Open Access Article
Open Access ArticleEffects of chemical pretreatment on the enzymatic hydrolysis of post-consumer waste viscose†
Edvin
Bågenholm-Ruuth
 a,
Mahla
Bagherigelvardi
b,
Caroline
Gustafsson
a,
Miguel
Sanchis-Sebastiá
a,
Mahla
Bagherigelvardi
b,
Caroline
Gustafsson
a,
Miguel
Sanchis-Sebastiá
 *b and
Ola
Wallberg
*b and
Ola
Wallberg
 a
a
aDivision of Chemical Engineering, Department of Process and Life Science Engineering, Lund University, Box 124, SE-221 00 Lund, Sweden
bShareTex AB, Erikslustvägen 55, 217 73 Malmö, Sweden. E-mail: miguel.sanchis@sharetex.com
First published on 31st October 2024
Abstract
The pretreatment of post-consumer waste viscose before enzymatic hydrolysis was tested and evaluated to develop a tertiary recycling strategy for waste viscose, as no such strategy currently exists. There were differences between hydrolyzability of pre-consumer and post-consumer viscose, as we obtained 100% glucose yield from pre-consumer viscose while only 60–80% could be achieved from post-consumer, which we attributed to the inhibition – induced by contaminants that accumulated in the post-consumer viscose during its manufacture and use. Dilute alkali and acid pretreatment were applied to improve the hydrolysis of post-consumer viscose, although both of them proved unsuccessful strategies as alkali and acid pretreatment reduced glucose yield up to 28% and 44%, respectively. We concluded that avoiding pretreatment altogether was the most energy-efficient and resource-efficient alternative with regard to the saccharification of waste viscose. Thus, the economic viability of (enzymatically) saccharifying waste viscose depends entirely on the inherent hydrolyzability of the feedstock and its price.
Sustainability spotlightThe demand for viscose fibers continue to rise, but efficient recycling strategies are still lacking, which leads to vast amounts of textile waste accumulating in landfills worldwide. Due to the low degree of polymerization of viscose, alternatives to fiber-to-fiber recycling must be implemented. In this work, we identify challenges with recycling viscose post the use phase and evaluate different approaches to overcome these challenges. Ultimately, we identify avoiding pretreatment altogether as the most efficient strategy from an energy and resource perspective, for recycling post-consumer viscose via saccharification. This work aligns closely with the 12th Sustainable Development Goal identified by the UN in that it contributes to improving resource utilization, and fosters sustainable consumption, in viscose manufactory. |
1 Introduction
Waste textiles are becoming an increasingly significant environmental problem. Approximately 113 million tons of fibers were produced globally in 2021, most of which was used for apparel.1 Although much effort is being directed toward developing processes to recycle waste textiles, such a practice remains limited. In 2017, the Ellen MacArthur Foundation reported that less than 1% of textile fibers that were dedicated to the production of apparel were derived from recycled textiles. Further, only 12% of waste textiles were recycled into a lower-value application, whereas 73% were landfilled or incinerated.2Viscose production suffers from having a high environmental impact due to its reliance on toxic processing chemicals and its intensive chemical, water, and energy use.2 Nevertheless, the demand for MMCFs, such as viscose, is estimated to grow significantly.1 Thus, the environmental incentives for developing efficient methods for recycling post-consumer waste viscose are substantial. Although fiber-to-fiber recycling is preferred, not every type of waste textile can be recycled in such a manner. For instance, viscose, the most ubiquitous manmade cellulosic fiber (MMCF) on the market,1 has limited potential for fiber-to-fiber recycling due to its low degree of polymerization.3
The hydrolysis of waste viscose into glucose for the production of valuable chemicals is an attractive potential alternative pathway toward valorization.4 Viscose can be hydrolyzed chemically and enzymatically.4,5 Chemical hydrolysis methods, such as acid hydrolysis, are typically faster than enzymatic hydrolysis, which, conversely, can be performed at lower temperatures and generate a biocompatible broth.5–7
Post-consumer textiles are more recalcitrant to hydrolysis and generally harder to recycle than pre-consumer textiles,4,8–12 further complicating the saccharification of post-consumer textiles. This complexity arises from the incorporation of various additives into apparel during processing, the accumulation of contaminants throughout the lifecycle of the garment, and degradation due to wear and tear. For example, certain finishers effect significant resistance against the hydrolysis of textiles,13 and reactive dyes impair the enzymatic depolymerization of cellulose.14
Various pretreatment methods are commonly applied to improve the sugar yields of hard-to-hydrolyze materials in biorefineries.15,16 For example, alkaline treatment of cellulose-based textiles can be employed for many reasons, including improving enzymatic digestibility, targeting the removal of textile finishing agents, and separating fiber types in fiber blends.6,13,17–23 Sodium hydroxide (NaOH) is a popular agent for pretreating cellulosic materials, but its strong hydrolyzing ability often damages the raw material and can lead to losses in material.16,17,23 Such losses during pretreatment with NaOH are especially notable in the pretreatment of viscose, wherein even dilute concentrations of NaOH decrease viscose fiber content.6 Alternatively, sodium carbonate (Na2CO3) can be used as an alkaline agent during pretreatment, resulting in less loss of material when applied to cellulosic waste textiles, compared with NaOH.16,23
In this study, we report that post-consumer waste viscose has inherent resistance toward hydrolysis, evaluating various pretreatment methods that are typically used in biorefining with regard to minimize this resistance and increasing the sugar yield. The pretreatment approaches comprised an acidic (sulfuric acid) and alkaline (sodium carbonate) method, each with and without gradual washing of the pretreated waste viscose. The results of this study will facilitate and provide useful insights into the development of tertiary recycling alternatives for post-consumer waste viscose.
2 Materials and methods
2.1 Collection and preparation of raw materials
The waste viscose was post-consumer 100% viscose that was purchased from a textile sorter (Lounais-Suomen Jätehuolto, Turku, Finland). The waste viscose was separated into a colored fraction and a white fraction. Textile trimmings that were not made of viscose, including seams, labels, buttons, and decorative details, were removed from the garments using a circular textile knife (Ø 45 mm, Stoff & Stil, Malmö, Sweden). The textiles were then reduced to ∼10 × 10 mm pieces in a textile shredder with a 170 × 107 mm chamber and 15 mm blades (Filamaker, Kaufungen, Germany).The control material was white pre-consumer 100% virgin viscose fabric (woven) (Textil & Metallskrot Skroten AB, Kinna, Sweden) and medicinal cotton (VWR International, Radnor, PA, USA). The virgin viscose was cut into smaller pieces using the circular textile knife, which were then shredded into ∼10 × 10 mm pieces in the textile shredder. The medicinal cotton was not treated in any way and it was directly subjected to enzymatic hydrolysis in all experiments.
2.2 Enzymatic hydrolysis of nontreated viscose
The enzyme blend was Cellic CTec 2 (Novozymes, Bagsværd, Denmark). The citrate–sodium citrate buffer (0.1 M and pH 5) was prepared in 5 L batches: 43.08 g of citric acid monohydrate and 86.77 g of sodium citrate dihydrate in 4 L distilled water. The pH was adjusted to 5 with sulfuric acid and sodium hydroxide as needed, and distilled water was added to a total volume of 5 L.
2.3 Pretreatment of waste viscose
Pretreated viscose was subjected to enzymatic hydrolysis in Falcon tubes, with a total mass of 20 g; the mass of pretreated viscose corresponded to a solids loading of 5 wt% dry textiles, and enzymes were added to 0.05, 0.10, or 0.15 g enzyme blend per g dry viscose. Samples that contained medicinal cotton and untreated colored waste viscose were also hydrolyzed as controls. The tubes were incubated for 96 h at 50 ± 3 °C and 270 ± 10 rpm; the enzymatic hydrolysis was performed in duplicate. After hydrolysis, the contents were vacuum-filtered through a filter cloth with a pore size of 100 μm. Samples of the liquid product were collected and analyzed.
2.4 Gradual washing of pretreated waste viscose
White and colored waste viscose was pretreated as described in Sections 2.3.1 and 2.3.2, except for the wash steps. Pretreated viscose was washed with diluted pretreatment solution that contained 3 wt% Na2CO3 or 10 wt% H2SO4, depending on the pretreatment. After the first wash, the materials were vacuum-filtered through a filter cloth (pore size 100 μm) and washed with 1 wt% Na2CO3 or 5 wt% H2SO4 solution for the alkali-pretreated and acid-pretreated viscose, respectively. After the second wash, the materials were vacuum-filtered, washed with deionized water, and vacuum-filtered again before being subjected to enzymatic hydrolysis, as detailed in Section 2.3.1.2.5 Analyses
The samples for this analysis were evaluated by high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) on an ICS3000 system (Dionex, Sunnyvale, CA, USA) equipped with a CarboPac PA1 column, GP50 gradient pump, and AS50 autosampler. The HPAEC-PAD analysis was performed at 30 °C and an eluent flow rate of 1 mL min−1. Deionized and degassed water, degassed sodium hydroxide solution (200 mmol per L NaOH), and degassed sodium hydroxide and sodium acetate solution (200 mmol per L NaOH, 170 mmol per L NaAc) were used as eluents. The chemicals for the eluents were purchased from Merck (Merck KGaA, Darmstadt, Germany). In cases in which the HPAEC-PAD data indicated a cellulose content near 100%, the cellulose content of the samples was calculated by subtracting the mass of the dried unhydrolyzed solid residue from the oven-dried weight of the raw materials. All compositional analyses were performed in triplicate.
Samples obtained from the enzymatic hydrolysis were passed through a syringe filter (pore size 0.22 μm) and analyzed for sugar content on a Shimadzu LC-20 AD high-performance liquid chromatography (HPLC) system that was equipped with a Shimadzu RID 10A refractive index detector (Shimadzu Corporation, Kyoto, Japan) and a Carbosep CHO 782 column (Concise Separations, San Jose, CA, USA), which was operated at 70 °C and 50 bar, with 0.6 mL min−1 deionized water as eluent.
The supernatants were centrifuged before being subjected to protein quantification. The concentration of unbound proteins in the samples was measured using Coomassie Plus (Bradford) Assay Reagent (Thermo Fisher Scientific, Waltham, MA, USA), and the absorbance at 595 nm was measured on a UV-1800 UV-VIS spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The concentrations were determined against bovine serum albumin (BSA).
3 Results and discussion
3.1 Material composition
The waste viscose (colored and white) had a TS content of 95.8% (±0.1%) and consisted of 99.1% cellulose (standard deviation 0.3%). The virgin viscose had a TS content of 94.0% (±0.8%) and a cellulose content of 99.6% (standard deviation 0.4%). The TS content of the medicinal cotton was 93.4% (±0.5%), with a cellulose content of 98.9% (standard deviation 0.5%). Data for the pretreated waste viscose can be found in Table S1.†3.2 Effect of enzyme loading
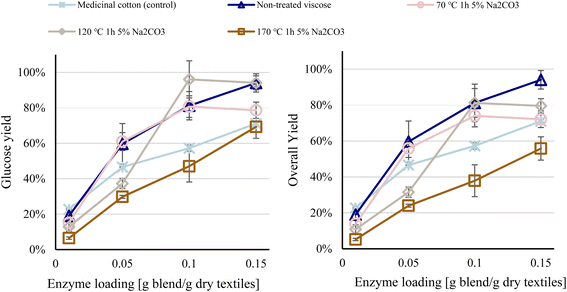
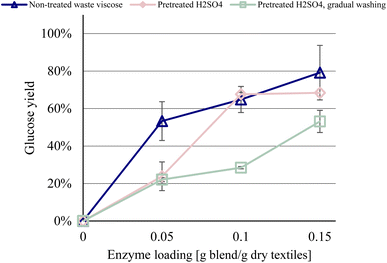
Increased enzyme loadings resulted in higher glucose yields for all raw materials, although 0.3 g enzyme blend per g dry textiles was insufficient to hydrolyze the waste viscose completely. The virgin viscose achieved near-total hydrolysis at an enzyme loading of 0.15 g enzyme blend per g dry textiles, whereas the glucose yields for the colored and white waste viscose samples peaked at 80% and 60%, respectively (Fig. 1). Increasing the enzyme loading improves the hydrolysis yield of lignocellulosic biomass to a plateau, after which additional enzyme loading does not enhance yields significantly.15,25 Consequently, it is not possible for glucose yields of the waste viscose samples to approach that of virgin viscose through increases in enzyme loading.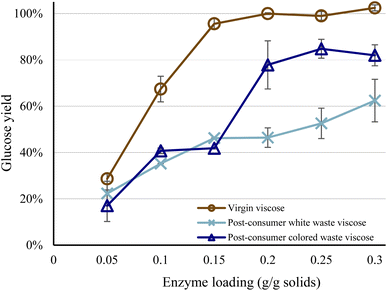 | ||
| Fig. 1 Glucose yield as a function of enzyme loading, at a residence time of 96 h. Error bars show the spread between duplicates. | ||
That post-consumer waste viscose was more recalcitrant toward hydrolysis was not unexpected. Many finishing agents can be added to the fabric between the spinning of the fibers and the sale of the garment. The type of finisher depends on the customer's requirements and thus differs between fabrics,26 and certain finishing agents can significantly inhibit enzymatic activity against textiles.13 Further, worn textiles come into contact with contaminants during use, such as oily organic substances in perspiration, human skin, sebum, and clay minerals, as well as oils from food, cosmetics, and machinery.27,28 Whereas some of these contaminants are readily removed during washing, others, such as sebum, do not desorb and thus accumulate in textiles,28,29 suggesting that these contaminants caused the recalcitrance of waste viscose samples toward hydrolysis.
The origin of the recalcitrance of post-consumer waste viscose toward hydrolysis was examined by analyzing the adsorption of enzyme on samples of colored and white waste viscose and virgin viscose. The adsorption of enzyme was similar across all samples (Table 1), indicating that their resistance to hydrolysis did not stem from the inaccessibility of enzymes to waste viscose. Instead, we attributed the recalcitrance of waste viscose to inhibition of the active site.
| Sample | Protein concentration (mg mL−1) |
|---|---|
| White pre-consumer viscose | 0.52 (±0.03) |
| White post-consumer viscose | 0.50 (±0.01) |
| Colored post-consumer viscose | 0.54 (±0.02) |
3.3 Pretreatment of waste viscose
The most efficient method of improving glucose yields beyond the point at which they have been maximized by enzyme loading is pretreatment.15 The effects of alkaline and acidic pretreatment were thus examined with regard to overcoming the resistance of post-consumer waste viscose to hydrolysis compared with virgin viscose. A consolidated table presenting the different pretreatment conditions tested as well as the cellulose recovery and glucose yield obtained at each condition can be found in Appendix B of the ESI Table S2.†As shown above, pretreatment with Na2CO3 effected losses in cellulose prior to enzymatic hydrolysis. Thus, because pretreatment did not improve enzymatic activity, the overall yields (based on the cellulose in the starting material) were lower for all pretreated samples versus nontreated material (Fig. 2). Losses in cellulose during alkali pretreatment of waste cotton have been reported,23 but we observed such decreases under milder pretreatment conditions, due to the lower degree of polymerization and crystallinity of waste viscose. Thus, based on its greater proclivity to degrade during pretreatment, waste viscose can only be pretreated under mild conditions to avoid unacceptable cellulose losses; such conditions, however, are insufficient to increase the amenability to enzymes. As a result, alkali pretreatment is not a feasible strategy for overcoming the resistance of waste viscose.
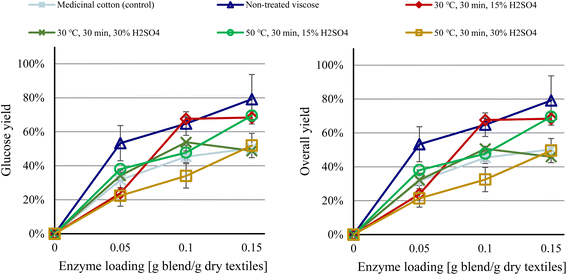 | ||
| Fig. 3 Glucose and overall yields for pretreated colored post-consumer waste viscose after acidic pretreatment. | ||
Pretreatment with 15% H2SO4 led to no extensive loss in cellulose, whereas cellulose levels fell by roughly 5% with 30% H2SO4. As a result, the overall yield approximated the glucose yield in all cases (Fig. 3). Exposure of waste cotton to higher concentrations of sulfuric acid (60% to 80%) induces extensive cellulose degradation.8 Because waste viscose is significantly more susceptible to degradation than waste cotton,6 it is unlikely that increased acid concentrations will improve the efficiency of the process. As with alkali pretreatment, the results indicate that waste viscose can only be pretreated under mild conditions to avoid unacceptable losses in cellulose, but these conditions are insufficient to increase enzymatic activity.
The results of the acid pretreatment should be interpreted with caution, because the enzymatic hydrolysis was performed using the same batch of enzyme blend as for the alkaline pretreatment studies, albeit several months later. The performance of the enzymes declined during this period, as evidenced by the 16% lower glucose yield for the control material (medicinal cotton) between the 2 experiments. Thus, despite the lower yields in Fig. 3versusFig. 2, the 2 pretreatment methods performed similarly, with the optimal condition for each method resulting in approximately the same overall yield as nontreated waste viscose.
3.4 Post-pretreatment gradual washing
We hypothesized that the unexpected negative effect of chemical pretreatment on waste viscose was attributed to the formation of a boundary layer that results from washing immediately with deionized water following pretreatment, impeding the activity of enzymes against the pretreated material. Gradual washing could thus reduce the evolution of such a boundary layer by decreasing the concentration gradient to which the pretreated waste viscose is exposed during the wash. Gradual washing is common in the textile and pulp industries, which frequently apply countercurrent washing to limit the generation of wastewater. Thus, a series of experiments was conducted, in which the pretreated materials were washed twice with increasingly dilute solutions of Na2CO3 and H2SO4 for the alkaline and acidic pretreated materials, respectively, before a third wash with deionized water, followed by enzymatic hydrolysis.The incorporation of a gradual wash step after the pretreatment of waste viscose with Na2CO3 increased glucose yields compared with pretreated waste viscose that did not undergo a gradual wash (Fig. 4). Without gradual washing, the pretreated material performed slightly worse than the nontreated material but performed modestly better with it. This improvement demonstrates that this post-pretreatment procedure is an important determinant of the efficiency of the process and influences the effect of pretreatment on waste viscose. It also strengthens the hypothesis that gradual washing reduces the formation of a boundary layer that impedes enzymatic hydrolysis and explains the lower glucose yields in the previous experiments.
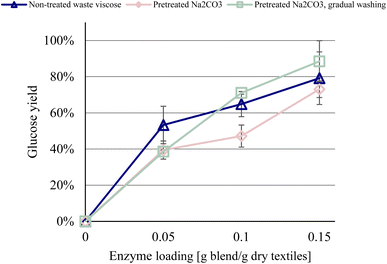 | ||
| Fig. 4 Glucose yield of alkali pretreated post-consumer waste viscose (70 °C, 1 h, 5% Na2CO3) with and without gradual washing. | ||
Gradual washing with H2SO4 after acidic pretreatment decreased glucose yields compared with no gradual washing (Fig. 5). This decline coincided with a drop in pH on addition of washed pretreated material to the buffer, indicating the presence of residual H2SO4 in the washed pretreated material, explaining the lower enzymatic activity. We conclude that the gradual washing procedure in this case results in insufficient acid removal prior to enzymatic hydrolysis, necessitating a prolonged sequence with additional wash steps.
 | ||
| Fig. 5 Glucose yield of acid pretreated post-consumer waste viscose (30 °C, 30 min, 15% H2SO4) with and without gradual washing. | ||
Despite the improved efficiency due to gradual washing, the increase in glucose yield remained limited compared with the non-treated material, when applying alkali pretreatment. Further, application of acid pretreatment effected no clear improvements, and gradual washing in the post-pretreatment step would need to be prolonged to completely remove residual acid. Thus, although post-pretreatment is beneficial in certain cases, the inhibition that is associated with waste viscose cannot be overcome through chemical pretreatment.
3.5 Comparison with other cellulosic feedstocks
Post-consumer waste viscose exhibited mass losses even under mild pretreatment conditions, negating any improvement in hydrolyzability. This behavior eliminates the benefit of pretreatment before enzymatic hydrolysis, because it fails to improve the overall yield of the process. To ensure that no mass losses occur, the severity of the pretreatment must be lowered to a level at which there is no increase in the hydrolyzability of the post-consumer waste viscose. This property is notable, because it distinguishes post-consumer waste viscose from other types of cellulosic feedstocks in biorefinery applications, such as lignocellulosic biomass.There are abundant previous studies that demonstrate the positive effect of pretreatment on other types of materials. For example, pretreating cotton with 20% NaOH at 100 °C increasing glucose yield from 26.2% to 98.6%5 while pretreatment with 5% Na2CO3 (the same chemical used in this study) at 200 °C increased the glucose yield from 30% to 80%.23 There is a stark difference between these results and those obtained for viscose in this study, as pretreatment with 5% Na2CO3 at 170 °C actually led to a decrease of the glucose yield which is the opposite behavior as that observed for cotton. There are also clear differences between viscose and other lignocellulosic feedstocks beyond textile fibers. For example, pretreating bamboo with sulfuric acid at 180 °C led to an increase in glucose yield from 2.5% to 49.4%30 while glucose yield for viscose samples treated with sulfuric acid was lower than for untreated viscose in this study. Once again, viscose exhibits opposite changes in hydrolyzability upon pretreatment and it is therefore clear that this feedstock has a unique behavior in biorefinery applications that requires adjusting process concepts and conditions in a different manner compared to previously studied feedstocks.
Post-consumer waste viscose contains cellulose II, the thermodynamically more stable crystal lattice and the confirmation that is assumed by the regenerated cellulose that is recovered.32 Cellulose II is more susceptible to hydrolytic degradation than cellulose I, which is the naturally occurring crystal lattice in plant biomass.6,32,33 However, the difference in hydrolyzability between cotton and viscose textiles is greater6 than what is expected from the disparities between cellulose I and II; further, we have shown that this difference is more pronounced when comparing post-consumer waste cotton23 with post-consumer waste viscose, although this difference depends on the presence of textile additives.
Previous studies show that it would also be possible to convert waste cotton into glucose via acid hydrolysis.4 Acid hydrolysis delivered higher yields than those obtained in this study or, alternatively, similar yields at higher concentration of glucose.4 However, the mixture obtained from enzymatic hydrolysis would be biocompatible, that is, it would be possible to directly ferment it without any treatment while the mixture from acid hydrolysis would require a separation step to remove the acid, for which there are no commercially attractive alternatives. Thus, in spite of the lower efficiency in the production of glucose, enzymatic hydrolysis seems a more promising technology due to advantages in downstream processing and biocompatibility of the product.
Post-consumer waste viscose is a unique second-generation cellulosic feedstock, because its valorization by enzymatic hydrolysis does not benefit from pretreatment. Thus, the price of the downstream product, glucose, and the economics of the process depend entirely on the intrinsic characteristics of the feedstock—i.e., its hydrolyzability and cost. Consequently, efficient and low-cost sourcing of the feedstock are paramount to the economic viability of sugar production from waste viscose, and even if such feedstock becomes available, the process and economics would nevertheless rely heavily on the substances that are introduced to the material during the manufacturing and use phases.
4 Conclusions
Post-consumer waste viscose is resistant to enzymatic hydrolysis. This recalcitrance was noted for white and colored waste viscose, of which the former was more resistant. In contrast, pre-consumer virgin viscose fabric was completely hydrolyzed at moderate enzyme loadings above 0.15 g Cellic CTec 2 per g dry textiles. The recalcitrance of post-consumer waste viscose likely stems from inhibition of the viscose by contaminants that are introduced during garment manufacture and use, such as textile finishers and sebum.The recalcitrance against enzymatic hydrolysis of post-consumer waste viscose can be partially overcome by increasing the enzyme loading, although it is insufficient to hydrolyze the material completely. Pretreatment of waste viscose with Na2CO3 or H2SO4 over a range of conditions, followed by a wash with deionized water prior to hydrolysis, does not improve glucose yields. Gradual washing after the pretreatment increased the glucose yield when using Na2CO3 (70 °C, 1 h, 5%) and decreased it with H2SO4, compared with the hydrolysis of nontreated waste viscose. Thus, only one of the pretreatment procedures that were tested can overcome the inhibition and effect higher enzymatic activity and glucose yields.
However, the improvement in glucose yield following the combination of pretreatment and gradual washing is counteracted by mass losses during pretreatment with Na2CO3. This property is notable, differentiating post-consumer waste viscose from other second-generation cellulosic feedstocks, because otherwise effective pretreatment methods fail to improve the overall yield of saccharification. Thus, the cost of the glucose that is produced via enzymatic hydrolysis of post-consumer waste viscose depends heavily on the price of the feedstock and its inherent hydrolyzability. Consequently, higher enzyme loadings and lower process efficiency will be associated with post-consumer waste viscose compared with its pre-consumer counterpart, prompting us to conclude that (enzymatically) saccharifying this waste stream will become more expensive after the use phase.
Data availability
Data used in this study is contained within the article.Author contributions
Edvin Bågenholm-Ruuth: conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, visualization, writing – original draft, writing – review & editing. Mahla Bagherigelvardi: data curation, investigation, validation, writing – original draft. Caroline Gustafsson: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization. Miguel Sanchis-Sebastiá: conceptualization, formal analysis, methodology, project administration, supervision, validation, visualization, writing – review & editing. Ola Wallberg: conceptualization, funding acquisition, project administration, resources.Conflicts of interest
Authors Mahla Bagherigelvardi and Miguel Sanchis-Sebastiá are employed by ShareTex AB. Authors Edvin Bågenholm-Ruuth, Miguel Sanchis-Sebastiá, and Ola Wallberg owns shares in ShareTex AB. Author Caroline Gustafsson declares no financial interests.Acknowledgements
The work was funded by Vinnova (reference number 2021-04404) within the BioInnovation Program, and the Swedish Energy Agency (project number 51217-1).References
- S. Opperskalski, A. Franz, A. Patanè, S. Siew and E. Tan, Preferred Fiber & Materials Market Report Textile Exchange, 2022 Search PubMed.
- Ellen MacArthur Foundation, A New Textiles Economy: Redesigning Fashion's Future, 2017 Search PubMed.
- H. Wedin, M. Lopes, H. Sixta and M. Hummel, Evaluation of post-consumer cellulosic textile waste for chemical recycling based on cellulose degree of polymerization and molar mass distribution, Text. Res. J., 2019, 89, 5067–5075, DOI:10.1177/0040517519848159.
- E. Ruuth, M. Sanchis-Sebastiá, P. T. Larsson, A. Teleman, A. Jiménez-Quero, S. Delestig, V. Sahlberg, P. Salén, M. Sanchez Ortiz, S. Vadher and O. Wallberg, Reclaiming the value of cotton waste textiles: a new improved method to recycle cotton waste textiles via acid hydrolysis, Recycl, 2022, 7, 57, DOI:10.3390/recycling7040057.
- A. Jeihanipour, K. Karimi, C. Niklasson and M. J. Taherzadeh, A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles, Waste Manage., 2010, 30, 2504–2509, DOI:10.1016/j.wasman.2010.06.026.
- K. Safartalab, F. Dadashian and F. Vahabzadeh, Fed batch enzymatic hydrolysis of cotton and viscose waste fibers to produce ethanol, Univers. J. Chem., 2014, 2, 11–15, DOI:10.13189/ujc.2014.020103.
- S. Mihalyi, M. Tagliavento, E. Boschmeier, V.-M. Archodoulaki, A. Bartl, F. Quartinello and G. M. Guebitz, Simultaneous saccharification and fermentation with Weizmannia coagulans for recovery of synthetic fibers and production of lactic acid from blended textile waste, Resour., Conserv. Recycl., 2023, 196, 107060, DOI:10.1016/j.resconrec.2023.107060.
- M. Sanchis-Sebastiá, E. Ruuth, L. Stigsson, M. Galbe and O. Wallberg, Novel sustainable alternatives for the fashion industry: a method of chemically recycling waste textiles via acid hydrolysis, Waste Manage., 2021, 121, 248–254, DOI:10.1016/j.wasman.2020.12.024.
- A. Hussain, N. Kamboj, V. Podgurski, M. Antonov and D. Goliandin, Circular economy approach to recycling technologies of postconsumer textile waste in Estonia: a review, Proc. Est. Acad. Sci., 2021, 80–90 CrossRef.
- E. Büyükaslan, S. Jevsnik and F. Kaloğlu, A sustainable approach to collect post-consumer textile waste in developing countries, Marmara Journal of Pure and Applied Sciences, 2015, 1, 107–111 Search PubMed.
- G. Coşkun and F. N. Başaran, Post-consumer textile waste minimization: a review, J. Strateg. Res. Soc. Sci., 2019, 5, 1–18 Search PubMed.
- E. McCauley and I. Jestratijevic, Exploring the business case for textile-to-textile recycling using post-consumer waste in the US: Challenges and opportunities, Sustain, 2023, 15, 1473, DOI:10.3390/su15021473.
- J. Egan, S. Wang, J. Shen, O. Baars, G. Moxley and S. Salmon, Enzymatic textile fiber separation for sustainable waste processing, Resour. Environ. Sustain., 2023, 13, 100118, DOI:10.1016/j.resenv.2023.100118.
- M. Czilik, É. Pászt, I. Réczey, J. Alt, I. Rusznák, É. Kárpáti and A. Víg, Effects of reactive dyes on the enzymatic depolymerization of cellulose, Dyes Pigm., 2002, 54, 95–106, DOI:10.1016/S0143-7208(02)00042-6.
- C. Tengborg, M. Galbe and G. Zacchi, Influence of enzyme loading and physical parameters on the enzymatic hydrolysis of steam-pretreated softwood, Biotechnol. Prog., 2001, 17, 110–117, DOI:10.1021/bp000145+.
- M. Galbe and O. Wallberg, Pretreatment for biorefineries: a review of common methods for efficient utilisation of lignocellulosic materials, Biotechnol. Biofuels, 2019, 12, 294, DOI:10.1186/s13068-019-1634-1.
- P. N. Abhyankar, K. R. Beck, C. M. Ladisch and J. G. Frick, Effect of different catalysts on the DMDHEU-cotton cellulose reaction, Text. Res. J., 1986, 56, 551–555, DOI:10.1177/004051758605600904.
- P. N. Abhyankar, K. R. Beck, C. M. Ladisch and S. P. Rowland, A new and effective method for removing DMDHEU crosslinks from cotton, Text. Res. J., 1985, 55, 444–448, DOI:10.1177/004051758505500708.
- P. N. Abhyankar, K. R. Beck, C. M. Ladisch and N. R. Bertoniere, Stability of DMDHEU and alkylated crosslinking finishes towards acidic and alkaline hydrolysis, Text. Res. J., 1987, 57, 395–400, DOI:10.1177/004051758705700704.
- L. V. Haule, C. M. Carr and M. Rigout, Investigation into the removal of an easy-care crosslinking agent from cotton and the subsequent regeneration of lyocell-type fibres, Cellulose, 2014, 21, 2147–2156, DOI:10.1007/s10570-014-0225-3.
- C.-H. Kuo, L. Po-Ju, Y.-Q. Wu, L. Ye, D.-J. Yang, C.-J. Shieh and C.-K. Lee, Simultaneous saccharification and fermentation of waste textiles for ethanol production, BioResources, 2014, 9, 2866–2875, DOI:10.15376/biores.9.2.2866-2875.
- A. Palme, A. Peterson, H. de la Motte, H. Theliander and H. Brelid, Development of an efficient route for combined recycling of PET and cotton from mixed fabrics, Textiles and Clothing Sustainability, 2017, 3, 1–9, DOI:10.1186/s40689-017-0026-9.
- M. Sanchis-Sebastiá, V. Novy, L. Stigsson, M. Galbe and O. Wallberg, Towards circular fashion – transforming pulp mills into hubs for textile recycling, RSC Adv., 2021, 11, 12321–12329, 10.1039/d1ra00168j.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Determination of Structural Carbohydrates and Lignin in Biomass, Laboratory Analytical Procedure, NREL, 2011 Search PubMed.
- W. Sattler, H. Esterbauer, O. Glatter and W. Steiner, The effect of enzyme concentration on the rate of the hydrolysis of cellulose, Biotechnol. Bioeng., 1989, 33, 1221–1234, DOI:10.1002/bit.260331002.
- W. D. Schindler and P. J. Hauser, Chemical Finishing of Textiles, Woodhead Publishing, Sawston, 1st edn, 2004 Search PubMed.
- M. Krifa, S. Rajaganesh and W. Fahy, Perspectives on textile cleanliness – detecting human sebum residues on worn clothing, Text. Res. J., 2019, 89, 5226–5237, DOI:10.1177/0040517519855323.
- Y.-S. Chi and S. K. Obendorf, Aging of oily soils on textile materials: a literature review, J. Surfactants Deterg., 1998, 1, 407–418, DOI:10.1007/s11743-998-0044-0.
- A. Møllebjerg, L. G. Palmén, K. Gori and R. L. Meyer, The bacterial life cycle in textiles is governed by fiber hydrophobicity, Microbiol. Spectrum, 2021, 9, e01185, DOI:10.1128/spectrum.01185-21.
- Z. Li, B. Fei and Z. Jiang, Comparison of dilute organic and sulfuric acid pretreatment for enzymatic hydrolysis of bamboo, BioResources, 2014, 9, 5652–5661, DOI:10.15376/biores.9.3.5652-5661.
- S. Wu, S. Shi, R. Liu, C. Wang, J. Li and L. Han, The transformations of cellulose after concentrated sulfuric acid treatment and its impact on the enzymatic saccharification, Biotechnol. Biofuels Bioprod., 2023, 16, 36, DOI:10.1186/s13068-023-02293-4.
- A. M. Donald, Polysaccharide Crystallization, in Encyclopedia of Materials: Science and Technology, ed. K. H. J. Buschow, R. W. Cahn, M. C. Flemings, B. Ilschner, E. J. Kramer, S. Mahajan and P. Veyssière, Elsevier, Oxford, 2001, pp. 7714–7718, DOI:10.1016/B0-08-043152-6/01385-1.
- M. Wada, M. Ike and K. Tokuyasu, Enzymatic hydrolysis of cellulose I is greatly accelerated via its conversion to the cellulose II hydrate form, Polym. Degrad. Stab., 2010, 95, 543–548, DOI:10.1016/j.polymdegradstab.2009.12.014.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00486h |
| This journal is © The Royal Society of Chemistry 2024 |