Open Access Article
Open Access ArticleHigh-performance deep-blue electroluminescence from multi-resonance TADF emitters with a spirofluorene-fused double boron framework†
Ke
Xu
,
Nengquan
Li
 ,
Zeyuan
Ye
,
Yuxi
Guo
,
Yuxin
Wu
,
Chenghao
Gui
,
Xiaojun
Yin
,
Zeyuan
Ye
,
Yuxi
Guo
,
Yuxin
Wu
,
Chenghao
Gui
,
Xiaojun
Yin
 ,
Jingsheng
Miao
,
Xiaosong
Cao
,
Jingsheng
Miao
,
Xiaosong
Cao
 * and
Chuluo
Yang
* and
Chuluo
Yang

Shenzhen Key Laboratory of New Information Display and Storage Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, China. E-mail: xcao@szu.edu.cn
First published on 4th October 2024
Abstract
The development of multi-resonance thermally activated delayed fluorescence (MR-TADF) materials in the deep-blue region is highly desirable. A usual approach involves constructing an extended MR-TADF framework; however, it may also intensify aggregate-caused quenching issues and thereby reduce device efficiency. In this study, we develop a molecular design strategy that fuses the MR-TADF skeleton with 9,9′-spirobifluorene (SF) units to create advanced deep-blue emitters. The SF moiety facilitates high-yield one-shot bora-Friedel–Crafts reaction towards an extended skeleton and mitigates interchromophore interactions as a steric group. Our findings reveal that orbital interactions at the fusion site significantly influence the electronic structure, and optimizing the fusion mode allows for the development of emitters with extended conjugation length while maintaining non-bonding character. The proof-of-concept emitter exhibits narrowband emission in the deep-blue region, a near-unity photoluminescence quantum yield, and a fast kRISC of 2.4 × 105 s−1. These exceptional properties enable the corresponding sensitizer-free OLED to achieve a maximum external quantum efficiency (EQEmax) of 39.0% and Commission Internationale de l'Eclairage (CIE) coordinates of (0.13, 0.09). Furthermore, the hyperfluorescence device realizes an EQEmax of 40.4% with very low efficiency roll-off.
Introduction
The recent conceptual advancements in molecular design have facilitated the transition of organic light-emitting diodes (OLED) from academic to commercial applications. To meet the increasing performance demands of ultrahigh-resolution displays, significant efforts have been directed towards enhancing the intrinsic color purity of organic emitters, while ensuring high efficiency and long-term operational stability. The inadequate color purity of current commercial emitters necessitates the development of new emitters with small linewidth. Recently, boron- and nitrogen-doped polycyclic heteroaromatics with multi-resonance thermally activated delayed fluorescence (MR-TADF) have garnered significant interest.1,2 These emitters offer narrowband emissions even surpassing those of perovskite and quantum-dot light-emitting compounds.3–5 The separation of the frontier molecular orbitals (FMOs) in these materials effectively suppresses structural relaxation and vibronic coupling, leading to high photoluminescence quantum yield (ΦPL) and superior color purity. Numerous studies have since focused on revolutionizing molecular design and optimizing synthetic methodologies, significantly accelerating the development of MR-TADF materials for practical applications.Among the efforts to modulate the optoelectronic properties of MR-TADF emitters, extending the π-skeleton has been identified as a cutting-edge strategic design, as theoretically predicted by Olivier et al.6,7 This design principle has proven effective in increasing the reverse intersystem crossing rate constant (kRISC), which is crucial for avoiding severe efficiency roll-off at high brightness.8–10 The accelerated RISC is facilitated by short-range charge transfer (SR-CT) within the extended framework, resulting in a reduction in exchange energy. Importantly, with rational molecular design, the enlarged ring-fused structure can further promote the ΦPL and generate even narrower emission linewidths. Consequently, multi-boron-embedded MR-TADF emitters currently outperform single boron-embedded MR-TADF materials in OLEDs.11–20
Nonetheless, the rigid planar structure of π-extended MR-TADF emitters can result in severe aggregation-caused quenching (ACQ) and spectral broadening in the solid state due to strong π–π interactions. As a result, a low doping ratio is typically employed during device fabrication to mitigate intermolecular interactions between chromophores, often leading to insufficient energy transfer and pronounced phase separation.21,22 Furthermore, achieving precise and consistent doping ratios adds complexity to production processes and increases manufacturing costs. To address this bottleneck, it is crucial to introduce steric hindrance into the extended fused-ring structure to specifically hinder intermolecular interactions and further improve OLED performance.23–30
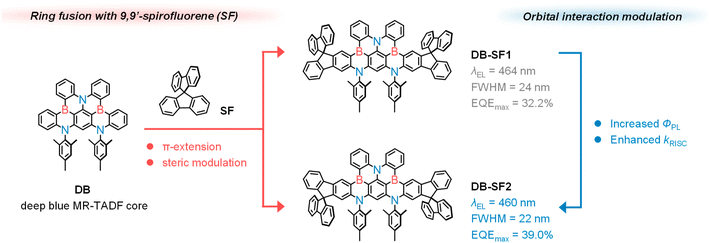
To address this issue, we propose herein a 9,9′-spirobifluorene (SF)-fused strategy aimed at developing narrowband deep-blue emitters with high efficiency and low efficiency roll-off (Fig. 1). The incorporation of SF units serves a dual purpose: promoting π-electron delocalization and sterically shielding the emitting skeleton.31 Unlike previous studies that attached bulky units to the MR-TADF core via a single bond, which may trigger undesirable structural relaxation during the electronic transition process, our approach yields emitters with high structural rigidity. It should be noted that embedding SF in the MR-TADF skeleton in a proper manner is challenging, as its π-bonding orbitals may deteriorate the non-bonding nature and enhance the conjugation length. Therefore, we designed two regioisomeric emitters to investigate the influence of fusion mode on the photophysical properties. Our results show that excellent emitters can be derived with extended conjugation length and well-retained non-bonding character by optimizing the fusion mode. Benefiting from the extended skeleton and suppressed chromophore interactions, the sensitizer-free device achieved maximum external quantum efficiencies (EQEmax) of up to 39.0% and a small full width at half maximum (FWHM) of 22 nm in the deep-blue region with Commission Internationale de l′Eclairage (CIE) coordinates of (0.13, 0.09). Moreover, with the assistance of a TADF sensitizer, the hyperfluorescent device achieved state-of-the-art efficiency with an EQEmax/1000 of 40.4% and 28.4%.
Molecular design, synthesis and characterization
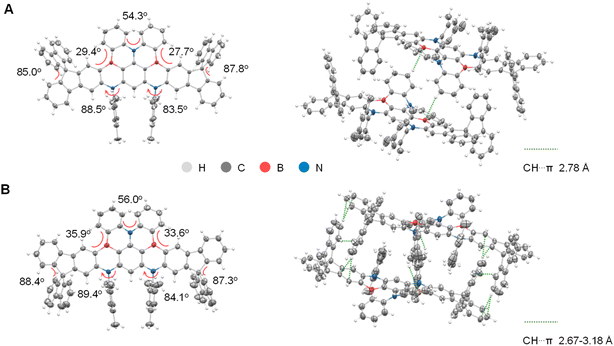
As illustrated in Fig. 1, the DB featuring a double-boron-embedded framework was selected as the parent emitter due to its deep-blue emission and a relatively high kRISC of 1.3 × 105 s−1.32,33 Additionally, the skeleton can be synthesized via a lithium-free bora-Friedel–Crafts reaction with good yield and can be easily derivatized by modifying the terminal aryl units of the amine precursor. The synthetic routes of DB-SF1 and DB-SF2 are similar and straightforward, as depicted in Scheme S1.† The corresponding precursors were synthesized through multi-step Buchwald–Hartwig C–N coupling reactions, and the final products were obtained via a one-shot borylation reaction with moderate yield (∼40%) (Fig. S1–S11†). Notably, due to the high electrophilicity at the C2 position of SF, the borylation step of DB-SF1 did not produce other possible regioisomers. Meanwhile, the large steric hindrance at the C1 position of SF also ensured the exclusive formation of DB-SF2. Both products were fully confirmed by NMR spectroscopy and high-resolution mass spectrometry (HR-MS), validating that the regioselectivity of the reacting sites greatly enhanced the synthetic yields of the targeted compounds. As shown in Fig. S12,†DB-SF1 and DB-SF2 exhibited significantly higher thermal decomposition temperatures (Td, corresponding to a 5% weight loss) of 466 and 469 °C, respectively, compared to the parent molecule DB (386 °C), according to thermogravimetric analysis (TGA). Clearly, the introduction of SF contributes to better thermal stabilities and allows the emitters to be refined and further processed by vacuum sublimation.To acquire a comprehensive understanding of the impact induced by the introduction of SF groups on molecular conformations and packing modes, single crystals of DB-SF1 and DB-SF2 were obtained by slow liquid diffusion of methanol into a chlorobenzene solution. Both emitters belonged to the triclinic space group P1, and the crystallographic data are summarized in Tables S1 and S2.† As displayed in Fig. 2, the linear π-extension of the DB core with SF moieties enhanced the overall structural planarity of both compounds but induced significant twisting at the molecular edges. Specifically, the spatial fluorene and mesitylene units displayed nearly perpendicular conformations with the MR-TADF skeleton, featuring dihedral angles in the range of 85.0° to 88.4°. The distorted geometry of the DB center remained intact in the presence of multiple B,N-doped-[4]helicenes, which not only mitigated intermolecular π–π stacking interactions but also triggered large spin–orbit coupling (SOC) and benefitted the RISC process according to previous reports.34,35 The packing patterns indicated that the steric effect prevented the formation of π–π interactions between the dimers of DB-SF1 and DB-SF2, enhancing the quenching-resistant properties of the emitters. Furthermore, adjacent molecules were interlocked via multiple C–H⋯π interactions below 3.2 Å, potentially minimizing nonradiative energy losses in the solid state.
Theoretical calculations
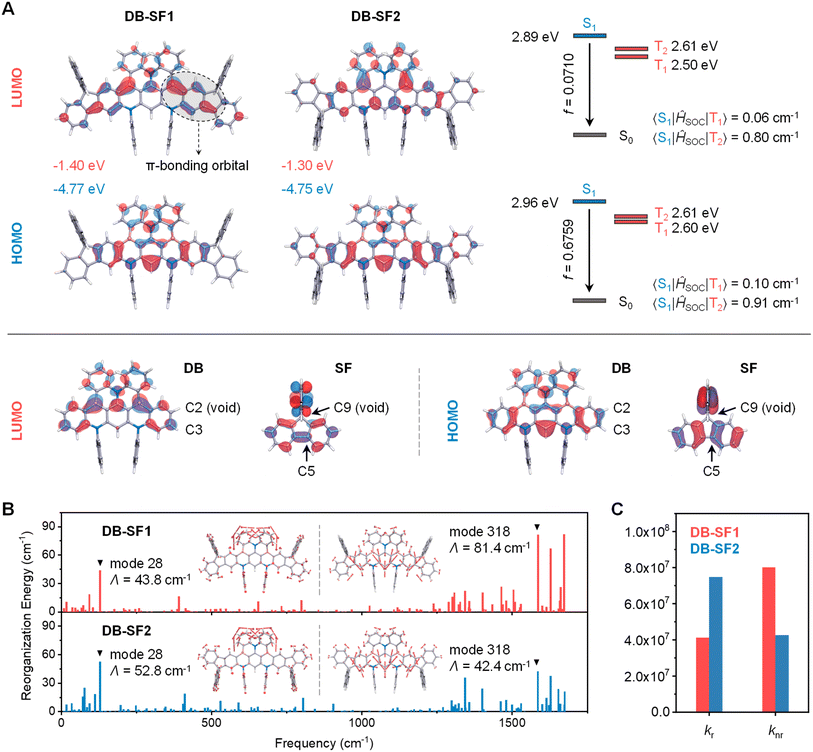
To unveil the effect of isomeric skeletons on the geometric and optoelectronic properties, we conducted analyses of their molecular orbitals using density functional theory (DFT) and time-dependent DFT (TD-DFT) at the B3LYP/6-31G(d,p) level (Fig. 3A and Tables S3–S7†). The electron density distributions on the HOMO were similar for both emitters, with DB-SF2 showing slightly more extended delocalization to the fluorene segments. In contrast, their LUMO distributions differed significantly: DB-SF2 primarily involved non-bonding orbitals across the fused-ring framework, suppressing undesired stretching vibrations, whereas DB-SF1 exhibited hybridized π-bonding/non-bonding orbital characteristics. Clearly, the distinct π-bonding orbital on the boron and fluorene segments in DB-SF1 weakened the resonance effect.36 These findings thus suggested a significant influence of the fusion mode on the electron density distribution of FMOs, preserving desirable MR-TADF properties in DB-SF2. Consequently, both the HOMO and LUMO energy levels of DB-SF2 were more destabilized than those of DB-SF1, and the net effect resulted in a higher energy bandgap (Eg) of DB-SF2 (3.45 eV) compared to DB-SF1 (3.37 eV).To gain a deeper understanding of how the fusing site affects optoelectronic properties at the molecular level, the FMOs of DB and SF fragments were subsequently studied (Fig. 3A). The FMOs of SF exhibited a dominant π character on the biphenyls. In the fusion mode of DB-SF1, the C2(C3) atom of DB was linked with the C9(C5) atom of SF, respectively, while in the case of DB-SF2, the C3(C2) atom of DB was linked with the C9(C5) atom of SF. In a simplified manner, the different LUMO distributions between the two isomers can be understood as follows: in the case of DB-SF2, the C2 atom of DB could be regarded as a void position, perturbing the covalent π-bonding structure of the biphenyl site. In contrast, the C3 atoms of DB and the C5 atom of SF possessed the same bonding character in their LUMOs and thus could form a more delocalized π-conjugation, which deteriorated the MR-TADF character.
The natural transition orbitals (NTOs) (Fig. S14†) of S1 closely resembled the FMO results, reflecting the electronic inertness of the spatial fluorene units. Consistent with the trend of Egs, DB-SF1 and DB-SF2 exhibited S1 energy levels of 2.89 eV and 2.96 eV, respectively. Despite the enlarged π-systems, the S1 of DB-SF2 only decreased moderately from DB (3.12 eV), indicating that the dominant non-bonding characters of the MR-TADF skeleton hindered effective conjugation length, thereby preserving deep-blue emission. Notably, DB-SF2 showed reduced ΔEST values (0.36 eV) and increased fosc values (0.6759) compared to DB (ΔEST = 0.41 eV, fosc = 0.0618) and DB-SF1 (ΔEST = 0.39 eV, fosc = 0.0710), indicating enhanced SR-CT transition due to extended wavefunction delocalization and restricted π-bonding orbital formation. To account for electron correlation involving double excitations, we conducted higher-level RI-SCS-CC2 calculations to more accurately estimate excited-state energies.6 These calculations produced smaller ΔEST values of 0.15 eV for DB-SF1 and 0.09 eV for DB-SF2 (Table S8†), which are consistent with the trends observed in TD-DFT calculations and show good agreement with the photophysical measurements. Furthermore, like DB, the two new emitters presented large spin–orbit coupling (SOC) values induced by the embedded twisted helicene subunits (e.g., <S1|ĤSOC|T1> = 0.06 cm−1 and <S1|ĤSOC|T2> = 0.80 cm−1 for DB-SF1; <S1|ĤSOC|T1> = 0.10 cm−1 and <S1|ĤSOC|T2> = 0.91 cm−1 for DB-SF2, with T2 close to S1 and T1 for both emitters). These significant SOC matrix elements were expected to convert into high kRISCs according to Fermi′s golden rule.37–39
Next, the reorganization energy (Λ) and Huang-Rhys factors (HRF) of DB-SF1 and DB-SF2 were simulated and analyzed based on the optimal S0 and S1 geometries using the molecular materials property prediction package (MOMAP, Fig. 3B). The structural relaxation during the excitation-emission process was more suppressed in DB-SF2, as indicated by its apparent reduction of total Λ (1506 cm−1) compared to DB-SF1 (1836 cm−1). The relationships of Λ with normal vibration modes for the S1 → S0 transition showed that although the intensities of low-frequency vibrations between the two emitters were comparable, DB-SF2 exhibited much suppressed vibronic coupling strength of high-frequency modes around 1500 cm−1. These dominant high-frequency modes were associated with the stretching of C–C bonds across the entire molecular framework, and the intensity decrease in DB-SF2 was attributed to its predominantly non-bonding character. Ultimately, the reduced Λ combined with increased fosc translated into a larger radiative rate constant (kr) and a smaller non-radiative rate constant (knr) in DB-SF2, as illustrated in Fig. 3C. These findings confirmed that the orbital interactions at the fusion site within these emitters played a decisive role in their electronic structure.
Photophysical properties
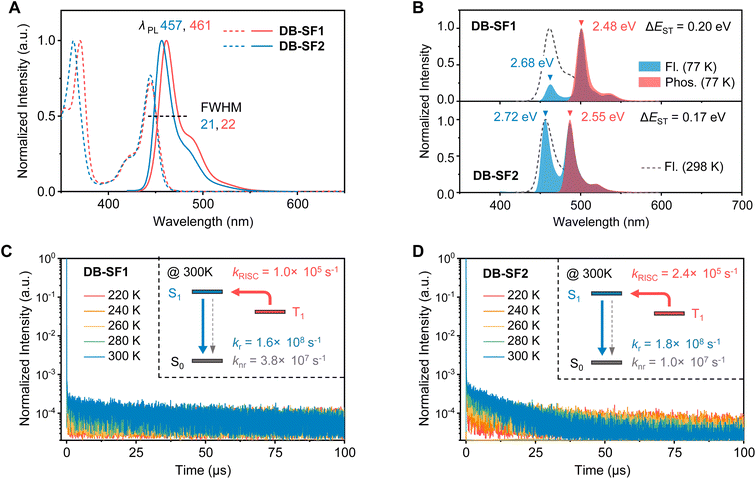
The photophysical properties in dilute toluene solution (1 × 10−5 M) were subsequently characterized, with the results displayed in Fig. 4 and summarized in Table 1. Both emitters′ absorption spectra featured a combination of n–π*/π–π* transitions (below 400 nm) and intense SR-CT bands (above 400 nm). Based on the onsets of the absorption spectra, the optical Eg of DB-SF1 (2.71 eV) was estimated to be marginally higher than that of DB-SF2 (2.69 eV). Correspondingly, the solution fluorescence spectra of DB-SF2 displayed a blue-shifted emission maximum (457 nm) compared to DB-SF1 (461 nm). The minimized Stokes shift (Δλ = 0.07 eV) in DB-SF2 indicated a lower reorganization energy between the ground and excited state configurations than DB-SF1 (Δλ = 0.10 eV). For the same reason, DB-SF2 exhibited a smaller FWHM value (21 nm) with a reduced intensity of the low-energy peak shoulder than that of DB-SF1 (FWHM = 22 nm). Such spectral profile rendered DB-SF2 (CIEy = 0.076) with better color purity compared to DB-SF1 (CIEy = 0.097) (Fig. S15A†). Furthermore, we investigated the fluorescence spectra in different polar solvents. When varying the solvents from n-hexane to dichloromethane, the FWHMs for both emitters remained narrow, and the solvatochromic effect was moderate, indicating SR-CT characteristics (Fig. S16 and Table S9†). To estimate the key energy levels of the two molecules, we measured their fluorescence and phosphorescence spectra in frozen toluene at 77 K (Fig. 4B). The S1/T1 energy levels for DB-SF1 were determined to be 2.68/2.48 eV and for DB-SF2 as 2.72/2.55 eV based on the peak maxima of fluorescence and phosphorescence, resulting in ΔEST values of 0.20/0.17 eV. The smaller ΔEST value for DB-SF2 suggested its potential for a more effective triplet exciton upconversion process.| Emitter | λ abs (nm) | λ em (nm) | FWHMa (nm meV−1) | ΔESTb (eV) | Φ PL (%) | Φ PF (%) | Φ DF (%) | τ PF (ns) | τ DF (μs) | k r (108 s−1) | k nr (106 s−1) | k RISC (105 s−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Peak of absorption (λabs) and fluorescence (λem, 298 K) spectra, as well as color coordinates in the CIE 1931 chromaticity diagram and full-width at half-maximum (FWHM) of fluorescence. b S1-T1 energy gap (ΔEST) determined from the onset of low-temperature fluorescence and phosphorescence spectra measured in 1 × 10−5 M toluene solution. c Absolute photoluminescence quantum yield (ΦPL), and quantum yields of prompt and delayed fluorescence (ΦPF and ΦDF). d Lifetimes of prompt and delayed fluorescence (τPF and τDF), the percentage value given in parentheses refers to the fractional intensity. e Rate constants of singlet radiative decay (kr), non-radiative decay (knr), intersystem crossing (kISC), and reverse intersystem crossing (kRISC) measured in the PMMA film with 2 wt% doping ratio. | ||||||||||||
| DB-SF1 | 445 | 461 | 22/130 | 0.20 | 81 | 36 | 45 | 2.2 | 22.3 | 1.6 | 38.0 | 1.0 |
| DB-SF2 | 445 | 457 | 21/125 | 0.17 | 95 | 40 | 55 | 2.2 | 10.1 | 1.8 | 9.6 | 2.4 |
To verify the TADF properties, doped films (2 wt% in polymethyl methacrylate, PMMA) were prepared. The associated kinetic parameters are summarized in Table 1. Both emitters exhibited similar fluorescence spectra compared to their toluene solutions, despite slightly broadened emission bands caused by the microenvironment change (Fig. S17†). The steric hindrance groups minimized molecular aggregation, resulting in good ΦPL values (81% for DB-SF1 and 95% for DB-SF2). Temperature-dependent transient decay curves (Fig. 4C and D) revealed that DB-SF1 and DB-SF2 in doped films exhibited pure TADF character with clear bi-exponential decay. The delayed components intensified as the temperature increased. Notably, DB-SF2 demonstrated a much shorter delayed lifetime of 10.1 μs compared to DB-SF1′s 22.3 μs at room temperature. This shorter lifetime rendered a more rapid kRISC of 2.4 × 105 s−1 for DB-SF2versus 1.0 × 105 s−1 for DB-SF1, nearly double that of the parent DB molecule (kRISC = 1.3 × 105 s−1). Further analysis showed that DB-SF2 exhibited a faster kr of 1.8 × 108 s−1 and a smaller knr of 9.6 × 106 s−1 compared to DB-SF1 (kr = 1.6 × 108 s−1, knr = 3.8 × 107 s−1), as deduced from the transient decay curves and ΦPL values. The combination of higher ΦPL values, shorter delayed lifetimes, and more efficient kRISC and kr rates in DB-SF2 underscores its superior TADF properties compared to DB-SF1 and the parent DB molecule, confirming the effectiveness in molecular design. These advantages arose from the promoted π-electron delocalization and prevalent non-bonding orbital character in DB-SF2, consistent with in silico predictions.
Electroluminescent properties
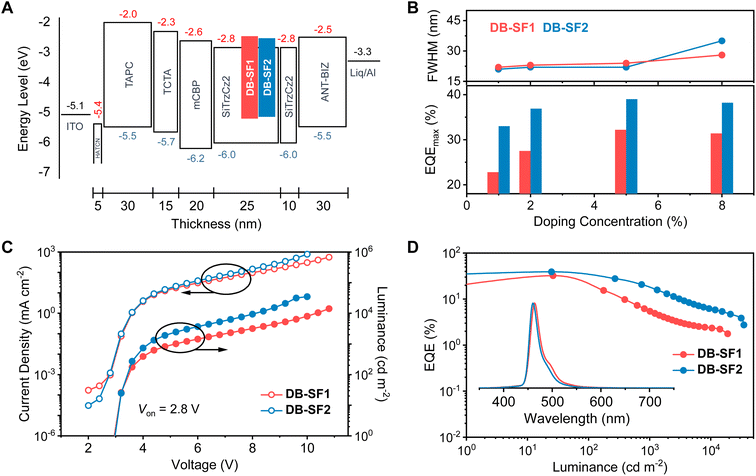
To assess the electroluminescent (EL) performance, we fabricated and characterized devices based on a structure comprising ITO/HAT-CN (5 nm)/TAPC (30 nm)/TCTA (15 nm)/mCBP (20 nm)/SiTrzCz2: x wt% DB-SF1 or DB-SF2 (25 nm)/SiTrzCz2 (10 nm)/ANT-BIZ (30 nm)/Liq (2 nm)/Al (100 nm). In this structure, ITO (indium tin oxide) and Al (aluminum) served as the anode and cathode, respectively. HAT-CN (dipyrazino[2,3-f:2′,3′-h]quinoxaline-2,3,6,7,10,11-hexacarbonitrile) and Liq (8-quinolinolato lithium) functioned as hole- and electron-injection layers, respectively. TAPC (1,1-bis((di-4-tolylamino)phenyl)-cyclohexane) and TCTA (4,4′,4′′-tris(N-carbazolyl)-triphenylamine) served as hole-transporting layers, while ANT-BIZ (1-(4-(10-([1,1′-biphenyl]-4 yl)anthracen-9-yl)phenyl)-2-ethyl-1H-benzo[d]imidazole) acted as the electron-transporting layer. mCBP (3,3-di(9H-carbazol-9-yl)biphenyl) was employed as the exciton-blocking layer. SiTrzCz2 (9,9′–(6-(3-(triphenylsilyl)phenyl)-1,3,5-triazine-2,4-diyl)bis(9H-carbazole)) was chosen as the host material due to its matched energy levels with adjacent functional layers, suitable triplet energy level, and appropriate spectral overlap between the host emission and dopant absorption. The device configurations and performances are depicted in Fig. 5 (the HOMO/LUMO levels of DB-SF1 and DB-SF2 in the energy level diagram were deduced by cyclic voltammetry (CV) measurements and absorption onset values, Fig. S13†), with key EL parameters summarized in Table 2.| Device (emitter) | V on (V) | λ EL (nm) | FWHMc (nm meV−1) | L max (cd m−2) | CEmax/1000e (cd A−1) | EQEmax/1000f (%) | CIEg (x, y) |
|---|---|---|---|---|---|---|---|
| a Turn-on voltage recorded at the luminance of 1 cd m−2. b Maximum EL wavelength. c FWHM of EL spectra. d Maximum brightness. e Current efficiency at maximum and 1000 cd m−2. f Efficiency at maximum and 1000 cd m−2. g EL color coordinates in the CIE 1931 chromaticity diagram recorded at 6 V. | |||||||
| DB-SF1 | 2.8 | 464 | 24/135 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911 911 |
34.5/5.7 | 32.2/6.1 | (0.13, 0.12) |
| DB-SF2 | 2.8 | 460 | 22/129 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851 851 |
31.6/16.8 | 39.0/20.7 | (0.13, 0.09) |
| DB-SF1 HF | 3.2 | 468 | 32/179 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 048 |
42.3/18.5 | 34.2/15.1 | (0.13, 0.16) |
| DB-SF2 HF | 3.2 | 462 | 29/168 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 163 163 |
45.1/30.2 | 40.4/28.4 | (0.13, 0.13) |
The doping ratio of DB-SF1 or DB-SF2 in the emitting layer (EML) was adjusted from 1 to 8 wt% to evaluate concentration-dependent characteristics (Fig. S18, S19 and Table S10†). The EQEmax and FWHM versus dopant concentration relationships are shown in Fig. 5B. For both emitters, energy transfer was not complete until the 5 wt% doping ratio, and the maximum device efficiency was achieved at this point. In the 1–5 wt% range, the spectra of DB-SF1 showed a marginal red-shift of the emission maxima from 461 to 464 nm with negligibly increased FWHMs from 22 to 24 nm. Similarly, a small FWHM increase from 21 nm (1 wt%, λEL = 459 nm) to 22 nm (5 wt%, λEL = 460 nm) was recorded for DB-SF2. Although further concentration increases can induce the formation of detrimental aggregates/excimers, leading to declined efficiency and color purity, it is noteworthy that previously reported π-extended MR-TADF emitters (such as v-DABNA) typically required less than 2 wt% to achieve optimal device performance.11,40 Thus, the relative concentration independence for both emitters validates the feasibility of our molecular design strategy for spatial regulation. Remarkably, efficient OLED performances were realized without light out-coupling technologies, offering EQEmax values of 32.2% and 39.0% for DB-SF1 and DB-SF2 at 5 wt%, accompanied by high maximum current efficiency (CEmax) of 34.5 and 31.6 cd A−1, respectively. The corresponding CIE coordinates were (0.13, 0.12) for DB-SF1 and (0.13, 0.09) for DB-SF2, with the latter approaching the blue point (0.14, 0.08) defined by the National Television System Committee (NTSC). The significantly higher EQEmax value of DB-SF2 was associated with its improved ΦPL, while the better CEmax of DB-SF1 could be attributed to its red-shifted EL spectrum. With a more efficient RISC process and therefore reduced triplet exciton density, DB-SF2 also showed moderate efficiency roll-off behavior at practical luminance, maintaining an EQE of 20.7% at a luminance of 1000 cd m−2, which was significantly higher than that of DB-SF1 (6.1% at 1000 cd m−2). As another crucial parameter, operational stability was subsequently assessed using OLEDs with a 5 wt% doping ratio (Fig. S20†). DB-SF2 manifested a 2.3-fold increase in operational half-life (LT50 of 88.6 h) at an initial luminance of 100 cd m−2 compared to DB-SF1 (LT50 of 38.5 h). Given the similar emission energies of the two emitters, the enhanced stability of DB-SF2 is attributed to its superior triplet-harvesting capability. These results highlight the importance of optimizing orbital interactions at the fusion site to minimize detrimental π-bonding character in deep-blue emitters.
To further enhance EL performance, hyperfluorescence (HF) OLEDs were fabricated with a structure of ITO/HATCN (5 nm)/TAPC (30 nm)/TCTA (15 nm)/mCBP (10 nm)/EML (25 nm)/DPFPO (10 nm)/ANT-BIZ (30 nm)/Liq (1 nm)/Al (150 nm). The EML was a ternary blended film DBFPO: 20 wt% mMDBA-DI: 2 wt% emitters (Fig. 6A), where mMDBA-DI (5-(3,11-dimethyl-5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracen-7-yl)-10,15-diphenyl-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole) was selected as a sensitizer due to its well-matched PL spectra (Fig. S21A†) with the absorption spectra and high kRISC. Meanwhile, DBFPO (2,8-bis(diphenylphosphoryl)dibenzo[b,d]furan) was employed as the host and hole/exciton-blocking material due to its higher T1 energy. In this device configuration, the generated triplet excitons were harvested by the sensitizer and then transferred to the terminal emitter through efficient long-range Förster resonance energy transfer (FRET). Combined with the close-to-unity ΦPL and large kr of DB-SF2, the HF device exhibited notable improvement in device efficiency while retaining narrowband emission (Fig. 6B, S21 and S22†). The EL spectrum was slightly red-shifted with emission peaking at 462 nm, an FWHM of 29 nm, and CIE coordinates of (0.13, 0.13), due to the large polarity of DBFPO and mMDBA-DI mixed matrixes. An impressive EQEmax of 40.4% was achieved, along with a CEmax of 45.1 cd A−1, representing one of the highest efficiencies among reported OLED devices based on blue MR-TADF materials. Furthermore, this device showed alleviated roll-offs, maintaining an EQE of 28.4% at a luminance of 1000 cd m−2. It is noteworthy that while DB-SF2 primarily served as a fluorescence emitter in this HF device, the triplet population on the terminal emitter was still possible. In this regard, the fast RISC process of DB-SF2 remained beneficial herein. For comparison, the HF device based on DB-SF1 exhibited inferior EQEmax and more pronounced roll-off, underscoring the advantage of the higher kRISC of DB-SF2.
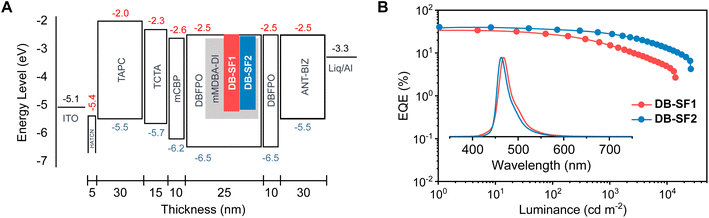 | ||
| Fig. 6 (A) Device configurations and energy-level diagrams of the hyperfluorescence (HF) devices. (B) EQE-luminance curves with electroluminescence spectra at 6 V as the inset. | ||
Conclusion
In summary, we designed and constructed an isomeric pair of deep-blue MR-TADF emitters, DB-SF1 and DB-SF2, by fusing a double-boron embedded framework with 9,9′-spirobifluorene. This approach aimed to extend π-conjugated skeletons while mitigating interchromophore quenching issues. Theoretical simulations and photophysical measurements highlighted the crucial role of orbital interactions between fused-ring segments on the electronic structure, providing a guideline for modulating π-bonding and non-bonding orbital features. Due to enhanced π-electron delocalization and well-retained non-bonding character, DB-SF2 exhibited hypsochromic shifted emission, smaller reorganization energy, and a reduced ΔEST compared to DB-SF1. These attributes led to a near-unity ΦPL and a fast RISC process (kRISC = 2.4 × 105 s−1) for DB-SF2 in the deep-blue region in the solid state, demonstrating the effectiveness of π-extension combined with structural steric hindrance improvement. Benefitting from its excellent MR-TADF properties, the non-sensitized device based on DB-SF2 exhibited narrowband electroluminescence (FWHM = 22 nm) with CIE coordinates of (0.13, 0.09) and EQEmax/1000 of 39.0%/20.7%. The remarkable device efficiency surpassed those of the previously reported binary deep-blue OLEDs with CIEy < 0.12 (Table S11†). Additionally, the hyperfluorescent OLED demonstrated a further improved EQEmax of 40.4% and a lower efficiency roll-off at high luminance (EQE1000 = 28.4%). Our design strategy thus provides a promising pathway to achieve high-performance narrowband blue MR-TADF emitters, aligning with the requisites of ultrahigh-definition OLED displays.Data availability
All data supporting the findings of this study are presented in the article and ESI.† Additional data are available from the corresponding author upon reasonable request.Author contributions
X. Cao conceived the idea and supervised the project. K. Xu wrote the original draft. X. Cao and C. Yang reviewed and edited the manuscript. K. Xu, Y. Wu, C. Gui and X. Yin conducted the synthesis, basic property characterization, and theoretical calculations. N. Li performed the fabrication and characterization of the devices. Z. Ye and J. Miao analysed the data. All the authors participated in the discussion of the results.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (22375130, 52130308, and 52373192), the Shenzhen Science and Technology Program (JCYJ20230808105603008), the Research Team Cultivation Program of Shenzhen University (2023DFT004), and the Guangdong Basic and Applied Basic Research Foundation (2024A1515030199). We also thank the Instrumental Analysis Center of Shenzhen University for analytical support.References
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef PubMed.
- S. Madayanad Suresh, D. Hall, D. Beljonne, Y. Olivier and E. Zysman-Colman, Adv. Funct. Mater., 2020, 30, 1908677 CrossRef.
- Y. Liu, J. Cui, K. Du, H. Tian, Z. He, Q. Zhou, Z. Yang, Y. Deng, D. Chen, X. Zuo, Y. Ren, L. Wang, H. Zhu, B. Zhao, D. Di, J. Wang, R. H. Friend and Y. Jin, Nat. Photonics, 2019, 13, 760–764 CrossRef CAS.
- T.-H. Han, K. Y. Jang, Y. Dong, R. H. Friend, E. H. Sargent and T.-W. Lee, Nat. Rev. Mater., 2022, 7, 757–777 CrossRef.
- T. Kim, K.-H. Kim, S. Kim, S.-M. Choi, H. Jang, H.-K. Seo, H. Lee, D.-Y. Chung and E. Jang, Nature, 2020, 586, 385–389 CrossRef CAS.
- A. Pershin, D. Hall, V. Lemaur, J.-C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne and Y. Olivier, Nat. Commun., 2019, 10, 597 CrossRef CAS PubMed.
- Y.-J. Pu, D. Valverde, J. C. Sancho-García and Y. Olivier, J. Phys. Chem. A, 2023, 127, 10189–10196 CrossRef CAS.
- K. R. Naveen, H. I. Yang and J. H. Kwon, Commun. Chem., 2022, 5, 149 CrossRef CAS.
- H. Jiang, J. Jin and W.-Y. Wong, Adv. Funct. Mater., 2023, 33, 2306880 CrossRef CAS.
- X.-F. Luo, X. Xiao and Y.-X. Zheng, Chem. Commun., 2024, 60, 1089–1099 RSC.
- Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Nat. Photonics, 2019, 13, 678–682 CrossRef CAS.
- Y. Sano, T. Shintani, M. Hayakawa, S. Oda, M. Kondo, T. Matsushita and T. Hatakeyama, J. Am. Chem. Soc., 2023, 145, 11504–11511 CrossRef.
- S. Uemura, S. Oda, M. Hayakawa, R. Kawasumi, N. Ikeda, Y.-T. Lee, C.-Y. Chan, Y. Tsuchiya, C. Adachi and T. Hatakeyama, J. Am. Chem. Soc., 2023, 145, 1505–1511 CrossRef PubMed.
- J. Jin, C. Duan, H. Jiang, P. Tao, H. Xu and W.-Y. Wong, Angew. Chem., Int. Ed., 2023, 62, e202218947 CrossRef.
- X.-C. Fan, F. Huang, H. Wu, H. Wang, Y.-C. Cheng, J. Yu, K. Wang and X.-H. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202305580 CrossRef PubMed.
- X. Cai, Y. Pu, C. Li, Z. Wang and Y. Wang, Angew. Chem., Int. Ed., 2023, 62, e202304104 CrossRef PubMed.
- K. R. Naveen, J. H. Oh, H. S. Lee and J. H. Kwon, Angew. Chem., Int. Ed., 2023, 62, e202306768 CrossRef PubMed.
- Y. Zhang, J. Wei, L. Wang, T. Huang, G. Meng, X. Wang, X. Zeng, M. Du, T. Fan, C. Yin, D. Zhang and L. Duan, Adv. Mater., 2023, 35, 2209396 CrossRef CAS PubMed.
- X. Huang, J. Liu, Y. Xu, G. Chen, M. Huang, M. Yu, X. Lv, X. Yin, Y. Zou, J. Miao, X. Cao and C. Yang, Natl. Sci. Rev., 2024, 11, nwae115 CrossRef PubMed.
- L. Yuan, J.-W. Xu, Z.-P. Yan, Y.-F. Yang, D. Mao, J.-J. Hu, H.-X. Ni, C.-H. Li, J.-L. Zuo and Y.-X. Zheng, Angew. Chem., Int. Ed., 2024, 63, e202407277 CrossRef.
- H. Liu, J. Zeng, J. Guo, H. Nie, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2018, 57, 9290–9294 CrossRef PubMed.
- Y. Xu, Z. Cheng, Z. Li, B. Liang, J. Wang, J. Wei, Z. Zhang and Y. Wang, Adv. Opt. Mater., 2020, 8, 1902142 CrossRef.
- Y. Xu, C. Li, Z. Li, Q. Wang, X. Cai, J. Wei and Y. Wang, Angew. Chem., Int. Ed., 2020, 59, 17442–17446 CrossRef PubMed.
- P. Jiang, J. Miao, X. Cao, H. Xia, K. Pan, T. Hua, X. Lv, Z. Huang, Y. Zou and C. Yang, Adv. Mater., 2022, 34, 2106954 CrossRef PubMed.
- Y. Zhang, J. Wei, D. Zhang, C. Yin, G. Li, Z. Liu, X. Jia, J. Qiao and L. Duan, Angew. Chem., Int. Ed., 2022, 61, e202113206 CrossRef PubMed.
- Y.-K. Qu, D.-Y. Zhou, F.-C. Kong, Q. Zheng, X. Tang, Y.-H. Zhu, C.-C. Huang, Z.-Q. Feng, J. Fan, C. Adachi, L.-S. Liao and Z.-Q. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202201886 CrossRef CAS PubMed.
- T. Fan, Y. Zhang, L. Wang, Q. Wang, C. Yin, M. Du, X. Jia, G. Li and L. Duan, Angew. Chem., Int. Ed., 2022, 61, e202213585 CrossRef CAS.
- F.-M. Liu, Z.-H. Qu, P. Zuo, Y.-J. Yu, M.-T. Li, L.-S. Liao, D.-Y. Zhou and Z.-Q. Jiang, ACS Mater. Lett., 2024, 6, 1380–1387 CrossRef CAS.
- X.-F. Luo, H.-X. Ni, X. Liang, D. Yang, D. Ma, Y.-X. Zheng and J.-L. Zuo, Adv. Opt. Mater., 2023, 11, 2203002 CrossRef CAS.
- E. Ravindran, H. E. Baek, H. W. Son, J. H. Park, Y.-H. Kim and M. C. Suh, Adv. Funct. Mater., 2023, 33, 2213461 CrossRef CAS.
- Y.-K. Qu, Q. Zheng, J. Fan, L.-S. Liao and Z.-Q. Jiang, Acc. Mater. Res., 2021, 2, 1261–1271 CrossRef.
- K. Matsui, S. Oda, K. Yoshiura, K. Nakajima, N. Yasuda and T. Hatakeyama, J. Am. Chem. Soc., 2018, 140, 1195–1198 CrossRef PubMed.
- Z. Ye, H. Wu, Y. Xu, T. Hua, G. Chen, Z. Chen, X. Yin, M. Huang, K. Xu, X. Song, Z. Huang, X. Lv, J. Miao, X. Cao and C. Yang, Adv. Mater., 2024, 36, 2308314 CrossRef.
- X.-C. Fan, K. Wang, Y.-Z. Shi, Y.-C. Cheng, Y.-T. Lee, J. Yu, X.-K. Chen, C. Adachi and X.-H. Zhang, Nat. Photonics, 2023, 17, 280–285 CrossRef.
- B. Lei, Z. Huang, S. Li, J. Liu, Z. Bin and J. You, Angew. Chem., Int. Ed., 2023, 62, e202218405 CrossRef PubMed.
- X. Zeng, L. Wang, H. Dai, T. Huang, M. Du, D. Wang, D. Zhang and L. Duan, Adv. Mater., 2023, 35, 2211316 CrossRef.
- J.-L. Brédas, D. Beljonne, V. Coropceanu and J. Cornil, Chem. Rev., 2004, 104, 4971–5004 CrossRef PubMed.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Nat. Commun., 2016, 7, 13680 CrossRef CAS PubMed.
- P. K. Samanta, D. Kim, V. Coropceanu and J.-L. Brédas, J. Am. Chem. Soc., 2017, 139, 4042–4051 CrossRef CAS PubMed.
- K. Stavrou, A. Danos, T. Hama, T. Hatakeyama and A. Monkman, ACS Appl. Mater. Interfaces, 2021, 13, 8643–8655 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 23690102369013. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04835k |
| This journal is © The Royal Society of Chemistry 2024 |