Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Beyond waste: cellulose-based biodegradable films from bio waste through a cradle-to-cradle approach
Mai N.
Nguyen
a,
Minh T. L.
Nguyen
bc,
Marcus
Frank
 cd and
Dirk
Hollmann
cd and
Dirk
Hollmann
 *bc
*bc
aSchool of Chemistry and Life Sciences (SCLS), Hanoi University of Science and Technology, No. 1 Dai Co Viet Street, 10000 Hanoi, Vietnam
bDepartment of Chemistry, University of Rostock, Albert-Einstein-Straße 3A, 18059 Rostock, Germany. E-mail: dirk.hollmann@uni-rostock.de
cDepartment Life, Light & Matter, Faculty for Interdisciplinary Research, University of Rostock, Albert-Einstein-Straße 25, 18059 Rostock, Germany
dMedical Biology and Electron Microscopy Centre, University Medicine Rostock, 18059 Rostock, Germany
First published on 6th November 2024
Abstract
The basis of circular economy is the use and valorization of renewable raw materials. Especially in developing countries, crop waste such as straw and rice straw have high potential for further utilization. Within this report, we present a holistic strategy including the selective isolation of cellulose via simple, environmental benign two-step process. Rice straw was easily dissolved in a non-derivatizing electrolyte solvent such as aqueous solution of tetrabutylphosphonium hydroxide (TBPH) (50 wt%) at room temperature followed by precipitation in water. Quantitative amount of raw cellulose was recovered within a short period of time without heating or cooling enabling a further application of biomass material. The structure and characterization of the raw cellulose were investigated by nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and by scanning (SEM) and transmission electron microscopy (TEM). This method could be an excellent alternative to the current extraction methods such as the KRAFT process. Indeed, the same chemicals as for the isolation can be used to prepare regenerated cellulose film of high purity with the raw cellulose. Due to its sustainability and exceptional biodegradability, these films have a great potential for applications in environment, textile, and separation industry. No modification of the cellulose during the extraction and preparation process occurs thus, these films are no plastics and thus can be used without regulations. In general, a full “circular economy” process is provided: valuable raw materials (cellulose) are recovered selectively from natural resources such as rice straw and further to enable products with high applicability in life (cellulose packaging film). The cradle-to-cradle process is closed by fast biodegradation of the used products.
Sustainable spotlightAs the focus of society increasingly shifts to protecting our environment and creating sustainable lifestyles, the importance of innovative research that addresses these challenges continues to grow. The development of technologies and methods that are not only environmentally friendly but also economically viable is a crucial step towards a more sustainable future. The use and valorization of resources that were previously unused or considered as waste plays a key role in this context. The goal is to transform the conventional boundaries of waste management and materials science from a linear economy to a circular economy. So a holistic approach such as a cradle-to-cradle process is particularly important: from nature to nature, without polluting it. Our research aims to make an important contribution to overcoming one of the most pressing challenges of our time: the efficient and sustainable utilization of agricultural waste. By developing a simple but highly effective method of extracting high-purity cellulose from rice straw – a resource that is widely used but underutilized, especially in agricultural countries such as Vietnam – we are paving the way for a new paradigm of waste recycling that is both ecologically and economically viable. Rapid biodegradability in the soil, but also in water, is particularly relevant in order to minimize or even prevent environmental damage. This research aligns with the United Nations Sustainable Development Goals (SDGs), particularly those focusing on responsible consumption and production (SDG 12), climate action (SDG 13) and life on land (SDG 15). The practical and scalable application of sustainable circular chemistry in the conversion of agricultural waste into valuable raw materials is important for global debate and application. Especially in developing countries, simple processes can create added value. The use of renewable resources allows the support of economic development in agricultural communities and can thus protect our planet for future generations. |
Main
The challenge of sustainable re-use of waste products has become increasingly important in the global discussion on sustainability1 and the circular economy.2 It is aligned with the United Nations Sustainable Development Goals (SDGs), in particular the goal of responsible consumption and production, thus reduces the environmental impact of conventional waste disposal methods. Implementing the re-use of waste in agricultural countries such as Vietnam could not only reduce pollution, but also stimulate new economic sectors and thus contribute to achieving several SDGs. Rice straw has been recognized as a potential alternative for a sustainable, biodegradable and renewable resource. In Vietnam, rice straw is abundant throughout the year and it has been estimated that approximately 67 million tons are produced annually.3 Its main components are cellulose, hemicellulose and lignin, extractive/volatiles as well as a number of minor inorganic compounds such as silica.4 The standard treatment mainly comprises the direct burning of rice straw in the farmland because of the quickest and most economical preparation of the fields for the coming growing season. However, this approach not only wastes biomass but also contributes to environmental pollution, destroy public buildings and limit visibility: total rice residue burning emissions as 2.24 Gg high concentrations of fine-particulate matter PM 2.5, together with 36.54 Gg CO and 567.79 Gg CO2 for Hanoi Province.5,6 In contrast, the utilization of straw as a bioenergy or biomass source remains insufficient.7 Thus, managing rice straw is not only a challenge but also an opportunity to utilize the available resource and reduce agriculture's climate footprint in agricultural developing countries such as Vietnam.Cellulose and its derivatives are intensively used in numerous areas such as textiles, packaging, paper production, environment, filtration, agriculture, and medicine.8–13 However, the utilization and development of the great potential of cellulose still attracts the attention of scientists based on plenty of attractive features. In recent years, researchers have focused more on the separation of cellulose from biomass, which enables a wide range application in green chemistry.14 Recently advances the isolation of cellulose can be achieved by various methods, which have advantages and disadvantages in terms of final composition and structural characteristics. These methods can be divided into three main processes, which are physical, biological and chemical or a combination of these processes.15 Physical treatment such as steam explosion16 can be carried out to enhance the surface area and accessibility of the biomass for acid or enzyme degradation.17,18 While biological treatment uses bacteria and fungi to decompose lignin which has gained popularity due to its advantages over other methods. A biological method is an environmentally friendly and energy-saving process that ensures a high yield of the desired product without the need for special solvents and chemical reagents.19 However, biological treatment has some disadvantages such as the process takes a long time and requires careful attention and detailed control of microbial growth conditions. Chemical treatment has become one of the most promising methods to degrade lignin in biomass, which decreases the polymerization and crystallinity structure of cellulose. Thus, removal of lignins will help to increase the available surface area and pore volume of the substrate. Acidic and alkali pretreatments have shown of great promise in biomass solubilization.16 The use of acid aims at dissolving the hemicellulose, leaving lignin and cellulose as solid, while the addition of alkali, usually NaOH, targets mainly lignin, leaving mainly cellulose as solid with hemicelluloses.20 Indeed, the well-known Kraft process is used without changes over the past 60–80 years producing millions of tonnes of raw cellulose also called Kraft cellulose. They have several benefits, such as simple equipment, easy handling and low cost. However, they induce the formation of toxic by-products such as furfural and hydroxymethyl furfural, potent inhibitors of microbial fermentation. Further disadvantages of the acidic method are the high toxicity and the strong corrosive effect due to the extremely low pH-value, which requires special materials for the reactor design.21 Recently a new method involving a one-pot route to achieve cellulose dissolution via derivatization were described.22 Ionic liquid immersed as a potential alternative method such as [Emim][acetate] and recently [Emim][formate] plus glycerol.23,24 However, no recycling of the ionic liquid has been demonstrated, the role of silica is often neglected in such studies, and high temperatures are required.25,26
Recently it was reported that aqueous electrolyte solution such as TBPH (30 to 60 wt%) readily dissolves cellulose,27 as well as some woody biomass without heating.28 In our previous research, we investigated further co-solvents to enhance processability. However, some solvents were not applicable and precipitation occurs automatically.29 Further testing indicated no precipitation of hemicellulose and lignin with these solvents.
Therefore, in this study, we propose a simple and fast method to dissolve rice straw followed by easy and selective precipitation of cellulose without special equipment or conditions. With the same electrolyte solution (TBPH 50 wt%), the raw cellulose can be used to fabricate the films by the MDCell (abbreviation for the inventors Mai Ngoc Nguyen and Dirk Hollmann cellulose) process.30 The films were characterized in terms of Fourier transform infrared (FTIR), X-ray diffraction (XRD), solid-state dynamic nuclear polarization-enhanced nuclear magnetic resonance spectroscopy (DNP-NMR). The morphology of the synthesized films was investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). To highlight the possibility of a cradle-to-cradle approach we further focused on the biodegradability in soil.
Results and discussion
Isolation of cellulose from rice straw
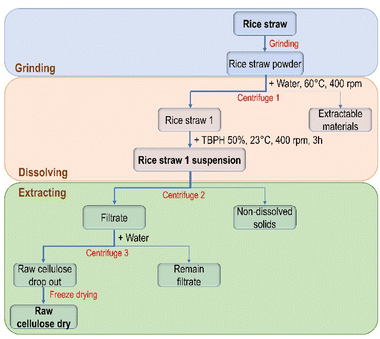
The detailed procedure for isolation of cellulose using electrolyte solution is illustrated in Fig. 1.First, to increase the accessible surface between rice straw with solvents, rice straw was grinded in a planetary ball mill. Mechanochemistry is considered as a promising method, easy approach and environmentally friendly which can reduce the particle size, increase the contact surface, reduce time for solving, and decrease the degree of crystallinity.31,32 In order to produce ultrafine powders, a suitable grinding conditions were obtained by carrying out an evaluation of grinding process. Good distribution and homogeneous powder were found by using pre-cut rice straw (size 0.5 cm in lengths) under grinding conditions: 400 rpm, 180 min in agate material with 15 balls. Then, the finely grinded rice straw was washed in hot distilled water at 60 °C followed by drying at 50 °C in the oven to remove water-soluble extractives such as non-structural sugars and proteins. The dried powder was dissolved in TBPH (50 wt%) at room temperature to get a homogeneous rice straw solution. It has been reported that lignin, hemicellulose as well as cellulose can be dissolved by TBPH (50 wt%).27,33,34 To separate the insoluble compounds from the dissolved cellulose, hemicellulose and lignin, the rice straw solution was centrifuged at high speed (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). The residue contained mainly inorganic compound. Note, with the alkaline method such as using sodium hydroxide, the removal of silica was inefficient and affected the purity and quality of cellulose.35 The filtrate was added into a large amount of water resulting in the selectivity precipitation of cellulose as fine fiber (Fig. 2). Water react as an anti-solvent for cellulose solution which weaken and even destroy the hydrogen bonds formed between cellulose and TBPH.29 The precipitated cellulose can be isolated by centrifugation and washing with water. This was proven by a study of the precipitation behavior of dissolved cellulose, hemicellulose and lignin from aqueous TBPH (50 wt%) solution. If these solutions are added to an excess of water, only cellulose was precipitated from the solution. This result can be explained by their structure. In contrast to cellulose, which is made only from glucose, hemicellulose is an amorphous heteropolymer consisting of several different carbohydrates, including xylose, mannose, glucose and galactose in the main chain and arabinose, galactose and 4-O-methyl-D-glucuronic acid in the side chain. They are water-soluble due to their branched structure.36 Lignin is a three-dimensional amorphous polymer containing ionized phenolic groups making the lignin soluble in a water solution. Lignin precipitation is usually carried out by acidification due to the protonation of the ionized phenolic groups on the lignin molecules.37,38
000 rpm). The residue contained mainly inorganic compound. Note, with the alkaline method such as using sodium hydroxide, the removal of silica was inefficient and affected the purity and quality of cellulose.35 The filtrate was added into a large amount of water resulting in the selectivity precipitation of cellulose as fine fiber (Fig. 2). Water react as an anti-solvent for cellulose solution which weaken and even destroy the hydrogen bonds formed between cellulose and TBPH.29 The precipitated cellulose can be isolated by centrifugation and washing with water. This was proven by a study of the precipitation behavior of dissolved cellulose, hemicellulose and lignin from aqueous TBPH (50 wt%) solution. If these solutions are added to an excess of water, only cellulose was precipitated from the solution. This result can be explained by their structure. In contrast to cellulose, which is made only from glucose, hemicellulose is an amorphous heteropolymer consisting of several different carbohydrates, including xylose, mannose, glucose and galactose in the main chain and arabinose, galactose and 4-O-methyl-D-glucuronic acid in the side chain. They are water-soluble due to their branched structure.36 Lignin is a three-dimensional amorphous polymer containing ionized phenolic groups making the lignin soluble in a water solution. Lignin precipitation is usually carried out by acidification due to the protonation of the ionized phenolic groups on the lignin molecules.37,38
Thus, with this simple process, cellulose is precipitated easily and selective. Raw cellulose contents 25.9–30.7 wt% of the rice straw were achieved. It corresponds nicely to the theoretical maximum amount of cellulose in rice straw.
Structural characteristics and chemical composition of raw cellulose
To prove the success of the raw cellulose, the rice straw after removing insoluble compounds (Fig. 3a), the raw cellulose (Fig. 3b) and the remaining filtrate (Fig. 3c) were analyzed by the liquid state 13C NMR measurement (Fig. 3).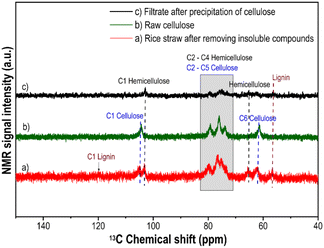 | ||
| Fig. 3 LS-13C NMR spectra of (a) rice straw after removing insoluble compounds, (b) raw cellulose and (c) filtrate after precipitation of cellulose. | ||
The rice straw solution shows the signal of cellulose, hemicellulose and lignin as expected (Fig. 3a). However, the intensity of C1 lignin is very weak due to the small amount of lignin. After separation, cellulose with distinct characteristic peaks was obtained (Fig. 3b). The remaining filtrate (Fig. 3c) featured only the signals of hemicellulose and lignin at 103.3 ppm, 65.8 ppm and 56.6 ppm, respectively. No cellulose was detected.
As second method, solid state 13C-NMR was applied to check the amount of lignin (Fig. 4). However, even with this method only traces of lignin and no hemicellulose were detected in the raw cellulose (Fig. 4A). Therefore, a purity of >95% can be estimated. Notably, the signals from 180 to 120 ppm are attributed to the aromatic carbons of lignin, from 58 to 50 ppm are related to methoxyl groups (–O–CH3) of lignin39,40 and the signal from 30 to 20 ppm are assigned to acetyl groups (–CO–CH3) of hemicellulose.40,41 These results indicate the high purity of the precipitated cellulose.
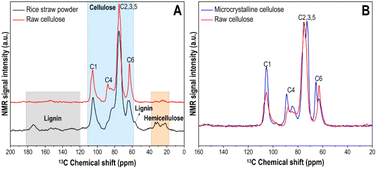 | ||
| Fig. 4 SS-13C NMR (A) between rice straw powder and raw cellulose; (B) between commercially microcrystalline cellulose and raw cellulose. | ||
To gain a more comprehensive idea of the structure of raw cellulose, the SS 13C NMR signals of the raw cellulose were compared with commercially available microcrystalline cellulose from cotton linters (Sigma-Aldrich). In Fig. 4B, according to previous studies,42–44 the shifts of the C6 but especially the C4 peaks indicating the amorphous structure of the raw cellulose.41 The changes suggested that hydrogen bonds in crystalline structure of rice straw was disrupted to some extent.
Furthermore, the chemical structure was investigated by FTIR spectroscopy. In rice straw, all major compounds such as cellulose, hemicellulose and lignin are detected (Fig. 5a). The peak at 1728 cm−1 (position 1) was assigned to aliphatic esters in lignin and/or hemicellulose in the rice straw.45,46 The peak at 1640 cm−1 (position 2) can be attributed to the bending mode of the absorbed water and also to carbonyl groups of hemicelluloses.47 The peaks at 1512 and 1460 cm−1 in the rice straw are indicative of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch in lignin (position 3 and 4).48,49 The FTIR spectra of isolated raw cellulose don't show any of these signals indicating again the removal of hemicelluloses and lignin after treatment. Indeed, the FTIR spectra between the isolated raw cellulose (Fig. 5b) and commercially microcrystalline cellulose (Fig. 5c) revealed the similarities. No difference between the spectra were detected. These results suggested that the main component of the precipitated raw material is pure cellulose.
C stretch in lignin (position 3 and 4).48,49 The FTIR spectra of isolated raw cellulose don't show any of these signals indicating again the removal of hemicelluloses and lignin after treatment. Indeed, the FTIR spectra between the isolated raw cellulose (Fig. 5b) and commercially microcrystalline cellulose (Fig. 5c) revealed the similarities. No difference between the spectra were detected. These results suggested that the main component of the precipitated raw material is pure cellulose.
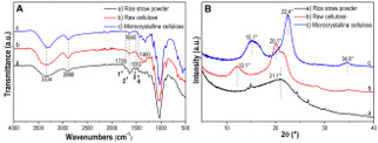 | ||
| Fig. 5 (A) FTIR spectra and (B) XRD patterns of (a) rice straw powder, (b) raw cellulose and (c) microcrystalline cellulose. | ||
To obtain further information about the macroscopic structure, the XRD patterns (Fig. 5B) of rice straw powder, raw cellulose isolated from rice straw and microcrystalline cellulose were analyzed. The Rice straw composed of crystalline and amorphous regions.50 After grinding rice straw in the ball mill, the rice straw powder's spectrum is broad distribution indicating a large amount of amorphous substances. No distinct polymorphs can be observed. This could be due to the effect of ball mill which change the morphological and structural properties of cellulose.51
Analyzing the raw cellulose samples revealed the presence of reflections (2θ = 12.1° (101); 20.1° (102)) which can be assigned to cellulose II. However, the sharper and narrower diffraction peak at (102) clearly demonstrated the increase in the crystallinity of raw cellulose compared with rice straw. During the extraction process, amorphous hemicellulose and lignin were readily dissolved, while leaving the remaining cellulose of a higher degree of crystallinity.45 For references as native cellulose, microcrystalline cellulose was analyzed which is composed of cellulose I with three characteristic diffraction peaks (2θ = 15°, 22.4°, and 34.6°).
Utilization as regenerated cellulose films and biodegradability
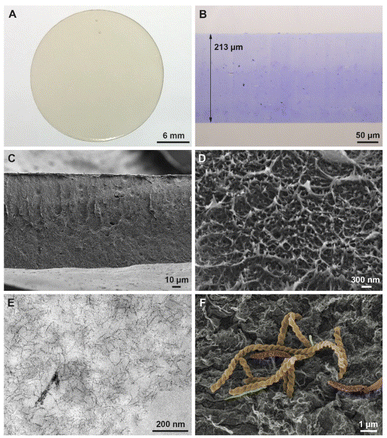
Recently, we reported a method for fabricating regenerated cellulose films using commercial microcrystalline cellulose.30 This method can now be extended to the utilization of the precipitated raw cellulose. The films consist of 10 wt% raw cellulose in TBPH (50 wt%). Indeed, no difference in chemical composition (detected by FT-IR spectroscopy, Fig. 6A) and the crystallinity (detected by XRD, Fig. 6B) between RC films from raw cellulose or from microcrystalline cellulose (MCC) were observed. In addition, for the membrane application the mechanical durability based on the hydraulic pressure of the flow is important. Indeed, the RC films from raw cellulose collapsed at a pressure of 2.0 bar which corresponds to a maximum flow rate of around 4.0 ml min−1. This was in the range of the cellulose from MCC (2.5 bar/4.5 ml min−1).30 The film has a smooth and homogeneous surface suggesting a good regeneration of the raw cellulose through the hydrogen-bonding rearrangement of the cellulose macromolecules and fast penetrates of the coagulation reagent propylene carbonate. Noticeable, the film has pale yellow color (Fig. 7A) suggesting a tiny amount of lignin which cannot be detected by the other spectroscopic methods mentioned before.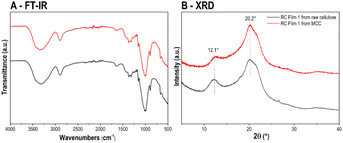 | ||
| Fig. 6 (A) FT-IR spectra, (B) XRD patterns of the RC films from raw cellulose and microcrystalline cellulose. | ||
This forced us to invest the structure and morphology of the films applying Light Microscopy (LM), Transmission Electron Microscopy (TEM), and Scanning Electron Microscopy (SEM). In Fig. 7 a summary of the surface characterization techniques for the most important RC film 1 is shown.
First, the wet RC films were inspected by LM with toluidine blue (Fig. 7B). Here, a homogeneous distribution of the TB over the entire size of regenerated cellulose was detected. The thickness of the wet films decreased to 213 μm, which is nearly the same height as for RC film obtained by microcrystalline cellulose (220 μm).30 The reduction in thickness (casting high 500 μm) was probably caused by the coagulation process and dehydration during the embedding and resin infiltration process of the membrane for subsequent LM and TEM preparation. Again, no capillary pores were visible. However, small more intensely stained particles of unknown origin distributed homogeneously throughout the complete film were observed.
These particles can be detected by SEM (Fig. 7C and D) and more specifically in detail using high resolution TEM imaging as well (Fig. 7E). It seems that still some small undissolved remains of former cellulose fibers or microfibrils are incorporated. Fibrillar composition, i.e. the cellulose microfibrils are best visualized with contrast reagents such as lead citrate and uranyl acetate (Fig. 7E). Here, an irregular, net-like structure of branched microfibrils was observed, with a size of the net meshes in the range of ∼30–100 nm. Furthermore, the film surface appears relatively smooth by TEM inspection at the border to the resin. Under SEM conditions (dried in air or critical point dried), an almost perfect surface with a compact, very uniform, dense, but layered structure was detected, with a superior structure preservation in critical point dried samples (Fig. 7C). Again, no capillary pores were observed, however the film thickness was decreased below 200 μm. The layers and microfibrils observed with high resolution SEM correspond well to the regular distribution cellulose microfibrils seen in TEM. Taken together all microscopic results indicate a fast and homogeneous distribution of the cellulose fibers, similar to earlier observations in films from pure microcrystalline cellulose.
Interestingly after a prolonged time of storage (2–6 months) in (distilled) water we were able to detect multiple bacteria species hosted on and densely attached to the film surface (Fig. 7F). This indicates a descent biodegradability of the membranes even under less favourable conditions in distilled water. Further investigation on the origin and nature of the bacteria are in progress.
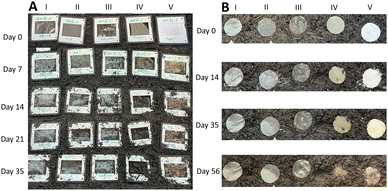
This detection was of great importance, as it shows how well the film can degrade even under very low bacterial conditions. Therefore, we were particularly interested in the biodegradability of the film in soil. The test was performed at 20 degrees inside the soil (Fig. 8B, depth 2 cm) and at 20 degrees on the surface (Fig. 8A). For the soil test, the samples were clamped in a Dia frame, buried and excavated as well as photographed after 7 days. LDPE film, PLA film, cellophane film and paper were tested for comparison.
Compared to all these films, inside the soil our film degrades completely within 4 weeks. This occurs with no release of microplastics. Which would also be unlikely under these conditions since the film consists only of cellulose. Surprisingly, the Cellophane NatureFlex (Fig. 8A(III) and B(III)), which is currently sold as a sustainable cellulose material, has not degraded a single bit. Note that MCC cellulose materials is also degrading even in water.52 On the surface, the degradation takes a longer since bacteria can only attack from one side and decompose the film. Complete decomposition occurs after 2 months. Further water biodegradability test in our laboratory and under real conditions are in progress.
Conclusion
In this study, we presented an easy, simple, and fast strategy to approach selectively high purity cellulose from renewable bio waste such as rice straw. By selectively isolating cellulose from rice straw and converting it into biodegradable films, we offer an innovative solution for upgrading biowaste.The cellulose obtained is nearly free of contaminated lignin and hemicelluloses. No special requirements, heating or conditions are necessary. The chemicals used are non-toxic and aqueous solvents can be used. The dissolution of all rice straw material in TBPH 50 wt% simply followed by precipitation in water enables the recovery of a quantitative amount of cellulose. The same chemicals can be used to utilize the raw cellulose obtained towards to high quality regenerated cellulose films. These films exhibit an excellent biodegradability compared to other known packaging materials. Future investigations and applications are under progress. This is a future-oriented green “Cradle-to-Cradle” alternative to known industrial processes to valorize biomass but especially bio waste. It represents a significant step forward in the development of a circular economy by converting a waste product into a valuable raw material.
Methods
Procedures
The procedure for isolation of cellulose using electrolyte solution is illustrated in Fig. 9 according to the following 3 steps: grinding, dissolving and extraction.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm in 20 minutes. The solid was washed 1–2 times with TBPH (50 wt%) and combined with the filtrate. The filtrate was added dropwise into water under vigorous mixing enabling the selective precipitation of cellulose. The raw cellulose was washed many times with pure water, followed by freeze drying.
000 rpm in 20 minutes. The solid was washed 1–2 times with TBPH (50 wt%) and combined with the filtrate. The filtrate was added dropwise into water under vigorous mixing enabling the selective precipitation of cellulose. The raw cellulose was washed many times with pure water, followed by freeze drying.
Materials
Rice straw was harvested from a local farm in Thai Binh Province, Vietnam and was cut into small pieces of about 0.5 cm by a crusher. Tetrabutylphosphonium hydroxide (TBPH) containing 40 wt% in water was purchased from Acros and concentrated through rotary evaporation under 70 bar, 40 °C to get a higher concentration (50 wt%). Microcrystalline cellulose (MCC, powder, 20 μm), propylene carbonate (PC, 99.7% purity) and dimethyl sulfoxide (DMSO) were supplied from Sigma-Aldrich.Characterizations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 accumulations, 2 s pulse delay, at 25 °C).
000 accumulations, 2 s pulse delay, at 25 °C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetone and resin o/n, followed by pure resin for 4 h. Specimens were transferred to rubber molds and the resin was allowed to cure at 60 °C for 2 days. The membranes were exposed from the resin blocks with a trimming tool (Leica EM Trim 2, Leica Microsystems, Wetzlar, Germany). Semithin sections (approx. 0.5 μm) and thin sections (approx. 50–70 nm) were cut on an ultramicrotome (Ultracut S, Reichert, Wien, Austria) with a diamond knife (Diatome, Biel, Switzerland). Semithin sections were stained with an aqueous solution of toluidine blue to visualize specimens for further trimming prior to thin sectioning and ultrastructural inspection. Thin sections were transferred to copper grids and were either examined directly without further contrasting agents or were alternatively contrasted with uranyl acetate and lead citrate. The sections were examined on a Zeiss EM902 electron microscope operated at 80 kV (Carl Zeiss, Oberkochen, Germany). Digital images were acquired with a side-mounted 1 × 2k FT-CCD Camera (Proscan, Scheuring, Germany) using iTEM camera control and imaging software (Olympus Soft Imaging Solutions, Münster, Germany).
1 mixture of acetone and resin o/n, followed by pure resin for 4 h. Specimens were transferred to rubber molds and the resin was allowed to cure at 60 °C for 2 days. The membranes were exposed from the resin blocks with a trimming tool (Leica EM Trim 2, Leica Microsystems, Wetzlar, Germany). Semithin sections (approx. 0.5 μm) and thin sections (approx. 50–70 nm) were cut on an ultramicrotome (Ultracut S, Reichert, Wien, Austria) with a diamond knife (Diatome, Biel, Switzerland). Semithin sections were stained with an aqueous solution of toluidine blue to visualize specimens for further trimming prior to thin sectioning and ultrastructural inspection. Thin sections were transferred to copper grids and were either examined directly without further contrasting agents or were alternatively contrasted with uranyl acetate and lead citrate. The sections were examined on a Zeiss EM902 electron microscope operated at 80 kV (Carl Zeiss, Oberkochen, Germany). Digital images were acquired with a side-mounted 1 × 2k FT-CCD Camera (Proscan, Scheuring, Germany) using iTEM camera control and imaging software (Olympus Soft Imaging Solutions, Münster, Germany).
Data availability
The data set to this publication: Beyond waste: cellulose-based biodegradable films from bio waste through a cradle-to-cradle approach [data set] can be accessed via Zenodo https://doi.org/10.5281/zenodo.13867404.Author contributions
M. N. N. concepted and performed the experiments for the isolation of cellulose and the synthesis of cellulose films. M. T. L. N. concepted and performed the biodegradability test of the films. M. F. concepted and performed the SEM, TEM and the LM measurements. D. H. formulated and coordinated the overall research goal. This work was supported by funding acquisition through D. H. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the RoHan Project funded by the German Academic Exchange Service (DAAD, No. 57315854 and 57560571) and the Federal Ministry for Economic Cooperation and Development (BMZ) inside the framework “SDG Bilateral Graduate School Program”. We acknowledge the expert technical support during EM sample preparation of Karoline Schulz, Ute Schulz and Dr Armin Springer. We thank Dr Victoria Aladin and Prof. Björn Corzilius for the solid-state NMR measurements. Further we acknowledge Dr Henrik Lund for the XRD measurements.Notes and references
- H. Kopnina, R. Padfield and J. Mylan, Sustainable Business, Tayler and Francis Group, 2023 Search PubMed.
- G. Kaur, K. Uisan, K. L. Ong and C. S. K. Lin, Curr. Opin. Green Sustainable Chem., 2018, 9, 30–39 CrossRef.
- World Rice Production 2019/2020, United States Department of Agriculture (USDA), 2020 Search PubMed.
- W. D. Jong and J. R. V. Ommen, in Biomass as a Sustainable Energy Source for the Future, John Wiley & Sons, Inc., Hoboken, New Jersey, United States of America, 2014, ch. 2, pp. 36–68, DOI:10.1002/9781118916643.ch2.
- R. Gupta, SANDEE Working Papers, 2012, vol. 66 Search PubMed.
- K. Lasko, K. P. Vadrevu, V. T. Tran, E. Ellicott, T. T. N. Nguyen, H. Q. Bui and C. Justice, Environ. Res. Lett., 2017, 12, 085006 CrossRef PubMed.
- V. B. Agbor, N. Cicek, R. Sparling, A. Berlin and D. B. Levin, Biotechnol. Adv., 2011, 29, 675–685 CrossRef CAS PubMed.
- K. H. Gardner and J. Blackwell, Biopolymers, 1974, 13, 1975–2001 CrossRef CAS.
- H. Zhu, Z. Fang, C. Preston, Y. Li and L. Hu, Energy Environ. Sci., 2014, 7, 269–287 RSC.
- C. Huang, Y. Gao, Y. Chen, Y. Shen and H.-Y. Yu, J. Clean. Prod., 2024, 451, 142107 CrossRef CAS.
- S. Wang, A. Lu and L. Zhang, Prog. Polym. Sci., 2016, 53, 169–206 CrossRef CAS.
- N. Sharma, B. J. Allardyce, R. Rajkhowa and R. Agrawal, Bioengineered, 2023, 14, 2242124 CrossRef PubMed.
- M. Yang, Y. Chen, S. Y. H. Abdalkarim, X. Chen and H.-Y. Yu, Int. J. Biol. Macromol., 2024, 276, 133799 CrossRef CAS PubMed.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- D. Ussiri and R. Lal, Biofuels, 2015, 5, 1–30 Search PubMed.
- N. Sharma, B. J. Allardyce, R. Rajkhowa and R. Agrawal, Sci. Rep., 2023, 13, 16327 CrossRef CAS PubMed.
- Y. Sun and J. Cheng, Bioresour. Technol., 2002, 83, 1–11 CrossRef CAS PubMed.
- L. Cadoche and G. D. López, Biol. Wastes, 1989, 30, 153–157 CrossRef CAS.
- A. Hatakka, FEMS Microbiol. Rev., 1994, 13, 125–135 CrossRef CAS.
- V. S. Chang and M. T. Holtzapple, Appl. Biochem. Biotechnol., 2000, 84, 5–37 CrossRef PubMed.
- W. E. Karr and M. T. Holtzapple, Biotechnol. Bioeng., 1998, 59, 419–427 CrossRef.
- Y. Chen, C. Huang, Z. Miao, Y. Gao, Y. Dong, K. C. Tam and H.-Y. Yu, ACS Nano, 2024, 18, 8754–8767 CrossRef CAS PubMed.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- J. S. Moulthrop, R. P. Swatloski, G. Moyna and R. D. Rogers, Chem. Commun., 2005, 1557–1559, 10.1039/B417745B.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
- C. G. Yoo, Y. Pu and A. J. Ragauskas, Curr. Opin. Green Sustainable Chem., 2017, 5, 5–11 CrossRef.
- M. Abe, Y. Fukaya and H. Ohno, Chem. Commun., 2012, 48, 1808–1810 RSC.
- M. Abe, T. Yamada and H. Ohno, RSC Adv., 2014, 4, 17136–17140 RSC.
- M. N. Nguyen, U. Kragl, D. Michalik, R. Ludwig and D. Hollmann, ChemSusChem, 2019, 12, 3458–3462 CrossRef CAS PubMed.
- M. N. Nguyen, U. Kragl, I. Barke, R. Lange, H. Lund, M. Frank, A. Springer, V. Aladin, B. Corzilius and D. Hollmann, Commun. Chem., 2020, 3, 116 CrossRef CAS PubMed.
- C. C. Piras, S. Fernández-Prieto and W. M. De Borggraeve, Nanoscale Adv., 2019, 1, 937–947 RSC.
- D. J. Schell and C. Harwood, Appl. Biochem. Biotechnol., 1994, 45, 159–168 CrossRef.
- H. Yamada, H. Miyafuji, H. Ohno and T. Yamada, Bioresources, 2016, 11, 839–849 CAS.
- U. Hyväkkö, A. W. T. King and I. Kilpeläinen, Bioresources, 2014, 9, 13 CrossRef.
- L. Luduena, D. Fasce, V. A. Alvarez and P. M. Stefani, Bioresources, 2011, 6, 1440–1453 CAS.
- G. Brunner, in Supercritical Fluid Science and Technology, ed. G. Brunner, Elsevier, 2014, vol. 5, pp. 395–509 Search PubMed.
- M. A. Gilarranz, F. Rodriguez, M. Oliet and J. A. Revenga, Sep. Sci. Technol., 1998, 33, 1359–1377 CrossRef CAS.
- W. Zhu and H. Theliander, Bioresources, 2015, 10, 19 Search PubMed.
- Y. Lu, Y.-C. Lu, H.-Q. Hu, F.-J. Xie, X.-Y. Wei and X. Fan, J. Spectrosc., 2017, 2017, 8951658 Search PubMed.
- K. M. Holtman, N. Chen, M. A. Chappell, J. F. Kadla, L. Xu and J. Mao, J. Agric. Food Chem., 2010, 58, 9882–9892 CrossRef CAS PubMed.
- W. Cao, Z. Wang, Q. Zeng and C. Shen, Appl. Surf. Sci., 2016, 389, 404–410 CrossRef CAS.
- H. Kono, T. Erata and M. Takai, Macromolecules, 2003, 36, 5131–5138 CrossRef CAS.
- H. Yamamoto and F. Horii, Macromolecules, 1993, 26, 1313–1317 CrossRef CAS.
- E. R. Alonso, C. Dupont, L. Heux, D. D. S. Perez, J.-M. Commandré and C. Gourdon, Energy, 2016, 97, 381–390 CrossRef.
- E. Abraham, B. Deepa, L. A. Pothan, M. Jacob, S. Thomas, U. Cvelbar and R. Anandjiwala, Carbohydr. Polym., 2011, 86, 1468–1475 CrossRef CAS.
- S. Sen, J. D. Martin and D. S. Argyropoulos, ACS Sustain. Chem. Eng., 2013, 1, 858–870 CrossRef CAS.
- X. Chen, J. Yu, Z. Zhang and C. Lu, Carbohydr. Polym., 2011, 85, 245–250 CrossRef CAS.
- D. Watkins, M. Nuruddin, M. Hosur, A. Tcherbi-Narteh and S. Jeelani, J. Mater. Res. Technol., 2015, 4, 26–32 CrossRef CAS.
- F. Xu, J.-X. Sun, R. Sun, P. Fowler and M. S. Baird, Ind. Crops Prod., 2006, 23, 180–193 CrossRef CAS.
- M. K. M. Haafiz, S. J. Eichhorn, A. Hassan and M. Jawaid, Carbohydr. Polym., 2013, 93, 628–634 CrossRef PubMed.
- M. Ago, T. Endo and K. Okajima, Polym. J., 2007, 39, 435–441 CrossRef CAS.
- M. Bading, O. Olsson and K. Kümmerer, Chemosphere, 2024, 352, 141298 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |