Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stormwater alters the resistome of urban surface water, an impact that can be mitigated by green stormwater infrastructure†
Kassidy
O'Malley
 ,
Walter
McDonald
and
Patrick
McNamara
,
Walter
McDonald
and
Patrick
McNamara
 *
*
Department of Civil, Construction, and Environmental Engineering, Marquette University, 1637 W Wisconsin Ave, Milwaukee, WI 53233, USA. E-mail: patrick.mcnamara@marquette.edu; Tel: +414 288-2188
First published on 22nd July 2024
Abstract
Antibiotic resistance poses an escalating threat to global health, with environmental reservoirs being pivotal areas of concern, as well as opportunities for potential mitigation. Stormwater systems are an important type of environmental reservoir in the urban water cycle with a dearth of research related to impacts on antibiotic resistance. In particular, there has been limited research exploring the impact of diverse antibiotic resistance genes (ARGs) carried by stormwater from various land uses on surface water, nor has there been an examination of the role played by green stormwater infrastructure (GSI) in mitigating this impact. Therefore, this study sought to elucidate the variability of ARGs across diverse land uses and evaluate the efficacy of GSI in mitigating ARG dissemination. Five distinct stormwater samples—representing mixed, residential, urban, and GSI-treated effluents—were taken to assess variations in ARG resistomes based on land use types. The ARGs in stormwater collected from different land uses were found to be similar in composition and represent a similar level of diversity. A GSI system with a rock swale and bioretention cell connected in series, was also sampled to see how GSI impacted ARGs, and this GSI system did substantially alter the diversity of ARGs. Moreover, the bioretention cell was found to reduce ARG concentrations by 30%. This research also sought to assess the impact of all five stormwater samples on the resistome of surface water via lab-scale microcosm experiments. The urban and residential stormwater significantly (p < 0.05) altered the resistome of surface water, while the mixed-land use sample did not. This finding underscored stormwater's pivotal role in introducing distinct ARG resistome compositions into downstream waters, heightening the chances for development of antibiotic resistant bacteria. The effluent stormwater from the GSI system, however, had less of an impact on the resistome of surface water in the microcosm experiments in comparison to the influent (untreated) stormwater. In managing stormwater runoff through GSI systems, this study's findings highlight the potential of GSI designs and practices to limit the dissemination of diverse and abundant ARGs, safeguard public health, and contribute to sustainable stormwater management by minimizing the impact on downstream surface waters.
Environmental significanceThe study addresses the pressing issue of antibiotic resistance gene (ARG) dissemination through stormwater runoff into surface water bodies. Understanding this phenomenon is crucial due to the potential risks posed by the emergence of antibiotic resistant bacteria in aquatic ecosystems. The key finding reveals that stormwater, especially from residential and urban areas, significantly impacts surface water resistomes, potentially leading to long-term consequences for environmental and public health. This underscores the urgency of implementing effective mitigation strategies, such as green stormwater infrastructure (GSI), to reduce ARG dissemination. Moreover, this work reveals that GSI systems, particularly bioretention cells, can reduce ARG concentrations and alter stormwater's resistome, ultimately lessening the immediate risk posed to aquatic ecosystems. |
1. Introduction
Antibiotic resistance is a public health crisis that presents a growing concern in the environment, due to the interconnectedness of environmental and human health.1 The environment is a reservoir for antibiotic resistance genes (ARGs) and can contribute to the development of resistance in environmental and pathogenic bacteria.2–4 Stormwater has recently been identified as an area of concern for the environmental occurrence of antibiotic resistance,5–7 due to its capacity to transport ARGs from various sources, such as urban soil environments and impervious surfaces, into downstream environments.8–10 Moreover, stormwater contains concentrated and diverse ARGs,11,12 in addition to other resistance elements, specifically mobile genetic elements (MGEs), and selective pressures (e.g., heavy metals) that can contribute to resistant bacteria propagation.8,13,14Stormwater is a concern for antibiotic resistance in the environment particularly for the role it plays in spreading ARGs across an urban environment.9 As stormwater runs off of urban surfaces, it can accumulate a unique composition of ARGs and subsequently deposit them into downstream urban surface waters.8,15 Urban surface waters, as such, can serve as a critical area in the public health crisis of antibiotic resistance, acting as dynamic environments where the convergence of human, environmental, and bacterial interactions heighten the risk and dissemination of antibiotic resistance.16 However, a notable gap in the literature is the downstream impact that stormwater has on the antibiotic resistance profile of receiving surface waters. This knowledge gap hinders a comprehensive understanding of the environmental implications of ARGs in stormwater and the subsequent risks they pose to aquatic ecosystems. In understanding the risks stormwater poses as it mixes with urban surface waters, specific management strategies can be proposed on how stormwater should be managed to mitigate antibiotic resistance. Green stormwater infrastructure (GSI) is a specific management system that can be utilized to mitigate the transport of pollutants via stormwater runoff in urban settings.17 These systems are designed to capture and filter stormwater, removing pollutants before transport to downstream waters.18 As it relates to ARGs, GSI systems have very scarcely been investigated for their ability to remove ARGs from stormwater runoff. In one field-scale biofilter, a 0.9 and 2.5-log reduction in the ARGs sul1 and ermB was achieved, respectively.19 Consequently, it is possible that GSI systems play a role in shifting stormwater's resistome prior to mixing with urban surface waters.
Stormwater is a further concern for antibiotic resistance in the environment due to the concentration and diversity of elements that contribute to the development of resistance in microbial communities.5,15,20 ARGs, MGEs, and selective pressures in stormwater, however, are not homogenous and exhibit significant variations across geographical locations.21 These variations lead to distinctions and region-specific pressures on the microbial communities in stormwater, differentially influencing antibiotic resistance propagation.22 Stormwater has thus been found to vary in ARG concentrations across land uses.12,23 Specifically, stormwater from residential areas was found to harbor a higher concentration of ARGs compared to commercial areas.23 Moreover, specific land uses have been found to result in the occurrence of distinct ARGs, such as the blaSHV-02 ARG which was only detected in samples collected from a campus setting.12
Research has not clarified the diversity of ARGs in stormwater across land uses. Diversity, which indicates the variety and composition of ARGs in an environment, is crucial to providing insights into the complexity and potential evolution of antibiotic resistance.24,25 By understanding these variations, a deeper comprehension of environmental resistomes and the specific environmental factors shaping them can be gained. Such knowledge is essential for predicting resistance patterns and informing strategies to mitigate the risks associated with antibiotic resistance dissemination in various ecosystems.
The aim of this research was to elucidate how the dissemination of stormwater's resistome impacts the resistome of urban surface water. The impact of stormwater from different land uses was evaluated, as well as stormwater before and after treatment in a GSI system. With this knowledge, effective strategies can be developed for managing and mitigating the potential adverse effects of stormwater runoff on downstream surface water. Specifically in this study, microcosms were conducted in which surface water was combined with five different stormwater samples and monitored for five days. The stormwater samples included runoff collected at an outfall of a mixed, residential, and urban land use as well as stormwater from the effluent of a GSI rock swale and bioretention cell. The specific objectives of this research were to: (1) determine how the diversity of ARGs in stormwater varies across land uses, (2) investigate the impact of stormwater from varying land uses on the resistome of a receiving surface water, and (3) determine how a GSI bioretention cell changes the impact of stormwater on the resistome of urban surface water.
2. Methods
2.1. Sampling locations and events
One surface water and five stormwater locations were targeted for sample collection for the microcosm experiments (Fig. S1†). The surface water sample was taken downstream of all stormwater locations at the convergence of the Menomonee, Milwaukee, and Kinnikinic Rivers in Lake Michigan. The first sampling location was a stormwater outfall in Wauwatosa, WI, USA, that collects runoff from a roughly 1.10 km2 drainage area of mixed land use. The second location was a stormwater outfall also in Wauwatosa, WI, USA that discharges to the Menominee River and collects stormwater from a 4.4 km2 drainage area that is dominated by residential land use. The third sampling location was the downspout of a highway overpass that discharges into a GSI system and that collects urban runoff exclusively from a highway surface. The fourth and fifth samples were collected at sequential locations within the GSI system. The fourth location was the effluent of a rock swale and the fifth location was the effluent of a bioretention cell at the most downstream end of the GSI treatment system. Land use characteristics of each sampling location were defined using the National Land Cover Database and the City of Milwaukee's Land Use Citywide Policy Plan.26 Surface water samples were collected from August 3rd through August 13th, 2023, after an antecedent dry weather period of 5 days.27 Each of the five stormwater sites were then sampled from on August 14th, 2023.2.2. Sample collection and microcosm processing
A microcosm, which combined surface water with five stormwater samples separately, was conducted to evaluate the impact of stormwater from various land use types on the downstream surface water bacterial community and resistomes (Fig. S2†).28,29 Prior to the microcosm, surface water was collected for 10 days to characterize the baseline microbial community (designated as days −10 through −1). All surface water samples were grab samples collected at the same time each day and transported back to the lab and analyzed immediately. On day 0 a rain event occurred, and grab stormwater samples were collected at all five locations. Upon transport to the lab, the stormwater samples were analyzed immediately for their initial day 0 condition. Then the microcosms were assembled, in which the stormwater samples were mixed with an equal volume of surface water and placed on a shaker table (100 rpm) for 5 days at ambient temperatures. To determine the mixing ratio, streamflow data from USGS stream gages across Milwaukee County was evaluated during rainfall events. It was determined that during peak flows, the streamflow could more than quadruple during intense rainfall. Therefore, the decision to mix stormwater and surface water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by volume, simulating a doubling in streamflow, was made to simplify the experimental setup and enhance reproducibility, as it provides a clear, standardized condition under which the response of surface water to stormwater introduction can be systematically monitored and analyzed. The surface water that was mixed with the stormwater was the sample collected for day −1. The day −1 surface water was kept in a refrigerator (5 °C) for 24 hours after collection and a sample was collected from that surface water (designated as surface water day 0) prior to mixing with stormwater to analyze any changes in the surface water community within the time that it was stored in the lab. Samples were collected from the mixed stormwater/surface water microcosms immediately after mixing, as well as on days 1, 3, and 5 thereafter. The duration of the experiment was set at five days based on prior research finding that ARG concentrations in surface water return to a pre-rain state five days after a rainfall event.27 Therefore, samples were taken on days 1, 3, and 5 to track the evolving changes during this period. From each sample collected, water quality analyses were performed, DNA was extracted, and metagenomic sequencing was completed. Microcosm controls were also included in which surface water and each stormwater location were run by themselves to monitor any changes due to time without mixing of waters. All conditions, the mixed microcosms and controls, were run in triplicate (n = 3).
1 ratio by volume, simulating a doubling in streamflow, was made to simplify the experimental setup and enhance reproducibility, as it provides a clear, standardized condition under which the response of surface water to stormwater introduction can be systematically monitored and analyzed. The surface water that was mixed with the stormwater was the sample collected for day −1. The day −1 surface water was kept in a refrigerator (5 °C) for 24 hours after collection and a sample was collected from that surface water (designated as surface water day 0) prior to mixing with stormwater to analyze any changes in the surface water community within the time that it was stored in the lab. Samples were collected from the mixed stormwater/surface water microcosms immediately after mixing, as well as on days 1, 3, and 5 thereafter. The duration of the experiment was set at five days based on prior research finding that ARG concentrations in surface water return to a pre-rain state five days after a rainfall event.27 Therefore, samples were taken on days 1, 3, and 5 to track the evolving changes during this period. From each sample collected, water quality analyses were performed, DNA was extracted, and metagenomic sequencing was completed. Microcosm controls were also included in which surface water and each stormwater location were run by themselves to monitor any changes due to time without mixing of waters. All conditions, the mixed microcosms and controls, were run in triplicate (n = 3).
2.3. DNA extraction and ARG quantification
For each sample collected prior to and during the microcosm experiment, DNA was extracted by first filtering the sample through a 0.22 μm Merck Millipore Express Plus® membrane filter and then extracting the DNA from the filters via FastDNA Spin Kit (MP Biomedicals, Santa Ana, CA). The manufacturer's protocol was followed with the addition of three liquid nitrogen freeze–thaw cycles for cell lysis as described previously.29,30 Subsequently, qPCR was utilized to quantify three ARGs, sul1, tetW, and ermF, and the 16S rRNA gene which were selected based on their reported frequency and abundances in stormwater.5 The protocol followed for qPCR has been previously described.31–332.4. Metagenomic sequencing and bioinformatics
Samples from this work were sequenced at the SeqCenter (previously Microbial Genome Sequencing Center, Pittsburgh, PA) on the Illumina NextSeq 2000 platform (151-bp paired end) at a sequencing depth of 650 Mbp. The raw metagenomic sequencing data have been deposited in the Sequence Read Archive under accession number PRJNA1026961. The sequenced reads were first quality filtered using Trimmomatic to remove adaptors and low-quality sequences.34 The quality filtered reads were utilized to assign taxonomy using Metaphlan.35 Next, reads were de novo assembled into contigs using metaSPAdes.36 After each sample had its own assembly created using metaSPAdes, these assemblies were individually annotated using resistance gene identifier (RGI) to predict ARGs.37 To estimate abundances of annotated ARGs, quality-filtered reads were mapped FASTA sequences from RGI output files using Kallisto.38 Following, relative gene abundance was calculated for each ARG as the mapped reads per kilo base of gene length per million total reads (RPKM).392.5. Water quality analysis
Water quality analysis for each sample included pH, conductivity, dissolved organic carbon (DOC), metals (chromium, iron, copper, zinc, cadmium, nickel) and ions (sodium and magnesium), total phosphate, nitrate, and ammonia. In detail, pH and conductivity were measured with Thermo Scientific Orion probes (Thermo Fisher Scientific, Waltham, MA). The US EPA Method 415.3 was employed to measure dissolved organic carbon (DOC) using a TOC-VCSN analyzer (Shimadzu, Kyoto, JP). Metals and ions were quantified through inductively coupled plasma mass spectrometry (ICP-MS) as previously described.40 Furthermore, total phosphate was determined using the Hach Phosphate Color Disc Test Kit (0–40 ppm PO4), nitrate levels were assessed with the Hach Nitrate Color Disc Test Kit (0–10 ppm NO3–N), and ammonia concentrations were quantified with the Hach TNTplus Vial Test (0.015–2.000 ppm NH3–N).2.6. Statistical analyses
All genes and water quality parameters were measured in technical triplicates from each microcosm, which were completed in experimental triplicates. Error between replicate values was calculated through the standard deviation of the mean, and statistically significant relationships across sampling locations was evaluated with one-way analysis of variance (ANOVA) with the post hoc Tukey's multiple comparisons test. Significant relationships were assessed at a p-value ≤ 0.05 and all analysis were completed using GraphPad Prism 7® (GraphPad Software, La Jolla, CA). Gene relative abundances were determined by dividing the gene's absolute concentration by the 16S rRNA gene absolute concentration.Statistical analyses for metagenomic results were also performed in R (v.4.2.2). The package phyloseq (v.1.42.0) was used to calculate the alpha and beta diversities of the ARG. The metric utilized for alpha diversity was the Shannon diversity index. Alpha diversity analysis was completed to measure the diversity in each sample individually, accounting for the number of different ARGs and their relative abundance. The metric utilized for beta diversity was the Bray–Curtis dissimilarity index. Beta diversity analysis was completed to comparatively analyze the number and relative abundance of ARGs in one sample versus another sample to determine the degree of similarity. Principle coordinate analysis (PCoA) was further used to ordinate and plot the beta diversity dissimilarity distances. To assess statistical significance between samples, an ANOVA test with the Tukey's post hoc multiple comparison test was applied for alpha diversity and the permutational multivariate analysis of variance (PERMANOVA) (999 permutations) test adonis2 (vegan package v.2.6.4) was applied to the Bray–Curtis distance matrices. Both were assessed at a significance level of p-values ≤ 0.05.
3. Results and discussion
3.1. Stormwater contains a more diverse and distinct ARG profile than surface water: grab sample comparison
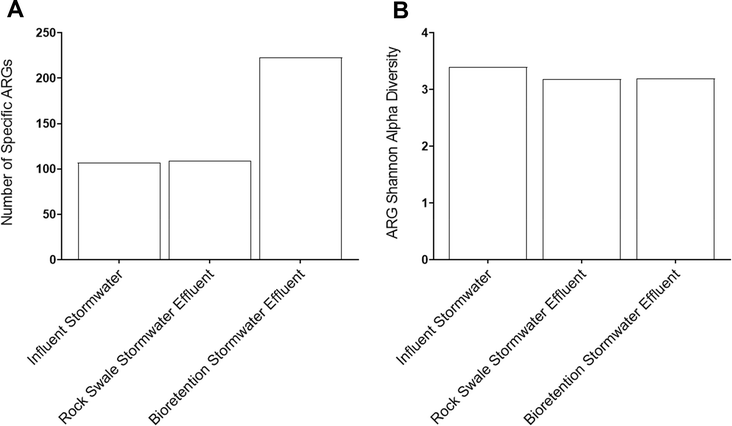
Diversity analyses were completed on individual samples to define the initial condition of the stormwater and surface water collected in this work, prior to mixing. First, the number of different ARGs identified in the metagenomic datasets were quantified (Fig. 1A). Only the mixed land use stormwater sample had a greater number of uniquely identified ARGs in comparison to the surface water sample. The mixed land use was followed by the residential site in terms of the number of uniquely identified ARGs, and then the urban site had the least. Despite the lower number of uniquely identified ARGs in the mixed and residential land use stormwater samples in comparison to the surface water sample, the shared proportion of ARGs between the surface water and all stormwater samples was relatively low (Fig. S3†). This finding carries implications for the potential unique composition that is created when stormwater is transported into downstream surface waters. Moreover, as the ARGs introduced by stormwater into surface water are new and unique, further risks arise for the development of new antibiotic resistant bacteria (ARB) and resistance mechanisms from the potential exchange and mutations of ARGs among the microbial communities in aquatic environments.41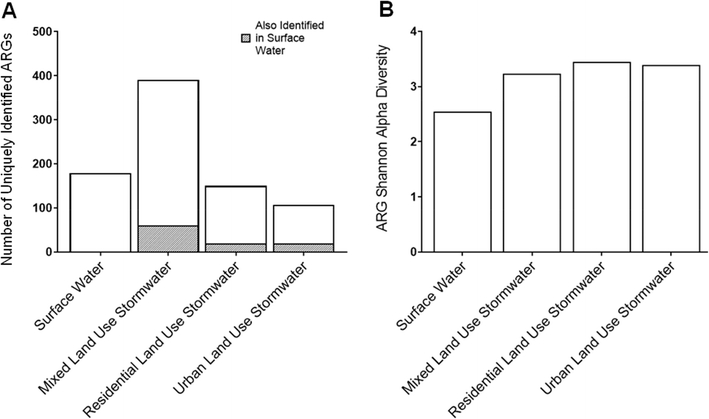 | ||
| Fig. 1 (A) The number of uniquely identified ARGs identified in surface water and stormwater collected from a mixed, residential, and urban land use area and (B) Shannon alpha diversity indices. | ||
Alpha diversity analysis indicates that stormwater harbors a greater diversity of ARGs in comparison to surface water (Fig. 1B). All stormwater samples exhibited similar diversity values, with the diversity index ranging from 3.19 to 3.45. Though the difference was minimal, the residential stormwater sample had the highest diversity, followed by the urban stormwater, and then the mixed land use stormwater. The higher diversity of ARGs in residential areas could have been the result of a multitude of sources, such as pet waste left on surfaces and lawns, as well as fecal matter contamination when transported through the storm sewer network from possible cross contamination with wastewater sources such as leakages in broken pipes or joints.23,42,43 Furthermore, the residential stormwater sample location had the largest drainage area, indicating that ARGs accumulated from a broad geographical region. In addition, the alpha diversity of the resistomes in the residential and urban land uses samples were greater than the diversity of the resistome in the surface water, even though the surface water had a higher number of unique ARGs (e.g., richness). This contrasting trend suggests that the higher diversity in the stormwater samples from the residential and urban areas was driven primarily by a greater evenness of ARGs in the resistomes.
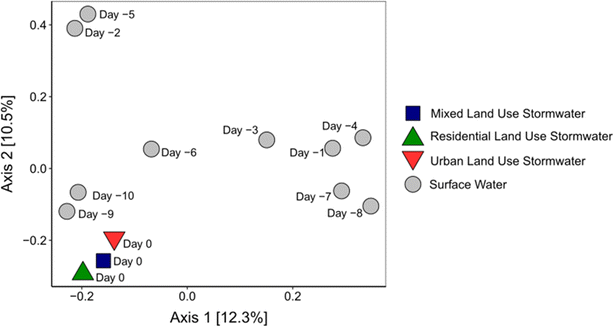
Beta diversity analysis – comparing the composition of ARGs identified in each sample – revealed statistically significant differences (PERMANOVA, p < 0.05) in the mixed, residential, and urban land use stormwater grab samples relative to surface water (Fig. 2). As indicated in Fig. 2, there are notably significant variations between the ARG profiles of the surface water samples collected over a period of 10 days and those of stormwater collected on Day 0. Conversely, when comparing the diversity of ARGs across the stormwater samples themselves, there is minimal difference, suggesting a relatively stable ARG composition in stormwater collected from this rainfall event. The findings of this study contradict prior research, such as the studies conducted by Ahmed et al., 2018 and X. Zuo et al., 2022, which identified varying concentrations of ARGs in stormwater based on different land uses. Moreover, ARG diversity has also been found to vary by land use in other environments, including soil and surface water environments.44–46 As such, it is likely that quantitative analysis of a limited set of ARGs may not be sufficient in drawing broad conclusions about the nature of ARGs in stormwater runoff, and also that stormwater might maintain a more uniform profile of ARGs in comparison to other environments. This conclusion is supported by previous research which found that land use had less of an impact on ARG diversity in surface water during the rainy season in comparison to the dry season. This was the result of stormwater's ability to dilute physicochemical parameters that are land use specific and can indirectly affect microbial activity and ARG diversity.47 This work, however, only represents one stormwater event and a relatively small spatial scale. Therefore, future work should aim to assess these results over a larger temporal scale and include additional land uses (e.g., agriculture, industrial, and undisturbed). Importantly, the beta diversity analysis, when paired with the alpha diversity results, indicates that the ARGs in stormwater collected from different land uses are similar in composition and represent a similar level of diversity.
Interestingly, the diversity of ARGs in the surface water samples collected across 10 days varied significantly (Fig. S4†). A hierarchical clustering analysis was completed to determine which samples were most similar in terms of ARG diversity (Fig. S5†) and no trend was observed based on time. A statistical difference in ARG diversity was however observed between samples collected 1, 3, 4, 7, and 8 days prior to the rain event and samples collected 2, 5, 6, 9, and 10 days prior to the rain event (PERMANOVA, p = 0.005). Consequently, other factors, such as pollution sources (e.g., fecal contamination, surface sediments, or biofilms) or upstream dynamics, may be leading to the substantial difference in ARG diversity.48 To the authors' knowledge, no other study has sequenced a surface water's resistome daily during a dry period. Seasonal and monthly sampling has been conducted, revealing variations in ARG concentrations and diversity on account of differences in water quality and rainfall conditions.8,49–51 Further work will be needed to confirm what factors are influencing the diversity of ARGs in surface water over a daily period.
3.2. Stormwater collected from varying land uses have a distinct impact on the resistome of surface water: microcosm experiments
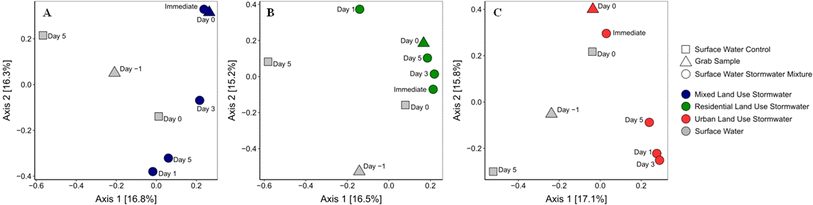
Despite the similarities in ARG composition in the original stormwater samples, the different stormwater had distinct impacts on surface water (Fig. 3). After collecting the stormwater, the samples were mixed with surface water to monitor the impact on the resistome. The mixed land use stormwater had an immediate impact on the diversity of ARGs in the surface water, as the sample collected immediately after mixing had a similar diversity of ARGs to the stormwater day 0 sample, that was statistically different (PERMANOVA, p < 0.05) from the surface water samples (grab and control). The significant change in the surface water's resistome diversity was not maintained throughout the 5 day experimental period as no statistical difference was observed in the ARG diversity in the surface water grab and controls samples from the mixed surface–stormwater samples (days 1, 3, and 5) (PERMANOVA, p > 0.05). Residential stormwater on the other hand had an immediate and steady impact on the composition of ARGs in surface water. Statistically, a significant difference (PERMANOVA, p < 0.05) in ARG diversity was found between the surface water samples (grab and controls) and the residential stormwater impacted surface water (immediate, day 1, 3, and 5). Finally, the urban land use did not have an immediate impact on the diversity of ARGs in surface water, p > 0.05 between surface water (grab and control) and the urban stormwater grab sample (day 0) and immediately mixed sample. However, a statistical difference (PERMANOVA, p < 0.05) was found between the surface water (grab and control) and the mixed surface–stormwater samples (days 1, 3, and 5). In addition, as can be observed in Fig. 3, the beta diversity of the surface water did vary throughout the 5 day experiment. All statistical analyses were thus performed with all surface water control samples (days 0, −1, and 5) to incorporate this variability into the analyses.ARG concentrations have previously been shown to increase in downstream receiving environments after rainfall events but return to a baseline condition shortly after;27,52,53 this is the first instance in which the diversity of ARGs in surface water was monitored for their response to stormwater from various land uses. The implications of these results are that stormwater from residential and urban areas could be having a lasting impact on the resistome of surface water, altering the profile of ARGs for some time after a stormwater event. The stormwater from the mixed-land use, while impacting the diversity of ARGs in surface water immediately, did not have an impact that lasted beyond 5 days. Further research will be needed to determine how these results apply to real-world environmental systems. This includes sampling and evaluating the duration of the impact of residential and urban stormwater in situ, and assessing whether the resistome ultimately returns to a pre-rain state or if the community is permanently altered.
3.3. The role of GSI in shifting the concentration and diversity of ARGs in stormwater and in altering the impact of stormwater on surface water
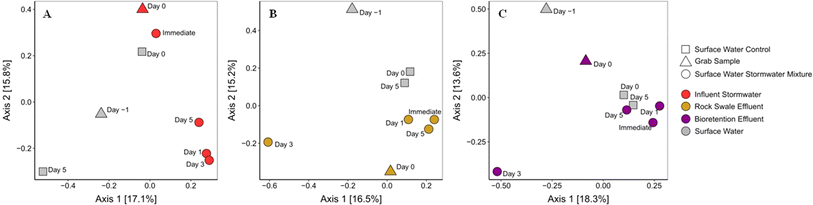
Through the microcosm experiments conducted, we observed that stormwater can significantly alter the resistome of surface water in the short-term aftermath of rainfall events. Given the increasing implementation of GSI in urban settings for managing stormwater volume and quality, it is crucial to investigate its potential in mitigating such impacts.54,55 Thus, in this work, a bioretention cell was sampled to analyze the capability of GSI to remove ARGs and change the resistome. In addition, the stormwater effluent of GSI was similarly mixed with surface water to determine if the impact of stormwater on surface water can be lessened by passing the stormwater through GSI.Metagenomic ARG annotation has revealed that, within the bioretention cell, the fate of ARGs includes both removal (ARGs in influent and not in effluent) and release (ARGs in effluent and not in influent). Removal is likely due to soil adsorption, whereas release might stem from factors like surface competition and desorption, findings consistent with observations related to other bacterial contaminants in GSI such as E. coli and fecal indicator bacteria.59–62 It is also a possibility that these ARGs, which are subject to mobilization from the soil, may have been formed within the GSI, potentially due to the presence of metals or other selective pressures in the soil. This suggests that ARGs don't solely originate from stormwater inputs but could also be locally generated within the GSI environment. Crucially, when mixed with surface water, the effluent from the GSI system had less of a prolonged impact compared to the influent. Consequently, even though there is mobilization within the bioretention cell and a subsequent rise in the number of ARGs detected in the effluent stormwater, the ARGs are not causing substantial effects on the downstream surface waters. To explain this result, the relative abundance of the ARGs identified was calculated as RPKM (Fig. S9†). The relative abundance notably declined from 8460 RPKM in the influent to 5608 RPKM in the effluent. This decrease could potentially explain the limited change in ARG diversity in the surface water after its mixing with the effluent stormwater. Such findings underscore the potential of GSI systems in mitigating the proliferation of ARB and highlight their role in safeguarding public health. The implications of these results suggest that optimizing GSI designs and practices could further enhance their efficacy in ensuring water quality, thereby reinforcing their value in sustainable stormwater management.
4. Conclusion
In unveiling the dynamics of ARGs in stormwater, this work sought to specifically explore the impact of stormwater's resistome on the resistome of urban surface water. Initially, stormwater exhibited a richer and more diverse profile of ARGs than surface water, suggesting that the introduction of stormwater into downstream surface waters can result in a unique composition of ARGs, posing potential risks for the emergence of ARB. Furthermore, the ARG profile in stormwater varied in the number of unique ARGs based on land use types. However, the diversity of ARGs in the stormwater samples did not vary across the residential, mixed, and urban land uses, highlighting that this geographical factor had minimal impact on shaping ARG diversity in stormwater runoff.Stormwater originating from different land uses did lead to distinct impacts on the resistome of surface water. Interestingly, while stormwater from mixed land use areas had an immediate impact on the resistome of surface water, its influence did not last beyond its initial impact on day 0. In contrast, stormwater from residential and urban areas had a more lasting impact on the resistome of surface waters, suggesting potential long-term consequences for aquatic ecosystems. This observation only reflects one stormwater event however, and thus further research is necessary to ascertain the duration and extent of this impact, and whether these changes have a long-lasting effect on surface water's resistome.
Additionally, this study delved into the role of GSI in modulating the diversity and concentration of ARGs in stormwater runoff. Results indicated that, within the GSI system monitored, the rock swale process promoted the dissemination of ARGs while the bioretention cell reduced ARG concentrations, and through the entire system the resistome of stormwater was significantly altered. Importantly, the shifts in the resistome observed between the influent and the effluent of the GSI system led to a lessened impact on the resistome of surface water. This result suggests that bioretention cells can be effective in removing ARGs from the environment and the observed modifications in the resistome profiles indicate a crucial role for GSI in limiting the dissemination of diverse ARGs into aquatic ecosystems.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship [grant number 2041851]. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. This work was also supported by the Lafferty Family Charitable Foundation. The assistance of graduate researcher Veronika Folvarska and undergraduate researchers Maya Adelgren, Laura Johnson, Maya Martinez-Bates, and Sasha Razin is also greatly appreciated.References
- United Nations Environment Programme, Bracing for Superbugs: Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance, 2023 Search PubMed.
- R. L. Finley, P. Collignon, D. G. J. Larsson, S. A. Mcewen, X.-Z. Li, W. H. Gaze, R. Reid-Smith, M. Timinouni, D. W. Graham and E. Topp, Clin. Infect. Dis., 2013, 57, 704–710 CrossRef PubMed.
- G. D. Wright, Curr. Opin. Microbiol., 2010, 13, 589–594 CAS.
- H. K. Allen, J. Donato, H. H. Wang, K. A. Cloud-Hansen, J. Davies and J. Handelsman, Nat. Rev. Microbiol., 2010, 8, 251–259 CAS.
- K. O'Malley, W. McDonald and P. McNamara, Environ. Sci., 2023, 9, 2188–2212 Search PubMed.
- E. Garner, R. Benitez, E. von Wagoner, R. Sawyer, E. Schaberg, W. C. Hession, L. A. H. Krometis, B. D. Badgley and A. Pruden, Water Res., 2017, 123, 144–152 CrossRef CAS PubMed.
- S. Lee, M. Suits, D. Wituszynski, R. Winston, J. Martin and J. Lee, Sci. Total Environ., 2020, 723, 138033 CrossRef CAS PubMed.
- K. O'Malley, P. J. McNamara and W. M. McDonald, Environ. Sci.: Adv., 2022, 1, 380–390 Search PubMed.
- D. Baral, B. I. Dvorak, D. Admiraal, S. Jia, C. Zhang and X. Li, Environ. Sci. Technol., 2018, 52, 9033–9044 CrossRef CAS PubMed.
- S. Zhang, S. Pang, P. F. Wang, C. Wang, N. Han, B. Liu, B. Han, Y. Li and K. Anim-Larbi, Environ. Sci. Pollut. Res., 2016, 23, 9984–9992 CAS.
- K. O'Malley, W. McDonald and P. McNamara, J. Environ. Eng., 2022, 148, 04022017 CrossRef.
- X. Zuo, P. Suo, Y. Li and Q. Xu, Environ. Pollut., 2022, 292, 118470 CAS.
- S. Saifur and C. M. Gardner, Water Sci. Technol., 2021, 83, 2863–2885 CrossRef CAS PubMed.
- L. Hou, J. Li, H. Wang, Q. Chen, J. Q. Su, M. Gad, W. Ahmed, C. P. Yu and A. Hu, Environ. Int., 2022, 168, 107457 CrossRef CAS PubMed.
- K. A. Hamilton, E. Garner, S. Joshi, W. Ahmed, N. Ashbolt, G. Medema and A. Pruden, Curr. Opin. Environ. Sci. Health., 2020, 16, 101–112 CrossRef.
- S. Lee, M. Suits, D. Wituszynski, R. Winston, J. Martin and J. Lee, Sci. Total Environ., 2020, 723, 138033 CrossRef CAS.
- M. Deeb, P. M. Groffman, J. L. Joyner, G. Lozefski, A. Paltseva, B. Lin, K. Mania, D. L. Cao, J. McLaughlin, T. Muth, B. Prithiviraj, J. Kerwin and Z. Cheng, Ecol. Eng., 2018, 125, 68–75 Search PubMed.
- B. Bodus, K. O. Malley, G. Dieter, C. Gunawardana and W. Mcdonald, Sci. Total Environ., 2024, 906, 167195 CrossRef CAS.
- M. B. Rugh, S. B. Grant, W.-C. Hung, J. A. Jay, E. A. Parker, M. Feraud, D. Li, S. Avasarala, P. A. Holden, H. Liu, M. A. Rippy, L. C. Van De Werfhorst, T. Kefela, J. Peng, S. Shao, K. E. Graham, A. B. Boehm, S. Choi, S. K. Mohanty and Y. Cao, Water Res., 2022, 219, 118525 CAS.
- W. Ahmed, K. Hamilton, S. Toze, S. Cook and D. Page, Sci. Total Environ., 2019, 692, 1304–1321 CrossRef CAS.
- H. J. Beck and G. F. Birch, Water, Air, Soil Pollut., 2012, 223, 1005–1015 CrossRef CAS.
- A. Selvakumar and M. Borst, J. Water Health, 2006, 4, 109–124 CrossRef.
- W. Ahmed, Q. Zhang, A. Lobos, J. Senkbeil, M. J. Sadowsky, V. J. Harwood, N. Saeidi, O. Marinoni and S. Ishii, Environ. Int., 2018, 116, 308–318 CrossRef CAS.
- J. Bengtsson-Palme, E. Kristiansson and D. G. J. Larsson, FEMS Microbiol. Rev., 2018, 42, 68–80 CrossRef CAS PubMed.
- S. Sengupta, M. K. Chattopadhyay and H. P. Grossart, Front. Microbiol., 2013, 4, 1–13 Search PubMed.
- D. of C. Development, Land Use, 2016 Search PubMed.
- N. L. R. Williams, N. Siboni, S. L. McLellan, J. Potts, P. Scanes, C. Johnson, M. James, V. McCann and J. R. Seymour, Environ. Pollut., 2022, 307, 119456 CrossRef CAS PubMed.
- L. K. Kimbell, E. Lou Lamartina, S. Kohls, Y. Wang, R. J. Newton and P. J. Mcnamara, mSphere, 2023, 8(5), e00307 CrossRef PubMed.
- K. R. Harrison, A. D. Kappell and P. J. Mcnamara, Environ. Pollut., 2020, 257, 113472 CrossRef CAS.
- K. O'Malley, P. McNamara and W. McDonald, J. Water Health, 2021, 19, 885–894 CrossRef.
- L. K. Kimbell, E. Lou Lamartina, A. D. Kappell, J. Huo, Y. Wang, R. J. Newton and P. J. Mcnamara, Environ. Sci.: Water Res. Technol., 2021, 7, 584 RSC.
- A. D. Kappell, L. K. Kimbell, M. D. Seib, D. E. Carey, M. J. Choi, T. Kalayil, M. Fujimoto, D. H. Zitomer and P. J. McNamara, Environ. Sci.: Water Res. Technol., 2018, 4, 1783–1793 CAS.
- L. K. Kimbell, A. D. Kappell and P. J. McNamara, Environ. Sci.: Water Res. Technol., 2018, 4, 1807–1818 RSC.
- A. M. Bolger, M. Lohse and B. Usadel, Bioinformatics, 2014, 30, 2114–2120 CrossRef CAS PubMed.
- A. Blanco-Míguez, F. Beghini, F. Cumbo, L. J. McIver, K. N. Thompson, M. Zolfo, P. Manghi, L. Dubois, K. D. Huang, A. M. Thomas, W. A. Nickols, G. Piccinno, E. Piperni, M. Punčochář, M. Valles-Colomer, A. Tett, F. Giordano, R. Davies, J. Wolf, S. E. Berry, T. D. Spector, E. A. Franzosa, E. Pasolli, F. Asnicar, C. Huttenhower and N. Segata, Nat. Biotechnol., 2023, 41, 1633–1644 CrossRef.
- S. Nurk, D. Meleshko, A. Korobeynikov and P. A. Pevzner, Genome Res., 2017, 27, 824–834 CrossRef CAS PubMed.
- B. P. Alcock, A. R. Raphenya, T. T. Y. Lau, K. K. Tsang, M. Bouchard, A. Edalatmand, W. Huynh, A. L. V. Nguyen, A. A. Cheng, S. Liu, S. Y. Min, A. Miroshnichenko, H. K. Tran, R. E. Werfalli, J. A. Nasir, M. Oloni, D. J. Speicher, A. Florescu, B. Singh, M. Faltyn, A. Hernandez-Koutoucheva, A. N. Sharma, E. Bordeleau, A. C. Pawlowski, H. L. Zubyk, D. Dooley, E. Griffiths, F. Maguire, G. L. Winsor, R. G. Beiko, F. S. L. Brinkman, W. W. L. Hsiao, G. V. Domselaar and A. G. McArthur, Nucleic Acids Res., 2020, 48, D517–D525 CrossRef CAS PubMed.
- N. L. Bray, H. Pimentel, P. Melsted and L. Pachter, Nat. Biotechnol., 2016, 34, 525–527 CrossRef CAS PubMed.
- K. O’Malley, P. McNamara, C. W. Marshall, E. Lou LaMartina, T. Lam, N. Ali and W. McDonald, J. Hazard. Mater., 2024, 469, 133923 Search PubMed.
- E. K. Maher, K. N. O’Malley, M. E. Dollhopf, B. K. Mayer and P. J. McNamara, Environ. Eng. Sci., 2020, 37(2), 99–108 CrossRef CAS.
- H. Sanderson, C. Fricker, R. S. Brown, A. Majury and S. N. Liss, Environ. Rev., 2016, 24, 205–218 CrossRef.
- S. L. McLellan, E. J. Hollis, M. M. Depas, M. Van Dyke, J. Harris and C. O. Scopel, J. Great Lake. Res., 2007, 33, 566–580 CrossRef.
- A. K. Salmore, E. J. Hollis and S. L. McLellan, J. Water Health, 2006, 4, 247–262 CrossRef CAS.
- J. Wu, S. Guo, H. Lin, K. Li, Z. Li, J. Wang, W. H. Gaze and J. Zou, J. Environ. Manage., 2023, 344, 118920 CrossRef PubMed.
- P. A. Fernanda, S. Liu, T. Yuan, B. Ramalingam, J. Lu and R. Sekar, Front. Microbiol., 2022, 13, 1–17 Search PubMed.
- C. E. Sanderson, J. T. Fox, E. R. Dougherty, A. D. S. Cameron and K. A. Alexander, Front. Microbiol., 2018, 9, 1–13 CrossRef.
- L. Zhang, L. Ji, X. Liu, X. Zhu, K. Ning and Z. Wang, Water Res., 2022, 215, 118279 CrossRef CAS PubMed.
- G. Reichert, S. Hilgert, J. Alexander, J. C. Rodrigues de Azevedo, T. Morck, S. Fuchs and T. Schwartz, Sci. Total Environ., 2021, 768, 144526 CrossRef CAS.
- H. Jiang, R. Zhou, M. Zhang, Z. Cheng, J. Li, G. Zhang, B. Chen, S. Zou and Y. Yang, Ecotoxicol. Environ. Saf., 2018, 161, 64–69 CrossRef CAS PubMed.
- I. Herrig, S. Fleischmann, J. Regnery, J. Wesp, G. Reifferscheid and W. Manz, PLoS One, 2020, 15(4), e0232289 CrossRef CAS PubMed.
- C. Dang, Y. Xia, M. Zheng, T. Liu, W. Liu, Q. Chen and J. Ni, Environ. Int., 2020, 136, 105449 CrossRef CAS PubMed.
- R. L. Carney, M. Labbate, N. Siboni, K. A. Tagg, S. M. Mitrovic and J. R. Seymour, Water Res., 2019, 167, 115081 CrossRef CAS PubMed.
- A. Di Cesare, E. M. Eckert, M. Rogora and G. Corno, Environ. Pollut., 2017, 226, 473–478 CrossRef CAS PubMed.
- A. R. McFarland, L. Larsen, K. Yeshitela, A. N. Engida and N. G. Love, Environ. Sci.: Water Res. Technol., 2019, 5, 643–659 CAS.
- M. Keeley, A. Koburger, D. P. Dolowitz, D. Medearis, D. Nickel and W. Shuster, Environ. Manag., 2013, 51, 1093–1108 CrossRef.
- S. A. Ekka, H. Rujner, G. Leonhardt, G. T. Blecken, M. Viklander and W. F. Hunt, J. Environ. Manage., 2021, 279, 111756 CrossRef CAS.
- X. Zuo, Q. Xu, Y. Li and K. Zhang, J. Hazard. Mater., 2022, 429, 128336 CrossRef CAS PubMed.
- L. Poirel, J. D. Pitout and P. Nordmann, Future Microbiol., 2007, 2, 501–512 CrossRef CAS PubMed.
- S. K. Mohanty, A. A. Torkelson, H. Dodd, K. L. Nelson and A. B. Boehm, Environ. Sci. Technol., 2013, 47, 10791–10798 CrossRef CAS.
- B. P. Kranner, A. R. M. Nabiul Afrooz, N. J. M. Fitzgerald and A. B. Boehm, PLoS One, 2019, 14, 1–23 CrossRef.
- S. K. Mohanty, K. B. Cantrell, K. L. Nelson and A. B. Boehm, Water Res., 2014, 61, 288–296 CrossRef CAS PubMed.
- P. Shen, A. Deletic, C. Urich, G. I. Chandrasena and D. T. McCarthy, Sci. Total Environ., 2018, 630, 992–1002 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00111g |
| This journal is © The Royal Society of Chemistry 2024 |