 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low-water-permeability foils based on bio-renewable cellulose derivatives†
Tanner J.
Hickman
 a,
Li
Tao
b,
Natalie
Stingelin
ab and
J. Carson
Meredith
*a
a,
Li
Tao
b,
Natalie
Stingelin
ab and
J. Carson
Meredith
*a
aGeorgia Institute of Technology, School of Chemical and Biomolecular Engineerring, Atlanta, GA, USA. E-mail: carson.meredith@chbe.gatech.edu
bGeorgia Institute of Technology, School of Materials Science and Engineering, Atlanta, GA, USA
First published on 24th September 2024
Abstract
Packaging is one of the largest contributors to plastic waste. Hence, polymers produced from renewable sources have become attractive to substitute or fully replace petroleum-based plastics in packaging materials. However, the properties of some of the prime candidates—e.g., cellulose and its derivatives—rapidly deteriorate already at a modest relative humidity rendering them impractical for use in packaging products. Here, we show by the example of carboxymethyl cellulose that chemical crosslinking with citric acid can be exploited to precisely control the moisture sensitivity of cellulose-based structures. Specifically, we demonstrate that the water vapor transmission rate (WVTR) of carboxymethyl cellulose can be manipulated in a controlled fashion over three orders of magnitude. Thereby, the lowest WVTR value, obtained for an optimal crosslinker content, is one order of magnitude lower than that measured for poly(ethylene terephthalate) even at a relatively humidity of up to 65%. Our work, thus, clearly illustrates that cellulose-based materials can be made insensitive to humidity, which is not only of great importance for providing a solution towards more sustainable plastic packaging but, generally, for expanding the scope of applications of cellulose and its derivatives, allowing us to leverage their natural abundance, chemical versatility, and biodegradability.
Sustainability spotlightA sustainable future requires single-use plastics that can be completely reclaimed through recycling or biodegradation. Primary packaging materials rarely fulfill either requirement, in part due to the lack of renewably sourced materials with sufficient water barrier performance. We show that a simple crosslinking approach can be used to produce cellulose-based structures with commercially competitive water vapor barrier properties. Our success opens the door to more widespread implementation of cellulose and other polysaccharides for barrier packaging applications, thus reducing our dependence on petroleum-derived plastics, and contributing to the UN's Sustainable Development Goals to ensure sustainable consumption and production patterns (SDG 12) and take urgent action to combat climate change and its impacts (SDG 13). |
Introduction
Petroleum-sourced plastics, including single-use packaging materials, have become ubiquitous in our daily life because of their low-cost manufacturability, versatility, and, in many cases, their soft nature. Indeed, a world without plastics is nowadays difficult to imagine. Yet, our strong dependence on this materials class has led to drastic amounts of plastic waste finding its way into landfills and the environment.1 It is clear that reducing the use of plastics on the consumer side is not foreseeable in the near future. Hence, alternative materials systems that are renewable and easily degradable need to be advanced to meet the stringent property requirements of packaging materials. This includes ease of processing, biodegradability, mechanical properties, as well as water and oxygen permeability that both need to be low in a broad range of environments.Cellulose-based systems have attracted increasing attention for use in packaging, among other reasons, because these relatively green plastics can feature excellent barrier properties, at least in the dry state.2–5 However, their highly hygroscopic nature renders cellulose materials extremely sensitive to humidity.6–11 This sensitivity can be reduced by reducing the materials' propensity to interact with water, e.g., by hornification or physical crosslinking by multivalent ions, but these processes are specific to certain morphologies and chemistries, and therefore not generalizable for different cellulose materials.12–14 Straight-forward chemical crosslinking of cellulose-based materials may offer another efficient route to control the sensitivity of cellulose materials to relative humidity (RH) and, generally, render the properties of these renewable plastics less sensitive to the environment. Some evidence consistent with this has been provided in literature, but by using uncontrolled or narrow ranges of crosslinker content and RH.15–19 We use here carboxymethyl cellulose (CMC) as a model system and explore whether crosslinking with citric acid (CA) via esterification, a reaction that leads to crosslinks as illustrated in Fig. 1A, can be exploited to limit the water vapor transmission characteristics of cellulose materials. The process is straight-forward and results in low water vapor transmission rates (WVTR) up to relative humidity levels of 65%.
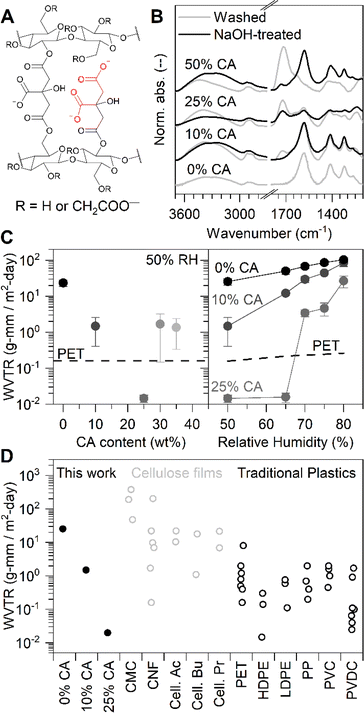 | ||
| Fig. 1 Carboxymethyl cellulose (CMC) crosslinked by citric acid (CA) with very low water vapor transmission rate (WVTR). (A) Structure of CMC crosslinked by CA with full (left) and partial (right) crosslinks. (B) FTIR spectra of CMC/CA films with 0, 10, 25, and 50 wt% CA before (grey) and after (black) deprotonation of free acid groups via NaOH treatment. Note that neat CMC does not feature the characteristic 1718 cm−1 ester vibration, and therefore does not form crosslinks with itself. All spectra are normalized by their maximum absorbance. (C) WVTR of CMC/CA films and PET at 23 °C and 50% RH (left), and at different RH (right). Error bars represent the standard deviation of at least two measurements. (D) Summary of WVTR for various films and testing conditions showing that the lowest WVTR of CMC with 25% CA is comparable to commercial water barrier plastics. References and detailed experimental conditions are listed in Table S1.† | ||
Results and discussion
We begin our discussion with CMC/CA film preparation. First, 1.0 wt% CMC solutions were produced in deionized water. Subsequently, CA was added at room temperature at different concentrations. The resulting CMC/CA solutions were then cast at ambient conditions into either polystyrene (typically, CA content of <35 wt%) or polytetrafluoroethylene dishes (CA content of 25 and 35 wt%). These films were left to dry for approximately two weeks at ambient conditions, leading to homogenous structures of 5 × 5 cm2 dimensions and a thickness of approximately 50 to 100 microns, as the photographs in Fig. 2 illustrate. To remove water and promote crosslinking, the films were after that dried in an oven at 120 °C for 2 hours. | ||
| Fig. 2 Photographs of CMC/CA films dried in polystyrene (PS) and polytetrafluoroethylene (PTFE) dishes. | ||
We first assessed whether CA successfully crosslinked CMC under these conditions. For this we employed infrared (IR) spectroscopy and monitored vibrations characteristic for ester bonds (and, hence, crosslinking) focusing on the stretching vibration at 1718 cm−1. Vice versa, the signature feature for carboxylic acid moieties at 1585 cm−1 was also used as they are only present in CMC or unreacted CA.20–22 Our data is summarized in Fig. 1B, gray spectra. More details are given in the ESI (see Section 2.2).
A few observations can be made. Clear evidence of esterification can already be made in films of a CA content of 10 wt% only. Specifically, distinctive signals for ester moieties become apparent at 1718 cm−1 upon introduction of this relatively small amount of CA – a feature that becomes more intense for samples where more CA was added (Fig. 1B, gray spectra). In parallel, the 1585 cm−1 vibration characteristic for carboxylic acid moieties is decreasing in intensity with increasing CA content, further supporting the view of successful crosslinking.
Reassuringly, systems with even modest CA content display drastically decreased WVTR values – by more than three orders of magnitude (Fig. 1C). As a result, at 50% RH and 25 wt% CA, the WVTR value is 10 times lower than that recorded for poly(ethylene terephthalate) (PET), one of the most used barrier materials in packaging (Fig. 1C, left panel). This performance is maintained up to a RH of 65% (Fig. 1C, right panel), demonstrating how the simple tool of crosslinking via esterification can be exploited to render cellulose-materials such as CMC rather insensitive to the environment. Indeed, only high-density polyethylene (HDPE) and poly(vinylidene chloride) (PVDC) have similar performance (Fig. 1D),4–6,12,19,23–33 but these polymers are non-biodegradable and substantial contributors to plastic waste pollution.
The question remains why an optimal composition/CA content is found at 25 wt%. At higher content, the WVTR values increase significantly (Fig. 1C, left). We attribute this observation to the presence of unreacted or only partially reacted crosslinker, highlighted in red in Fig. 1A. In order to gain some additional insights, we exposed our CMC/CA films to a sodium hydroxide (NaOH) treatment to deprotonate remaining free acid groups so that unreacted or partially reacted CA can be detected.
The IR spectra of such treated CMC/CA films are displayed in Fig. 1B, black spectra. The comparison of the CMC/CA (25 wt%) and CMC/CA (50 wt%) systems is telling. For the former, no drastic spectral changes are observed after NaOH exposure, indicating that in these films, most carboxylic acid groups in the crosslinker are consumed. In contrast, for CMC/CA (50 wt%), after the NaOH treatment, the intensity of the 1585 cm−1 vibration notably increases while the 1718 cm−1 feature essentially is lost – an observation from which we conclude that in these films a considerable amount of excess CA or partially reacted CA is present.
Collectively, our data shows that at 25 wt% CA content, i.e. 1 mol CA per 2.5 mol CMC monomer units, nearly complete crosslinking is reached, i.e. two out of three carboxylic acid groups react with CMC. At higher crosslinker content, some CA stays un- or partially reacted. As a result, a higher fraction of such films remains soluble when placed in deionized water compared to films to which the optimal CA amount was added (Fig. 3A, top panel, black markers). In contrast, the amount of material that remains soluble is around 20 wt% at a CA content of 10 wt% to 30 wt%.
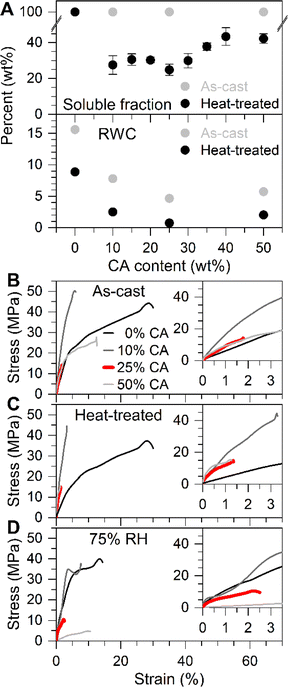 | ||
| Fig. 3 Accumulation of unreacted and partially reacted citric acid (CA) above the maximum crosslink density leads to increased residual water content (RWC) and hydration of CA. (A) Soluble fraction (top panel) and residual water content (bottom panel), for CMC/CA films before (grey markers) and after (black markers) heat treatment. Both the soluble fraction and residual water content show a non-monotonic behavior with a minimum at 25 wt% CA, the composition corresponding to the maximum crosslink density. Error bars represent the standard deviation of at least three measurements. (B) Tensile properties of CMC/CA films before heat treatment, (C) after heat treatment, and (D) after heat treatment and aging at 75% RH for seven days. The data shown here represents a single measurement, see Table S2† for average results of repeat measurements. Insets highlight the data in the low strain regime. | ||
The un- or partially reacted CA leads to other undesirable effects, including a high residual water content (RWC), extracted from the mass loss of dried films at 150 °C (Fig. 3A, bottom panel; see the ESI, Section 2.5, for more detail), which contributes to the increased WVTR observed in films with more than 25 wt% CA. While the difference between CMC/CA systems of 25 wt% and 50 wt% seems small (RWC content of 1 and 2 wt%), the implications are considerable. This is reflected in the mechanical behavior of CMC/CA foils. While as-cast films of a CA content of 50 wt% plastically deform, structures of optimal CA content (25 wt%) fail in the linear regime as a result of nearly complete to complete crosslinking (Fig. 3B). However, the total solubility of as-cast films in deionized water indicate that their crosslinks are not permanent, and thus non-covalent in nature (Fig. 3A, top panel, grey markers). After a heat-treatment at 120 °C for 2 hours, this picture changes as crosslinks become irreversible via esterification, leading the CMC/CA films to fail in the elastic regime independent of CA content (Fig. 3C). Despite the fact that heat-treated films of 25 and 50 wt% CA are mechanically essentially identical, indicating similar degrees of crosslinking, the RWC of the latter remains about 1% higher than the former, pointing to the conclusion that un- or partially reacted CA is responsible for the greater affinity for water and higher WVTR in films of 50 wt% CA content. This view is corroborated by the mechanical response to changes in relative humidity, highlighted in Fig. 3D, which is notably more pronounced in films of 50 wt% CA compared to 25 wt% CA, considering their increased breaking strain of approximately 500% and 100%, respectively. Clearly, there are substantial differences in the mechanical properties of crosslinked CMC compared to packaging plastics such as PET, which has an upper tensile strength and strain-at-break of 55 to 75 MPa and 65 to 320%, respectively.34 However, barrier packaging applications do not necessarily require the same performance. Thus, a compromise may eventually be sought to maximize strain at break, while minimizing the WVTR and residual water content. This possibly can be achieved in structures produced of alternating neat CMC and CMC/CA layers.
Conclusions
In conclusion, we demonstrated that CA-crosslinked CMC displays excellent water barrier properties with a WVTR of less than 0.02 g mm per m2 day (one order of magnitude less than PET) up to 65% RH. Our results illustrate that the lowest attainable WVTR using crosslinked CMC is much less than inferred from similar studies, which have achieved only relatively small reductions in WVTR of approximately 0–60% compared to neat CMC, due to the addition of plasticizers or limitation to high testing humidities.17,20,35 In contrast, our work shows that the WVTR can be reduced by over 99.9% by crosslinking CMC with CA. The formation of ester crosslinks, which are conclusively identified by the 1718 cm−1 vibration of NaOH-treated films, is critical to the barrier performance of the material. We highlight that it is important to precisely control the crosslink density. At too high CA content, unreacted and partially reacted crosslinker accumulates and leads to undesired water uptake/hydration and, in turn, an increase in WVTR.We expect that this crosslinking approach can be applied to any cellulose-based system with sufficient hydroxyl side groups available for reaction with citric acid, such as hydroxyethyl cellulose, hydroxypropyl cellulose, or CMC with different degrees of substitution. After all, it is well established in the literature that citric acid effectively crosslinks hydroxylated polymers with diverse structures, including nanocellulose, starch, chitosan, and polyvinyl alcohol.15–19 Conversely, this approach would likely be ineffective using any cellulose derivative with very few or no hydroxyl side groups, such as highly substituted cellulose acetate, methyl cellulose, or nitrocellulose. In these cases, the absence of hydroxyl side groups prevents the esterification of the cellulose-based material with citric acid.
Beneficially, the process is straight-forward and provides a promising alternative route towards cellulose-based coatings and films with robust barrier properties, compared to, e.g., direct chemical modification and functionalization of the cellulose that usually requires introduction of petroleum-based high-molecular weight hydrocarbons or fluoropolymers into the systems, or production of nanocomposites, which is limited by nanoparticle aggregation. Unlike these approaches, we utilize only biodegradable components, thus delivering a high water vapor barrier material that is likely biodegradable, as demonstrated in previous studies of similar materials systems.36 Clearly, further insight is required to fully exploit simple crosslinking of cellulose-based materials for packaging applications. More broadly, our work promises to expand the possible applications of cellulose-based films and enables targeted materials design towards more sustainable plastic alternatives.
Data availability
Data for this article, including all raw and processed data, are available at the Georgia Tech Digital Repository at https://hdl.handle.net/1853/75447.Author contributions
Tanner Hickman: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, visualization, writing – original draft, writing – review & editing; Li Tao: investigation; Natalie Stingelin: conceptualization, funding acquisition, project administration, supervision, writing – review & editing; J. Carson Meredith: conceptualization, funding acquisition, project administration, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Georgia Tech Renewable Bioproducts Institute (GTF114000085) and the U. S. Department of Defense through the National Defense Science and Engineering Graduate Fellowship Program.References
- Facts and Figures about Materials, Waste and Recycling, United States Environmental Protection Agency, 2022 Search PubMed.
- S. S. Ahankari, A. R. Subhedar, S. S. Bhadauria and A. Dufresne, Carbohydr. Polym., 2021, 255, 117479 CrossRef CAS PubMed.
- F. Silva, F. Dourado, M. Gama and F. Pocas, Nanomater., 2020, 10, 2041 CrossRef CAS PubMed.
- J. Vartiainen, P. Lahtinen, T. Kaljunen, V. Kunnari, M. S. Peresin and T. Tammelin, O Papel., 2015, 76, 51–54 Search PubMed.
- S. Sharma, X. Zhang, S. S. Nair, A. Ragauskas, J. Zhu and Y. Deng, RSC Adv., 2014, 4, 45136–45142 RSC.
- J. Wang, D. J. Gardner, N. M. Stark, D. W. Bousfield, M. Tajvidi and Z. Cai, ACS Sustain. Chem. Eng., 2017, 6, 49–70 CrossRef.
- C. C. Satam, C. W. Irvin, A. W. Lang, J. C. R. Jallorina, M. L. Shofner, J. R. Reynolds and J. C. Meredith, ACS Sustain. Chem. Eng., 2018, 6, 10637–10644 CrossRef CAS.
- G. Fotie, L. Amoroso, G. Muratore and L. Piergiovanni, Food Packag. Shelf Life, 2018, 18, 62–70 CrossRef.
- M. Martínez-Sanz, A. Lopez-Rubio and J. M. Lagaron, Carbohydr. Polym., 2013, 98, 1072–1082 CrossRef PubMed.
- C. Aulin, M. Gällstedt and T. Lindström, Cellulose, 2010, 17, 559–574 CrossRef CAS.
- I. Siró, D. Plackett, M. Hedenqvist, M. Ankerfors and T. Lindström, J. Appl. Polym. Sci., 2011, 119, 2652–2660 CrossRef.
- J. Xia, Z. Zhang, W. Liu, V. C. F. Li, Y. Cao, W. Zhang and Y. Deng, Cellulose, 2018, 25, 4057–4066 CrossRef CAS.
- M. Shimizu, T. Saito and A. Isogai, J. Membr. Sci., 2016, 500, 1–7 CrossRef CAS.
- C. Chen, W. Sun, L. Wang, M. Tajvidi, J. Wang and D. J. Gardner, ACS Sustain. Chem. Eng., 2022, 10, 9419–9430 CrossRef CAS.
- Q. J. Chen, L. L. Zhou, J. Q. Zou and X. Gao, Int. J. Biol. Macromol., 2019, 132, 1155–1162 CrossRef CAS PubMed.
- M. A. Herrera, A. P. Mathew and K. Oksman, Cellulose, 2017, 24, 3969–3980 CrossRef CAS.
- V. Beghetto, V. Gatto, S. Conca, N. Bardella, C. Buranello, G. Gasparetto and R. Sole, Carbohydr. Polym., 2020, 249, 116810 CrossRef CAS PubMed.
- L. Zhuang, X. Zhi, B. Du and S. Yuan, ACS Omega, 2020, 5, 1086–1097 CrossRef CAS PubMed.
- P. G. Ponnusamy, J. Sundaram and S. Mani, J. Appl. Polym. Sci., 2021, 139(17), 52017 CrossRef.
- T. Nongnual, N. Butprom, S. Boonsang and S. Kaewpirom, Int. J. Biol. Macromol., 2024, 267, 131135 CrossRef CAS PubMed.
- H. Awada, D. Montplaisir and C. Daneault, Ind. Eng. Chem. Res., 2014, 53, 4312–4317 CrossRef CAS.
- A. Quellmalz and A. Mihranyan, ACS Biomater. Sci. Eng., 2015, 1, 271–276 CrossRef CAS PubMed.
- E. Rincon, L. Serrano, A. M. Balu, J. J. Aguilar, R. Luque and A. Garcia, Materials, 2019, 12, 2356 CrossRef CAS PubMed.
- A. A. Oun and J. W. Rhim, Carbohydr. Polym., 2015, 127, 101–109 CrossRef CAS PubMed.
- N. Zabihollahi, A. Alizadeh, H. Almasi, S. Hanifian and H. Hamishekar, Int. J. Biol. Macromol., 2020, 160, 409–417 CrossRef CAS PubMed.
- S. S. Nair, J. Zhu, Y. Deng and A. J. Ragauskas, Sustainable Chem. Processes, 2014, 2, 23 CrossRef.
- G. de Souza, M. N. Belgacem, A. Gandini and A. J. F. Carvalho, Cellulose, 2021, 28, 1617–1632 CrossRef CAS.
- O. L. Sprockel, W. Prapaitrakul and P. Shivanand, J. Pharm. Pharmacol., 1990, 42, 152–157 CrossRef CAS PubMed.
- J. Lange and Y. Wyser, Packag. Technol. Sci., 2003, 16, 149–158 CrossRef CAS.
- L. K. Massey, Film Properties of Plastics and Elastomers, William Andrew Publishing, 2nd edn, 2004 Search PubMed.
- P. E. Keller and R. Kouzes, Water Vapor Permeation in Plastics, Pacific Northwest National Laboratory, 2016 Search PubMed.
- L. W. McKeen, Permeability Properties of Plastics and Elastomers, Elsevier Science & Technology Books, 2011 Search PubMed.
- M. D. Steven and J. H. Hotchkiss, Packag. Technol. Sci., 2002, 15, 17–27 CrossRef CAS.
- R. Nisticò, Polym. Test., 2020, 90, 106707 CrossRef.
- K. M. Tavares, A. Campos, B. R. Luchesi, A. A. Resende, J. E. Oliveira and J. M. Marconcini, Carbohydr. Polym., 2020, 246, 116521 CrossRef CAS PubMed.
- Q. Yu, L. Yang, S. Wang, L. Zhang and D. Sun, Cellulose, 2023, 30, 10273–10284 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00425f |
| This journal is © The Royal Society of Chemistry 2024 |
