- J. J. Stewart, Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters, J. Mol. Model., 2013, 19, 1–32 Search PubMed.
- D. Alberico, M. E. Scott and M. Lautens, Aryl–Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation, Chem. Rev., 2007, 107, 174–238 Search PubMed.
- W. Hagui, H. Doucet and J.-F. Soulé, Application of Palladium-Catalyzed C(sp2)–H Bond Arylation to the Synthesis of Polycyclic (Hetero)Aromatics, Chem, 2019, 5, 2006–2078 Search PubMed.
- L.-C. Campeau, M. Parisien, M. Leblanc and K. Fagnou, Biaryl Synthesis via Direct Arylation: Establishment of an Efficient Catalyst for Intramolecular Processes, J. Am. Chem. Soc., 2004, 126, 9186–9187 Search PubMed.
- D. García-Cuadrado, A. A. C. Braga, F. Maseras and A. M. Echavarren, Proton Abstraction Mechanism for the Palladium-Catalyzed Intramolecular Arylation, J. Am. Chem. Soc., 2006, 128, 1066–1067 Search PubMed.
- K. E. Yamada, I. A. Stepek, W. Matsuoka, H. Ito and K. Itami, Synthesis of Heptagon-Containing Polyarenes by Catalytic C-H Activation, Angew Chem. Int. Ed. Engl., 2023, 62, e202311770 Search PubMed.
- H. Xie, Z. Xiao, Y. Song, K. Jin, H. Liu, E. Zhou, J. Cao, J. Chen, J. Ding, C. Yi, X. Shen, C. Zuo and L. Ding, Tethered Helical Ladder-Type Aromatic Lactams, J. Am. Chem. Soc., 2024, 146, 11978–11990 Search PubMed.
- K. Takagi, D. Miyamoto, H. Yamaguchi and I. Azumaya, Toward the Synthesis of a Belt-Shaped Cyclic π-Conjugated System Comprising para-Phenylene Framework and Amide Bridging Unit, Bull. Chem. Soc. Jpn., 2022, 95, 47–51 Search PubMed.
- F. P. Gasparro and N. H. Kolodny, NMR determination of the rotational barrier in N,N-dimethylacetamide. A physical chemistry experiment, J. Chem. Educ., 1977, 54, 258–261 Search PubMed.
- V. Štejfa, S. Chun, V. Pokorný, M. Fulem and K. Růžička, Thermodynamic study of acetamides, J. Mol. Liq., 2020, 319, 114019 Search PubMed.
- P. Kiraly, G. Dal Poggetto, L. Castanar, M. Nilsson, A. Deák and G. A. Morris, Broadband measurement of true transverse relaxation rates in systems with coupled protons: application to the study of conformational exchange, Chem. Sci., 2021, 12, 11538–11547 Search PubMed.
- N. G. Malliaros and M. Orfanopoulos, One-step photocatalyzed functionalization of [60]Fullerene: A study on the direct incorporation of amides and lactams to C60, J. Phys. Chem. Solids, 2023, 178, 111305 Search PubMed.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion, Chem. Rev., 2005, 105, 3842–3888 Search PubMed.
- P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. van Eikema Hommes, Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe, J. Am. Chem. Soc., 1996, 118, 6317–6318 Search PubMed.
- M. Krzeszewski, H. Ito and K. Itami, Infinitene: A Helically Twisted Figure-Eight [12]Circulene Topoisomer, J. Am. Chem. Soc., 2022, 144, 862–871 Search PubMed.
- M. Toya, T. Omine, F. Ishiwari, A. Saeki, H. Ito and K. Itami, Expanded [2,1][n]Carbohelicenes with 15- and 17-Benzene Rings, J. Am. Chem. Soc., 2023, 145, 11553–11565 Search PubMed.
- S. E. Wheeler, K. N. Houk, P. V. R. Schleyer and W. D. Allen, A hierarchy of homodesmotic reactions for thermochemistry, J. Am. Chem. Soc., 2009, 131, 2547–2560 Search PubMed.
- M. Tang, Y. Liang, X. Lu, X. Miao, L. Jiang, J. Liu, L. Bian, S. Wang, L. Wu and Z. Liu, Molecular-strain engineering of double-walled tetrahedra, Chem, 2021, 7, 2160–2174 Search PubMed.
- P. K. Saha, A. Mallick, A. T. Turley, A. N. Bismillah, A. Danos, A. P. Monkman, A.-J. Avestro, D. S. Yufit and P. R. McGonigal, Rupturing aromaticity by periphery overcrowding, Nat. Chem., 2023, 15, 516–525 Search PubMed.
- Y. Tanaka, K. Tajima, R. Kusumoto, Y. Kobori, N. Fukui and H. Shinokubo, End-to-End Bent Perylene Bisimide Cyclophanes by Double Sulfur Extrusion, J. Am. Chem. Soc., 2024, 146, 16332–16339 Search PubMed.
- M. M. Safont-Sempere, P. Osswald, M. Stolte, M. Grune, M. Renz, M. Kaupp, K. Radacki, H. Braunschweig and F. Würthner, Impact of molecular flexibility on binding strength and self-sorting of chiral π-surfaces, J. Am. Chem. Soc., 2011, 133, 9580–9591 Search PubMed.
- B. Mahlmeister, T. Schembri, V. Stepanenko, K. Shoyama, M. Stolte and F. Würthner, Enantiopure J-Aggregate of Quaterrylene Bisimides for Strong Chiroptical NIR-Response, J. Am. Chem. Soc., 2023, 145, 13302–13311 Search PubMed.
- X. Yang, M. Brückner, F. Rominger, T. Kirschbaum and M. Mastalerz, Dispersion-driven formation of chiral twisted PAH double helices, Chem, 2024, 10, 832–842 Search PubMed.
- T. Lu and Q. Chen, Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems, J. Comput. Chem., 2022, 43, 539–555 Search PubMed.
- C. Lefebvre, G. Rubez, H. Khartabil, J. C. Boisson, J. Contreras-García and E. Henon, Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density, Phys. Chem. Chem. Phys., 2017, 19, 17928–17936 Search PubMed.
- T. Lu and F. Chen, Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 Search PubMed.
- W. Humphrey, A. Dalke and K. Schulten, VMD: Visual molecular dynamics, J. Mol. Graphics Modell., 1996, 14, 33–38 Search PubMed.
- T. Lu and Q. Chen, Interaction Region Indicator: A Simple Real Space Function Clearly Revealing Both Chemical Bonds and Weak Interactions, Chem.: Methods, 2021, 1, 231–239 Search PubMed.
- F. Neese, Software update: The ORCA program system—Version 5.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed.
- S. Grimme, A. Hansen, S. Ehlert and J. M. Mewes, r(2)SCAN-3c: A "Swiss army knife" composite electronic-structure method, J. Chem. Phys., 2021, 154, 064103 Search PubMed.
- T. Gasevic, J. B. Stuckrath, S. Grimme and M. Bursch, Optimization of the r(2)SCAN-3c Composite Electronic-Structure Method for Use with Slater-Type Orbital Basis Sets, J. Phys. Chem. A, 2022, 126, 3826–3838 Search PubMed.
- J. B. Birks, Excimers, Rep. Prog. Phys., 1975, 38, 903–974 Search PubMed.
- H. Saigusa and E. C. Lim, Excimer Formation in van der Waals Dimers and Clusters of Aromatic Molecules, Acc. Chem. Res., 1996, 29, 171–178 Search PubMed.
- F. M. Winnik, Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media, Chem. Rev., 1993, 93, 587–614 Search PubMed.
- H. Liu, L. Shen, Z. Cao and X. Li, Covalently linked perylenetetracarboxylic diimide dimers and trimers with rigid "J-type" aggregation structure, Phys. Chem. Chem. Phys., 2014, 16, 16399–16406 Search PubMed.
- J. M. Robertson and J. G. White, 72, The crystal structure of pyrene. A quantitative X-ray investigation, J. Chem. Soc., 1947, 358–368 Search PubMed.
- J. Ferguson, Absorption and Fluorescence Spectra of Crystalline Pyrene, J. Chem. Phys., 1958, 28, 765–768 Search PubMed.
- J. B. Birks, A. A. Kazzaz and T. A. King, Excimer’ Fluorescence - IX. Lifetime Studies of Pyrene Crystals, Proc. R. Soc. London, Ser. A, 1997, 291, 556–569 Search PubMed.
- F. Würthner, Z. Chen, V. Dehm and V. Stepanenko, One-dimensional luminescent nanoaggregates of perylene bisimides, Chem. Commun., 2006, 1188–1190, 10.1039/b517020f.
- Z. Chen, U. Baumeister, C. Tschierske and F. Würthner, Effect of core twisting on self-assembly and optical properties of perylene bisimide dyes in solution and columnar liquid crystalline phases, Chem.–Eur. J., 2007, 13, 450–465 Search PubMed.
- L. Arrico, L. Di Bari and F. Zinna, Quantifying the Overall Efficiency of Circularly Polarized Emitters, Chem.–Eur. J., 2021, 27, 2920–2934 Search PubMed.
- H. Tanaka, Y. Inoue and T. Mori, Circularly Polarized Luminescence and Circular Dichroisms in Small Organic Molecules: Correlation between Excitation and Emission Dissymmetry Factors, ChemPhotoChem, 2018, 2, 386–402 Search PubMed.
- Y. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao and Z. Tang, Circularly polarized luminescence in chiral materials, Matter, 2022, 5, 837–875 Search PubMed.
- Z. L. Gong, Z. Q. Li and Y. W. Zhong, Circularly polarized luminescence of coordination aggregates, Aggregate, 2022, 3, e177 Search PubMed.
- T. Zhang, Y. Zhang, Z. He, T. Yang, X. Hu, T. Zhu, Y. Zhang, Y. Tang and J. Jiao, Recent Advances of Chiral Isolated and Small Organic Molecules: Structure and Properties for Circularly Polarized Luminescence, Chem.–Asian J., 2024, 19, e202400049 Search PubMed.
- K. Takaishi, S. Murakami, F. Yoshinami and T. Ema, Binaphthyl-Bridged Pyrenophanes: Intense Circularly Polarized Luminescence Based on a D2 Symmetry Strategy, Angew Chem. Int. Ed. Engl., 2022, 61, e202204609 Search PubMed.
- J. Wang, G. Zhuang, M. Chen, D. Lu, Z. Li, Q. Huang, H. Jia, S. Cui, X. Shao, S. Yang and P. Du, Selective Synthesis of Conjugated Chiral Macrocycles: Sidewall Segments of (-)/(+)-(12,4) Carbon Nanotubes with Strong Circularly Polarized Luminescence, Angew Chem. Int. Ed. Engl., 2020, 59, 1619–1626 Search PubMed.
- W. L. Zhao, M. Li, H. Y. Lu and C. F. Chen, Advances in helicene derivatives with circularly polarized luminescence, Chem. Commun., 2019, 55, 13793–13803 Search PubMed.
- J. L. Ma, Q. Peng and C. H. Zhao, Circularly Polarized Luminescence Switching in Small Organic Molecules, Chem.–Eur. J., 2019, 25, 15441–15454 Search PubMed.
- Y. Liu, Z. Ma, Z. Wang and W. Jiang, Boosting Circularly Polarized Luminescence Performance by a Double π-Helix and Heteroannulation, J. Am. Chem. Soc., 2022, 144, 11397–11404 Search PubMed.
- Z. Wei, Y. Yang and L. Liu, Chiral Organic Optoelectronic Materials and Circularly Polarized Light Luminescence and Detection, Acta Chim. Sin., 2022, 80, 970–992 Search PubMed.
- M. Hasegawa, C. Hasegawa, Y. Nagaya, K. Tsubaki and Y. Mazaki, Multiply Twisted Chiral Macrocycles Clamped by Tethered Binaphthyls Exhibiting High Circularly Polarized Luminescence Brightness, Chem.–Eur. J., 2022, 28, e202202218 Search PubMed.
- A. Morisaki, R. Inoue and Y. Morisaki, Synthesis of Two Novel Optically Active #-Shaped Cyclic Tetramers Based on Planar Chiral [2.2]Paracyclophanes, Chem.–Eur. J., 2023, 29, e202203533 Search PubMed.
- Y. J. Shen, N. T. Yao, L. N. Diao, Y. Yang, X. L. Chen and H. Y. Gong, A -Extended Pentadecabenzo[9]Helicene, Angew Chem. Int. Ed. Engl., 2023, 62, e202300840 Search PubMed.
- W. Niu, Y. Fu, Z. L. Qiu, C. J. Schurmann, S. Obermann, F. Liu, A. A. Popov, H. Komber, J. Ma and X. Feng, π-Extended Helical Multilayer Nanographenes with Layer-Dependent Chiroptical Properties, J. Am. Chem. Soc., 2023, 145, 26824–26832 Search PubMed.
- Y. Liu, Z. Li, M. W. Wang, J. Chan, G. Liu, Z. Wang and W. Jiang, Highly Luminescent Chiral Double π-Helical Nanoribbons, J. Am. Chem. Soc., 2024, 146, 5295–5304 Search PubMed.
- A. Bedi and O. Gidron, The Consequences of Twisting Nanocarbons: Lessons from Tethered Twisted Acenes, Acc. Chem. Res., 2019, 52, 2482–2490 Search PubMed.
- Y. Liang, M. Tang and Z. Liu, Molecular Bows—Strained Bow-shaped Macrocycles, Chem. Lett., 2020, 49, 1329–1336 Search PubMed.
- J. Liu, M. Wu, L. Wu, Y. Liang, Z. B. Tang, L. Jiang, L. Bian, K. Liang, X. Zheng and Z. Liu, Infinite Twisted Polycatenanes, Angew. Chem., Int. Ed., 2023, 62, e202314481 Search PubMed.
- L. Jiang, Z. Peng, Y. Liang, Z. B. Tang, K. Liang, J. Liu and Z. Liu, Strain-Driven Formal [1,3]-Aryl Shift within Molecular Bows, Angew. Chem., Int. Ed., 2023, 62, e202314481 Search PubMed.
- M. Tang, Y. Liang, J. Liu, L. Bian and Z. Liu, Mechanical Trapping of the Phlorin Intermediate, CCS Chem., 2022, 4, 3230–3237 Search PubMed.
- M. Tang, Y. Liang, J. Liu, L. Wu, S. Wang, L. Bian, L. Jiang, Z. B. Tang and Z. Liu, Mechanical trapping and in situ derivatization of the porphodimethene intermediate, Mater. Today Chem., 2022, 24, 100868 Search PubMed.
- L. Wu, M. Tang, L. Jiang, Y. Chen, L. Bian, J. Liu, S. Wang, Y. Liang and Z. Liu, Synthesis of contra-helical trefoil knots with mechanically tuneable spin-crossover properties, Nat. Synth., 2022, 2, 17–25 Search PubMed.
- K. Liang, Y. Liang, M. Tang, J. Liu, Z.-B. Tang and Z. Liu, π-Diamond: A Diamondoid Superstructure Driven by π-Interactions, Angew. Chem., Int. Ed., 2024, 63, e202409507 Search PubMed.
- P. Osswald, M. Reichert, G. Bringmann and F. Würthner, Perylene Bisimide Atropisomers: Synthesis, Resolution, and Stereochemical Assignment, J. Org. Chem., 2007, 72, 3403–3411 Search PubMed.
- B. Sadowski, D. Mierzwa, S. Kang, M. Grzybowski, Y. M. Poronik, A. L. Sobolewski, D. Kim and D. T. Gryko, Tuning the aromatic backbone twist in dipyrrolonaphthyridinediones, Chem. Commun., 2022, 58, 3697–3700 Search PubMed.
- A. Bedi and O. Gidron, Chiroptical Properties of Twisted Acenes: Experimental and Computational Study, Chem.–Eur. J., 2019, 25, 3279–3285 Search PubMed.
- A. Bedi, L. J. W. Shimon and O. Gidron, Helically Locked Tethered Twistacenes, J. Am. Chem. Soc., 2018, 140, 8086–8090 Search PubMed.
- A. Bedi, G. Schwartz, U. Hananel, A. Manor Armon, I. Shioukhi, G. Markovich and O. Gidron, The effect of axial and helical chirality on circularly polarized luminescence: lessons learned from tethered twistacenes, Chem. Commun., 2023, 59, 2011–2014 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2354646 and 2354656–2354661. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04796f |
| This journal is © The Royal Society of Chemistry 2024 |

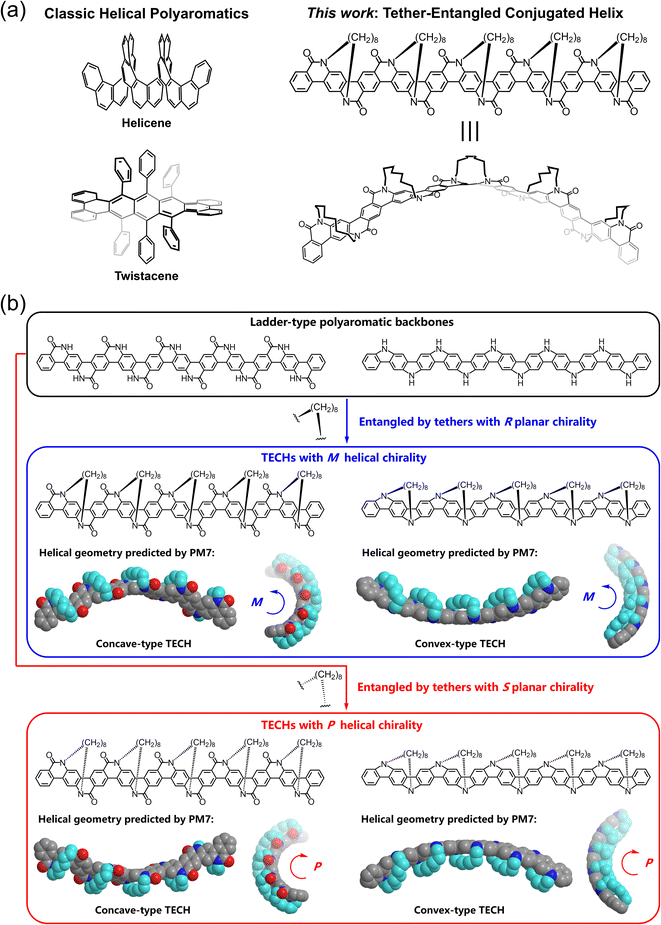
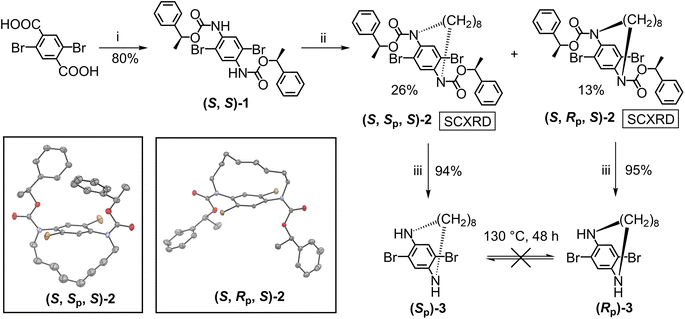
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ON3/Et3N/(
ON3/Et3N/(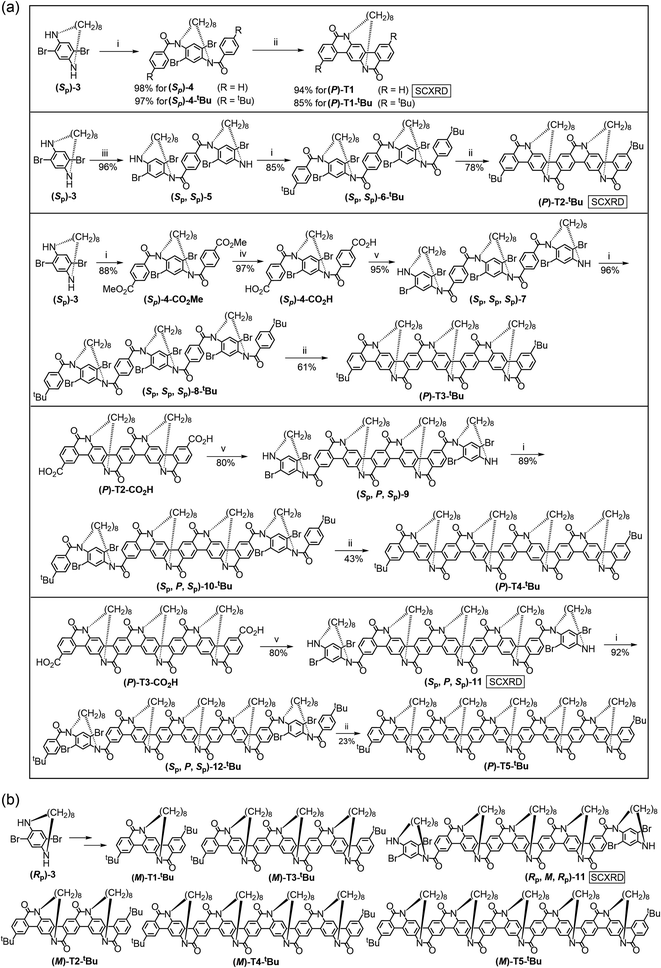
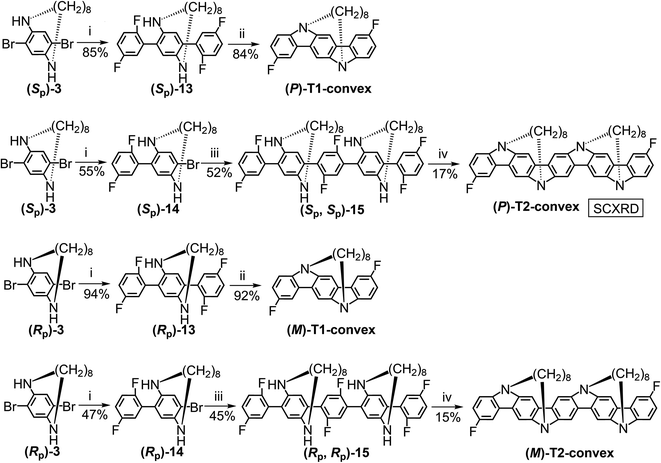
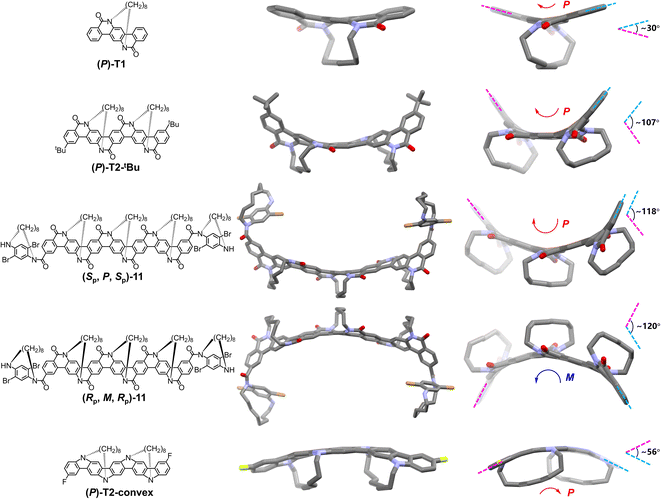
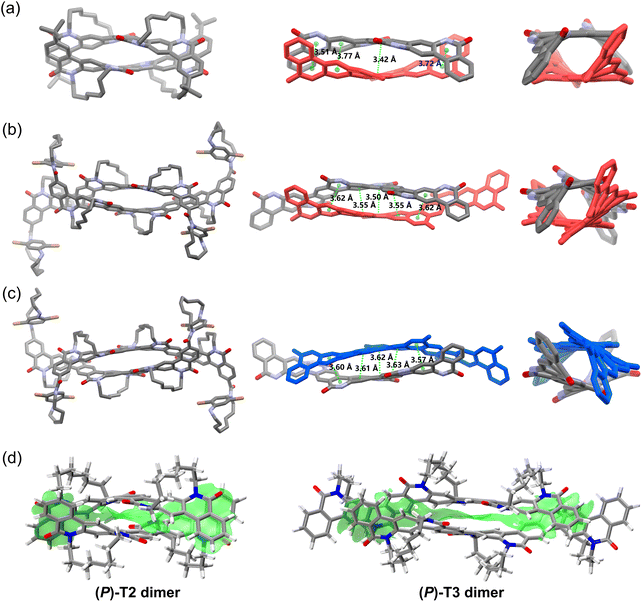
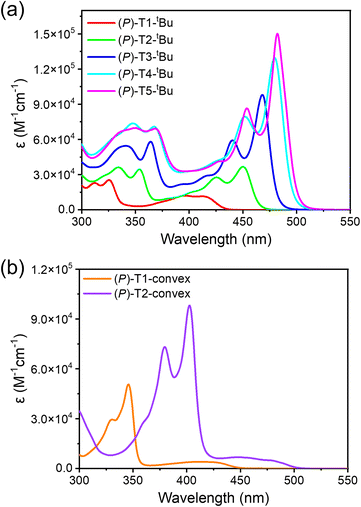
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: