Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Using soapnut extract as a natural surfactant in green chemistry education: a laboratory experiment aligning with UN SDG 12 for general chemistry courses†
Zi
Wang
a,
Carter
McLenahan
b and
Liza
Abraham
 *a
*a
aGordon College, 255 Grapevine Rd., Wenham, MA 01984, USA. E-mail: liza.abraham@gordon.edu
bAmbrose University, 150, Ambrose Cir SW, Calgary, AB T3H 0L5, Canada
First published on 18th September 2024
Abstract
Green chemistry education has gained significant momentum, with its emphasis on sustainable practices and the 12 principles of green chemistry. These principles aim to reduce waste, use safer solvents, and promote renewable resources in chemical processes. Integrating these principles into laboratory curricula fosters critical thinking about chemical impacts on the environment and society. The experiment involved extracting saponin from soapnut, testing its surfactant properties, and applying it in DNA extraction, and micellar extractions of pollutants like hexavalent chromium and methylene blue dye. Utilization of soapnut as a sustainable alternative to synthetic surfactants aligns with UN Sustainable Development Goal 12-Responsible Consumption and Production. Overall, this laboratory activity integrates green chemistry principles, sustainable development goals, and environmental stewardship, offering students practical experience in environmentally friendly practices and supporting SDG 4 (Quality Education) by enhancing learning through hands-on experimentation.
Sustainability spotlightThe laboratory experiment using soapnut extract as a natural surfactant represents a significant advancement in green chemistry education and aligns with UN Sustainable Development Goal (SDG) 12 – Responsible Consumption and Production. This experiment addresses the critical need to reduce reliance on synthetic surfactants, which have significant environmental impacts due to their persistence and potential toxicity. By extracting saponin from soapnut and demonstrating its surfactant properties through various tests, including foam formation, surface tension reduction, and emulsification, students gain practical insights into sustainable chemical practices. Moreover, the application of soapnut extract in environmental remediation, such as removing hexavalent chromium and methylene blue dye from water through micellar extractions, underscores its potential as a cleaner alternative. This integration of green chemistry principles not only enhances students' understanding of chemical processes but also prepares them to innovate towards sustainable solutions, contributing directly to SDG 4 – Quality Education by fostering critical thinking and practical skills in green chemistry and environmental stewardship. |
Introduction
Green chemistry education1–20 has experienced tremendous growth in recent years. It has become increasingly relevant to teach students the 12 principles of green chemistry21 with the goal to prevent waste, design processes with atom economy, avoid hazardous reactants/reagents/products, utilize safer solvents, and renewable raw materials. It is strategic to train students with a system thinking22–26 and a holistic approach to design and conduct chemical reactions and processes that are sustainable, and safer for both human health and environment.By purposefully incorporating green chemistry principles into the laboratory curriculum,27–34 students are afforded the opportunity to think critically about the impact of chemicals on the environment and society, which they may not otherwise have. This can inspire them to develop skills in waste reduction, pollution prevention, and the utilization of renewable resources.
It is equally important to educate students about the UN Sustainability Development Goals (SDGs)35–41 and to encourage them to critically reflect on these goals, particularly in relation to their daily choices and the environmental impact of common substances like surfactants. By examining the use of surfactants, students can reflect on their own consumption patterns and explore how natural alternatives, such as soapnut extract, contribute to more sustainable practices. This reflection is crucial for understanding the environmental and societal impacts of chemicals.
Utilizing greener and more sustainable approaches in the laboratory curriculum27,30,31 directly contributes to achieving these SDGs. One key SDG, goal 12,42 focuses on ensuring sustainable consumption and production patterns. This goal aims to promote efficient use of resources and reduce dependence on non-renewable sources such as petroleum.
Incorporating renewable chemicals and materials into laboratory practices is essential for promoting responsible consumption and production. This approach not only aligns with goal 12 but also contributes to broader efforts in advancing sustainable development worldwide. By educating students on these principles and integrating them into practical applications, we empower future generations to contribute positively to global sustainability efforts.
Surfactants43 are surface-active compounds that lower the surface tensions at the interface. Surfactants are amphiphilic molecules, they align themselves at the interface with the hydrophobic part oriented in air, and the hydrophilic part is in water. Surfactants can be neutral, anionic, cationic or zwitterionic. The amphiphilic nature of surfactants makes them suitable for use in several industrial products, including medicines,44 textiles,45 polymers,46 pesticides,47 paper,48 personal care products,49 corrosion inhibitors50 for protecting steel and other corrosive metals, detergents,51 de-emulsifiers,52 wetting agents,53 oil recovery enhancers,54 pharmaceutical formulations55 and drug delivery56. The global market size of surfactants is currently about 42.1 billion US dollars, and it is projected to reach $52.4 bn by 2025.57 Surfactants are among the most challenging emerging contaminants that are consistently released into the environment via wastewater treatment plants.58,59 Their widespread use means that surfactants are routinely introduced into water bodies, even after undergoing treatment in wastewater facilities.60
Connecting surfactants to the United Nations Sustainable Development Goals (SDGs), several goals are particularly relevant: goal 6: Clean Water and Sanitation – surfactants in wastewater can compromise water quality61 and make it more challenging to achieve sustainable water management and sanitation goals.
Goal 14: Life Below Water – surfactants can harm aquatic ecosystems, including marine life and biodiversity, thereby affecting the health and sustainability of ocean and freshwater habitats.62
Goal 12: Responsible Consumption and Production – addressing surfactant contamination involves promoting more sustainable production practices,63,64 reducing environmental impact, and optimizing wastewater treatment technologies.
The soapnut tree65 is indigenous to India and the southern part of Asia. The fruit of the soapnut tree is known for its saponin content,66 and traditionally used as a cleaning agent.67 Soapnut is also valued for its medicinal properties68,69 and has been used in traditional medicine to treat various ailments. Utilizing the fruit of the soapnut tree as a natural surfactant offers a greener alternative to synthetic surfactants. Furthermore, soapnut contributes to more sustainable surfactant production due to its biodegradable nature and sustainable harvesting practices.
Saponins70 are natural surfactants of plant origin and are classified as neutral surfactants. Saponins occur in the roots, leaves, fruit, pericarp, flowers and seeds of several plants. Before humankind started using soaps, clothes were cleaned by lathers from these plants' parts, which functioned similarly to soaps. For example, the pericarp of the fruits of soapnuts (Sapindus mukorossi) growing in tropical and subtropical regions, has been used as detergents for thousands of years. The hydrophilic part is sugar molecules, and the hydrophobic part is a steroid or triterpenoid.71
In addition to their benefits, it is important to consider potential risks associated with soapnut-derived saponins. Research indicates that while saponins from soapnuts are generally less harmful to humans, they can exhibit toxicity to aquatic organisms at certain concentrations.72,73 Therefore, a comprehensive assessment of their environmental impact and safety is crucial to avoid misconceptions that ‘natural’ substances are inherently safe and ‘synthetic’ substances are inherently harmful.
Hexavalent chromium [Cr(VI)] is a toxic pollutant found worldwide.74,75 It increases risk for several types of cancer and recognized as a neurotoxicant. Industrial effluents/wastewater is the main source of [Cr(VI)].76 It's a major environmental concern due to persistent nature and toxicity to living things. Like [Cr(VI)], methylene blue is a wastewater pollutant. In humans, methylene blue may induce methemoglobinemia in those with glucose-6-phosphate dehydrogenase deficiency, cause cytotoxicity and hypersensitivity reactions, and pose teratogenic risks during pregnancy. In animals, it can lead to methaemoglobin formation, oxidative damage to red blood cells, regenerative anaemia, and developmental issues such as premature delivery and teratogenic effects in fish.77 Another environmental concern is oil spill cleanup. Oil spills harm marine animals, including birds, sea turtles, and mammals.78 Additionally, DNA extraction requires the use of detergents for isolation.
Integrating the United Nations Sustainable Development Goals (SDGs) and Green Chemistry principles into laboratory activities (Fig. 1) enables students to see how their scientific knowledge contributes to global sustainability efforts. By aligning practical laboratory work with these principles, students not only enhance their understanding of chemistry but also develop a deeper awareness of their role in addressing environmental challenges. This approach equips future scientists and professionals with the skills and ethical grounding needed to implement innovative, sustainable solutions, ultimately fostering positive change and progress toward achieving global sustainability goals.
In this laboratory activity, students will investigate:
• Extraction of saponin from soapnut.
• The surfactant properties of soapnut extract (saponin).
○ Foam test.
○ Surface tension.
○ Soapnut as an emulsifier.
•Test for triterpenoid.
○ Test for reducing sugars.
• The environmental remediation applications of soapnut extract, including:
○ Hexavalent chromium [Cr(VI)] through reverse micellar extraction.
○ Methylene blue dye through reverse micellar extraction.
○ Water insoluble pollutants through micellar extraction.
• DNA extraction using soapnut extract.
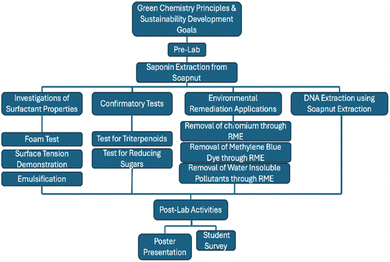
Scheme 1 provides a comprehensive overview of the laboratory activities performed by students at each stage of the experiment. It visually summarizes the various tasks and processes undertaken by students, detailing each step involved in their laboratory work.
Course details
The paper outlines a laboratory experiment developed for General Chemistry course at Ambrose University in Calgary, Alberta. CHE 103 is the second general chemistry course taken by first-year students, with an average enrollment of about 45 students. Students in CHE 103 already familiar with or have the background to designate bond polarities, identify polar and non-polar molecules, identify the intermolecular forces (IMF) present within pure samples and mixtures, to apply their understanding of IMF to explain or predict relative boiling points, viscosities, and solubility and understand the behaviour of soap molecules in water and why they lower the surface tension. The students also have a general understanding of the 12 principles of green chemistry21,79–81 and familiarity with UN Sustainable Development Goals.82The laboratory experiment outlined in the paper builds upon these foundational concepts and skills. It aims to deepen students' practical understanding of these principles by engaging them in hands-on activities that integrate chemistry concepts with real-world applications and sustainability principles. Through this experiment, students are encouraged to apply their knowledge to address contemporary challenges and explore sustainable solutions within the context of chemical processes and environmental stewardship.
Introduction to the experiment
The pre-lab assignment, which constitutes 10% of the lab grade, is designed to prepare students with crucial knowledge and critical thinking skills for effective engagement with the experiment. Students answer questions on the 12 Principles of Green Chemistry, including how the use of soapnut extract aligns with these principles by promoting renewable resources, reducing toxicity, and minimizing waste. The assignment prompts students to identify relevant SDGs associated with natural surfactants and explain how the laboratory experiment addresses these goals. It encourages students to reflect on the broader implications of using natural versus synthetic surfactants in terms of environmental impact and sustainability. The assignment includes research tasks where students analyse substances such as hexavalent chromium, methylene blue dye, and pesticides. They fill out a table assessing these substances' environmental and health impacts, sources of pollution, and regulatory limits. This task aims to illustrate how these factors influence the choice of surfactants in the laboratory.The post-lab poster presentation (90% of the lab mark) is a collaborative effort conducted in pairs, where students create a scientific poster to showcase their laboratory findings and discussions. To guide them, a structured framework is provided, outlining key sections to be included in their posters. This framework ensures that each poster covers essential aspects such as the experiment's objectives, methods employed, results obtained, discussions on findings, and conclusions drawn.
In addition, the poster presentation includes a dedicated section for reflecting on the sustainability aspects of their findings. Students are guided with specific questions to prompt their reflection:
• How does the use of synthetic surfactants in your daily life compared to the use of natural alternatives like soapnut extract?
• What are the potential environmental impacts of the synthetic surfactants you use frequently?
• How might transitioning to natural surfactants influence sustainability?
Following the completion of their posters, students upload them onto the Moodle course management system for evaluation and grading. A detailed rubric is utilized for assessing the posters, which provides clear criteria for evaluation encompassing content comprehensiveness, scientific accuracy, clarity of presentation, graphical quality, and adherence to the provided framework. To facilitate learning and improvement, students are first required to submit their posters for pre-assessment and feedback. Based on the feedback received, students then make necessary corrections and enhancements to their posters before the final submission.
Additionally, developing a poster necessitates critical thinking skills. Students must analyse experimental data, draw meaningful conclusions, and discuss the implications of their findings. The poster presentation cultivates effective communication skills essential in scientific and professional contexts. Working in pairs promotes collaborative learning. The iterative process of submitting posters for pre-assessment, receiving feedback, and making revisions fosters a culture of continuous improvement.
The laboratory activity spans across two three-hour lab periods, during which students engage in practical experiments related to soapnut extract. This laboratory experiment is designed to provide students with a multifaceted exploration into various aspects of soapnut, spanning from its extraction process to the investigation of its surfactant properties and practical applications in environmental remediation and biological sciences.
Firstly, students engage in the extraction of saponin from soapnut (Expt. 1). Secondly, they investigate the surfactant properties of the soapnut extract through several experimental tests (Expt. 2.1–2.3). Thirdly, confirmatory tests are conducted to identify the structural units present in saponin (Expt. 3.1–3.2). Fourthly, they explored the practical applications of soapnut extract in environmental remediation. Specifically, students examine its efficacy in extracting heavy metals like hexavalent chromium [Cr(VI)] (Expt. 4.1) and persistent dyes such as methylene blue (Expt. 4.2) from water. Students also engaged in a model activity to assess the effectiveness of soapnut extract in extracting water-insoluble pollutants, such as pesticides (Expt. 4.3). Lastly, the experiment includes a segment on DNA extraction from strawberries using soapnut extract (Expt. 5). Table 1 summarizes these activities along with their corresponding experimental procedures.
| Experiment | Experiment description (ESI†) |
|---|---|
| Expt. 1 extraction of soapnut | Gently heat soapnut powder in distilled water for 5 minutes. Filter the mixture while still hot and allow it to cool down |
| Expt. 2.1 foam test | Shake the diluted extract vigorously with water for 2 minutes and make observations. Record the height of the foam |
| Expt. 2.2 surface tension demonstration | Take a clean beaker and fill it with water. Place a piece of tissue paper that is slightly larger than the paperclip on the surface of the water. Next, carefully lay the paperclip on top of the tissue paper. The paper towel will sink eventually, leaving the paperclip floating on top of the water. Lastly, add a few drops of saponin solution to the water and observe what happens |
| Expt. 2.3 emulsification | Shake a test tube containing saponin solution and oil vigorously. Determine the emulsion stability by noting the time taken for the saponin solution to separate from the emulsion |
| Expt. 3.1 test for triterpenoid | Heat a mixture of soap nut extract, vanillin, and sulfuric acid at 60 °C until a purple-red colour change is observed |
| Expt. 3.2 test for reducing sugars | Gently boil a solution of soap nut extract in H2SO4. Make the solution alkaline before adding Benedict's reagent. Observe the changes that occur |
| Expt. 4.1 reverse micellar extraction of [Cr(VI)] | Mix soapnut extract in butanol with a solution of dichromate at pH 2 and shake the mixture before allowing the layers to separate |
| Expt. 4.2 reverse micellar extraction of methylene blue dye | Mix the saponin extract in butanol with an aqueous solution of methylene blue dye. Shake the mixture and let the layers separate |
| Expt. 4.3 modelling micellar extraction of water insoluble pollutants | Mix soapnut extract in water with a solution 2,4-dinitrophenylhydrazine in ethyl acetate and shake the mixture before allowing the layers to separate |
| Expt. 5 strawberry DNA extraction | The dish detergent in the standard DNA extraction procedure was substituted with soap nut extract |
Experiment details
Experiment 1 extraction of soapnut
Soapnut are natural sources of saponins, which are bioactive compounds with surfactant properties. Saponins can be extracted from soapnut using various solvents, each offering different advantages based on their polarity and extraction efficiency. Among the solvents commonly used are ethanol, methanol, n-butanol, acetone, ethyl acetate, dichloromethane, and mixtures of these solvents.71,83The powdered soapnut is mixed with water as the solvent and gently heated with stirring to facilitate the extraction process. After extraction, the mixture is filtered to remove the solid particles and labelled it as “soapnut extract” for use in further experiments.
Experiment 2.1 foam test
Foaming is a key property of surfactants.84–86 When a surfactant is mixed with water, it reduces the surface tension between water and air, allowing bubbles to form and stabilize. The foam height test (Fig. 2) is a simple and quick method to evaluate the foaming properties of a surfactant solution. In this case, the soapnut extract was subjected to the foam height test, which involved shaking the extract and measuring the height of the foam produced. The result indicated that the soapnut extract produced an adequate amount of foam with good stability.Experiment 2.2 surface tension demonstration
Surface tension is a cohesive force between molecules in liquid, the force gives the liquid surface a stretched elastic membrane like behaviour. The paper clip test87 is an uncomplicated experiment for surface tension. To conduct the paper clip test, gently place a paper clip on the surface of the liquid being tested, such as water. The paper clip will float on the surface due to surface tension that resists its weight. The introduction of soap nut extract can disrupt the surface tension and cause the paper clip to sink. Surface tension and its effects can be demonstrated simply yet effectively (Fig. 3), which making it an appropriate educational tool for quick demonstrations (see ESI† for a video demonstration of surface tension using soapnut extract).Experiment 2.3 soapnut as emulsifier
To test the emulsifying ability88 of soap nut extract, a simple test can be performed by adding a small amount of oil to a test tube containing saponin solution and shaking it vigorously to create an emulsion (Fig. 4). The emulsion stability can then be determined by noting the time taken for the saponin solution to separate from the emulsion. The longer it takes for the saponin solution to separate, the more stable the emulsion. The examination provides a comprehension about soapnut extract surfactant properties and ability to interact with water and oil. | ||
| Fig. 4 Soapnut as an emulsifier: soapnut extract with oil after vigorous shaking and settling (left); water and oil after vigorous shaking and settling (right). | ||
Experiment 3.1 test for terpenoids
Saponins are a group of naturally occurring glycosides, which are composed of a steroid or triterpenoid aglycone and one or more sugar chains.83 The structure of saponins in soap nut contains a triterpenoid linked to a sugar chain, usually glucose.65 To test for the presence of terpenoids in soap nut extract, the vanillin assay89 was used. The test involves heating a mixture of soap nut extract, vanillin, and sulfuric acid. The appearance of a purple red colour indicates the presence of terpenoids (Fig. 5).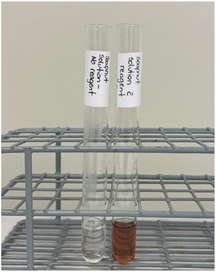 | ||
| Fig. 5 Test for triterpenoids after heating a mixture of soap nut extract, vanillin, and sulfuric acid at 60 °C. | ||
Experiment 3.2 test for reducing sugars
The Benedict's test is a standard test for confirming the presence of a reducing sugar. In this test, Benedict's reagent is added to the sample and heated. The absence of colour change from blue to green, yellow, orange, or red in the saponin may be a non-reducing sugar. In such cases, a pre-hydrolysis step is performed using concentrated sulfuric acid, which breaks down the glycosidic bond in the sugar molecule, resulting in the formation of reducing sugars. After pre-hydrolysis, the sample is treated with Benedict's reagent, and if reducing sugars are present, a colour change was observed (Fig. 6).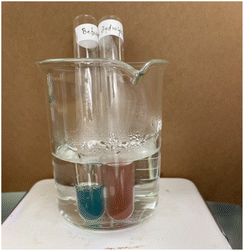 | ||
| Fig. 6 Test for reducing sugars before hydrolysis and heating with Benedict's reagent (left) and after hydrolysis and heating with Benedict's reagent (right). | ||
Experiment 4.1 reverse micellar extractions of hexavalent chromium
Reverse micellar extraction (RME) of hexavalent chromium was achieved using soapnut extract in butanol. Maximum extraction requires an acidic pH of the dichromate solution. After shaking and separation, the chromium ions are extracted into the reverse micellar phase, resulting in a colour change in the organic phase (Fig. 7).Experiment 4.2 reverse micellar extractions of dyes
Soapnut extract in organic solvent such as butanol forms reverse micelles. In these micelles, the hydrophilic polar heads of saponin align themselves in the interior, while the hydrophobic non-polar triterpenoid tails are on the surface—this is the reverse of the situation in a normal micelle. Reverse Micellar Extraction (RME)90–93 is a method used to extract substances from an aqueous solution by transferring them to an organic solvent.In this experiment, methylene blue was used as an illustrative dye to demonstrate the effectiveness of reverse micellar extraction. An aqueous solution of methylene blue dye was shaken with soapnut extract in butanol. After shaking the mixture and allowing it to settle, the blue coloration observed in the soapnut layer indicated the successful extraction of the dye molecules through reverse micellar extraction (Fig. 8).
 | ||
| Fig. 8 Reverse micellar extraction of methylene blue dye: a mixture of methylene blue in water with butanol (left); a mixture of methylene blue in water with soapnut extract in butanol (right). | ||
Experiment 4.3 modelling micellar extraction of water insoluble pollutants
Surfactant forms micelles in water at the concentration known as critical micelle concentration (CMC). The hydrophobic non-polar tails align themselves in the interior and the hydrophilic polar heads on the surface.Micellar extraction94,95 can be used to remove pollutants such as pesticide which have very little water solubility. Large amounts of pesticide residues remain in soils after their application. They leach into groundwater and are also found in run-off water. Micellar extraction of pesticides was modelled using a coloured organic compound, 2,4-dinitrophenyl hydrazine (DNP). A solution of DNP dissolved in ethyl acetate is shaken with solution of soapnut extract in water and allow the layers to separate. The yellow colour in the soapnut layer shows that soapnut extract can be used for pesticide and other water insoluble pollutants (Fig. 9).
 | ||
| Fig. 9 A solution of dinitrophenyl hydrazine in ethyl acetate and water (left) & a solution of dinitrophenylhydrazine in ethyl acetate and soapnut extract in water (right). | ||
Experiment 5 strawberry DNA extraction
In DNA extraction, detergents are important to solubilize cell membranes and denature the interfering proteins. Detergents disrupt the cell membrane, release the DNA, and denature proteins that can bind and protect them from degradation. Commonly used detergents involved DS, CTAB, Triton, and X-100, chosen depending on the sample and downstream application.The experiment demonstrated (see ESI† for a video demonstration of DNA extraction from strawberry) that soapnut extract can be a potential replacement for traditional detergents in DNA extraction from strawberry (Fig. 10). The experiment showed that soapnut extract can complete the important steps in DNA extraction: solubilizing cell membranes and denaturing proteins. Using soapnut extract as a natural and sustainable substitution to synthetic detergents can potentially reduce the negative environmental impacts. However, more research is needed to completely analyse the effectiveness of soapnut extract in DNA extraction for different types of samples and more downstream applications.
Student feedback
In an effort to assess the impact of the laboratory activities on students' understanding of green chemistry principles and attitudes towards sustainability, a survey was administered. Participants rated their responses on a scale from 1 to 5, with higher numbers indicating more positive responses. The full survey instrument and results are available in the ESI.†Discussion
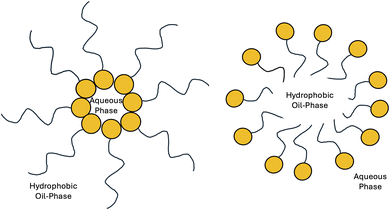
The laboratory activity provided students a novel opportunity to explore surfactant chemistry for the first time. They extracted saponin from soapnut, confirmed its structural features, investigated its surfactant properties, substituted it for synthetic detergents in DNA extraction, and applied it in micellar and reverse micellar extractions of environmental pollutants.Surfactants play a vital role in various industries due to their ability to lower surface tension and facilitate the formation of micelles. Formation of micelles occurs when the concentration of surfactant molecules exceeds a certain critical micelle concentration (CMC). In an aqueous solution, surfactant molecules such as saponin arrange themselves such that their hydrophilic heads (here the sugar moieties) face outward towards the water, while their hydrophobic tails (here the triterpenoid moieties) cluster inward away from the water (Fig. 11). Reverse micelles (Fig. 11), on the other hand, form in non-polar solvents rather than in water. They are typically composed of surfactant molecules arranged such that their hydrophilic heads are buried inside the core, shielding them from the non-polar solvent. The hydrophobic tails extend outward, forming a shell that interacts with the surrounding solvent. Formation of reverse micelles occurs when water is added to a non-polar solvent containing surfactants above their critical micelle concentration.
Soapnut contain saponins that act as effective surfactants. The experiments demonstrated that soapnut extract can produce stable foams, reduce surface tension in water, and emulsify oil effectively. These properties make soapnut extract a greener and sustainable alternative to synthetic surfactants.
This laboratory activity provided students with the opportunity to apply several of the 12 green chemistry principles throughout the experiments. The 12 green chemistry principles constitute the framework and gold standard for academic institutions and industries conducting chemical reactions and processes.
Ethanol, methanol, butanol, acetone, ethyl acetate, dichloromethane and a mixture of solvents are often used for the extraction of saponins from plant material.76,77 Among these, ethanol and n-butanol are the most used solvent. In this activity water is used for extraction in alignment with green chemistry principle 5.
Furthermore, the activity explored the environmental remediation applications of soapnut extract, particularly in the extraction of pollutants such as hexavalent chromium [Cr(VI)], methylene blue dye, and pesticides, all of which are pollutants of concern. The pre-lab assignment gave students the opportunity to find out the environmental and health hazards of these chemicals, along with their sources and regulatory limits. The use of soapnut extract yielded promising results in removing these contaminants.
The experiment on DNA extraction using soapnut extract highlighted its potential as a substitute for synthetic detergents traditionally used in this process. Soapnut extract effectively disrupted cell membranes and denatured proteins, which are essential steps in releasing DNA from cells. DNA extraction is a standard experiment in high school and higher education laboratory curricula.
The experimental design incorporates several green chemistry principles: renewable feedstocks (GCP 7), design for degradation (GCP 10), designing safer chemicals (GCP 4) and waste prevention (GCP 1). The United Nations' Sustainability Development Goals (SDGs) aims to ensure sustainable practices in resource use, including chemicals and waste management. The possibility of replacing synthetic detergents with soapnut extract aligns with SDG 12-Responsible Consumption and Production. Soapnut extract is biodegradable and sustainably sourced. Its use supports UNSDG 12 by encouraging the use of renewable resources and reducing the need for non-renewable petroleum-based chemicals.
By effectively removing pollutants from water, utilizing soapnut extract also contributes to cleaner water, aligning with UNSDG 6, Clean Water and Sanitation. In addition, the laboratory activity, which integrates green chemistry principles, sustainable development goals, and environmental stewardship, exemplifies SDG 4 – Quality Education for All. Moreover, students gain experience investigating surfactant properties, conducting structural confirmatory tests, performing micellar and reverse micellar extraction, and DNA extraction within the framework of environmentally friendly and sustainable practices.
The survey results demonstrate that the laboratory activities effectively enhanced students' understanding of green chemistry and increased their awareness of sustainability. Before the lab, understanding was rated lower, but after participation, the majority of students rated their understanding as 4 or 5, reflecting a significant gain in knowledge. Most students felt that their perception of the importance of sustainability had significantly changed as a result of the laboratory activities. Students indicated a strong likelihood of applying sustainable practices in their future work, with high ratings suggesting that the laboratory activities had a meaningful impact on their future professional behavior. The laboratory activities were generally found to be engaging, with positive feedback on their role in learning about sustainability. However, there was some variation in how well surfactant chemistry was explained, indicating a need for refinement in this area.
Conclusions
In this laboratory activity, students investigated surfactant properties of soapnut extract, substituted it for synthetic detergents in DNA extraction, and applied it in micellar and reverse micellar extractions of environmental pollutants. Overall, this laboratory activity integrated green chemistry principles, sustainable development goals, and environmental stewardship. It exemplifies SDG 4 – Quality Education for All by providing students with hands-on experience in environmentally friendly practices. Additionally, the use of soapnut extract aligns with SDG 12 – Responsible Consumption and Production by promoting the use of renewable resources and reducing dependence on non-renewable chemicals. It also contributes to SDG 6 – Clean Water and Sanitation by effectively removing pollutants from water.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Zi Wang received the 2024 Gordon College Undergraduate Research Funding to complete the work.References
- A. S. Cannon, J. C. Warner, J. L. Vidal, N. J. O'Neil, M. M. S. Nyansa, N. K. Obhi and J. W. Moir, Green Chem., 2024, 26, 6983–6993 RSC.
- L. B. Armstrong, L. M. Irie, K. Chou, M. Rivas, M. C. Douskey and A. M. Baranger, Chem. Educ. Res. Pract., 2024, 25, 115–132 RSC.
- V. G. Zuin, I. Eilks, M. Elschami and K. Kümmerer, Green Chem., 2021, 23, 1594–1608 RSC.
- J. A. Haack and J. E. Hutchison, ACS Sustain. Chem. Eng., 2016, 4(11), 5889–5896 CrossRef CAS.
- C. A. Marques and A. A. S. C. Machado, Found. Chem., 2021, 23(3), 299–328 CrossRef.
- M. Karpudewan, Z. Ismail and W. M. Roth, Chem. Educ. Res. Pract., 2021, 13, 120–127 RSC.
- S. M. Kernaghan, T. Coady, M. Kinsella and C. M. Lennon, RSC Sustainability, 2024, 2, 578–607 RSC.
- C. Zowada, N. Frerichs, V. G. Zuin and I. Eilks, Chem. Educ. Res. Pract., 2020, 21, 141–153 RSC.
- K. Grieger, B. Hill and A. Leontyev, Green Chems., 2022, 24, 8770–8782 RSC.
- E. L. Day, S. J. Petritis, H. McFall-Boegeman, J. Starkie, M. Zhang and M. M. Cooper, J. Chem. Educ., 2024, 101(5), 1847–1857 CrossRef CAS.
- K. Greiger and A. Leontyev, J. Chem. Educ., 2024, 101(7), 2644–2655 CrossRef PubMed.
- B. J. J. Timmer, F. Schaufelberger, D. Hammarberg, J. Franzén, O. Ramström and P. Dinér, J. Chem. Educ., 2018, 95(8), 1301–1306 CrossRef CAS.
- A. Parker, E. Noronha and A. Bongers, J. Chem. Educ., 2023, 100(5), 1728–1738 CrossRef CAS.
- S. Johnson, M. Meyers, S. Hyme and A. Leontyev, J. Chem. Educ., 2020, 97(2), 383–389 CrossRef CAS.
- R. Zidny and I. Eilks, Educ. Sci., 2022, 12(4), 227 CrossRef.
- M. Chen, E. Jeronen and A. Wang, Int. J. Environ. Res. Public Health, 2020, 17(21), 7876 CrossRef.
- J. Andraos and A. P. Dicks, Chem. Educ. Res. Pract., 2012, 13, 69–79 RSC.
- S. A. Kennedy, J. Chem. Educ., 2016, 93(4), 645–649 CrossRef CAS.
- R. A. Haley, J. M. Ringo, H. Hopgood, K. L. Denlinger, A. Das and D. C. Waddell, J. Chem. Educ., 2018, 95(4), 560–569 CrossRef CAS.
- C. Zowada, M. Linkwitz, A. Siol and I. Eilks, CHEMKON, 2020, 27(8), 365–372 CrossRef.
- P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- A. Jackson and G. A. Hurst, Chem. Educ. Res. Pract., 2021, 22, 855–865 RSC.
- Y. Gu, X. Zhang, B. Deal, L. Han, J. Zheng and H. Ben, Chem. Educ. Res. Pract., 2021, 22, 855–865 RSC.
- K. B. Aubrecht, M. Bourgeois, E. J. Brush, J. Mackellar and J. E. Wissinger, J. Chem. Educ., 2019, 96(12), 2872–2880 CrossRef CAS.
- A. R. Szozda, K. Bruyere, H. Lee, P. G. Mahaffy and A. B. Flynn, J. Chem. Educ., 2022, 99(7), 2474–2483 CrossRef CAS.
- D. J. C. Constable, C. Jiménez-González and S. A. Matlin, J. Chem. Educ., 2019, 96(12), 2689–2699 CrossRef CAS.
- T. Freese, N. Elzinga, M. Heinemann, M. M. Lerch and B. L. Feringa, RSC Sustainability, 2024, 2, 1300–1336 RSC.
- S. Ritter and L. Abraham, J. Chem. Educ., 2022, 99, 4134–4142 CrossRef CAS.
- L. Abraham, J. VanderZwaag and D. H. Chap, J. Chem. Educ., 2021, 98, 824–832 CrossRef.
- L. Abraham, J. Chem. Educ., 2020, 97(10), 3810–3815 CrossRef CAS.
- L. Abraham, L. Stachow and D. H. Chao, J. Chem. Educ., 2020, 97(10), 3797–3805 CrossRef CAS.
- L. B. Armstrong, M. C. Rivas, M. C. Rivas, Z. Zhou, L. M. Irie, G. A. Kerstiens, M. T. Robak, M. C. Douskey and A. M. Barange, J. Chem. Educ., 2019, 96(11), 2410–2419 CrossRef CAS.
- F. Xiong, R. Liu, X. Fan, M. Zhang, Y. She, W. Cao, C. Chen, T. Ding and S. Zhang, J. Chem. Educ., 2023, 100(12), 4686–4695 CrossRef CAS.
- D. Tan, M. Li, W. Xiong, Y. Xu, Y. Pan, W. Fan and W. Jiang, J. Chem. Educ., 2023, 100(2), 811–814 CrossRef CAS.
- United Nations, Transforming Our World. The 2030 Agenda for Sustainable Development. A/RES/70/1, New York, 2015. https://www.sdgs.un.org/es/goals Search PubMed.
- P. Lozano and E. García-Verdugo, Green Chem., 2023, 25, 7041–7057 RSC.
- M. Burmeister, F. Rauch and I. Eilks, Chem. Educ. Res. Pract., 2012, 13, 59–68 RSC.
- M. H. G. Prechtl, RSC Sustainability, 2023, 1, 1580–1583 RSC.
- J. E. Wissinger, A. Visa, B. B. Saha, S. A. Matlin, P. G. Mahaffy, K. Kümmerer and S. Cornell, J. Chem. Educ., 2021, 98(4), 1061–1063 CrossRef CAS.
- R. J. Petillion, T. K. Freeman and W. S. McNeil, J. Chem. Educ., 2019, 96(12), 2845–2851 CrossRef CAS.
- C. E. Karayel, M. Krug, L. Hoffmann, C. Kanbur, C. Barth and J. Huwer, J. Chem. Educ., 2023, 100(1), 102–111 CrossRef CAS.
- F. Roschangar, J. Li, Y. Zhou, W. Aelterman, A. Borovika, J. Colberg, D. P. Dickson, F. Gallou, J. D. Hayler, S. G. Koenig, M. E. Kopach, B. Kosjek, D. K. Leahy, E. O'Brien, A. G. Smith, M. Henry, J. Cook and R. A. Sheldon, ACS Sustainable Chem. Eng., 2022, 10(16), 5148–5162 CrossRef CAS.
- L. L. Schramm, E. N. Stasiuk and D. G. Marangoni, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2003, 99, 3–48 RSC.
- I. Anestopoulos, D. E. Kiousi, A. Klavaris, A. Galanis, K. Salek, S. R. Euston, A. Pappa and M. I. Panayiotidis, Pharmaceutics, 2020, 12(7), 688 CrossRef CAS.
- C. N. Sivaramakrishnan, Advances in the Dyeing and Finishing of Technical Textiles, 2013, pp. 199–235 Search PubMed.
- G. M. Aleid, A. S. Alshammari, D. B. Tripathy, A. Gupta and S. Ahmad, Macromol. Chem. Phys., 2023, 224(17), 2300107 CrossRef CAS.
- M. J. L. Castro, C. Ojeda and A. F. Cirelli, Environ. Chem. Lett., 2014, 12(1), 85–95 CrossRef CAS.
- T. Wang, D. Chang, D. Huang, Z. Liu, Y. Wu, H. Liu, H. Yuan and Y. Jiang, Appl. Microbiol. Biotechnol., 2021, 105, 7619–7634 CrossRef CAS PubMed.
- A. Karnwal, S. Shrivastava, A. R. M. S. Al-Tawaha, G. Kumar, R. Singh, A. Kumar, A. Mohan, N. Yogita and T. Malik, BioMed Res. Int., 2023, 21, 1–21 Search PubMed.
- Y. Zhu, M. L. Free, R. Woollam and W. Durnie, Prog. Mater. Sci., 2017, 90, 159–223 CrossRef CAS.
- S. Q. A. Rizvi, Surfactants and Detergents: Chemistry and applications, ASTM International Manual, 2021 Search PubMed.
- R. Zolfaghari, A. Fakhru'I-Razi, L. C. Abdullah, S. S. E. H. Elnashaie and A. Pendashteh, Surfactants in Upstream E&P, 2021, pp. 443–466 Search PubMed.
- S. M. Shaban, J. Kang and D. Kim, Compos. Commun., 2020, 22, 100537 CrossRef.
- W. Chen, X. Geng, W. Liu, B. Ding, C. Xiong, J. Sun, C. Wang and K. Jiang, Energy Fuels, 2023, 37(7), 4729–4750 CrossRef CAS.
- B. Das, B. Kumar, W. Begum, A. Bhattarai, M. H. Mondal and B. Saha, Chem. Afr., 2022, 5(3), 459–480 CrossRef CAS.
- M. Y. d. F. A. Reis, R. I. d. A. Rêgo, B. P. Rocha, G. G. Guedes, Í. M. d. M. Ramalho, A. L. d. M. Cavalcanti, G. P. Guimarães and B. P. G. d. L. Damasceno, Curr. Pharm. Des., 2021, 27(42), 4300–4314 CrossRef.
- S. O. Badmus, H. K. Amusa, T. A. Oyhan and T. A. Saleh, Environ. Sci. Pollut. Res., 2021, 28, 62085–62104 CrossRef CAS PubMed.
- J. Arora, A. Ranjan, A. Chauhan, R. Biswas, V. D. Rajput, S. Sushkova, S. Mandzhieva, T. Minkina and T. Jindal, J. Appl. Microbiol., 2022, 133, 1229–1244 CrossRef CAS.
- G. T. Trajano, O. M. S. R. Vasconcelos, L. C. M. Pataca and M. P. G. Mol, Environ. Monit. Assess., 2022, 194, 248 CrossRef CAS PubMed.
- P. Johnson, A. Trybala, V. Starov and V. J. Pinfield, Adv. Colloid Interface Sci., 2021, 288, 102340 Search PubMed.
- K. Obaideen, N. Shehata, E. T. Sayed, M. A. Abdelkareem, M. S. Mahmoud and A. G. Olabi, Energy Nexus, 2022, 7, 100112 CrossRef.
- R. K. Mishra, S. S. Mentha, Y. Misra and N. Dwivedi, Water-Energy Nexus, 2023, 6, 74–95 CrossRef CAS.
- V. S. Nagtode, C. Cardoza, H. K. A. Yasin, S. N. Mali, S. M. Tambe, P. Roy, K. Singh, A. Goel, P. D. Amin, B. R. Thorat, J. N. Cruz and A. P. Pratap, ACS Omega, 2023, 8, 11674–11699 CrossRef CAS.
- A. Bhadani, A. Kafle, T. Ogura, M. Akamatsu, K. Sakai, H. Sakai and M. Abe, Curr. Opin. Colloid Interface Sci., 2020, 45, 124–135 CrossRef CAS.
- M. Sochacki and O. Vogt, Plants, 2022, 11(1–24), 2355 CrossRef CAS.
- A. Krishnan, A. Saravana and A. Packirisamy, J. Mol. Struct., 2024, 1315 Search PubMed.
- B. N. Suhagia, I. S. Rathod and S. Sindhu, ResearchGate, 2011, 2(8), 1905–1913 CAS.
- A. Panda, A. Kumar, S. Mishra and S. S. Mohapatra, Sustainable Chem. Pharm., 2020, 17, 100297 CrossRef.
- S. Rawat, G. Gupta, A. Mishra, S. Pathak, L. Thangavelu, S. K. Singh, N. K. Jha, D. Kumar, P. Negi, A. P. Kumar, D. K. Chellappan and K. Dua, PubMed, 2022, 21, 354–359 Search PubMed.
- V. M. Devi, M. Rajakohila, L. A. M. Syndia, P. N. Prasad and V. Ariharan, Res. J. Pharmaceut. Biol. Chem. Sci., 2012, 3, 420–424 Search PubMed.
- S. Rai, E. Acharya-Siwakoti, A. Kafle, H. P. Devkota and A. Bhattarai, Sci, 2021, 3, 44–62 CrossRef.
- M.-P. Wei, J.-D. Qiu, L. Li, Y.-F. Xie, H. Yu, Y.-H. Guo and W.-R. Yao, J. Ethnopharmacol., 2021, 268, 113552 CrossRef CAS PubMed.
- M. Du, S. Huang, J. Zhang, J. Wang, L. Hu and J. Jiang, Open J. For., 2015, 05, 749–753 Search PubMed.
- F. M. Henretig, M. A. Kirk and C. A. McKay, N. Engl. J. Med., 2019, 380, 1638–1655 CrossRef CAS.
- P. Sharmaa, S. P. Singh, S. Ki. Parakha and Y. W. Tonga, Bioengineered, 2022, 13(3), 4923–4938 CrossRef PubMed.
- M. Mokarram, A. Saber and V. Sheykhi, J. Cleaner Prod., 2020, 277, 123380 CrossRef CAS.
- National Center for Biotechnology Information, PubChem Compound Summary for CID 6099, Methylene Blue, 2024, retrieved August 15, 2024 from https://www.pubchem.ncbi.nlm.nih.gov/compound/Methylene-Blue.
- A. Clifton, Environmental Issues, Prevention and Ecological Impacts, 2014 Search PubMed.
- Beyond Benign, About Green Chemistry – Beyond Benign, https://www.beyondbenign.org/about-green-chemistry/, accessed on Oct 25 2023 Search PubMed.
- Principles of Green Chemistry, American Chemical Society, https://www.acs.org/greenchemistry/principles/12-principles-of-green-chemistry.html, accessed on Aug 16, 2023 Search PubMed.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- The 17 Goals, https://www.sdgs.un.org/goals, accessed on Aug 16, 2023 Search PubMed.
- M. D. Köse and O. Bayraktar, World J. Adv. Res. Rev., 2016, 2, 89–93 Search PubMed.
- G. C. Sawicki, Colloids Surf., A, 2005, 263, 226–232 CrossRef CAS.
- R. A. Karthick, K. Jangir and P. Chattopadhyay, Tenside, Surfactants, Deterg., 2018, 55, 162–168 CAS.
- A. G. Deshmukh and B. B. Gogte, Int. J. Chem. Sci., 2014, 12(3), 933–940 Search PubMed.
- American Chemical Society, https://www.acs.org/content/dam/acsorg/msc/downloads/chapter-5/lesson-2/ch5-l2-lesson-plan.pdf, accessed on July 7, 2023.
- F. Ravera, K. Dziza, E. Santini, L. Cristofolini and L. Liggieri, Adv. Colloid Interface Sci., 2021, 288, 102344 Search PubMed.
- Z. Jiang, C. Kempinski and J. Chappell, Curr. Protoc. Plant Biol., 2016, 1, 345–358 CrossRef CAS.
- T. Maruyama, N. Ishizu, Y. Eguchi, T. Hosogi and M. Goto, Chem. Commun., 2016, 52, 12376–12379 RSC.
- C. Chen, H. Tian, S. Xing, C. Li, X. Zeng and L. He, J. Food Sci. Technol., 2019, 56(6), 2899–2908 CrossRef CAS.
- P. Pandit and S. Basu, Environ. Sci. Technol., 2004, 38(8), 2435–2442 CrossRef CAS PubMed.
- T. K. Chaturvedi, P. Pandit, S. Upadhyay and M. Vashishtha, Asian J. Water, Environ. Pollut., 2022, 19, 9–16 Search PubMed.
- C. P. Sanz, R. Halko, Z. S. Ferrera and J. J. S. Rodríguez, Anal. Chim. Acta, 2004, 524, 265–270 CrossRef.
- D. V. Moreno, Z. S. Ferrera and J. J. Santana Rodríguez, J. Agric. Food Chem., 2006, 54(20), 7747–7752 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00397g |
| This journal is © The Royal Society of Chemistry 2024 |