Multi-stimuli responsive photonic hydrogel based on a novel photonic crystal template containing gold nanorods†
Hanlin
Lv‡
,
Jin
Li‡
,
Zhengsheng
Hu
,
Yuhang
Wang
,
Yanjun
Chen
 * and
Yifeng
Wang
* and
Yifeng
Wang
 *
*
School of Materials Science and Engineering, Wuhan University of Technology, Wuhan, 430070, China. E-mail: yanjunchen@whut.edu.cn; yifengwang@whut.edu.cn
First published on 27th November 2023
Abstract
Multi-stimuli responsive photonic hydrogels (MRPHs) fabricated by doping nanoparticles into hydrogels show promising potential value in the fields of visual detection and drug delivery. However, complicated surface chemical modification is selected to improve the compatibility between nanoparticles and a pre-gel solution of hydrogel. Herein, we developed a simple and convenient vertical deposition method to prepare a novel photonic crystal (PC) template containing gold nanorods (Au NRs) (Au NRs/PC template), which could respond to near-infrared (NIR) light due to the conversion capability of Au NRs from NIR light to heat. Additionally, carboxyl groups on the surface of polystyrene (PS) colloids endowed the Au NRs/PC template with pH-stimulus responsiveness. Based on the Au NRs/PC template, MRPH film was fabricated by infiltrating the pre-gel solution of poly(N-isopropylacrylamide) (PNIPAM) hydrogel into the gap of a ‘sandwich’ structure through capillary forces and then polymerizing at 25 °C for 24 h. The obtained MRPH film could respond to NIR light, pH and temperature. Under the irradiation of NIR light, only the irradiated position lost structural color while the film volume had no distinct change. With the increase of ambient temperature, the whole MRPH film completely lost structural color and shrank significantly, which was greatly different from the phenomenon irradiated by NIR light. Besides, the structural color of the MRPH film exhibited a red shift from green to orange-red as the pH increased. Overall, both the Au NRs/PC template and the MRPH film may have potential applications in visual detection, due to their multi-stimuli responsiveness.
1. Introduction
Photonic crystal (PC) with a periodic structure formed by the periodic arrangement of different refractive index media has attracted extensive attention on account of brilliant structural color through Bragg diffraction.1–3 Therefore, a series of PC materials with vivid structural colors have been fabricated, which have significant application value in sensors,4–6 drug delivery,7–9 actuators,10–12 printing13–15 and other fields.16–18 A hydrogel is a stimulus-responsive material due to its elastic network that can shrink and swell, so it is usually combined with PC to fabricate stimulus-responsive photonic hydrogels, which realize a visual conversion to optical signals by changing structural colors in response to external stimuli, such as light,19,20 organic compounds and solvents,21–24 metal ions,25,26 temperature,27–29 and pH.30–32 For example, Zhao et al.33 combined a polymeric hydrogel with an SiO2 inverse opal structure to prepare an inverse opal hydrogel (IOH) sensor which can respond to pH, temperature and ultraviolet light. The polymeric hydrogel was composed of pH and temperature responsive poly(dimethylaminoethyl) methacrylate and a light responsive spiropyran-methacrylate segment. Matsubara et al.34 chose thermosensitive monomer N-isopropylacrylamide and light sensitive monomer 4-acryloylaminoazobenzene to prepare a thermally adjustable multicolor photochromic hydrogel.Stimulus-responsive photonic hydrogels can also be obtained by combining functional nanoparticles, such as graphene oxide quantum dots (GO QDs), gold nanorods (Au NRs), and carbon dots (CDs) with photonic hydrogels. Generally speaking, there are two methods to realize the combination of nanoparticles and photonic hydrogels. One is doping nanoparticles into hydrogel, followed by combining them with PC. For example, Xiao et al.35 modified Au NRs by thiol terminal poly(ethylene glycol) so that they could disperse stably in poly(N-isopropyl acrylamide-co-acrylamide) P(NIPAM-co-AAm) pre-gel solution. Then the pre-gel solution was infiltrated into a two glass slide sandwiched polystyrene (PS) photonic colloid by capillary force. After UV light-initiated polymerization, the Au NRs-polymer hybrid photonic hydrogel was fabricated, which exhibited a near-infrared (NIR) light response. Wang et al.36 dispersed graphene oxide quantum dots (GO QDs) into a biocompatible hydrogel of gelatin methacryloyl (GelMA) and hyaluronic acid methacryloyl (HAMA), and then combined the HAMA/GelMA hydrogel with SiO2 photonic crystals to prepare self-bonded hydrogel inverse opal particles. Attributed to the photothermal conversion capability of GO QDs, amoxicillin and vascular endothelial growth factors (VEGFs) encapsulated in the inverse opal particles were released under NIR light, which were applied for wound tissue healing and drug delivery monitoring. It is worth noting that the compatibility between nanoparticles and pre-gel solutions may limit the choice of hydrogel systems. The other method is to use nanoparticle-functionalized monodisperse colloids to prepare PC templates, followed by the combination of the PC templates and polymeric hydrogels. For example, Xie et al.37 grafted CDs on the surface of polystyrene@poly (methyl methacrylate-co-acrylic acid) (PS@P(MMA-AA)) colloidal particles through covalent bonding. The PC film from CDs/PS@P(MMA-AA) colloidal particles not only showed multiple structural color and fluorescence emission, but also illustrated the sensitive response toward stress and pH. However, nanoparticle-functionalized monodisperse colloids always require a complicated chemical preparation process.
Nanostructured metals such as Ag, Cu and Au, can be nice plasmonic nanostructures.38–40 Moreover, their mesoporous structures show nice plasmonic characteristics like a localized surface plasmon.38,39 Au NRs exhibit an excellent photothermal effect, derived from their plasmonic nanostructures and surface plasmon resonance properties.41 In this work, we present a multi-stimuli responsive photonic hydrogel (MRPH) film based on a novel PC containing an Au NR (Au NR/PC) template. Firstly, Au NR/PC templates are prepared using a simple and convenient vertical deposition co-assembly method. Attributed to the high efficiency of Au NRs in converting specific NIR light to heat, the Au NR/PC template can exhibit a NIR light response. Since the PS colloids used for preparing the Au NR/PC template are synthesized by copolymerization of styrene (St) and acrylic acid (AA), the surface of PS colloids has −COOH groups so that the Au NR/PC template can respond to pH. Next, a pre-gel solution of PNIPAM is infiltrated into a ‘sandwich’ structure formed by covering the Au NRs/PC template on the top of a glass slide. Finally, after polymerization for 24 h at 25 °C, the Au NR/PC template is combined with the PNIPAM hydrogel. Attributed to the temperature sensitivity of the PNIPAM hydrogel and the dual-stimuli responsiveness of the Au NR/PC template, the MRPH film can respond to NIR light, pH and temperature. Both the Au NR/PC template and the MRPH film are fabricated using low-cost and convenient methods. Thus, we can foresee that the MRPH film and the Au NR/PC template have potential application value in the field of visual detection.
2 Experimental section
2.1 Materials
Styrene (St), potassium persulfate (KPS), chloroauric acid (HAuCl4), hexadecyltrimethyl ammonium bromide (CTAB), ascorbic acid, silver nitrate (AgNO3), xanthan gum, N,N′-methylenebisacrylamide (MBA), N,N,N′,N′-tetramethylethylene diamine (TEMED), β-cyclodextrin and concentrated hydrochloric acid (HCl) were purchased from Sinopharm Chemical Reagent Co., Ltd. N-Isopropylacrylamide (NIPAM), acrylic acid (AA) and sodium borohydride (NaBH4) and were purchased from Aladdin Reagent Co., Ltd. Concentrated sulfuric acid was provided by Xinyang chemical reagents. Carbon dots (C-dots) were prepared through low-temperature hydrothermal carbonization.42 Gold nanorods (Au NRs) were prepared using the improved seed mediated growth procedure,43 in which the addition of 1.5 mL 1 mol L−1 HCl was used to prepare Au NRs. The Au NRs were dispersed in water to obtain a burgundy Au NR suspension (Fig. S1(a), ESI†). The TEM image of the Au NR indicates that the Au NRs had a uniform size and a rod-like microstructure (Fig. S1(b), ESI†). The average size of Au NRs analyzed using the software Image-Pro Plus was 60.96 nm × 15.39 nm (length × diameter), and the ratio of the length to diameter was 3.96. The location of the longitudinal surface plasmon resonance (LSPR) absorption peak of the prepared Au NRs was at 808 nm (Fig. S1(c), ESI†). Styrene was washed three times with 10 wt% NaOH solution before use. Deionized water was produced by deionization and filtration using a Millipore device (resistivity = 18.2 MΩ cm−1). The glass slides (76.2 × 25.4 mm) were treated using a mixed solution of concentrated sulfuric acid and H2O2 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) for 48 h to improve their hydrophilicity.
3) for 48 h to improve their hydrophilicity.
2.2 Preparation of monodisperse PS colloids
The preparation of monodisperse PS colloids with different particle sizes was achieved by soap-free emulsion polymerization. First, 0.6 g KPS and 0.65 g AA were dissolved in 170 mL deionized water in a four-necked flask equipped with a mechanical stirrer and stirred for 10 min at a speed of 300 rpm under the protection of N2 to mix the above solutions evenly and remove O2. Then, the reaction kettle was slowly heated to 75 °C in an oil bath, followed by adding 15 mL of refined St into a constant pressure dropping funnel, in which 1/3 of monomer St was slowly dropped into the reaction kettle. When blue light appeared in the reaction system, the rest of St was dropped into the mixture. After the polymerization continued for 8 h, monodisperse PS colloid latex was obtained. Five kinds of PS colloids with different particle sizes (258, 295, 340, 396, and 458 nm) were fabricated by changing the amount of St, AA and KPS, which were named as PS258, PS295, PS340, PS396 and PS458, respectively.2.3 Preparation of the Au NRs/PC template
Typically, 1 mL of a 0.5 mg mL−1 Au NR dispersion with an LSPR absorption peak at 808 nm and PS colloid latex were mixed in 80 mL of deionized water, in which the content of PS colloids was 0.1 wt%. Then the mixed dispersion was ultrasonically treated for 0.5 h, followed by vertically inserting the glass slide into the mixed dispersion. Then, the Au NRs/PC template was obtained through the vertical deposition self-assembled method at 60 °C. The Au NR/PC templates formed by depositing PS258, PS295, PS340, PS396 and PS458 were labeled as Au NRs/PC258, Au NRs/PC295, Au NRs/PC340, Au NRs/PC396 and Au NRs/PC458, respectively.2.4 Preparation of the MRPH film
Synthesis of the pre-gel solution of PNIPAM hydrogel: briefly, 0.02 g C-dots were dispersed in 8 mL deionized water, and ultrasonic dispersion was carried out for 0.5 h. Then, 0.02 g xanthan gum, 1.5 g NIPAM and 0.02 g MBA were sequentially added into the above dispersion until it was completely dissolved with the help of magnetic stirring. Then, the mixed solution was moved to an ice bath environment and stirred continuously for 1 h under the protection of N2. Additionally, 0.01 g KPS and 11 μL TEMED were added to the mixed solution and stirred vigorously for 1 min. Finally, the excess bubbles were removed by ultrasonic wave in an ice bath for 3 min and the pre-gel solution was obtained for later use.2.5 Characterization
The morphology of Au NRs was observed with a field emission high-resolution transmission electron microscope (TEM, JEM-2100F, Electronics, Japan) at an accelerating voltage of 80 kV. Before the observation, 10 μL of the Au NR dispersion was diluted with deionized water, and then dropped onto a copper net to be dried naturally.The characteristic plasmon resonance absorption peak of Au NRs was measured using an ultraviolet and visible spectrophotometer (UV-2550, Shimadzu, Japan) at room temperature. An Au NR dispersion was diluted three times before testing. The scanning wavelength range was 400–1000 nm and the incident light was perpendicular to the sample.
An optical fiber spectrometer (USB 2000+, Ocean Optical Company, USA) was used to record the reflection spectra of the Au NRs/PC template and the MRPH film at room temperature. The scanning wavelength range was 400–800 nm, and the incident light was perpendicular to the sample.
A cold field emission scanning electron microscope (SEM, JSM-7500F, Electronics, Japan) was used to observe the surface morphology of the Au NRs/PC template and the MRPH film at an accelerating voltage of 5.0 kV. Before observation, the MRPH film was frozen with liquid nitrogen, and then lyophilized for 24 h to obtain a completely freeze-dried film sample. All the samples were coated with gold using a sputter coater.
The 808 nm NIR laser (MDL-H-808-5W, Changchun New Industry Photoelectric Technology Co., Ltd, China) was used to explore the photothermal effect of Au NR/PC templates and MRPH films under different NIR intensities (1 W cm−2, 2 W cm−2 and 3 W cm−2). The distance between the sample and the light source probe was fixed at 5 cm. The temperature changes of the sample surface were recorded using an infrared thermometer (AT380, Xima industry, China). Before exploration, MRPH film was put into water (pH = 7) to reach swelling equilibrium and the water on the surface of the MRPH film was absorbed by filter paper.
After reaching the swelling equilibrium, the MRPH film was placed on a heating stage with a temperature controlling module to perform the thermosensitive test. The pH-sensitive test of MRPH films was carried out at 25 °C by touching aqueous solutions with different pH values.
The swelling ratio (SR) of MRPH film was calculated according to the mass change of hydrogel before and after swelling, as defined in formula (1)
 | (1) |
3 Results and discussion
3.1 Preparation and morphology of the Au NR/PC template
The vertical deposition method for the preparation of the Au NRs/PC template is schematically illustrated in Fig. 1(a). In this procedure, with the evaporation of water, PS colloids are uniformly self-assembled by capillary force on the glass slide. Since the average size of Au NRs is 60.96 nm × 15.39 nm and the widest voids of PS colloids are around 63 nm, Au NRs can be held in the voids of PS colloids.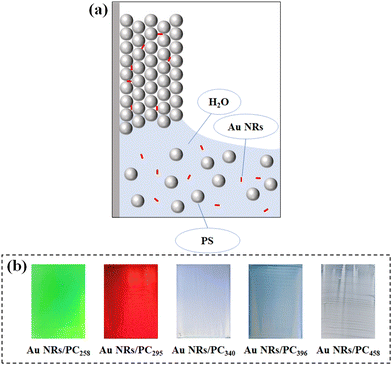 | ||
| Fig. 1 (a) Schematic diagram of the preparation for the Au NR/PC template. (b) Optical photos of the Au NR/PC template assembled by PS colloids with different particle sizes. | ||
Fig. 1(b) exhibits the structural colors of Au NR/PC templates formed using different PS colloid sizes. Visibly, Au NR/PC258 and Au NR/PC295 templates show bright green and red structural colors, respectively. The structural colors of Au NRs/PC340, Au NRs/PC396 and Au NRs/PC458 templates are pale blue, blue and white, respectively. With the increase of the particle size of PS colloids, the structural colors of Au NRs/PC templates become paler and even disappear. The theoretical calculation formula of the reflection wavelength is as follows:
| λmax = 2.397D | (2) |
Because the wavelength range of light visible to the naked eye is about 390–780 nm, the Au NR/PC template can show bright structural colors only when the theoretical particle size of the PS colloids is about 163–325 nm according to formula (1). The average sizes of PS258 and PS295 are between 163 and 325 nm so that Au NRs/PC258 and Au NRs/PC295 templates show bright green and red structural colors, respectively. However, the average sizes of PS340, PS396 and PS458 are beyond the theoretical range, so the structural colors of the Au NRs/PC templates formed by them are pale or even lost.
In addition, both the concentration of PS colloids and the amount of Au NRs in the assembly solution affect the structural color of the Au NR/PC templates. In order to investigate the influence of the PS colloids’ concentration, PS258 colloids were selected and the amount of Au NRs was fixed at 0.5 mg. As shown in Fig. 2, the Au NR/PC templates formed are flat and uniform, and also show bright and vivid structural colors, when the concentration of PS258 colloids is between 0.1 wt% and 0.5 wt%. However, with the concentration of PS258 colloids increasing from 0.7 wt% to 1.0 wt%, the Au NR/PC templates become rough and non-uniform, and the structural colors start disappearing, which is due to the irregular arrangement of PS colloids caused by the deposition acceleration with the increase of PS258 colloid concentration. As a consequence, 0.1 wt% is an optimal concentration of PS258 colloids and was selected for further studies.
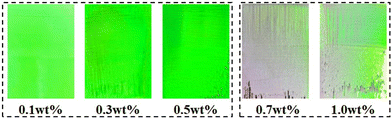 | ||
| Fig. 2 Optical photos of Au NR/PC258 templates assembled with different PS258 colloid concentrations. | ||
Fig. 3(a) shows the optical photos of the Au NRs/PC258 templates with different amounts of Au NRs when the concentration of PS258 colloids was fixed at 0.1 wt%. When the amount of Au NRs varies from 0 to 2.5 mg, the structural colors of the Au NR/PC258 templates are yellow-green and have little difference with each other. However, the structural colors of the Au NR/PC258 templates are completely lost when the amount of Au NRs reaches 3.5 mg and 4.5 mg. The reflection spectra of the Au NRs/PC258 templates with the corresponding amount of Au NRs were recorded in Fig. 3(b). It can be found that with the amount of Au NRs increasing from 0 mg to 4.5 mg, the reflection peaks of Au NRs/PC258 templates are about 572 nm, with no obvious red shift or blue shift. The reflection of the reflection peak decreases slightly first, but drops greatly when the amount of Au NRs is over 2.5 mg. It is indicating that when the amount of Au NRs exceeds the void capacity of PS colloids, redundant Au NRs stop the formation of the three-dimensional face-centered cubic structure of the Au NR/PC258 template, so the template impossibly presents structural colors.
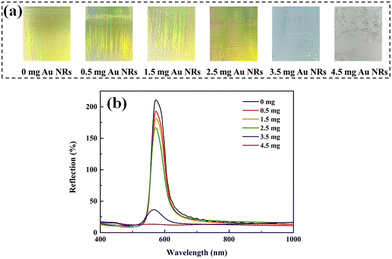 | ||
| Fig. 3 (a) Optical photos of the Au NR/PC258 template with different amounts of Au NRs. (b) The reflection spectra of the Au NRs/PC258 template under conditions with different amounts of Au NRs. | ||
As a result, the optimal concentration of PS258 colloids and the amount of Au NRs are 0.1 wt% and 0.5 mg, respectively, and Au NRs/PC258 templates are selected for further study of dual-stimuli responsiveness.
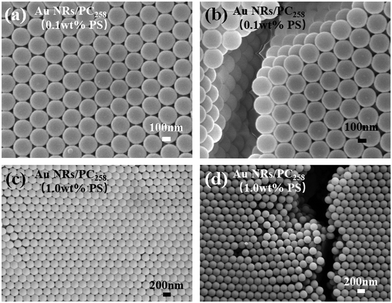
SEM images of Au NR/PC258 templates prepared under two different concentrations of PS258 colloids are shown in Fig. 4. When the concentration of PS258 colloids is 0.1 wt%, the PS258 colloids are uniform in size and arranged orderly in the Au NRs/PC258 template, as shown in Fig. 4(a). The average face center distance of PS colloids is 116 nm, calculated from Fig. 4(a). Fig. 4(b) presents a SEM cross-section image of the Au NRs/PC258 template. Distinctly, the superposition of multiple layers of regular PS colloids constitutes a three-dimensional face-centered cubic Au NR/PC258 template, which can illuminate the brilliant structural color of the template generated from 0.1 wt% PS258 colloids (Fig. 2). When the PS258 colloid concentration increases to 1.0 wt%, most PS258 colloids are still arranged orderly, as shown in Fig. 4(c). However, the sectioned SEM image (Fig. 4(d)) shows that there are irregular arrangements in some areas. These obvious defects give a reason why the template from 1.0 wt% PS258 colloids has a rough surface without brilliant structural color (Fig. 2).
3.2 Dual stimuli responsiveness of Au NR/PC templates
In order to explore the NIR-stimulus responsiveness of the Au NR/PC258 template, we put the Au NR/PC258 template under different NIR intensities and measured the temperature increase rate of the Au NR/PC258 template, as shown in Fig. 5. When the NIR intensity is 1 W cm−2, the temperature increase rate of the Au NR/PC258 template is only 0.16 °C s−1. When the NIR intensity is increased from 1 W cm−2 to 2.0 W cm−2, the temperature increase rate of the Au NR/PC258 template changes slightly from 0.16 °C s−1 to 0.88 °C s−1. In particular, the temperature increase rate of the Au NR/PC258 template changes significantly, from 0.88 °C s−1 to 3.85 °C s−1 when the NIR intensity reaches 3.0 W cm−2.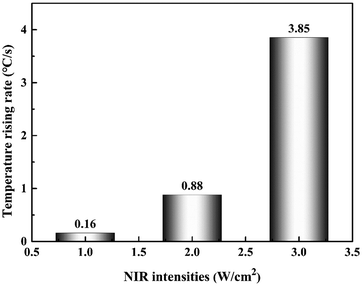 | ||
| Fig. 5 Histogram of the temperature increase rate of the Au NR/PC258 template under different NIR intensities. | ||
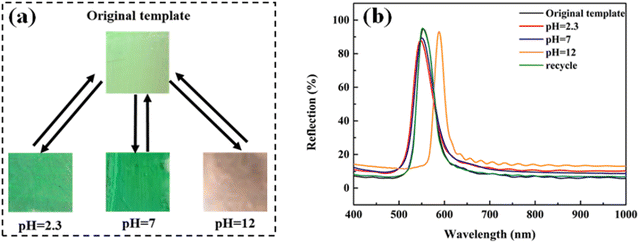
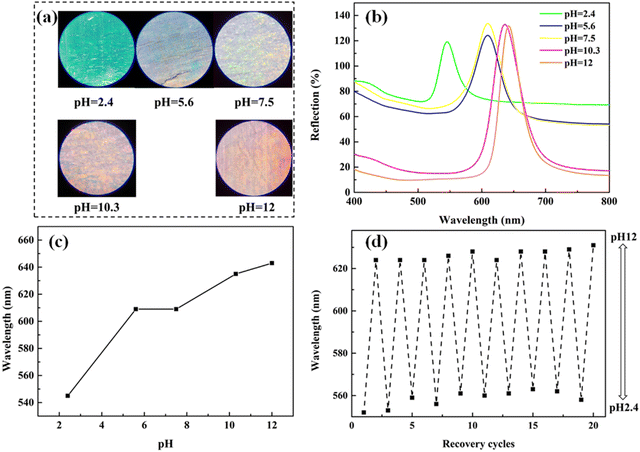
As PS colloids are prepared using a soap-free emulsion polymerization of St with a small amount of AA, the surface of PS colloids has hydrophilic −COOH groups, which provides the Au NR/PC258 template with pH-stimulus responsiveness. To study the pH-stimulus responsiveness of the Au NR/PC258 templates, aqueous solutions of pH 2.3, pH 7 and pH 12 were dropped on the Au NR/PC258 templates respectively. As shown in Fig. 6(a), after touching aqueous solutions of pH 2.3 or pH 7, the structural colors of the Au NR/PC258 templates change little, from light green to green. However, when the alkaline solution with a pH of 12 is dropped on the template, a great change happens, the structural color turns to orange. Moreover, the structural colors of the Au NR/PC258 templates return back to green after the aqueous solutions of pH 2.3, pH 7 and pH 12 evaporate completely. Fig. 6(b) displays the changes in the reflection spectra of the Au NR/PC258 templates under different pH conditions (2.3, 7 and 12). Compared to the original reflection peak of the Au NR/PC258 template (at 554 nm), the reflection peaks have no obvious shift at pH 2.3 and 7. However, the reflection peak of the template shifts to longer wavelengths (from 554 nm to 589 nm) at pH 12. After the alkaline solution on the template evaporates completely, the maximum reflection wavelength returns to 552 nm, close to the initial value. Because the refractive index of water is 1.33, according to the modified Bragg diffraction formula, the theoretical value of the reflection peak after filling aqueous solutions is 578 nm, which is due to the fact that the air in the voids of PS258 colloids is replaced by the water with a higher refractive index. However, the reflection peak moves to 589 nm at pH 12, because the −COOH groups on the surface of PS colloids are deprotonated to form carboxylate ions (−COO−) under alkaline conditions. The electrostatic repulsion between carboxylate ions causes the face-center distance between PS colloids in the template to increase, so the red shift of the reflection peak occurs. After the water evaporation of the alkaline solution, the electrostatic repulsion between PS colloids disappears. Therefore, the face-center distance between PS colloids returns to the initial value, which leads to a blue-shift of the reflection peak (from 589 nm back to 551 nm). Under the conditions of pH 7 or 2.3, the electrostatic repulsion between PS colloids is too small or disappears, so the structural color and the reflection peak of the Au NRs/PC258 template are not obviously affected.
3.3 Preparation and morphology of the MRPH film
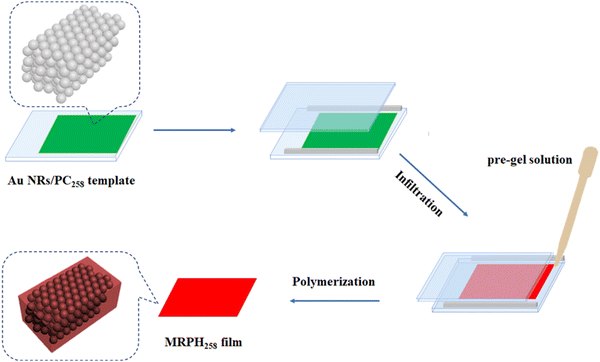
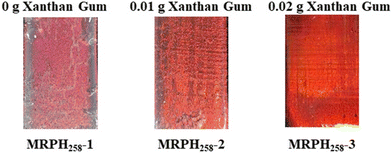
The schematic fabrication process of the MRPH film is shown in Fig. 7. We selected the green Au NR/PC258 template to fabricate the MRPH film. The pre-gel solution of PNIPAM hydrogel infiltrates the gaps of PS colloids under the capillary action. After a free radical polymerization of NIPAM, an orange-red MRPH258 film is obtained. The color difference between the Au NR/PC258 template and the MRPH258 film is due to the fact that the air in the voids of the PS colloids is replaced by the PNIPAM hydrogel with a greater refractive index than the air. Since PS258 colloids are physically stacked on the glass slide, the face-centered cubic structure formed by them is easily destroyed by an external force. Therefore, how to keep the integrity of the face-centered cubic structure is the key problem in the infiltrating procedure of the pre-gel solution. In order to solve this problem, xanthan gum is introduced into the hydrogel system to adjust the viscosity of the pre-gel solution of PNIPAM.The optical photos of the MRPH258 film prepared with different amounts of xanthan gum are shown in Fig. 8. The structural color of the MRPH258-1 film without xanthan gum is orange-red but not bright, and there is some white crazing in the middle area, indicating that the face-centered cubic structure of the template is damaged during the infiltrating process of the pre-gel solution. After adding 0.01 g xanthan gum, the structural color of the MRPH258-2 film becomes brighter and the crazing content decreases obviously. When the amount of xanthan gum increases to 0.02 g, a perfect MRPH258-3 film gum is formed, with a bright orange-red structural color and smooth surface. The reason is that the addition of xanthan gum increases the viscosity of the pre-gel solution appropriately, which prevents the face-centered cubic structure from being destroyed during the infiltrating process. Therefore, the optimal amount of xanthan gum is 0.02 g.
In addition, the amount of MBA can influence the structural color of the MRPH258 film through changing the crosslinking density of the MRPH258 film. With the addition of 0.02 g xanthan gum, and varying the amount of MBA from 0.01 to 0.05 g, MRPH258-4, MRPH258-5, MRPH258-6, MRPH258-7 and MRPH258-8 films can be obtained. As shown in Fig. 9(a), the structural colors of five MRPH258 films are all red, with few changes at different MBA amounts before the films swell. However, after reaching the swelling equilibrium, the MRPH258-4, MRPH258-5, MRPH258-6, MRPH258-7 and MRPH258-8 films exhibit different structural colors, white, red, orange, yellow and green, respectively. Fig. 9(b) shows the relationship between the swelling ratios of the MRPH258 films and MBA amount. With the amount of MBA increasing, the swelling ratio decreases gradually. According to previous research reports,33 the increase of swelling ratio can make the maximum reflection wavelength increase. When the amount of MBA is 0.01 g, the crosslinking density of the MRPH258-4 film is so low that the MRPH258-4 film absorbs more water and the swelling ratio is above 2.0. Therefore, the maximum reflection wavelength of the MRPH258-4 film is beyond the visible wavelength range and the structural color of the MRPH258-4 film is not seen by naked eyes. When the amount of MBA increases from 0.02 to 0.05 g, the increasing crosslinking densities of the MRPH258 films make the swelling ratio of the MRPH258 films decrease, which causes the maximum reflection wavelengths of the MRPH258 films to decrease and they are located in the visible wavelength range. Therefore, the MRPH258-5, MRPH258-6, MRPH258-7 and MRPH258-8 films show different bright structural colors, respectively. It is noteworthy that the structural color of the MRPH258-5 film is almost the same before and after swelling when the amount of MBA is 0.02 g. Therefore, the amount of both xanthan gum and MBA are fixed at 0.02 g in the following multi-stimuli responsiveness study.
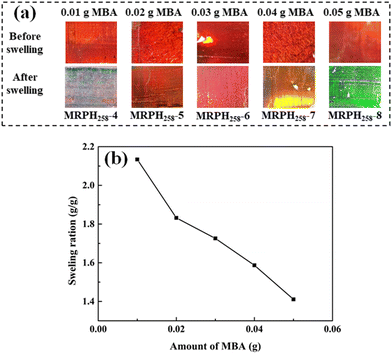 | ||
| Fig. 9 (a) Optical photos of the MRPH258-film with different amounts of MBA. (b) Swelling ratios of the MRPH258 film under different amounts of MBA. | ||
SEM images of the Au NR/PC258 template and MRPH258-5 film are shown in Fig. 10(a) and (b), respectively. Obviously, the voids of the PS258 colloids are completely filled with PNIPAM hydrogel. The PS colloids show a regular face-centered cubic structure, indicating that the structure of the Au NRs/PC258 template is completely preserved during the preparation process of the MRPH258-5 film. According to the calculations from Fig. 10(a) and (b), the average face center distance of PS colloids slightly increases from 116 nm to 118 nm, after the PNIPAM hydrogel fills the Au NR/PC258 template. As shown in Fig. 10(c), the maximum reflection wavelengths of the Au NR/PC258 template and MRPH258-5 film are at 570 nm and 600 nm, respectively, which is due to the fact that the air in the voids of the PS colloids are replaced by hydrogel with a higher refractive index than air.
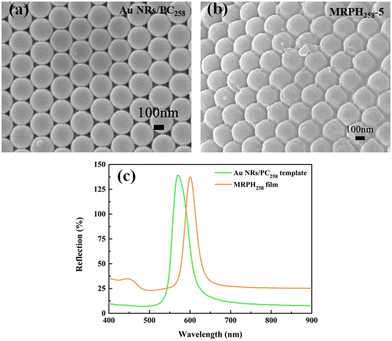 | ||
| Fig. 10 (a) SEM diagram of the Au NR/PC258 template. (b) SEM image of the MRPH258-5 film. (c) The reflection spectra of the Au NR/PC258 template and MRPH258-5 film. | ||
3.4 Multi-stimuli responsiveness of the MRPH film
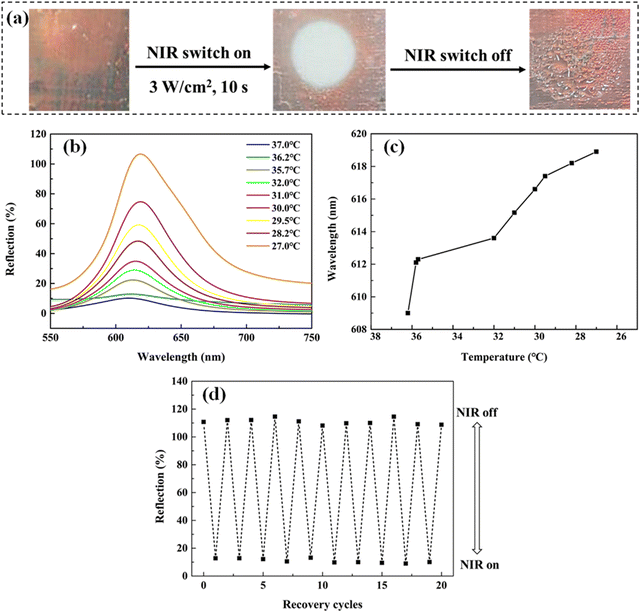
Under the irradiation of NIR light, the Au NRs embedded in the MRPH258-5 film at the irradiation position absorb NIR light and convert it into heat, so the surface temperature of the irradiation position increases rapidly and is higher than the LCST of the PNIPAM hydrogel in the MRPH258-5 film, which leads to the phase change of part of the PNIPAM hydrogel network from the hydrophilic state to hydrophobic state. Therefore, the MRPH258-5 film shows an opaque white color and the water in the film is “squeezed out”, just, in the irradiation position, but the positions without irradiation have no change. After the NIR light is removed, the surface temperature of the irradiation position drops, so the PNIPAM hydrogel network gradually turns from the hydrophobic state to the hydrophilic state. Squeezed-out water molecules are absorbed by the PNIPAM hydrogel again and the swelling ratio of the MRPH258-5 film changes back. Therefore, the orange-red structural color appears again and the maximum reflection wavelength of the MRPH258-5 film shifts to a longer wavelength during this recovery process.
Furthermore, we switch NIR light on or off 10 times to evaluate the recoverability of the NIR-stimulus responsiveness of the MRPH258-5 film. It is found that the appearance of the MRPH258-5 film has no change through observations with the naked eye. More importantly, the responsiveness of structural color remains quick as usual, and the reflection peak has no red or blue shift during 10 switching cycles. It is also found from Fig. 11(d) that the intensity of the maximum reflection peak at 619 nm has no obvious fluctuation. These results demonstrate that the NIR-stimulus responsiveness of the MRPH258-5 film has good recoverability.
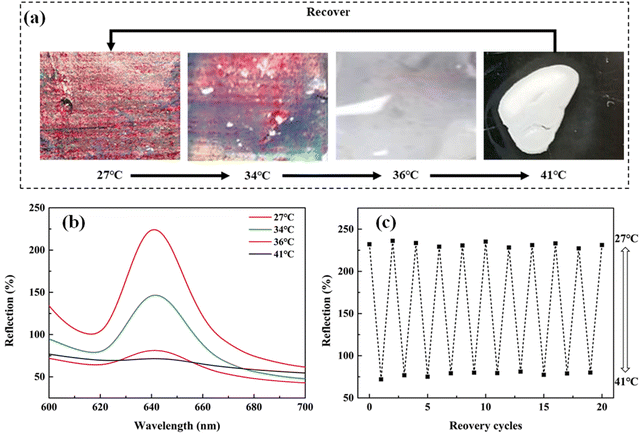
The difference in the temperature-stimulus responsiveness from NIR-stimulus responsiveness is that both the structural color and the film volume change with the increase of ambient temperature in the whole MRPH258-5 film. Since the irradiation of NIR light just causes local temperature increase, the changes just happen in the irradiation position of the film. However, when the ambient temperature exceeds 36 °C, the whole network of the PNIPAM hydrogel in the MRPH258-5 film changes from the hydrophilic state to hydrophobic state. A large amount of water in the film is squeezed out, so the volume of the whole film shrinks and the whole film exhibits a white color.
4 Conclusions
In summary, we have proposed a simple and convenient vertical deposition method for the fabrication of Au NR/PC templates, which can respond to NIR light and pH. When the voids of PS colloids were larger than the average size of the Au NRs, the Au NRs can be held in the voids of PS colloids. The optimal formulation for preparing the Au NR/PC template with bright structural color and a flat surface was that the concentration of PS258 was 0.1 wt% and the amount of Au NRs was 0.5 mg. Based on the Au NR/PC template, the MRPH film was prepared using the common method, infiltrating the pre-gel solution of PNIPAM into the Au NR/PC template and polymerizing under mild conditions (25 °C for 24 h). Typically, the introduction of xanthan gum was used to adjust the viscosity of the pre-gel solution of PNIPAM to prevent the face-centered cubic structure from being destroyed during the infiltrating process. The MRPH film was sensitive to various external stimuli including NIR light, temperature and pH. Under the irradiation of NIR light, the structural color was lost only at the irradiated position while the film volume had no obvious change. However, with the increase in ambient temperature, the whole MRPH258-5 film completely lost structural color and shrank significantly. Furthermore, the structural color of the MRPH258-5 film exhibited a red shift from green to orange-red as the pH increased. Therefore, both the Au NR/PC template and MRPH film have great potential application in visual detection materials. The simple and convenient fabrication method is beneficial to achieving industrial production of the Au NR/PC template and MRPH film.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China, China (Grant No. 51873167) and the National Innovation and Entrepreneurship Training Program for College Students (Grant No. CY202124).References
- R. K. Cersonsky, J. Antonaglia, B. D. Dice and S. C. Glotzer, Nat. Commun., 2021, 12, 2543 CrossRef CAS PubMed.
- P. Wu, J. Wang and L. Jiang, Mater. Horiz., 2020, 7, 338–365 RSC.
- Z. Cai, Z. Li, S. Ravaine, M. He, Y. Song, Y. Yin, H. Zheng, J. Teng and A. Zhang, Chem. Soc. Rev., 2021, 50, 5898–5951 RSC.
- S. Yoon, H. Park and W. Lee, Lab Chip, 2021, 21, 2997–3003 RSC.
- S. Chen, Q. Ren, K. Zhang, W. E. I. Sha, T. Hao, H. Xu, J. Zhao and Y. Li, Sens. Actuators, B, 2022, 347, 131326 CrossRef.
- J. Shin, S. G. Han and W. Lee, Sens. Actuators, B, 2012, 168, 20–26 CrossRef CAS.
- L. Z. Liu, X. Y. Sun, Z. Y. Yan and B. F. Ye, New J. Chem., 2021, 45, 7893–7899 RSC.
- X. Shou, Y. Liu, D. Wu, H. Zhang, Y. Zhao, W. Sun and X. Shen, Chem. Eng. J., 2021, 408, 127349 CrossRef CAS.
- X. Sun, L. Liu, H. Zou, C. Yao, Z. Yan and B. Ye, Int. J. Nanomed., 2020, 15, 4959–4967 CrossRef CAS PubMed.
- D. Zhang, J. Liu, B. Chen, Y. Zhao, J. Wang, T. Ikeda and L. Jiang, ACS Nano, 2018, 12, 12149–12158 CrossRef CAS PubMed.
- Z. Zhang, Z. Chen, Y. Wang, J. Chi, Y. Wang and Y. Zhao, Small Methods, 2019, 1900519 CrossRef CAS.
- R. Yu, L. Zhu, Y. Xia, J. Liu, J. Liang, J. Xu, B. Wang and S. Wang, Adv. Mater. Interfaces, 2022, 9, 220041 Search PubMed.
- Y. Wang, Q. Zhao and X. Du, Mater. Horiz., 2020, 7, 1341–1347 RSC.
- C. Zhao, H. Li, Y. Wang, K. Li, J. Hou, Y. Ma, M. Li and Y. Song, Adv. Opt. Mater., 2019, 1900127 CrossRef.
- A. T. L. Tan, S. Nagelberg, E. Chang-Davidson, J. Tan, J. K. W. Yang, M. Kolle and A. J. Hart, Small, 2020, 16, 1905519 CrossRef CAS PubMed.
- J. Hu, S. Yang, Z. Chen, Y. Chen and J. Wei, ACS Appl. Polym. Mater., 2023, 5, 1002–1013 CrossRef CAS.
- S. Wu, J. Nan, Y. Wu, Z. Meng and S. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 27048–27055 CrossRef CAS PubMed.
- Y. Shang, Z. Chen, F. Fu, L. Sun, C. Shao, W. Jin, H. Liu and Y. Zhao, ACS Nano, 2019, 13, 796–802 CrossRef CAS PubMed.
- Y. Xia, S. Gao, R. Yu, Z. Zeng, H. He, X. Zhou, Y. Hu, M. Cao and S. Wang, ACS Appl. Polym. Mater., 2021, 3, 757–764 CrossRef CAS.
- X. Fei, T. Lu, J. Ma, S. Zhu and D. Zhang, Nanoscale, 2017, 9, 12969–12975 RSC.
- H. Zhao, J. Gao, Z. Pan, G. Huang, X. Xu, Y. Song, R. Xue, W. Hong and H. Qiu, J. Phys. Chem. C, 2016, 120, 11938–11946 CrossRef CAS.
- M. Guo, X. Q. Yu, J. Zhao, J. W. Wang, R. K. Qing, J. D. Liu, X. Wu, L. Zhu and S. Chen, Sens. Actuators, B, 2021, 347, 130639 CrossRef CAS.
- Y. Zhang, J. Tang, K. Feng, H. Zhao, W. Yan, Z. Sun, F. Wu, Y. Sun and J. Gao, React. Funct. Polym., 2020, 148, 104504 CrossRef CAS.
- Y. Cao, G. Liu, B. Zheng, X. Wang, H. Li, G. Wang, L. Zhao and Y. Wang, Soft Matter, 2021, 17, 4969–4978 RSC.
- Y.-L. Wang, X. Wang, H.-R. Yu, T. Liang, X.-B. Lv and C.-J. Cheng, Soft Matter, 2023, 19, 4880–4890 RSC.
- W. Hong, X. Hu, B. Zhao, F. Zhang and D. Zhang, Adv. Mater., 2010, 22, 5043–5047 CrossRef CAS PubMed.
- C. Li, Q. Xue, Z. Ji, Y. Li, H. Zhang and D. Li, Soft Matter, 2020, 16, 3063–3068 RSC.
- K. Ueno, K. Matsubara, M. Watanabe and Y. Takeoka, Adv. Mater., 2007, 19, 2807–2812 CrossRef CAS.
- L. Cai, Y. Wang, L. Sun, J. Guo and Y. Zhao, Adv. Opt. Mater., 2021, 2100831 CrossRef CAS.
- M. L. Zhang, F. Jin, M. L. Zheng and X. M. Duan, RSC Adv., 2014, 4, 20567–20572 RSC.
- N. Griffete, H. Frederich, A. Maître, S. Ravaine, M. M. Chehimi and C. Mangeney, Langmuir, 2012, 28, 1005–1012 CrossRef CAS PubMed.
- J. Wang, Y. Hu, R. Deng, R. Liang, W. Li, S. Liu and J. Zhu, Langmuir, 2013, 29, 8825–8834 CrossRef CAS PubMed.
- W. Zhao, M. Quan, Z. Cao, Y. Zhang, J. Wen, D. Pan, Z. Dong, Z. Yang, D. Wang, H. Cao and W. He, Colloids Surf., A, 2018, 554, 93–99 CrossRef CAS.
- K. Matsubara, M. Watanabe and Y. Takeoka, Angew. Chem., Int. Ed., 2007, 46, 1688–1692 CrossRef CAS PubMed.
- X. Xiao, D. Shi, Z. Yang, Q. Yu, D. Kaneko and M. Chen, New J. Chem., 2021, 45, 4016–4023 RSC.
- L. Wang, L. Sun, F. Bian, Y. Wang and Y. Zhao, ACS Nano, 2022, 16, 2640–2650 CrossRef CAS PubMed.
- A. Q. Xie, J. Guo, L. Zhu and S. Chen, Chem. Eng. J., 2021, 415, 128950 CrossRef CAS.
- H. Lim, D. Kim, G. Kwon, H. J. Kim, J. You, J. Kim, M. Eguchi, A. K. Nanjundan, J. Na and Y. Yamauchi, J. Phys. Chem. C, 2020, 124, 23730–23737 CrossRef CAS.
- H. Lim, D. Kim, Y. Kim, T. Nagaura, J. You, J. Kim, H. J. Kim, J. Na, J. Henzie and Y. Yamauchi, J. Mater. Chem. A, 2020, 8, 21016–21025 RSC.
- H. Lim, T. Nagaura, M. Kim, K. Kani, J. Kim, Y. Bando, S. M. Alshehri, T. Ahamad, J. You, J. Na and Y. Yamauchi, RSC Adv., 2020, 10, 8309–8313 RSC.
- C. Han, M. Y. Qi, Z. R. Tang, J. Gong and Y. J. Xu, Nano Today, 2019, 27, 48–72 CrossRef CAS.
- M. Hu, Y. Yang, X. Gu, Y. Hu, J. Huang and C. Wang, RSC Adv., 2014, 4, 62446–62452 RSC.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sm01349a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |