Memristor based electronic devices towards biomedical applications
Jie
Zhang
 *a,
Junmei
Du
b,
Chuan
Yang
b,
Haotian
Liang
b,
Zelin
Cao
cd,
Xuegang
Duan
cd,
Wentao
Yan
cd,
Yong
Zhao
b and
Bai
Sun
*a,
Junmei
Du
b,
Chuan
Yang
b,
Haotian
Liang
b,
Zelin
Cao
cd,
Xuegang
Duan
cd,
Wentao
Yan
cd,
Yong
Zhao
b and
Bai
Sun
 *cd
*cd
aCollege of Mathematics and Physics, Chengdu University of Technology, Chengdu, Sichuan 610059, China. E-mail: jiezhang@cdut.edu.cn
bSchool of Physical Science and Technology, Key Laboratory of Advanced Technology of Materials, Southwest Jiaotong University, Chengdu, Sichuan 610031, China
cFrontier Institute of Science and Technology (FIST), Xi’an Jiaotong University, Xi’an, Shaanxi 710049, China. E-mail: baisun@xjtu.edu.cn
dMicro-and Nano-technology Research Center, State Key Laboratory for Manufacturing Systems Engineering, Xi’an Jiaotong University, Xi’an, Shaanxi 710049, China
First published on 22nd November 2023
Abstract
Memristors, as a novel type of electronic device with memory resistance characteristics, are attracting considerable interest in the field of biomedical applications. In the realm of biosensors and diagnostic technologies, memristors can interact with biomolecules, detecting and analyzing disease biomarkers, thus enabling highly sensitive early cancer detection and rapid virus diagnosis. Additionally, in the domain of neuromorphic computing and brain–machine interfaces, memristors’ ability to emulate biological neuron behavior opens up new possibilities for neuromorphic computing systems and neural-controlled prosthetics. Moreover, memristors are also applicable in simulating biological neurons and researching brain disorders, aiding the comprehension of complex brain functions and neurological diseases, and exploring novel treatment methods. This paper provides an overview of the basic principles of memristors, summarizes the latest achievements and advancements in biomaterial-based memristors, and discusses the challenges and prospects of employing biomaterial-based memristors in the biomedical field. Therefore, biomemristors hold tremendous potential for applications in biomedical research and therapy, offering new prospects for medical research and treatment.
1. Introduction
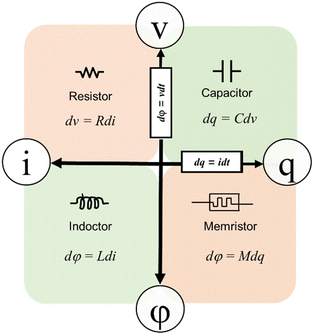
Organic materials have attracted much attention for their utility as functional layers in electronic devices because their tunable structures can be sustainably prepared from abundant precursors in an environmentally friendly manner.1 A memristor, also known as a memory resistor, is a special type of electronic device with fascinating and unique resistance characteristics. It was first proposed by scientist Leon Chua in 1971 and classified as the fourth fundamental circuit element alongside conventional resistors, capacitors, and inductors.2 The significance of memristors lies in their ability to remember past currents and adjust their resistance in the future, which makes them highly promising for information storage and processing.3 The distinctive features of memristors include memory effects, nonlinear resistance, programmability, high-speed response, and low energy consumption.4,5 These traits make memristors suitable for various applications in integrated circuit design,6 neural computing,7 artificial intelligence,8,9 and the Internet of Things.10 In the field of biomedical applications, memristors have attracted significant interest due to their unique functionalities. They can be utilized in biosensors and diagnostic technologies, interacting with biomolecules to enable high-sensitivity early cancer detection and rapid virus diagnosis.11 Additionally, memristors show great potential in the domain of neural computing and brain–machine interfaces, facilitating the construction of neuromorphic computing systems to simulate brain information processing and learning mechanisms.12A memristor is a non-linear two-terminal device, and its resistance varies as the input current or voltage accumulates.13 In 2008, Strukov et al. at Hewlett-Packard Labs successfully demonstrated a TiO2 nano-film device that functioned as a resistive device, following the predictions.14 A memristor is a circuit device that captures the correlation between charge (q) and magnetic flux (φ).15 These components are characterized by distinctive memory attributes, non-volatility, and non-linearity. Fig. 1 illustrates the existence of capacitors, resistors, inductors, and memristors as the four fundamental passive circuit elements, with voltage, current, charge, and magnetic flux serving as the four fundamental variables that define their interrelationships.16 In the proposed context of amnesia, when considering the fundamental primitive devices, amnesia, along with other passive basic circuit elements like resistors, capacitors, and inductors, forms a comprehensive set of quaternary basic circuit primitives. Since then, a substantial amount of research has been conducted on memristors. Memristors find widespread applications in various fields such as artificial neural networks, biomedicine, circuits and systems, new types of storage, and chaotic systems. However, one of the most intriguing areas of application is the integration of memristors with biomedicine.
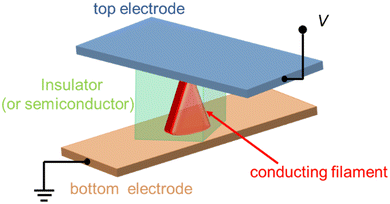
Typically, the unit structure of a memristor follows the metal/insulator/metal (MIM) configuration. The symbol “M” generally represents materials that are good electronic conductors, including active and inert metals, among others. On the other hand, “I” denotes insulator materials, which, in most cases, refer to good ionic conductors, such as oxides and organic compounds. Simultaneously, the upper and lower metal layers are referred to as the top electrode and the bottom electrode, respectively. Between these electrodes, there exists a resistive layer with memristive properties. The schematic structure of the memristor is shown in Fig. 2.17
Based on their rheostat switching characteristics, memristors can be categorized as binary or analog. Binary memristors are better suited for storing or processing binary data due to their two distinct resistance states, often representing 0 and 1. On the other hand, analog memristors are more appropriate for multi-resistance storage and analog calculations, as they can maintain a continuous range of resistance values.
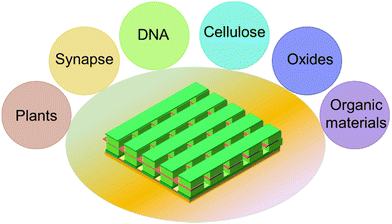
Depending on the resistive material used, memristors can be categorized into several types, they are simply classified as oxide memristors,18,19 solid-state electrolyte materials,20 calcium titanium oxide,19 memristors for 2D materials, and organic materials (e.g. egg white, blood serum).21,22 Conventional memristors offer several advantages, including miniaturization and robust read/write capabilities. The classification diagram of the memristor is shown in Fig. 3.
However, in terms of electrical performance, traditional memristors have certain drawbacks, such as discrete switching voltages caused by local conductive filaments and relatively weak durability. Yan et al. have been dedicated to the advancement of memristors and have achieved significant progress in brain-like synaptic bionics.19,23 Notably, they have made strides in areas such as long time-range potentiation (LTP), long time-duration inhibition (LTD), spike-timing-dependent plasticity (STDP), two-pulse facilitation (PPF), and paired-pulse depression (PPD). As a result, memristors have been further developed in the realm of brain neuromimetics. Pulse facilitation (PPF) and paired-pulse depression (PPD) have been further developed in the field of brain neuromimetics, alongside the progress made with amnesia-based memristors.
Since the initial realization of amnesia prototype devices in 2008, significant progress has been made in academia concerning amnesia-based memristors, achieving numerous noteworthy results. Nevertheless, due to the absence of a standardized set of evaluation criteria, there remains no uniform standard answer for selecting the amnesia material system and the mechanism model. As a consequence, research on amnesia is currently constrained to the fundamental research phase. Both the selection of the material system and the establishment of the mechanism model are still in the realm of individual exploration, with researchers striving to achieve a “perfect” amnesia device. The use of biomaterials in amnesia has generated significant research interest among a considerable number of scholars. This interest is primarily driven by the biomaterials' appealing characteristics, such as their lightweight nature, high flexibility, ease of processing, non-polluting properties, and recyclability. Currently, significant progress has been achieved in the biomedical application of amnesia. Fu et al. have made noteworthy advancements in this area. Protein nanowires derived from microbial soil bacteria enable electronic amnesia to function at biological voltages to replicate neuronal activity in the brain.24 This development holds promise for establishing genuine neuronal communication in biological systems. Wu et al. presented a novel implementation approach to create an artificial sensory neural system with habituation characteristics using amnesia.25 They developed a habituation impulse neural network, which adopts habituation as a biological learning rule. This neural network was successfully applied to robotic autonomous cruise obstacle avoidance, demonstrating the potential for integrating biological learning principles into artificial systems. These natural biomaterials hold potential to serve as the “ideal” memory blockers.
2. Research progress
2.1. Application of amnesia memristors in implantable electronic devices
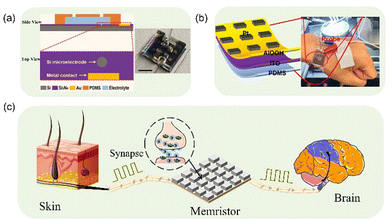
The memristor, an electronic component with unique electrical characteristics capturing the correlation between electric charge and magnetic flux, has garnered significant interest and exploration in recent years in the realm of biomedical applications. This has introduced new possibilities and innovations in the field. In 2009, Ho et al. introduced a read/write scheme for amnesia memories and addressed circuit design considerations concerning read/write stability and data integrity.26 In 2010, Jo et al. conducted an experimental demonstration of a nano-sized silicon-based amnesia device, illustrating that a hybrid system combining complementary metal–oxide–semiconductor neurons and amnesia synapses could support significant synaptic functions, such as pulse-time-dependent plasticity.13 This study offers evidence that incorporating amnesia-based synapses in neuromorphic circuits has the potential to deliver high connectivity and density needed for efficient computation. In 2011, Cong et al. introduced a reconfigurable FPGA architecture based on memristors.27 Utilizing amnesia to establish interconnections also facilitates capacitive shielding for unused routing paths, leading to a reduction in interconnect latency. In the same year, Sandro et al. introduced silicon nanowire sensors capable of cloning antibodies to detect antigens.28 The assay relies on the amnesic effect observed in silicon nanowire sensing materials. In 2012, Carrara et al. introduced a novel detection method for nanomemristors.29 The nanowires were fabricated using a process involving photolithography. Initially, a photoresist line was used to define the wire positions. Subsequently, silicon deep reactive ion etching was conducted to create sectorial grooves. Finally, the grooves were converted into suspended nanowires through wet oxidation. The resulting wires possess Schottky barrier contacts, which can be utilized for biomolecule detection on dry samples. Amnesic silicon nanowire devices are capable of detecting antigens, with a sensor sensitivity reaching up to 37 ± 1 mV fM−1.In 2013, Mohamad et al. presented the design of an amnesia sensor based on a TiO2 material for sensing applications.30 In 2014, Francesca Puppo et al. introduced an equivalent circuit that replicates the amnesic effect observed in the current–voltage (I–V) properties of silicon nanowires.31 Bequently, a human vascular endothelial growth factor (VEGF) antibody was employed to functionalize the functional layer of memristor device. The I–V characteristics of the nanowire were then investigated both before and after protein functionalization. The binding of biomolecules to the surface of the memristor was evidenced by the observed expansion of voltage intervals in the hysteresis curve. In 2015, Sun et al. introduced a novel ionic memristor that operates based on ionic currents in an aqueous solution with arbitrary ionic concentration.32 The ionic memristor is composed of silicon microelectrodes immersed in an aqueous solution environment, offering rapid and resilient switching with latch-up capabilities suitable for large-scale physiological ionic circuits as shown in Fig. 4a. The utilization of dielectric-immersed fluid-based sensors, actuators, and logic circuits eliminates the need for complex wireless connections with external solid-state integrated circuits. This advancement offers increased versatility and allows for larger signals in the development of future implantable and embedded smart medical devices. In the identical year, Tzouvadaki et al. produced collections of independent two-terminal Schottky barrier silicon nanowires exhibiting amnesic characteristics, aiming to acquire amnesic biosensors.33 The existence of biomolecules adhered to the nanostructures’ surface was assessed through the observation of a voltage gap in the memristive electrical properties. The system demonstrates promising potential for applications in molecular diagnostics, particularly owing to its capability for detection in the millimolar range, enabling early identification of cancerous diseases.
 | ||
| Fig. 4 (a) Schematic and image of a fluid-based ionic memristor.32 copyright 2015, Wiley-VCH. (b) Schematic illustration of AlOOH flexible memristive device and the physical display of the flexible device.39 copyright 2023, BioMed Central Ltd. (c) Schematic diagram of a memory synapse. | ||
In 2017, Volkov et al. introduced circuit-connected biosensors in plants and trees, exploring the various electrochemical components and devices that nature has developed within plants.34 Amnesia facilitates the electrical signal conduction between plant sensors and actuators. Electrical processes hold significant importance in the physiology of plants, trees, fruits, and seeds. The resistance of a memristor relies on its previous state, and this characteristic can be employed to emulate synaptic connections in the brain. In 2018, Dang et al. created a high-performance physical transient synapse utilizing memory resistors, designed for secure neuromorphic computing applications.35 They constructed physically transient and biodegradable amnesia devices using a W/MgO/ZnO/Mo configuration. The device exhibits exceptional analog switching characteristics and high reliability on silk protein substrates. It can be dissolved in deionized water for 7 minutes at room temperature, leading to synaptic device failure or disappearance, thereby emulating the process of biological neuronal cell death. In 2018, Barlucea et al. achieved the first detection of Ebola matrix proteins using nanoscale electronic sensors operating in memristor mode.36 In 2020, Mohamad et al. demonstrated the utility of non-structural protein 1 (NS1) as a widely adopted biomarker for early dengue diagnosis.37 In their research, they employed a fluid-based memristor sensor with label-free hair detection to identify NS1 protein. This sensor offers an alternative for label-free NS1 protein diagnosis under wet conditions, facilitating early dengue detection. In 2022, Mao et al. developed a magnetic field-regulated memristor to safeguard the well-being of specific populations.38 They achieved this by detecting the intensity of the surrounding magnetic field through the construction of an Ag/Cu/MnO/Ti memristor. This memristor was then incorporated into the design of an implantable detector that could be controlled and regulated by a magnetic field. In 2023, Chen et al. achieved significant potential for utilizing memristors as next-generation implantable multi-level resistive memory devices for long-term human health monitoring, as shown in Fig. 4b, through the construction of Pt/AlOOH/ITO memristor devices.39 The application of implantable electronic devices in synapses, neural systems, and sensory systems, among others. The memristor-based biosensing system is shown in Fig. 4c.
2.2. The application of memristors in brain–computer interface (BCI) technology
Memristors demonstrate significant potential in brain–computer interface (BCI) technology. BCIs aim to facilitate efficient communication between the brain and computers, aiding individuals with impaired mobility to regain motor function and even achieve direct brain–machine interaction. Integrating memristors into biological sensors or BCI devices enables the capture and transmission of neural signals. This integration enhances signal stability and precision, thereby improving the performance and reliability of BCI technology. In 2018, Li et al. utilized a large-scale amnesia crossbar for analog signal and image processing, using a reconfigurable amnesia crossbar composed of hafnium oxide amnesia on a metal oxide semiconductor transistor capable of performing analog vector matrix multiplication.40 In 2019, Sun et al. used 1T1R crossed arrays to solve matrix equations in parallel, showing that amnesia can directly process analog signals.41 The switching dynamics and electrical behavior of amnistors are similar to those of synapses and neurons, and these devices can be mounted in large arrays of crossbars to form neural networks that perform efficient in-memory computations with massive parallelism by directly exploiting the laws of physics. In the same year, Xia et al. summarized the progress, challenges, and possible solutions to achieve efficient brain-inspired computation with amnistors.42 In 2022, Tong et al. proposed an EEG signal analysis system based on 1T1R arrays, which utilizes the biological possibilities of amnesia to identify and classify brain electrical signals in the analog domain.43 The efficient analysis of EEG signals was realized using the amnesia array, which provides a new idea for the application of amnesia in biometric identification.2.3. The application of memristors in the field of biosensors
As an electronic component with the ability to mimic biological synapses, the application of memristors in the field of biosensors is gradually gaining attention. In 2010, Jo et al. constructed a nanoscale silicon-based amnesia and showed that a hybrid system consisting of complementary metal–oxide–semiconductor neurons and amnesic synapses can support important synaptic.13 In 2013, Indiveri et al. proposed a novel hybrid memristor-CMOS neuromorphic circuit that uses a memristor to directly simulate the biophysics and temporal dynamics of real synapses.6 In 2017, Wang et al. constructed a diffusive amnesic blocker based on silver nanoparticles in a dielectric film and then integrated these neurons with non-volatile amnesic synapses to construct a fully amnesic artificial neural network.7 In 2019, Fuller et al. introduced a polymer redox transistor-based ionic floating gate memory array (CBM).44In 2020, Jang et al. presented a one-transistor-two-memristor (1T2M) synaptic device, its array structure, and its operationalization in neurological applications, their proposed array structure was robust to the latent path problem, and superior pattern recognition accuracy was confirmed using artificial neural network simulations.45 In 2021, Pei et al. proposed a completely memristor-based artificial visual perception neural system (AVPNS) and utilized photonic memristors and threshold switching (TS) memristors on nanosheets to achieve synaptic and leaky integrate-and-fire (LIF) neuron functions, respectively. Their work demonstrates that the functionality of the biological visual neural system can be systemically emulated through a hardware system based on memristors.46 In 2022, they further developed an efficient multifunctional artificial vision system capable of recognition, memory, and self-protection through the utilization of a Sb2Se3/CdS-core/shell (SC) nanorod array optoelectronic memristor, a threshold-switching memristor, and an electrochemical actuator. When the photonic memristor is activated, it can initiate the movement of an electrochemical actuator, simulating the contraction of eye muscles and replicating the self-protective response of closing the eyes when exposed to intense light, akin to human eyes. This work provides a potential technological avenue for the application of memristors in biosensor systems.47
In 2023, Chen et al. proposed that CsPbBr3-based amnesia resistors with high on/off ratios, stable durability, and multilevel resistive memories could be used as artificial synapses to realize basic biological synaptic functions and neuromorphic computation based on controllable resistive modulation.48
In the same year, Yan et al. introduced a stable ferroelectric memristor based on the Pd/BaTiO3:Eu2O3/La0.67Sr0.33MnO3 structure grown on a silicon substrate with SrTiO3 serving as the buffer layer. The device exhibits a low coercive field voltage range of −1.3 to 2.1 V and robust endurance characteristics with approximately 1010 cycles achieved through optimization of the growth temperature. By integrating a pressure sensor, a photosensitive sensor, and a robotic arm, they reported, for the first time, a highly stable artificial multimodal sensory memory system with both visual and tactile functionalities.49 This work provides potential applications for memristor sensors in robotic perception systems.
2.4. Application of bio-memristors in medical image processing
As a cutting-edge electronic device, the bio-memory block has attracted great interest and potential in the field of medical image processing in recent years. Its unique characteristics make it ideal for exploring more efficient and accurate medical image processing methods. Medical image processing plays a key role in disease diagnosis, treatment planning, and surgical operations. Biological memristors bring new possibilities to the field of medical image processing due to their ability to mimic synaptic behavior and programmable features. In 2017, Wang et al. proposed a memristor-based image enhancement method that utilizes its synaptic simulation properties to successfully improve the clarity of low-quality images.7 In 2018, Li et al. demonstrated reconfigurable memristor crossbars consisting of hafnium oxide memristors on metal–oxide–semiconductor transistors, which allowed high output accuracy to be obtained and allowed the memristor to obtain a multistage steady state.40 They also demonstrated applications such as signal processing and image compression. In 2021, Zhu et al. introduced amnesia into the field of image enhancement for the first time, using the intrinsic properties of amnesia for secondary processing of images.50 The algorithm adopts the coarse transmission map and nonlinear amniotic properties, which is highly efficient and greatly reduces the computational cost.2.5. The application of memristors in the field of intelligent medicine
The application of memristors in the field of intelligent medicine is a promising research direction. By mimicking the synaptic connections between biological neurons, memristors can be used to build finer and smarter medical devices and systems, thus enhancing the efficiency of diagnosis, treatment, and monitoring. The application of amnesia in mobile health monitoring has the potential to help develop smarter and more efficient wearable devices that provide real-time monitoring of physiological parameters and assessment of health status. These devices can more accurately track a user's health status, thereby facilitating personalised healthcare management and health promotion. Memristors can be used to design more efficient sensors for physiological parameters such as heart rate, body temperature, and oxygen saturation. These sensors can be integrated into wearable devices to monitor the user's physiological status in real time. In 2015, Pang et al. developed a simple wearable capacitive pressure sensor with a bio-inspired design that achieves high sensitivity and reliability, which can show different patterns in waveforms taken from healthy people and heart patients, which in turn demonstrates the advantages of the device for the rapid diagnosis of cardiovascular and heart disease.51 Amnesia-powered wearable devices can monitor user's exercise and activity levels, helping them track fitness progress in real time and providing personalized health advice. Meanwhile, for patients suffering from chronic diseases, amnesia-powered wearables can regularly monitor the patient's physiological indicators and automatically alert doctors or patients themselves for early intervention and treatment.In 2020, Rajasekaran et al. introduced a TaOx/AlN-based flexible and transparent memristor with stability and durability under extreme conditions, and the device showed excellent flexibility under extreme bending conditions, which has excellent potential for wearable applications.52 In the same year, Liu et al. introduced a transparent flexible pressure sensor with microporous dielectrics recruited only by filling them with matching ionomers and demonstrated that the transparent flexible sensor could be used as a skin touch screen and smart interface.53 For cardiovascular medical applications, Chen et al. developed a flexible layered elastomer-tuned self-powered pressure sensor that achieves high sensitivity and a wide pressure range and also achieves fast response, high signal-to-noise ratio, and good stability. Fig. 5c shows a schematic diagram of cardiovascular monitoring.54
 | ||
| Fig. 5 Schematic diagram of smart healthcare applied to wearable devices: (a) schematic of in vivo diabetes diagnosis and treatment with smart contact lenses.56 copyright 2020, AMER ASSOC ADVANCEMENT SCIENCE. (b) The tester rotated his head from side to side at a frequency of 0.75 Hz.57 copyright 2021, Springer Nature. (c) Schematic diagram of the structure of HSPS and its use for real-time continuous cardiovascular monitoring.54 copyright 2020, Elsevier. (d) Structural design of muscle fibre-inspired piezoelectric textiles.58 copyright 2021, Wiley-VCH. (e) 3D hierarchical interleaved PME map based on PVDF/ZnO fibres for muscle behaviour monitoring.55 copyright 2020, Elsevier. (f) Walking sensor schematic.59 copyright 2021, Wiley-VCH. | ||
At the same time, they demonstrated that self-powered pressure sensors are not only sensitive for monitoring pulse, arterial, and cardiac conditions. In order to accurately monitor biological subtle signals, Yang et al. prepared a 3D hierarchical interlocking piezoelectric sensor based on PVDF/ZnO nanofibres by epitaxially growing ZnO nanorods. The muscle behavior monitoring schematic is shown in Fig. 5e.55 A three-dimensional hierarchically interlocked PVDF/ZnO nanofibre piezoelectric sensor was prepared by epitaxially growing ZnO nanorods on the surface of electrospun PVDF nanofibres, which resulted in a fiber-based physiological monitoring electronic device (PME) with good flexibility and high gas permeability. The PME designed on this basis can accurately monitor complex and subtle physiological signals such as respiration, wrist pulse, and muscle behaviour. In diabetes medicine, Keum et al. developed smart contact lenses that can be used for continuous glucose monitoring and diabetic retinopathy treatment. Fig. 5a illustrates a schematic diagram of their smart contact lens system for diagnosis and treatment.56 The smart contact lens device is made of a biocompatible polymer and contains ultrathin flexible circuitry and a microcontroller chip for real-time electrochemical biosensing, on-demand controlled drug delivery, wireless power management, and data communication. In a diabetes model, it can measure glucose levels in tears, validated by traditional invasive blood glucose tests, and trigger the release of drugs from the reservoir for the treatment of diabetic retinopathy. In 2021, Yu et al. developed an ultra-flexible BTS-GFF/PVDF composite film and highly sensitive sensors and demonstrated that BTS-GFF/PVDF sensors can detect very small forces (e.g., water droplet) and human body movements (e.g., head rotation). As shown in Fig. 5b, it displays a schematic illustration of the test subject rotating their head at different frequencies.57
In the same year, Su et al. developed a muscle fiber-inspired non-woven piezoelectric textile with adjustable mechanical properties for wearable physiological monitoring systems. The structural design and a functional schematic of piezoelectric textiles inspired by the MFP textile are shown in Fig. 5d.58 Meanwhile, Mariello et al. present a novel flexible, ultrathin wearable sensor that detects impulsive forces of sudden movements as well as slower microfriction phenomena with high sensitivity and a wide measurement range, ensuring stable and reproducible recognition of bio-signals. Fig. 5f shows a schematic diagram of gait and walking sensors.59 The versatility of the sensor is demonstrated by the recognition of gait walking and the monitoring of human joint movements. Feng et al. demonstrated that pearl-inspired enhancement of sensor sensitivity through spatially regulated cracking can be a pathway to developing sensors through crack engineering.60 In 2022, Zhou et al. reported flexible piezoelectric sensors based on self-assembled 10 nm BaTiO3 nanocubes on glass fibre fabrics (GFFs), which achieved high sensitivity and short response times and the sensors could intelligently identify note or keyboard users.61
In the same year, Li et al. designed a flexible pressure sensor with engineered microstructures on polydimethylsiloxane (PDMS) film, which has excellent bending and torsional strain detection properties, is mechanically durable, and is expected to be applied to wearable biosensor technology in healthcare.62 In 2023, Xie et al. summarised recent research advances in amnesia for smart healthcare as well as wearable applications.63 The application of wearable amnesia sensors is shown in Fig. 5.
The memristor exhibits significant potential advantages in the field of smart healthcare applications: (1) memristors can store information in wearable electronic devices and retain it even after power interruptions, reducing energy consumption. This is particularly crucial for implantable medical devices such as pacemakers, which require extended operation and minimize the need for battery replacements.64 (2) Memristors are of paramount importance for the storage of extensive patient data and medical images. Their high-density storage capacity can enhance the performance and efficiency of medical devices.65 (3) Memristors can emulate synaptic functions, enabling medical devices to learn and adapt to evolving patient needs and conditions.66 This feature enhances the precision and personalization of healthcare. Overall, memristors have tremendous potential in the field of smart healthcare, as they can enhance the performance, efficiency, and biocompatibility of medical devices, ultimately leading to improvements in medical treatment and patient care.
3. Conclusions and outlook
Due to the growing demand for renewable, environmentally friendly, biocompatible, and biodegradable materials, biomaterials has attracted significant attention in applications of biomemristors. These applications include the development of ultra-light and thin membranes, biodegradable materials, and biological DNA and silk protein amnesia. Nevertheless, the advancement of DNA and silk protein amnesia is currently at an early stage, and amnesia encounters various fundamental and technological hurdles in biomedical applications. The potential of biodegradable amnesia could be further explored, and researchers may consider developing composite biomaterials to enable the emergence of bioabsorbable or disposable electronics tailored for biomedical applications. Using genetic engineering and silk proteins, researchers are actively working on synthesizing and advancing the development of next-generation flexible electronic devices. These devices hold potential for various biosensor applications, opening up new possibilities in the field of biotechnology and healthcare. This objective can be attained through chemical modification and nanostructure engineering, offering potential applications in sensor integument and biocompatible robotics. The advancement of biomaterial memristors with a focus on environmental friendliness and sustainability has the potential to revolutionize the scientific community and society by enabling the production of high-performance deliverables. Xu et al. introduced a novel approach by combining oriented DNA molecules with silver nanoparticles, resulting in a low operating voltage, low operating power, and the achievement of high-performance textile memristors.67 This breakthrough offers a promising new direction for the advancement of next-generation memristors and textile electronics. Bio-memristors hold significant potential in the field of biomedicine, and certain bio-memristors demonstrate exceptional capabilities in emulating bionic synapses. Nonetheless, the application of bio-memristors still faces some challenges, including an unclear mechanism, poor reproducibility, limited life cycle, and retention time.The applications of memristors in artificial intelligence medicine primarily fall into three major categories: ultra-thin films, degradable memristors, and biodegradable memristors. They represent ultra-thin film based memristors.68,69 Ultra-thin film memristors have the capacity to minimize electronic waste and can be easily applied to curved and dynamic surfaces, akin to cling film. As such, they find applications in mobile electronic devices, artificial intelligence, healthcare, and biomedical systems, as shown in Fig. 6a–c. Fig. 6d and e represent bio-degradable memristors.70–72 With the rapid advancement of technology, there is a massive production scale of electronic components and an extremely fast pace of updates. This inevitably generates a significant amount of electronic waste, leading to severe environmental pollution. The introduction of degradable memristors effectively addresses these issues. Degradable memristors, when applied in implantable medical devices, can degrade or be absorbed by the human body after fulfilling their designated functions. Degradable memristors hold considerable application value in fields such as medicine, implantable equipment, and eco-friendly electronic products. They represent natural biomaterials based-memristors. The schematic diagram is shown in Fig. 6g–i.73–75 Natural biological materials are gifts from nature, and their utilization in the preparation and research of natural bio-memristors, such as soybeans, silk, and leaves, holds immense potential for applications in the manufacturing of implantable electronic devices and medical materials.76–80 The memristor devices hold significant potential applications in the field of biomedicine, simultaneously laying the foundation for the development of implantable neural systems.
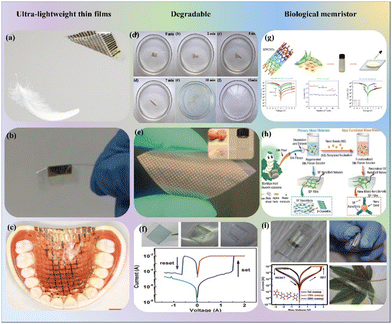 | ||
| Fig. 6 Memristors in artificial intelligence in medicine: (a) schematic of ultrathin plastic electronic foils and feather float.68 Copyright 2013, NPG. (b) The image of the memory foil can be sustained by human hair.69 Copyright 2016, Wiley-VCH. (c) The picture of tactile sensor sheet tightly conforming to a model of the human upper jaw.68 Copyright 2013, NPG. (d) The image of Mg/Ag-doped chitosan/ITO memristor dissolved in DI water.70 Copyright 2023, Wiley-VCH. Copyright 2023, Elsevier. (e) Photograph of the Ag/pectin/ITO memristor. Inset: Optical pictures of pectin powders and suspension extracted from orange peel.71 Copyright 2018, Wiley-VCH. (f) The silk protein resistive switching devices dissolved in deionized water or in phosphate-buffered saline.72 Copyright 2016, Wiley-VCH. (g) Schematic of Al/soybean MWCNT/ITO/glass memristor devices simulating the potentiation and suppression behavior of biological synapses.73 Copyright 2023, Elsevier. (h) Schematic of mesoscopic bioelectronic hybrid materials of silk fibroin (SF)-Ag nanoclusters (AgNCs@BSA; BSA: bovine serum albumin) memristors.74 Copyright 2019, Wiley-VCH. (i) Schematic of a memristor (flexible, transparent, and biocompatible resistive switching random access memory (ReRAM)) applied in wearable electronic devices.75 Copyright 2021, American Chemical Society. | ||
Currently, numerous challenges remain in realizing high-performance and multifunctional applications based on amnesia, including the following aspects: (1) there is still a lack of reliable technology to ensure the high performance of amnesia; (2) some biological amnesia can be easily denatured or even decomposed under high pressure, high temperature, or other harsh conditions, which impedes the preparation of structurally sophisticated amnesia; and (3) the mechanism for some biological amnesia is still not clear. Currently, the implementation of biological amnesia in smart healthcare and medical electronics remains a challenging issue.
To further enhance the application of amnesia in the biological domain, forthcoming researchers should emphasize the following aspects. Firstly, enhancing the performance of amnesia in the biomedical context can be achieved by developing multiple multilevel resistive storages, thereby significantly augmenting memory capacity. Concurrently, incorporating biocompatible and degradable functional materials can confer new capabilities to amnesia, such as photoelectric properties. Additionally, a combination of experimental and computational simulation methods should be employed to comprehensively analyze the memory resistance mechanism of the memristor. This would render amnesic resistors highly valuable in various fields, including logic operations, bionic synapses, neural networks, image recognition, in vivo diagnosis, and biomedical intelligent systems.81,82
Author contributions
Jie Zhang wrote and edited the paper. Junmei Du and Chuan Yang provided methodology and software guidance. Haotian Liang and Zelin Cao improved the reference resources for the paper. Xuegang Duan and Wentao Yan carried out the preliminary literature investigation. Bai Sun and Yong Zhao conceived the idea and corrected the manuscript. B. Sun made revisions. All authors gave their approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the Top Young Talent Project from Xi’an Jiaotong University (Grant No. 71211223010708) and the National Natural Science Foundation of China (Grant No. 52375575).Notes and references
- M. Berggren, D. Nilsson and N. D. Robinson, Nat. Mater., 2007, 6, 3–5 CrossRef CAS.
- L. Chua, IEEE Trans. Circuit Theory, 1971, 18, 507–519 Search PubMed.
- D. Ielmini and H. P. Wong, Nat. Electron., 2018, 1, 333–343 CrossRef.
- M. Khalid, Trans. Electr. Electron. Mater., 2019, 20, 289–298 CrossRef.
- O. Kavehei, A. Iqbal, Y. S. Kim, K. Eshraghian, S. F. Al-Sarawi and D. Abbott, Proc. R. Soc. A, 2010, 466, 2175–2202 CrossRef.
- G. Indiveri, B. Linares-Barranco, R. Legenstein, G. Deligeorgis and T. Prodromakis, Nanotechnology, 2013, 24, 384010 CrossRef PubMed.
- Z. Wang, S. Joshi, S. Savel Ev, W. Song, R. Midya, Y. Li, M. Rao, P. Yan, S. Asapu and Y. Zhuo, Nat. Electron., 2018, 1, 137–145 CrossRef.
- M. L. Sebastian and R. Gallo, Nat. Nanotechnol., 2020, 15, 529 CrossRef.
- M. Hu, C. E. Graves, C. Li, Y. Li, N. Ge, E. Montgomery, N. Davila, H. Jiang, R. S. Williams and J. J. Yang, Adv. Mater., 2018, 30, 1705914 CrossRef PubMed.
- K. C. Okafor and O. M. Longe, Int. J. Adv. Comput. Sci. Appl., 2022, 13, 896–912 Search PubMed.
- R. Homsi, N. Al-Azzam, B. Mohammad and A. Alazzam, Memristive Biosensors for Cancer Biomarkers Detection: A Review, IEEE Access, 2023, 19347–19361 Search PubMed.
- X. Zhang, Y. Zhuo, Q. Luo, Z. Wu, R. Midya, Z. Wang, W. Song, R. Wang, N. K. Upadhyay and Y. Fang, Nat. Commun., 2020, 11, 51 CrossRef CAS.
- S. H. Jo, T. Chang, I. Ebong, B. B. Bhadviya, P. Mazumder and W. Lu, Nano Lett., 2010, 10, 1297–1301 CrossRef CAS PubMed.
- D. B. Strukov, G. S. Snider, D. R. Stewart and R. S. Williams, Nature, 2008, 453, 80–83 CrossRef CAS.
- I. Abraham, Sci. Rep., 2018, 8, 10972 CrossRef PubMed.
- L. Chua, Semicond. Sci. Technol., 2014, 29, 104001 CrossRef.
- F. Pan, S. Gao, C. Chen, C. Song and F. Zeng, Mater. Sci. Eng., R, 2014, 83, 1–59 CrossRef.
- D. P. Sahu and S. N. Jammalamadaka, Sci. Rep., 2017, 7, 17224 CrossRef.
- X. B. Yan, Y. D. Xia, H. N. Xu, X. Gao, H. T. Li, R. Li, J. Yin and Z. G. Liu, Appl. Phys. Lett., 2010, 97, 112101 CrossRef.
- F. Longnos, E. Vianello, C. Cagli, G. Molas, E. Souchier, P. Blaise, C. Carabasse, G. Rodriguez, V. Jousseaume and B. De Salvo, Solid State Electron., 2013, 84, 155–159 CrossRef CAS.
- M. K. Choi, W. K. Kim, S. Sung, C. Wu, H. W. Kim and T. W. Kim, Sci. Rep., 2018, 8, 12275 CrossRef.
- N. G. Kang, B. Cho, B. G. Kang, S. Song, T. Lee and J. S. Lee, Adv. Mater., 2012, 24, 385–390 CrossRef CAS.
- X. Yan, J. Zhao, S. Liu, Z. Zhou, Q. Liu, J. Chen and X. Y. Liu, Adv. Funct. Mater., 2018, 28, 1705320 CrossRef.
- T. Fu, X. Liu, H. Gao, J. E. Ward, X. Liu, B. Yin, Z. Wang, Y. Zhuo, D. J. Walker and J. Joshua Yang, Nat. Commun., 2020, 11, 1861 CrossRef CAS PubMed.
- Z. Wu, J. Lu, T. Shi, X. Zhao, X. Zhang, Y. Yang, F. Wu, Y. Li, Q. Liu and M. Liu, Adv. Mater., 2020, 32, 2004398 CrossRef CAS.
- Y. Ho, G. M. Huang and P. Li, Nonvolatile memristor memory: Device characteristics and design implications, in Proceedings of the 2009 International Conference on Computer-Aided Design, 2009, 485–490.
- J. Cong and B. Xiao, mrFPGA: A novel FPGA architecture with memristor-based reconfiguration, in 2011 IEEE/ACM international symposium on Nanoscale Architectures, 2011, 1–8.
- D. Sacchetto, M. Doucey, G. De Micheli, Y. Leblebici and S. Carrara, Bionanoscience, 2011, 1, 1–3 CrossRef.
- S. Carrara, D. Sacchetto, M. Doucey, C. Baj-Rossi, G. De Micheli and Y. Leblebici, Sens. Actuators, B, 2012, 171, 449–457 CrossRef.
- N. S. Mohamad Hadis, A. A. Manaf and S. H. Herman, Microsyst. Technol., 2013, 19, 1889–1896 CrossRef CAS.
- F. Puppo, A. Dave, M. Doucey, D. Sacchetto, C. Baj-Rossi, Y. Leblebici, G. De Micheli and S. Carrara, IEEE Trans. NanoBiosci., 2014, 13, 19–30 Search PubMed.
- G. Sun, Z. Slouka and H. C. Chang, Small, 2015, 11, 5206–5213 CrossRef CAS.
- I. Tzouvadaki, N. Madaboosi, R. Soares, V. Chu, J. P. Conde, G. De Micheli and S. Carrara, Bio-functionalization study of memristive-biosensors for early detection of prostate cancer, in 2015 11th Conference on Ph. D. Research in Microelectronics and Electronics (PRIME), 2015, 17–20.
- A. G. Volkov, Int. J. Parallel, Emergent Distrib. Syst., 2017, 32, 44–55 CrossRef.
- B. Dang, Q. Wu, F. Song, J. Sun, M. Yang, X. Ma, H. Wang and Y. Hao, Nanoscale, 2018, 10, 20089–20095 RSC.
- B. Ibarlucea, T. Fawzul Akbar, K. Kim, T. Rim, C. Baek, A. Ascoli, R. Tetzlaff, L. Baraban and G. Cuniberti, Nano Res., 2018, 11, 1057–1068 CrossRef CAS.
- N. S. Mohamad Hadis, A. A. Manaf, M. F. A. Rahman, S. H. Ngalim, T. Hock Tang, M. Citartan, A. Ismail and S. H. Herman, Biosensors, 2020, 10, 143 CrossRef PubMed.
- S. Mao, B. Sun, G. Zhou, J. Qin, Y. Yang, Z. Rao, M. Liu, C. Ke and Y. Zhao, J. Colloid Interface Sci., 2023, 643, 38–46 CrossRef CAS PubMed.
- X. Chen, X. Zhao, X. Huang, X. Tang, Z. Sun, D. Ni, H. Hu and J. Yue, J. Nanobiotechnol., 2023, 21, 375 CrossRef CAS PubMed.
- C. Li, M. Hu, Y. Li, H. Jiang, N. Ge, E. Montgomery, J. Zhang, W. Song, N. Dávila and C. E. Graves, Nat. Electron., 2018, 1, 52–59 CrossRef.
- Z. Sun, G. Pedretti, E. Ambrosi, A. Bricalli, W. Wang and D. Ielmini, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 4123–4128 CrossRef.
- Q. Xia and J. J. Yang, Nat. Mater., 2019, 18, 309–323 CrossRef CAS PubMed.
- P. Tong, H. Xu, Y. Sun, Y. Wang, W. Wang and J. Li, AIP Adv., 2022, 12, 125108 CrossRef CAS.
- E. J. Fuller, S. T. Keene, A. Melianas, Z. Wang, S. Agarwal, Y. Li, Y. Tuchman, C. D. James, M. J. Marinella and J. J. Yang, Science, 2019, 364, 570–574 CrossRef CAS PubMed.
- J. T. Jang, D. Kim, W. S. Choi, S. Choi, D. M. Kim, Y. Kim and D. H. Kim, ACS Appl. Electron. Mater., 2020, 2, 2837–2844 CrossRef CAS.
- Y. Pei, L. Yan, Z. Wu, J. Lu, J. Zhao, J. Chen, Q. Liu and X. Yan, ACS Nano, 2021, 15, 17319–17326 CrossRef CAS PubMed.
- Y. Pei, Z. Li, B. Li, Y. Zhao, H. He, L. Yan, X. Li, J. Wang, Z. Zhao and Y. Sun, Adv. Funct. Mater., 2022, 32, 2203454 CrossRef CAS.
- D. Chen, X. Zhi, Y. Xia, S. Li, B. Xi, C. Zhao and X. Wang, Small, 2023, 2301196 CrossRef CAS.
- X. Yan, Y. Zhang, Z. Fang, Y. Sun, P. Liu, J. Sun, X. Jia, S. Sun, Z. Guo and Z. Zhao, InfoMat, 2023, e12429 CrossRef CAS.
- R. Zhu, Z. Tang, S. Ye, Q. Huang, L. Guo and S. Chang, IEEE Ttans. Electron. Dev., 2020, 68, 602–609 Search PubMed.
- C. Pang, J. H. Koo, A. Nguyen, J. M. Caves, M. G. Kim, A. Chortos, K. Kim, P. J. Wang, J. B. H. Tok and Z. Bao, Adv. Mater., 2015, 27, 634–640 CrossRef CAS PubMed.
- S. Rajasekaran, F. M. Simanjuntak, D. Panda, S. Chandrasekaran, R. Aluguri, A. Saleem and T. Tseng, ACS Appl. Electron. Mater., 2020, 2, 3131–3140 CrossRef CAS.
- Q. Liu, Z. Liu, C. Li, K. Xie, P. Zhu, B. Shao, J. Zhang, J. Yang, J. Zhang and Q. Wang, Adv. Sci., 2020, 7, 2000348 CrossRef CAS.
- S. Chen, N. Wu, S. Lin, J. Duan, Z. Xu, Y. Pan, H. Zhang, Z. Xu, L. Huang and B. Hu, Nano Energy, 2020, 70, 104460 CrossRef CAS.
- T. Yang, H. Pan, G. Tian, B. Zhang, D. Xiong, Y. Gao, C. Yan, X. Chu, N. Chen and S. Zhong, Nano Energy, 2020, 72, 104706 CrossRef.
- D. H. Keum, S. Kim, J. Koo, G. Lee, C. Jeon, J. W. Mok, B. H. Mun, K. J. Lee, E. Kamrani and C. Joo, Sci. Adv., 2020, 6, eaba3252 CrossRef CAS PubMed.
- D. Yu, Z. Zheng, J. Liu, H. Xiao, G. Huangfu and Y. Guo, Nano-Micro Lett., 2021, 13, 117 CrossRef CAS PubMed.
- Y. Su, C. Chen, H. Pan, Y. Yang, G. Chen, X. Zhao, W. Li, Q. Gong, G. Xie and Y. Zhou, Adv. Funct. Mater., 2021, 31, 2010962 CrossRef CAS.
- M. Mariello, L. Fachechi, F. Guido and M. De Vittorio, Adv. Funct. Mater., 2021, 31, 2101047 CrossRef CAS.
- B. Feng, X. Jiang, G. Zou, W. Wang, T. Sun, H. Yang, G. Zhao, M. Dong, Y. Xiao and H. Zhu, Adv. Funct. Mater., 2021, 31, 2102359 CrossRef CAS.
- P. Zhou, Z. Zheng, B. Wang and Y. Guo, Nano Energy, 2022, 99, 107400 CrossRef CAS.
- G. Li, D. Chen, C. Li, W. Liu and H. Liu, Adv. Sci., 2020, 7, 2000154 CrossRef CAS.
- L. Xie, Z. Zhang, Q. Wu, Z. Gao, G. Mi, R. Wang, H. Sun, Y. Zhao and Y. Du, Nanoscale, 2023, 15, 405–433 RSC.
- Z. Rao, X. Wang, S. Mao, J. Qin, Y. Yang, M. Liu, C. Ke, Y. Zhao and B. Sun, ACS Appl. Nano Mater., 2023, 6, 18645–18669 CrossRef CAS.
- H. Zhao, Z. Liu, J. Tang, B. Gao, Q. Qin, J. Li, Y. Zhou, P. Yao, Y. Xi and Y. Lin, Nat. Commun., 2023, 14, 2276 CrossRef CAS.
- S. Chen, T. Zhang, S. Tappertzhofen, Y. Yang and I. Valov, Adv. Mater., 2023, 35, 2301924 CrossRef CAS.
- X. Xu, X. Zhou, T. Wang, X. Shi, Y. Liu, Y. Zuo, L. Xu, M. Wang, X. Hu and X. Yang, Angew. Chem., Int. Ed., 2020, 59, 12762–12768 CrossRef CAS PubMed.
- M. Kaltenbrunner, T. Sekitani, J. Reeder, T. Yokota, K. Kuribara, T. Tokuhara, M. Drack, R. Schwödiauer, I. Graz and S. Bauer-Gogonea, Nature, 2013, 499, 458–463 CrossRef CAS PubMed.
- H. Wang, B. Zhu, H. Wang, X. Ma, Y. Hao and X. Chen, Small, 2016, 12, 3360–3365 CrossRef CAS.
- N. He, Y. Sun, Q. Yuan, Y. Wang and S. Zuo, Mater. Sci. Eng., B, 2023, 295, 116578 CrossRef CAS.
- J. Xu, X. Zhao, Z. Wang, H. Xu, J. Hu, J. Ma and Y. Liu, Small, 2019, 15, 1803970 CrossRef.
- H. Wang, B. Zhu, X. Ma, Y. Hao and X. Chen, Small, 2016, 12, 2715–2719 CrossRef CAS.
- L. Wang, W. Li and D. Wen, Microelectron. Eng., 2023, 267, 111911 CrossRef.
- C. Shi, J. Wang, M. L. Sushko, W. Qiu, X. Yan and X. Y. Liu, Adv. Funct. Mater., 2019, 29, 1904777 CrossRef CAS.
- N. Raeis-Hosseini and J. Rho, ACS Appl. Mater. Interfaces, 2021, 13, 5445–5450 CrossRef CAS.
- B. Sun, Y. Chen, G. Zhou, Y. Zhou, T. Guo, S. Zhu, S. Mao, Y. Zhao, J. Shao and Y. Li, Adv. Electron. Mater., 2023, 9, 2201017 CrossRef CAS.
- B. Sun, S. Ranjan, G. Zhou, T. Guo, Y. Xia, L. Wei, Y. N. Zhou and Y. A. Wu, Mater. Today Adv., 2021, 9, 100125 CrossRef CAS.
- B. Sun, G. Zhou, T. Guo, Y. N. Zhou and Y. A. Wu, Nano Energy, 2020, 75, 104938 CrossRef CAS.
- S. Zhu, B. Sun, Y. Chen, L. Tao, G. Zhou, H. Zhao, W. Mao and Y. Zhao, J. Mater. Chem. C, 2019, 7, 7593–7600 RSC.
- G. Ye, D. Song, J. Song, Y. Zhao and N. Liu, Adv. Funct. Mater., 2023, 34, 2303990 CrossRef.
- Z. Cao, B. Sun, G. Zhou, S. Mao, S. Zhu, J. Zhang, C. Ke, Y. Zhao and J. Shao, Nanoscale Horiz., 2023, 8, 716–745 RSC.
- M. Liu, Z. Cao, X. Wang, S. Mao, J. Qin, Y. Yang, Z. Rao, Y. Zhao and B. Sun, J. Mater. Chem. C, 2023, 11, 13167–13188 RSC.
| This journal is © The Royal Society of Chemistry 2024 |