Novel and high photovoltaic performance sensitizers of copolymeric sulfur coordination metal complexes of benzimidazolyl benzodithiophene derivatives†
Yu
Wang
,
Houpeng
Zhang
,
Yong
Tian
,
Yinfeng
Ma
,
Huimin
Liu
,
Jie
Yi
* and
Chaofan
Zhong
 *
*
Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan 411105, P. R. China. E-mail: zhongcf798@aliyun.com
First published on 15th November 2023
Abstract
To promote the ability of push–pull electrons in D–A′–π–A motif photoelectric sensitizers and to improve their photovoltaic performance, metal complexes with the sulfur coordination of benzimidazolyl benzodithiophene derivatives were used as the unit of auxiliary electron acceptors (A′) of sensitizers, and relevant five novel copolymeric sulfur coordination metal complexes (BDTT–BDTD–Ni, BDTT–BDTD–Cu, BDTT–BDTD–Zn, BDTT–BDTD–Cd, BDTT–BDTD–Hg) were designed, synthesized and characterized. In these sensitizer molecules, 8-quinolinol derivatives were used as a π-bridge and electron acceptor (A), and thienylbenzene-[1,2-b:4,5-b′] dithiophene (BDTT) was used as the electron donor (D). Under a light intensity of 100 mW cm−2 irradiation, the test results of photovoltaic performance for five copolymeric metal complex sensitizers were that the short circuit current densities (Jsc) are 11.49, 14.09, 15.54, 18.29 and 19.17 mA cm−2 respectively, and the power conversion efficiency (PCE) are 5.93%, 7.40%, 8.38%, 10.13% and 10.96%, respectively, and their thermal decomposition temperature (Td) are 302, 309, 295, 301 and 317 °C, respectively. The highest PCE reached 10.96% of BDTT–BDTD–Hg and exceeded 10%. The results showed that the Jsc and PCE values of the copolymeric complexes of the synperiodic metals increased successively from BDTT–BDTD–Ni to BDTT–BDTD–Cu to BDTT–BDTD–Zn, and the Jsc and PCE of the copolymeric complexes of metals in the identical cluster also increased successively from BDTT–BDTD–Zn to BDTT–BDTD–Cd to BDTT–BDTD–Hg, which indicates that the Jsc and PCE of copolymeric metal complexes are associated with the strength of the coordination bonds between the metal and sulfur coordination, and the stronger the coordination bonds, the higher the Jsc and PCE of the copolymeric sulfur coordination metal complexes. These findings provide a good basis for the development of relevant sensitizers in the future.
Introduction
In order to better use the abundant renewable energy source of solar energy, many photovoltaic technologies have appeared one after the other, such as perovskite solar cells (PSCs),1–3 organic solar cells (OSCs)4–6 and dye-sensitized solar cells (DSSCS). Among them, DSSCs have been widely researched because of their low cost and easy manufacturing.Photoelectric sensitizers, which are responsible for light absorption, excited state charge separation at semiconductor interfaces, and photoelectric regeneration are considered to be the most basic and important components that determine the photovoltaic performance of DSSCs,7–10 and they can be divided into two main categories: metal complex sensitizers and pure organic sensitizers.11–13 Early organic sensitizers were based on the simplest D–A motif. Organic D–A motifs usually do not have a large conjugate structure, which tends to cause charge aggregation and thus significantly attenuates the charge transport. The D–π–A motif was proposed, Yanagida and co-workers introduced double bonds on the basis of the D–A motif to synthesize TPA dyes with the D–π–A motif, and the PCE was improved from 3.3% to 5.3% in 2004,14 Jinxiang He et al. introduced thiopyridine and furan as π-bridges, and the PCE improved from 6.01% to 7.51%.15 The introduction of π-bridges not only solved the problem of charge buildup but also broadened the absorption spectrum and light response range, and the stability of the sensitizer molecule was also significantly improved;16–19 however, afterwards, there was no major breakthrough in enhancing PCE for a long time. In 2011, Zhu et al. synthesized the first dye molecule WS-2 with a D–A′–π–A motif by introducing benzothiadiazole (BTD) as an auxiliary electron acceptors (A′) between D and π of the dye molecule LS-1, and the PCE was improved from 4.4% to 8.7%,20 thus the D–A′–π–A motif emerged. The introduction of an auxiliary electron acceptor can enhance the electron withdrawing ability and reduce the recombination of electrons, thus promoting absorption spectrum redshift and exhibiting better photovoltaic performance.21–25 Since the proposal of D–A′–π–A motif, the focus of researching sensitizers has changed from the donor D and the acceptor A to auxiliary acceptor A′, because of strong electron-absorbing ability, benzothiadiazole (BTD),26,27 benzotriazole (BTZ),28–30 benzopyrazine (PPZ),31–33 and dipropylphthalata (DPP)34–36 have also been extensively studied for being used as auxiliary electron acceptors. During the study of the auxiliary electron acceptor A′, it was found that these pure organic auxiliary electron acceptors limit the ability of the intramolecular charge transfer (ICT), electron-withdrawing ability as well as the push–pull electron balance,37 and that the metal complexes were able to overcome these drawbacks by modulating the strength of the coordination bonds between the metal and the ligand. In recent years, metal complexes with N and O as coordinating atoms have been used as auxiliary electron acceptors, and their PCE has been increased to near 10%, but still not more than 10%, and it is also found that the stronger the coordination bond energy of the metals (Ni(II), Cu(II), Zn(II), Cd(II), and Hg(II)) with the coordinating atoms N and O is, the higher the PCE is ref. 37–40. According to the soft and hard acid–base principle,41,42 S atom is a softer base than O and N atoms, and the complexes of S coordinating with soft acids (Ni(II), Cu(II), Zn(II), Cd(II), and Hg(II)) will have stronger coordination bonds than those with O and N, this will facilitate the enhancement of the electron-withdrawing ability of the auxiliary electron acceptors (A′) and improve the push–pull electron balance within the sensitizer molecule, thereby improving the absorption of spectral range and intensity of the sensitizer and enhance the photovoltaic performance.
This study involved the investigation of the effect of the strength of ligating atoms S with transition metals (Ni(II), Cu(II), Zn(II), Cd(II), and Hg(II)) on improving the electron-withdrawing ability of the auxiliary electron acceptor, enhancing the light absorption properties and photovoltaic performance of sensitizers, five copolymeric sulfur coordination metal complexes, BDTT–BDTD–Ni, BDTT–BDTD–Cu, BDTT–BDTD–Zn, BDTT–BDTD–Cd, BDTT–BDTD–Hg were designed and synthesized, and their performances were tested.
Experimental section
Materials, instruments, and fabrication of DSSCs devices
The specific raw materials and equipment used and the detailed fabrication steps of DSSCs devices can be found in the ESI.†Synthesis of copolymeric sulfur coordination metal complexes
The specific synthesis route of the copolymeric sulfur coordination metal complexes is shown in Scheme 1. Detailed synthesis steps, characterization data, and the results of the photovoltaic performance for the five copolymeric sulfur coordination metal complexes, and corresponding figures can be found in the ESI.† | ||
| Scheme 1 Synthesis routes of the metal complexes and copolymeric sulfur coordination metal complexes. | ||
Results and discussion
Characterization of the metal complexes and copolymeric sulfur coordination metal complexes
The FT-IR spectra of the five metal complexes 6 (BDTD–Ni, BDTD–Cu, BDTD–Zn, BDTD–Cd and BDTD–Hg) and the five copolymeric sulfur coordination metal complexes 7 (BDTT–BDTD–Ni, BDTT–BDTD–Cu, BDTT–BDTD–Zn, BDTT–BDTD–Cd, BDTT–BDTD–Hg) are shown in Fig. 1.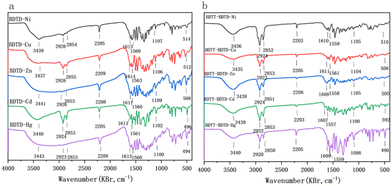 | ||
| Fig. 1 (a) Infrared spectra of BDTD–M (M = Ni, Cu, Zn, Cd, Hg) and (b) infrared spectra of BDTT–BDTD–M (M = Ni, Cu, Zn, Cd, Hg). | ||
As can be seen from Fig. 1, VS–Hg is more redshifted than the other four metal complexes, due to the larger reduced mass of mercury, which results in a smaller wave number. Meanwhile, the cyanide peak (V–CN), the carbon–carbon double bond peak (VC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), the carbon–nitrogen double bond peak (VC
C), the carbon–nitrogen double bond peak (VC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), and the two metal coordination peaks (VS–O–Metal, VS–Metal) of the copolymeric sulfur coordination metal complexes are redshifted by 3 to 5 nm compared to the metal complexes, which is due to the introduction of the electron donor BDTT. As an electron-rich group, the polymerization of BDTT with metal complexes results in an expansion of the degree of conjugation of the system and a weakening of the induction effect.43 The conjugation effect makes the system form large π-bonds, and the shortening of the single bond length and the elongation of the double bond length in the conjugated molecules together cause the red-shift phenomenon of various functional groups of the five copolymeric metal ligands.
N), and the two metal coordination peaks (VS–O–Metal, VS–Metal) of the copolymeric sulfur coordination metal complexes are redshifted by 3 to 5 nm compared to the metal complexes, which is due to the introduction of the electron donor BDTT. As an electron-rich group, the polymerization of BDTT with metal complexes results in an expansion of the degree of conjugation of the system and a weakening of the induction effect.43 The conjugation effect makes the system form large π-bonds, and the shortening of the single bond length and the elongation of the double bond length in the conjugated molecules together cause the red-shift phenomenon of various functional groups of the five copolymeric metal ligands.
The polymer molecular weight distribution was tested by gel permeation chromatography (GPC) measurement. The test results are shown in Table 1. From Table 1, it can be seen that the number average molecular weight (Mn) and heavy average molecular weight (Mw) of the five copolymeric sulfur coordination metal complexes are not high, and the polymerization degree (n) of the polymers is only 6–8. The reason may be that the metal complexes are not completely dissolved in the solvent or the metal complexes contain long alkyl chains with a certain potential resistance. Combining the above conclusions and the molecular weight distribution of the GPC, it can be seen that the required five metal complexes and the copolymeric sulfur coordination metal complexes were successfully synthesized.
| Polymer | M n [× 103] | M w [× 103] | PDI | n |
|---|---|---|---|---|
| a Determined by gel permeation chromatography with polystyrene as standard. | ||||
| BDTT–BDTD–Ni | 8.20 | 17.23 | 2.10 | 6 |
| BDTT–BDTD–Cu | 9.60 | 19.78 | 2.06 | 7 |
| BDTT–BDTD–Zn | 9.61 | 20.28 | 2.11 | 7 |
| BDTT–BDTD–Cd | 9.95 | 21.08 | 2.12 | 8 |
| BDTT–BDTD–Hg | 8.92 | 19.18 | 2.15 | 6 |
UV-vis absorption of metal complexes and copolymeric sulfur coordination metal complexes
UV-vis spectra of five metal complexes and five copolymeric sulfur coordination metal complexes were recorded. The results are shown in Fig. 2 and the relevant data are listed in Table 2.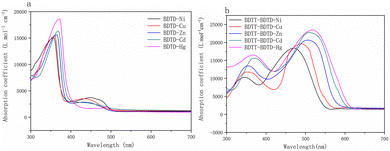 | ||
| Fig. 2 (a) UV-vis spectra of BDTD–M (M = Ni, Cu, Zn, Cd, Hg) and (b) UV-vis spectra of BDTT–BDTD–M (M = Ni, Cu, Zn, Cd, Hg). | ||
| Polymer | λ a,max (nm) | ε max (L mol−1 cm−1) | E red (V) | E ox (V) | HOMO (eV) | LUMO (eV) | E g (eV) |
|---|---|---|---|---|---|---|---|
| BDTT–BDTD–Ni | 473 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 225 |
−1.059 | 1.065 | −5.399 | −3.275 | 2.124 |
| BDTT–BDTD–Cu | 489 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595 595 |
−1.046 | 1.052 | −5.386 | −3.288 | 2.093 |
| BDTT–BDTD–Zn | 503 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630 |
−1.033 | 1.045 | −5.373 | −3.295 | 2.078 |
| BDTT–BDTD–Cd | 513 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718 718 |
−1.025 | 1.023 | −5.365 | −3.317 | 2.048 |
| BDTT–BDTD–Hg | 517 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 473 |
−1.012 | 1.009 | −5.352 | −3.331 | 2.021 |
As can be seen from Fig. 2, the maximum absorption wavelengths of the five metal complexes are located at 342 nm, 347 nm, 354 nm, 362 nm, and 368 nm, and the maximum absorption wavelengths of the five copolymeric sulfur coordination metal complexes are located at 473 nm, 489 nm, 503 nm, 513 nm, and 517 nm. Compared with the corresponding metal complexes, the absorption ranges of the five copolymeric sulfur coordination metal complexes are wider. This may be attributed to the introduction of the electron donor BDTT, which strengthens the conjugation system and the red-shifting of the maximum absorption peaks. The maximum absorption wavelengths of the five copolymeric sulfur coordination metal complexes increase regularly, which may be due to the enhancement of the coordination bond between the metal ions and the ligating atoms S, which improves intramolecular charge transfer (ICT) ability and thus broadens the absorption range. As can be seen from Table 2, the absorption coefficients of the five copolymeric sulfur coordination metal complexes are all above 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 and increase regularly, among which the absorption coefficient of BDTT–BDTD–Hg reaches 22
000 L mol−1 cm−1 and increase regularly, among which the absorption coefficient of BDTT–BDTD–Hg reaches 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 L mol−1 cm−1, which indicates that the photoelectric sensitizers have better absorption coefficients. In summary, It proves that the spectral response range can be broadened and the absorption coefficient can be increased by improving the ligand bond strength.
473 L mol−1 cm−1, which indicates that the photoelectric sensitizers have better absorption coefficients. In summary, It proves that the spectral response range can be broadened and the absorption coefficient can be increased by improving the ligand bond strength.
Cyclic voltammetry curves and molecular energy levels
CV curves (seen Fig. 3) of the five copolymeric metal complex dye sensitizers were measured by cyclic voltammetry, and the starting oxidation potential (Eox) and reduction potential (Ered) of the five copolymeric metal complex dye sensitizers were estimated from the CV curves. The HOMO and LUMO energy and Eg (energy gap) of the five copolymeric metal complex molecules can be worked out from the reference equation.44 The measured HOMO and LUMO energy levels and Eg could determine whether the five copolymeric metal complex dye sensitizers had sufficient ability to excite electrons from HOMO to LUMO by the absorptions sunlight, to inject electrons into TiO2 and to be reduced in the electrolyte (I−/I3−).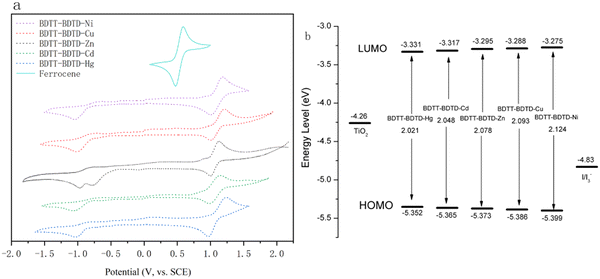 | ||
| Fig. 3 (a) Cyclic voltammetry curves of BDTT–BDTD–M (M = Ni, Cu, Zn, Cd, Hg) and (b) HOMO and LUMO energy levels and Eg of BDTT–BDTD–M (M = Ni, Cu, Zn, Cd, Hg). | ||
The HOMO energy levels of the five copolymeric metal complex dye sensitizers are −5.399 eV, −5.386 eV, −5.373 eV, −5.365 eV, and −5.352 eV, which are all lower than the standard electrode potential of the I−/I3− redox pair (−4.83 eV), which indicates that they meet the conditions for recycling and regenerative use in dye-sensitized solar cells and can ensure a certain service life. The LUMO energy levels are −3.275 eV, −3.288 eV, −3.295 eV, −3.317 eV, −3.331 eV, which are all higher than the Fermi energy level (−4.26 eV) of semiconductor (TiO2), which indicates that the five copolymeric metal complexes satisfy the sufficient driving force in the dye-sensitized solar cells to allow smooth injection of the electrons into the semiconductor.
From Fig. 3 and Table 2, the energy gaps follow the order BDTT–BDTD–Ni (2.124 eV) > BDTT–BDTD–Cu (2.093 eV) > BDTT–BDTD–Zn (2.078 eV) > BDTT–BDTD–Cd (2.048 eV) > BDTT–BDTD–Hg (2.021 eV). This data indicates that the five copolymeric sulfur coordination metal complexes can obtain enough energy to allow the electrons to be excited from the ground state to the excited state by absorbing light in the long wavelength region of higher energy. The coordination bonds of the five copolymeric sulfur coordination metal complexes are strengthened sequentially, and the Eg becomes smaller, which indicates that the absorption range of the photosensitizer is moving to the direction of the long wavelength, which is consistent with the change rule of the maximum absorption wavelength λmax. This means that more solar energy can be absorbed, which is conducive to increasing Jsc and PCE. BDTT–BDTD–Hg has the smallest Eg and therefore exhibits the longest λmax absorption range (517 nm) in the absorption spectrum.
Photovoltaic power conversion efficiency
The photocurrent–photovoltage (J–V) curves and the incident photon-to-current conversion efficiency (IPCE) curves of the five copolymeric sulfur coordination metal complexes are shown in Fig. 4, and the relevant data are listed in Table 3.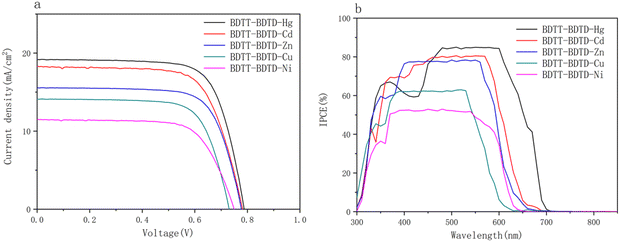 | ||
| Fig. 4 (a) J–V curves of BDTT–BDTD–M (M = Ni, Cu, Zn, Cd, Hg) and (b) IPCE curves of BDTT–BDTD–M (M = Ni, Cu, Zn, Cd, Hg). | ||
| Dye | Solvent | J sc (mA cm−2) | V oc (V) | FF (%) | η (%) |
|---|---|---|---|---|---|
| BDTT–BDTD–Ni | DMF | 11.49 | 0.75 | 68.92 | 5.93 |
| BDTT–BDTD–Cu | DMF | 14.09 | 0.73 | 71.63 | 7.40 |
| BDTT–BDTD–Zn | DMF | 15.54 | 0.78 | 71.63 | 8.38 |
| BDTT–BDTD–Cd | DMF | 18.29 | 0.78 | 71.00 | 10.13 |
| BDTT–BDTD–Hg | DMF | 19.17 | 0.79 | 72.35 | 10.96 |
| N719 | DMF | 17.68 | 0.75 | 74.00 | 9.29 |
From Fig. 4 and Table 3, the short circuit current densities (Jsc) of the five copolymeric sulfur coordination metal complexes follow the order BDTT–BDTD–Ni (11.49 mA cm−2) < BDTT–BDTD–Cu (14.09 mA cm−2) < BDTT–BDTD–Zn (15.54 mA cm−2) < BDTT–BDTD–Cd (18.29 mA cm−2) < BDTT–BDTD–Hg (19.17 mA cm−2). The open circuit voltages (Voc) are 0.75, 0.73, 0.78, 0.78, and 0.79 V. The PCEs are 5.93%, 7.40%, 8.38%, 10.13%, and 10.96%. The IPCE are 52.97%, 62.98%, 78.37%, 80.48%, and 85.01%. The Jsc of BDTT–BDTD–Ni, BDTT–BDTD–Cu, BDTT–BDTD–Zn, BDTT–BDTD–Cd, and BDTT–BDTD–Hg increased sequentially. The trends of PCE and IPCE are the same as that of Jsc. Among them, BDTT–BDTD–Hg shows the highest PCE of 10.96%, which is also related to the fact that BDTT–BDTD–Hg has the largest Jsc (19.17 mA cm−2). The Jsc, PCE, IPCE, and UV-vis absorption spectra of the five copolymeric sulfur coordination metal complexes showed the same pattern, which also proved that the stronger the coordination bond, the stronger the electron absorption ability and the better the test results.
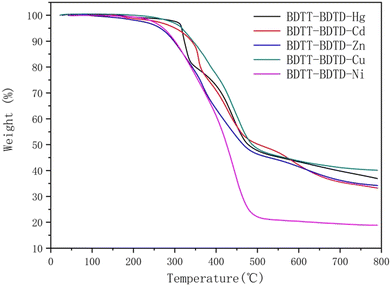
Glass transition temperature (Tg) and the thermal decomposition temperature (Td)
In this study, the glass transition temperature (Tg) and the thermal decomposition temperature (Td) of the five copolymeric sulfur coordination metal complexes were obtained through thermogravimetric analysis (TGA). The specific test results are shown in Fig. 5, and the relevant data are listed in Table 4.| Polymer | T d [°C] | T g [°C] |
|---|---|---|
| a The temperature at 5% weight loss under nitrogen. | ||
| BDTT–BDTD–Ni | 302 | 129 |
| BDTT–BDTD–Cu | 309 | 138 |
| BDTT–BDTD–Zn | 295 | 145 |
| BDTT–BDTD–Cd | 301 | 153 |
| BDTT–BDTD–Hg | 317 | 160 |
The glass transition temperatures of the five copolymeric sulfur coordination metal complexes are 129, 138, 145, 153, and 160 °C, and the thermal decomposition temperatures are 302, 309, 295, 301 and 317 °C.
The Tg of BDTT–BDTD–Ni, BDTT–BDTD–Cu, BDTT–BDTD–Zn, BDTT–BDTD–Cd and BDTT–BDTD–Hg increased sequentially. This may be attributed to the stronger bond energy, indicating a higher decomposition temperature. The ambient operating temperature of the photoelectric sensitizers is about 80 °C, while the Tg and Td of the five copolymeric sulfur coordination metal complexes are above 129 °C, and the Td of BDTT–BDTD–Hg even reaches 317 °C. This indicates that the five copolymeric sulfur coordination metal complexes are thermally stable and capable of meeting the practical application requirements of the photoelectric sensitizers.
Conclusions
In this study, five copolymeric sulfur coordination metal complexes (BDTT–BDTD–Ni, BDTT–BDTD–Cu, BDTT–BDTD–Zn, BDTT–BDTD–Cd, and BDTT–BDTD–Hg) were designed and synthesized. From the photovoltaic performance test results, the power conversion efficiency (PCE) of the five copolymeric sulfur coordination metal complexes is 5.93%, 7.40%, 8.38%, 10.13%, and 10.96%, where the complex with sulfur (BDTT–BDTD–Hg) was used as a photoelectric sensitizer, and the PCE exceeded 10%, reaching 10.96%. This is mainly because the ability of the ligand to bind to the metal affects the strength of the coordination bond. When using sulfur atoms, which are softer bases than N and O, to coordinate with soft acid metal ions, the strength of the coordination bond will be increased, which improves the electron absorption capacity of the auxiliary electron acceptor as well as the push–pull electron balance within the sensitizer molecule. This alteration reduces the energy gap of the copolymeric sulfur coordination metal complexes and induces the redshift of λmax, which improves Jsc and PCE. Meanwhile, the Tg and Td of the copolymeric sulfur coordination metal complexes are higher than 129 °C, showing good thermal stability. Therefore, this study provides a research basis for future studies on this type of photoelectric sensitizer.Author contributions
Chaofan Zhong and Jie Yi – supervision and writing (review and editing). Yu Wang, Houpeng Zhang, Yong Tian, Yinfeng Ma, and Huimin Liu – conceptualization, data curation, investigation, methodology, writing, and data analysis – (original draft). Yu Wang, Yinfeng Ma, and Huimin Liu – visualization and graphics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the financial support of the Open Project Program of the Key Laboratory of Environmentally Friendly Chemistry and Applications of the Ministry of Education, China (Grant No. 09HJYH10).Notes and references
- H. Li, C. Zhang, C. Gong, D. Zhang, H. Zhang, Q. Zhuang, X. Yu, S. Gong, X. Chen, J. Yang, X. Li, R. Li, J. Li, J. Zhou, H. Yang, Q. Lin, J. Chu, M. Grätzel, J. Chen and Z. Zang, Nat. Energy, 2023, 8, 946–955 CrossRef CAS.
- Q. Zhuang, H. Li, C. Zhang, C. Gong, H. Yang, J. Chen and Z. Zang, Adv. Mater., 2023, 35, 2303275 CrossRef CAS.
- C. Zhang, H. Li, C. Gong, Q. Zhuang, J. Chen and Z. Zang, Energy Environ. Sci., 2023, 16, 3825–3836 RSC.
- R. Ma, K. Zhou, Y. Sun, T. Liu, Y. Kan, Y. Xiao, T. A. D. Peña, Y. Li, X. Zou, Z. Xing, Z. Luo, K. S. Wong, X. Lu, L. Ye, H. Yan and K. Gao, Matter, 2022, 5, 725–734 CrossRef CAS.
- J. Xu, F. Lin, L. Zhu, M. Zhang, T. Hao, G. Zhou, K. Gao, Y. Zou, G. Wei, Y. Yi, A. K. Y. Jen, Y. Zhang and F. Liu, Adv. Energy Mater., 2022, 12, 2201338 CrossRef CAS.
- Y. Sun, L. Nian, Y. Kan, Y. Ren, Z. Chen, L. Zhu, M. Zhang, H. Yin, H. Xu, J. Li, X. Hao, F. Liu, K. Gao and Y. Li, Joule, 2022, 6, 2835–2848 CrossRef CAS.
- M. Mazumder, M. Akteruzzaman, H. Yeasmin and R. Islam, ChemRxiv, 2023, 9 Search PubMed.
- P. Li, H. Zhang and A. Troisi, J. Phys. Chem. C, 2018, 122, 23890–23898 CrossRef CAS.
- A. Sen, M. H. Putra, A. K. Biswas, A. K. Behera and A. Groβ, Dyes Pigm., 2023, 111087 CrossRef CAS.
- Z. Y. Yao, M. Zhang, H. Wu, L. Yang, R. Z. Li and P. Wang, Dye-Sensitized Sol. Cells, 2015, 137, 3799–3802 CAS.
- M. D. Ye, X. R. Wen, M. Y. Wang, J. Iocozzia, N. Zhang, C. J. Lin and Z. Q. Lin, Mater. Today, 2015, 18, 155–162 CrossRef CAS.
- Z. Yao, M. Zhang, R. Li, L. Yang, Y. Qiao and P. Wang, Angew. Chem., 2015, 127, 6092–6096 CrossRef.
- S. R. Bobe, A. Gupta, A. Rananaware, A. Bilic, W. C. Xiang, J. L. Li, S. V. Bhosale, S. V. Bhosale and R. A. Evans, Dyes Pigm., 2016, 134, 83–90 CrossRef CAS.
- T. Kitamura, M. Lkeda, K. Shigaki, T. Lnoue, N. A. Anderson, X. Ai, T. Lian and S. Yanagida, Chem. Mater., 2004, 16, 1806 CrossRef CAS.
- J. He, F. Guo, X. Li, W. Wu, J. Yang and J. Hua, Chem – Eur. J., 2012, 18, 7903–7915 CrossRef CAS PubMed.
- A. Sen and A. Groß, ACS Appl. Energy Mater., 2019, 2, 6341–6347 CrossRef CAS.
- H. Ding, Y. Chu, M. Xu, S. Zhang, H. Ye, Y. Hu and J. Hua, J. Mater. Chem. C, 2020, 8, 14864–14872 RSC.
- Y. K. Eom, J. Y. Hong, J. Kim and H. K. Kim, Dyes Pigm., 2017, 136, 496–504 CrossRef CAS.
- Y. K. Eom, I. T. Choi, S. H. Kang, J. Lee, J. Kim, M. J. Ju and H. K. Kim, Adv. Energy Mater., 2015, 5, 1500300 CrossRef.
- W. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z. Wang and H. Tian, Adv. Funct. Mater., 2011, 21, 756–763 CrossRef CAS.
- B. Hemavathi, V. Jayadev, P. C. Ramamurthy, R. K. Pai, N. Unni, T. N. Ahipa, S. Soman and R. G. Balakrishna, New. J. Chem., 2019, 43, 15673 RSC.
- Y. Z. Wu, W. H. Zhu, S. M. Zakeeruddin and M. Grätzel, ACS Appl. Mater. Interfaces, 2015, 7, 9307–9318 CrossRef CAS.
- W. W. Zhang, Y. Z. Wu, H. W. Bahng, Y. Cao, C. Y. Yi, Y. Saygili, J. S. Luo, Y. H. Liu, L. Kavan, J. E. Moser, A. Hagfeldt, H. Tian, S. M. Zakeeruddin, W. H. Zhu and M. Grätzel, Energy Environ. Sci., 2018, 11, 1779–1787 RSC.
- H. Jiang, Y. Ren, W. Zhang, Y. Wu, E. C. Socie, B. I. Carlsen, J. E. Moser, H. Tian, S. M. Zakeeruddin, W. H. Zhu and M. Grätzel, Angew. Chem., 2020, 132, 9410–9415 CrossRef.
- L. Han, J. Liu, Y. Liu and Y. Cui, J. Mol. Struct., 2019, 1180, 651–658 CrossRef CAS.
- J. M. Ji, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, Adv. Energy Mater., 2020, 10, 2000124 CrossRef CAS.
- H. Zhu, W. Li, Y. Wu, B. Liu, S. Zhu, X. Li, H. Agren and W. H. Zhu, ACS. Sustainable Chem. Eng., 2014, 2, 1026–1034 CrossRef CAS.
- J. Mao, F. Guo, W. Ying, W. Wu, J. Li and J. Hua, Chem. – Asian J., 2012, 7, 982–991 CrossRef CAS PubMed.
- Y. S. Yen, C. T. Lee, C. Y. Hsu, H. H. Chou, Y. C. Chen and J. T. Lin, Chem. – Asian J., 2013, 8, 809–816 CrossRef CAS.
- H. L. Cheng, Z. S. Huang, L. Wang, H. Meier and D. Cao, Dyes Pigm., 2017, 137, 143–151 CrossRef CAS.
- J. X. Cheng, Z. S. Huang, L. Wang and D. Cao, Dyes Pigm., 2016, 131, 134–144 CrossRef CAS.
- K. Pei, Y. Wu, A. Islam, S. Zhu, L. Han, Z. Y. Geng and W. H. Zhu, J. Phys. Chem. C, 2014, 118, 16552–16561 CrossRef CAS.
- H. Zhu, B. Liu, J. Liu, W. Zhang and W. H. Zhu, J. Mater. Chem. A, 2015, 3, 10603–10609 RSC.
- S. Qu, C. Qin, A. Islam, J. Hua, H. Chen, H. Tian and L. Han, Chem. Asian. J., 2012, 7, 2895–2903 CrossRef CAS.
- D. A. García, J. Warnan and E. Reisner, Chem. Sci., 2020, 11, 12769–12776 RSC.
- S. Fusco, M. Barra, M. Bonomo, A. Cassinese, R. Centore, F. Chiarella, F. Senneca and A. Carella, Dyes Pigm., 2021, 186, 109026 CrossRef CAS.
- Y. Ma, K. Wang, H. Liu, S. Tang, Y. Tian, H. Zhang, Y. Wang and C. F. Zhong, Dyes Pigm., 2022, 110771 CAS.
- Y. Tian, K. Wang, H. Zhang, X. Wu and C. Zhong, Tetrahedron, 2022, 113, 132756 CrossRef CAS.
- H. Zhang, X. Wu, Y. Tian, K. Wang, S. Tang and C. Zhong, J. Inorg. Organomet. Polym. Mater., 2022, 32, 1736–1743 CrossRef CAS.
- T. Wan, Y. Liu, C. Xia, Z. Xu, G. Wen, S. Tang, K. Wang and C. Zhong, J. Mater. Sci.: Mater. Electron., 2018, 29, 21170–21179 CrossRef CAS.
- K. W. Yeom, D. K. Lee and N. G. Park, Adv. Energy Mater., 2022, 12, 2202496 CrossRef CAS.
- Y. Liu, Z. Xing, X. Zhang and G. Liang, J. Solid State Chem., 2017, 246 CAS.
- J. K. Jin, J. K. Choi, B. J. Kim, H. B. Kang, S. C. Yoon, H. You and H. T. Jung, Macromolecules, 2011, 44, 502–511 CrossRef CAS.
- I. Chung, B. Lee, J. He, R. P. H. Chang and M. G. Kanatzidis, Nature, 2012, 485, 486–489 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tc03344a |
| This journal is © The Royal Society of Chemistry 2024 |