Open Access Article
Open Access ArticleStabilization of the surface and lattice structure for LiNi0.83Co0.12Mn0.05O2via B2O3 atomic layer deposition and post-annealing†
Jiawei
Li
 ,
Junren
Xiang
,
Ge
Yi
,
Zhijia
Hu
,
Xiao
Liu
,
Junren
Xiang
,
Ge
Yi
,
Zhijia
Hu
,
Xiao
Liu
 * and
Rong
Chen
* and
Rong
Chen
 *
*
State Key Laboratory of Intelligent Manufacturing Equipment and Technology, School of Mechanical Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, Hubei, People's Republic of China. E-mail: rongchen@mail.hust.edu.cn; xiaoliu@hust.edu.cn
First published on 22nd May 2024
Abstract
The Ni-rich LiNixCoyMn1−x−yO2 cathode (x ≥ 0.6) shows weak rate capability due to its deleterious surface lithium impurities and lattice defects. Herein, uniform ultrathin B2O3 coatings built by atomic layer deposition (ALD) are utilized to construct a B3+ doped single-crystal LiNi0.83Co0.12Mn0.05O2 (SC83) via post-annealing. LiOH is consumed due to reacting with B2O3 during the B2O3 ALD process, and then B2O3 is transformed into B3+ doping accompanied by the reduction of Li2CO3 during the post-annealing. Surface and bulk characterization results show that B3+ tends to diffuse into the bulk of the SC83 during the post-annealing, which expands the a and c axes and reduces the Li+/Ni2+ mixing of the SC83. When the B3+ content exceeds 0.54 wt%, B3+ segregation occurs on the surface of the SC83, which decreases the electronic conductivity of the SC83. B3+ doping at the content of 0.54 wt% gives the highest capacity of 177.6 mA h g−1 at 1C rate. The B2O3 ALD coupled with post-annealing builds a highly electronic and Li+ conductive surface and bulk for the SC83, which is the key to the improvement of the rate capability.
Introduction
Lithium-ion batteries with high charge speed are important for shortening the charge time of electric vehicles. The Ni-rich LiNixCoyMn1−x−yO2 cathode (NCM, x ≥ 0.6), with the advantages of high energy density, low cost, and environmental sustainability, has attracted much attention.1–3 However, the poor surface and crystal structure of the Ni-rich cathode work against the transfer of Li+ in the surface and bulk. Resistive surface lithium impurities (LiOH and Li2CO3), Li+/Ni2+ mixing, transition metal dissolutions, lattice shrinkage, and intergranular cracking all harm the rate capability of NCM.4,5 Benefitting from the good mechanical properties, single-crystal NCM shows excellent processability in the manufacture of batteries, which increases its usage in the industry. However, the large grain size of single-crystal NCM decreases its rate capability due to the longer pathways for Li+ transfer in the bulk.6,7 Hence, it is extraordinarily significant to enhance the Li+ diffusivity in single-crystal NCM for achieving the rapid charging of lithium-ion batteries.Reactive coating is regarded as an effective approach to improve the Li+ diffusivity in NCM. Co3(PO4)2, H3PO4, (NH4)2HPO4, H2C2O4, and Prussian blue have been used to transform surface lithium impurities into highly Li+ diffusive coatings.8–13 Although these methods are promising for enhancing Li+ diffusivity on the surface, they can only give a limited boost to the rate capability of single-crystal NCM, due to the low Li+ diffusivity in the bulk. Doping is an effective method for optimizing the bulk property of NCM via modifying the crystal structure.14 Various dopants, such as Na+, F−, Ce3+, Zn2+, and Mg2+, have been incorporated into the lattice of NCM.15–20 These dopants effectively improve the rate capability of NCM by enhancing the diffusivity of Li+ in the bulk. Simultaneously doping and reducing surface lithium impurities has recently been reported by coating and post-annealing as a promising method. Co3+, Al3+, Fe3+, and Ti4+ doped NCM are achieved via coating combined with post-annealing.21–23 The surface lithium impurities of all the doped cathodes are reduced, while their rate capabilities also declined. This is attributed to these large ions hardly diffusing into the bulk. They tend to segregate on the surface, which blocks the transfer of electrons and Li+. As an acidic oxide, B2O3 can react with alkaline surface lithium impurities. Moreover, with the advantages of small size but strong B–O bonds, B3+ is more likely to diffuse into the NCM from the surface and stabilize the lattice. B3+ doped NCM prepared by B2O3 coating and post-annealing have recently been reported.24–27 Although B3+ can diffuse into the bulk of NCM, B3+ segregation on the surface also occurs.28,29 B3+ segregation reduced the electronic conductivity of NCM, which is against the rate capability of NCM.29,30 Moreover, B3+ segregation induces the surface phase transformation of NCM from layered to rock-salt-like, which is detrimental to the Li+ transfer on the surface.31 Atomic layer deposition (ALD), with the virtue of sufficient gas–solid contacts and self-limiting reactions, can produce uniform ultrathin coatings with precise thickness control.32–36 It is a potential method to investigate the origin of B3+ segregation on the surface of NCM.
Here, we investigate the effect of reactive B2O3 coatings and B3+ doping content on the SC83 via B2O3 ALD coupled with post-annealing. The ALD growth characterization shows that B2O3 grows faster on the surface of LiOH. The surface characterization indicates that the surface LiOH reacts with B2O3 and forms LiBOx during the B2O3 ALD process. By performing the post-annealing treatment, the surface Li2CO3 is reduced, and B3+ diffuses into the bulk of the SC83. Moreover, the a and c axes of the SC83 expand, and the Li+/Ni2+ mixing of the SC83 decreases, which is influenced by the content of the B3+ bulk doping. B3+ surface segregation occurs after it saturates in the bulk of the SC83. The saturated B3+ bulk doping with virtually no B3+ surface segregation gives the highest improvement in the rate capability of the SC83, which results in enhanced electronic conductivity and Li+ diffusivity of the SC83.
Experimental section
Material preparation
The SC83 was synthesized by a high-temperature calcination method. LiOH·H2O (Shanghai Aladdin Biochemical Technology Co., Ltd) and Ni0.83Co0.12Mn0.05(OH)2 (Ningbo Gaosi New Energy Technology Co., Ltd) powders with the molar ratio of 1.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were mixed using a ball-milling method. Subsequently, the mixture was pre-heated at 500 °C for 5 h, then calcined at 850 °C for 12 h under an oxygen atmosphere. Finally, the calcination products are milled into powders to obtain the SC83 materials.
1 were mixed using a ball-milling method. Subsequently, the mixture was pre-heated at 500 °C for 5 h, then calcined at 850 °C for 12 h under an oxygen atmosphere. Finally, the calcination products are milled into powders to obtain the SC83 materials.
B2O3 coatings were coated on the SC83 particles using a homemade rotary ALD reactor. B2O3 ALD was performed at 120 °C using trimethyl borate (TMB, Shanghai Aladdin Biochemical Technology Co., Ltd) and H2O as precursors. In a typical B2O3 ALD cycle, 10 s TMB pulse, 60 s exposure time, 60 s N2 purge, 3 s H2O pulse, 60 s exposure time, and 60 s N2 purge are alternately operated. The SC83 particles were coated with B2O3 in various ALD cycles. For instance, 0, 2, 6, and 12 ALD cycles of B2O3 on the SC83 particles were marked as bare, 2B, 6B, and 12B SC83 particles. After the B2O3 ALD process, post-annealing was performed at 500 °C in an oxygen atmosphere for 3 h; the particles were marked as 2B-A, 6B-A, and 12B-A SC83 particles.
Material characterization
The concentration of B was measured via an Avio 220 Max (PerkinElmer) inductively coupled plasma-optical emission spectrometer (ICP-OES). The mass uptake during the B2O3 ALD process was measured by an SQM-160 (Inficon) quartz crystal microbalance (QCM). For QCM measurement, SC83 particles or Al2O3 are loaded on the QCM sensors. The by-products during the B2O3 ALD process were monitored by a Dycor LC-D200 (AMETEK) quadruple mass spectrometer (QMS). For QCM and QMS measurements, 1 s TMB pulse, 30 s N2 purge, 1 s H2O pulse, and 30 s N2 purge are alternately operated. The crystalline structure of SC83 was characterized by an X-ray diffractometer (PANalytical X'Pert) with a Cu Ka radiation source. The morphology of SC83 was characterized using a Quanta650 FEG (FEI instrument) scanning electron microscope (SEM) and a Tecnai G2 F20 (FEI instrument) transmission electron microscope (TEM). The surface compositions were detected by an AXIS-ULTRA (Shimadzu-Kratos Co.) X-ray photoelectron spectrometer (XPS). All XPS spectra were aligned by calibrating the C 1s peak at 285 eV. For pH measurements, 5 g of SC83 particles were evenly mixed with 25 ml of deionized water in a beaker to make a slurry. A S210-K pH meter (METTLER TOLEDO) was used to measure the pH of the slurry. For electronic conductivity measurements, SC83 particles were pressed into a mold under a pressure of 20 MPa, then measurements were conducted via a multimeter.Electrochemical measurements
The SC83 cathode slurry was prepared using a N-methyl pyrrolidine (NMP) based slurry containing 80 wt% SC83, 10 wt% carbon black, and 10 wt% polyvinylidene fluoride (PVDF). The SC83 cathode slurry was cast on carbon-coated Al foils, then dried in a vacuum oven at 120 °C overnight. The loading of SC83 was approximately 3 mg cm−2. The electrolyte was 1 M LiPF6 in the solvent containing ethylene carbonate (EC), dimethyl carbonate (DMC), and ethyl methyl carbonate (EMC) with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Microporous polypropylene membranes (Celgard 2400) were used as the separator. Li foils were used as the anode. The coin cells (CR2025) were assembled in an argon-filled glovebox (MB-Unilab). A Land CT3002A battery tester was used to perform the charge–discharge tests with a voltage range from 3.0 to 4.5 V at the desired current density (1C corresponds to 200 mA g−1) in the temperature of 21 °C. The galvanostatic intermittent titration technique (GITT) measurements were carried out on a Land CT3002A battery tester. The coin cells were firstly given a galvanostatic current of 0.1C in 20 minutes, then followed by a relaxation time of 60 minutes. Electrochemical impedance spectra (EIS) were obtained via an Autolab PGSTAT302N electrochemical workstation (Metrohm) over the frequency range from 0.01 Hz to 100 kHz with an amplitude perturbation of ±5 mV.
1. Microporous polypropylene membranes (Celgard 2400) were used as the separator. Li foils were used as the anode. The coin cells (CR2025) were assembled in an argon-filled glovebox (MB-Unilab). A Land CT3002A battery tester was used to perform the charge–discharge tests with a voltage range from 3.0 to 4.5 V at the desired current density (1C corresponds to 200 mA g−1) in the temperature of 21 °C. The galvanostatic intermittent titration technique (GITT) measurements were carried out on a Land CT3002A battery tester. The coin cells were firstly given a galvanostatic current of 0.1C in 20 minutes, then followed by a relaxation time of 60 minutes. Electrochemical impedance spectra (EIS) were obtained via an Autolab PGSTAT302N electrochemical workstation (Metrohm) over the frequency range from 0.01 Hz to 100 kHz with an amplitude perturbation of ±5 mV.
Results and discussion
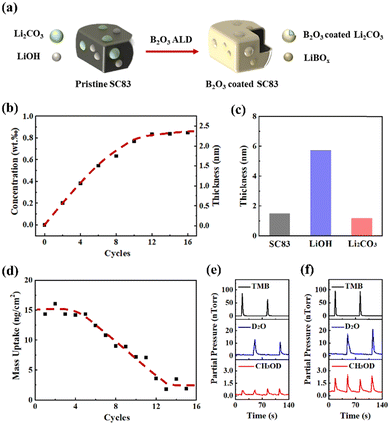
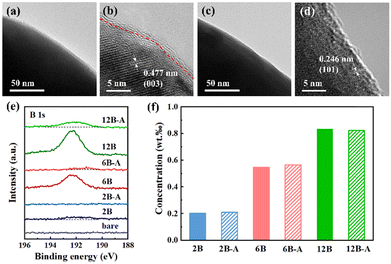
Fig. 1a illustrates the B2O3 ALD process on the SC83 particles. The details are described and analyzed in the following text. ICP-OES is used to measure the B concentration in the B2O3-coated SC83. As shown in Fig. 1b, the B concentrations are linear with the ALD cycles in the first 6 ALD cycles. After 6 ALD cycles, the growth rate of B2O3 gradually decreases. The B2O3 slowly and steadily grows after 12 ALD cycles. Although ALD is regarded as a linear growth process in thin film deposition, actually, the surface compositions of the substrate influence the ALD growth rates in the initial stage.37,38 Surface LiOH and Li2CO3 could induce the variation of the B2O3 growth rates on the SC83 particles. Fig. 1c shows the calculated thicknesses of B2O3 coatings on the SC83, LiOH, and Li2CO3 particles with 6 ALD cycles (the calculation method and relative parameters are shown in the ESI†). The calculated thickness of the B2O3 coatings on the LiOH particles is much higher than the theoretical thickness of 6 B2O3 molecules (approximately 1.2 nm), suggesting subsurface reactions during the B2O3 ALD on LiOH particles. In order to further verify the ALD growth behavior of B2O3 on the SC83 particles, in situ QCM is used to detect the mass change during the B2O3 growth on the SC83 particles. Fig. S1 (ESI†) shows the mass uptake during the B2O3 ALD on the SC83 particles. The mass increases in a stair-like shape after each ALD cycle. Fig. 1d shows the mass uptake in each B2O3 ALD cycle from Fig. S1 (ESI†). It shows that the mass uptake is constant in the initial stage, and then gradually decreases after the 6th ALD cycle. The mass uptake is low and constant after 12 ALD cycles, indicating a low growth rate of B2O3 ALD. These results are consistent with the above ICP-OES results. TMB is easy to chemisorb on the basic surfaces, while it is hard to chemisorb on acidic surfaces, which leads to a gradually decreased growth rate of the B2O3 ALD.34 Fig. S2 (ESI†) shows that the mass uptake from each B2O3 ALD cycle on Al2O3 gradually decreases to a low and constant value, which further indicates the growth behavior of the B2O3 ALD. The difference is that the mass uptake of B2O3 ALD on the SC83 particles exhibits a plateau in the initial stage, which may be attributed to the higher surface basicity of SC83 delaying the growth inhibition of the B2O3 ALD. For evaluating the reaction intensity of the B2O3 ALD on the SC83 and LiOH particles, in situ QMS is used to monitor the concentration of the reactants and byproducts during the B2O3 ALD process. As shown in Fig. 1e, the black and blue peaks indicate the pulse of TMB and D2O during the B2O3 ALD on the SC83 particles. It is found that the peaks of CH3OD occur during the TMB and D2O pulse, which come from the byproducts of the B2O3 ALD reactions. A similar phenomenon is found during the B2O3 ALD on the LiOH particles, as shown in Fig. 1f. The peaks of CH3OD for B2O3 ALD on the LiOH particles are stronger than that on the SC83 particles, which indicates that the reaction intensity of the B2O3 ALD on the LiOH particles is more violent than that on the SC83 particles.The morphology of the bare, 6B, and 6B-A SC83 particles is observed using SEM. As depicted in Fig. S3 (ESI†), the SC83 particles show polygonal shapes ranging from 2 to 3 μm, and their morphology remains after the B2O3 ALD and post-annealing treatment. To examine the B2O3 coatings, TEM is performed to characterize the surface morphology of the 6B and 6B-A SC83 particles. As shown in Fig. 2a, the edges of the 6B SC83 particles are smooth. The surface morphology of the 6B SC83 particles is shown in Fig. 2b using high-resolution TEM (HR-TEM). The measured lattice spacing is 0.477 nm, which is assigned to the (003) plane of the SC83.39,40 The amorphous B2O3 coatings with the thickness of 1.4 nm are observed on the surface of the SC83 particles, which agrees with the calculated thickness derived from the ICP-OES results. B is detected at the surface of the 6B SC83 particles, while the atomic concentration of B in the bulk of the 6B SC83 particles is much lower, which further validates the existence of B2O3 coatings (Fig. S4a, ESI†). Besides, amorphous B2O3 coatings with the thickness of 2.3 nm are observed on the 12B SC83 particles (Fig. S5, ESI†). As shown in Fig. 2c, the edges of the 6B-A SC83 particles are smooth. The surface morphology of the 6B-A SC83 particles is shown in Fig. 2d. The measured lattice spacing is 0.246 nm, which is assigned to the (101) plane of SC83. No coating is observed on the surface of the 6B-A SC83 particles. Besides, the EDS analysis (Fig. S4b, ESI†) also shows that the atomic concentration of B on the surface of the 6B-A SC83 particles is much lower than that of the 6B SC83 particles. The decreased atomic concentration of B could be attributed to B3+ diffusing into the bulk of the SC83 particles after post-annealing. XPS characterization has been performed to investigate the surface composition of the SC83 particles after B2O3 ALD and post-annealing. As shown in Fig. 2e, for 2B SC83 particles, a small and broad B 1s peak occurs around 192.0 eV. It is lower than the B 1s peak of B2O3 at 192.6–193.0 eV,41–43 but higher than the B 1s peak of LiBOx at 190.9–191.8 eV,44–46 which indicates that B2O3 and LiBOx coexist on the surface of the 2B SC83 particles. The formation of LiBOx is attributed to B2O3 ALD on the surface LiOH. Fig. S6 (ESI†) shows that the B 1s peak occurs at 191.5 eV and 192.7 eV for B2O3 coated LiOH and Li2CO3, respectively. This indicates that LiBOx and B2O3 formed on LiOH and Li2CO3, respectively. As the number of ALD cycles increases, the B 1s peak strengthens, indicating the accumulation of B on the surface. Moreover, the B 1s peak gradually becomes sharp and shifts to a higher position, which indicates that the ratio of B2O3 increases in the B2O3/LiBOx hybrid coatings. In the first 2 ALD cycles, much LiBOx forms due to the subsurface reactions between the B2O3 and the LiOH. After the LiOH is gradually consumed, no LiBOx forms in the subsequent ALD cycles, while the B2O3 continuously accumulates. After post-annealing, the B 1s peak decreases, indicating the reduction of the B content on the surface. To investigate the whereabouts of the surface B, ICP-OES was conducted to measure the B content in the SC83 particles. As shown in Fig. 2f, the ICP-OES results show the B content in the SC83 particles before and after post-annealing is almost the same. Combined with the XPS results, this indicates that surface B3+ diffuses into the bulk of SC83 particles during the post-annealing. For 2B-A SC83 particles, no B 1s peak is observed, which indicates that all the surface B3+ diffuses into the bulk. However, as the content of the B2O3 coatings increases, B3+ segregation occurs. For 6B-A SC83 particles, only a very small and broad B 1s peak is observed. Moreover, the B 1s peak occurs at the lower position, which indicates that the B2O3 coatings are transformed into B3+ doping. For 12B-A SC83 particles, the B 1s peak strengthens, which indicates that the B3+ segregation escalates. XPS characterization also demonstrates that the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak intensity of the SC83 particles noteworthily declines after B2O3 ALD and post-annealing, reflecting that the content of the surface Li2CO3 decreases47,48 (Fig. S7, ESI†). The decreased pH of the SC83 slurry after B2O3 ALD and post-annealing further validates the reduction of surface lithium impurities (Fig. S8, ESI†).
O peak intensity of the SC83 particles noteworthily declines after B2O3 ALD and post-annealing, reflecting that the content of the surface Li2CO3 decreases47,48 (Fig. S7, ESI†). The decreased pH of the SC83 slurry after B2O3 ALD and post-annealing further validates the reduction of surface lithium impurities (Fig. S8, ESI†).
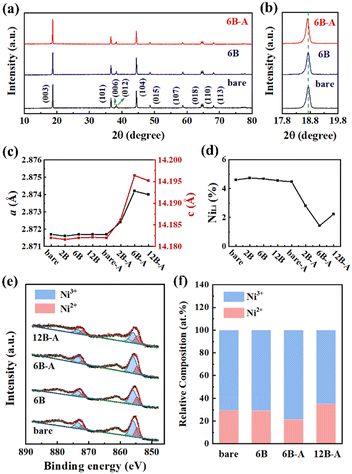
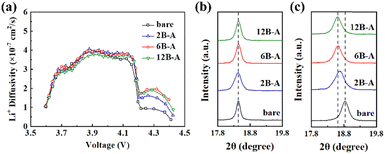
The crystal structures of bare, 6B, and 6B-A SC83 particles are analyzed via XRD. As shown in Fig. 3a, all samples show well-separated (006)/(012) peaks and (108)/(110) peaks, implying a typical α-NaFeO2 layered structure. No extra peaks and no peak shifts are detected in the XRD patterns of the 6B SC83 particles, indicating that the B2O3 ALD process does not change the crystal structure of the SC83. As shown in Fig. 3b, the (003) peak of the 6B-A SC83 particles shifts to the lower position, which reflects the increase of the (003) interplanar spacing. The (003)/(104) ratios of the bare, 6B and 6B-A SC83 particles are 1.37, 1.35 and 1.87, respectively. The B2O3 ALD process barely changes the (003)/(104) ratios, but combined with the post-annealing, the (003)/(104) ratios increase much, which reflects the decrease of the Li+/Ni2+ mixing. The lattice parameters of the bare, B2O3 ALD coated, annealed, and B2O3 ALD coated coupled with annealed SC83 are calculated from the XRD patterns using Rietveld refinement (Fig. S9 and Table S2, ESI†). As shown in Fig. 3c, the a and c axis lengths of the bare, 2B, 6B, 12B, bare-A SC83 are almost the same, which indicates that B2O3 ALD or annealing barely influences the lattice of the SC83. For B3+ doped SC83, the a and c axes expand, which is attributed to the B3+ being likely to occupy the tetrahedral gaps of the packed oxygen in the Li and TM layers, thus expanding the lattice parameters of the SC83.49,50 Moreover, B3+ doping decreases the Li+/Ni2+ mixing of SC83 initially (shown in Fig. 3d), which could be attributed to the B3+ blocking the migration pathway of Ni ions to the Li sites through the tetrahedral sites in the Li layers.51 However, as the content of B3+ doping further increases, the expansion of the a and c axes and the reduction of the Li+/Ni2+ mixing slightly declines for 12B-A SC83 due to the excess B3+ accelerating Ni3+ to Ni2+ transformations for charge compensation.27 Furthermore, XPS analysis is carried out to compare the valence states of surface Ni for the bare, 6B, 6B-A, and 12B-A SC83 particles. As shown in Fig. 3e, two main peaks at 855.5 eV and 873.0 eV can be assigned to Ni 2p3/2 and Ni 2p1/2, which can be split into two pairs of binding energies corresponding to Ni2+ (854.7 eV and 872.2 eV) and Ni3+ (856 eV and 873.5 eV). The relative composition of Ni2+ and Ni3+ is calculated according to the peak area and shown in Fig. 3f. The B2O3 ALD does not change the Ni2+ content on the surface. An obvious reduction of Ni2+ content occurs after post-annealing, which indicates the reduction of the surface Li+/Ni2+ mixing. However, the surface Ni2+ content increases for the 12B-A SC83 particles, which further illustrates that the excess B3+ will induce Li+/Ni2+ mixing.
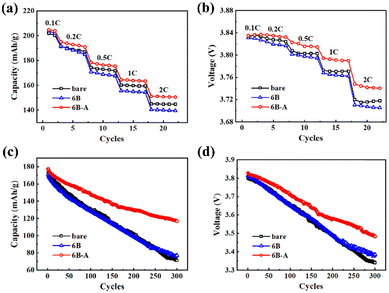
The electrochemical performance of the bare and coated SC83 cathodes was measured. Fig. 4a shows the rate capability of the bare, 6B, and 6B-A SC83 cathodes. As the charge–discharge rate increases, the gaps of the capacity among each cathode become more pronounced, and the 6B-A SC83 cathode gives the highest capacity. Fig. S10a (ESI†) also shows the rate capability of the 2B, 6B, and 12B SC83 cathodes, all of which exhibit lower capacity compared to the bare SC83 cathode. As the B2O3 ALD cycles increase, the rate capability of the SC83 cathodes further decays, which could be attributed to the regressive electronic conductivity of the SC83 particles (Fig. S11, ESI†). Besides, the B2O3 coatings may also block the transfers of Li+. After post-annealing, the rate capability of the B2O3 coated SC83 cathodes is improved (Fig. S10b, ESI†), which could be attributed to the enhanced electronic conductivity of the SC83 particles (Fig. S11, ESI†). Moreover, B2O3 ALD coupled with post-annealing enlarges the a and c axes and reduces the Li+/Ni2+ mixing of SC83, which is beneficial to the Li+ diffusivity. The 6B-A SC83 cathode shows the largest c axis with the lowest Li+/Ni2+ mixing, which is responsible for the best rate capability. Although the 12B-A SC83 cathode shows similar lattice parameters to the 6B-A SC83 cathode, inferior electronic conductivity may limit its capacity release. Fig. 4b shows the mean discharge voltage of the bare, 6B, and 6B-A SC83 cathodes at 0.1–2C rate. As the charge–discharge rate increases, the mean discharge voltage difference among each cathode increases, and the 6B-A SC83 cathode shows the highest mean discharge voltage, indicating the lowest polarizations. A cycling test is performed to evaluate the electrochemical stability of the SC83 cathodes. As shown in Fig. 4c, the capacity of the bare, 6B, and 6B-A SC83 cathodes starts at 173.5, 171.2 and 177.6 mA h g−1 and ends at 71.4, 77.2 and 116.6 mA h g−1, corresponding to the capacity retention of 41.2, 45.1, and 65.7% after 300 charge–discharge cycles. The 6B-A SC83 cathode shows much higher capacity retention than the bare and 6B SC83 cathodes. The cycling stability of the B2O3 coated SC83 cathodes with different ALD cycles is similar and almost the same as the bare SC83 cathode (Fig. S10c, ESI†). This may originate from the insufficient reaction of TMB during the B2O3 ALD inducing defects in the B2O3 coatings, which make it not dense enough to protect the SC83 cathode from the erosion of the electrolyte.34 After post-annealing, the B2O3 coated SC83 cathodes show enhancements in cycling stability (Fig. S10d, ESI†).
The capacity retention of the SC83 cathodes increases as the number of B2O3 ALD cycles increases from 2 to 6. The 12B-A SC83 cathode shows similar capacity retention to the 6B-A SC83 cathode, while the 12B-A SC83 cathode gives lower capacity after cycling due to the lower initial capacity. Fig. 4d shows the mean discharge voltage during cycling. The 6B-A SC83 cathode shows the highest mean discharge voltage from the beginning to the end, and exhibits the slowest voltage decay. Fig. S12 (ESI†) shows the discharge curves of bare, 6B, and 6B-A SC83 cathodes during cycling. The 6B-A SC83 cathode exhibits visible plateaus even after 300 charge–discharge cycles. The capacity release ability of the 6B-A SC83 cathodes is great compared with NCM modified by other methods reported in the literature (Table S3, ESI†). Moreover, the repeated rate capability and cycling stability results of the bare and 6B-A SC83 cathodes show that their capacity release ability is stable (Fig. S13, ESI†). The 6B-A SC83 cathode also shows better storage stability at high temperature compared to the bare SC83 cathode (Fig. S14, ESI†). Besides, the rate capability and cycling stability of the bare-A SC83 cathode are almost the same as those of the bare SC83 cathode, which indicates that the performance improvements should be attributed to the transformations of the B2O3 coatings into B3+ doping during the annealing (Fig. S15, ESI†).
In order to investigate the origin of the enhanced rate capability of the B3+ doped SC83 cathodes, GITT tests were performed to measure the Li+ diffusivity. The GITT curves of the bare and B3+ doped SC83 cathodes (Fig. S16, ESI†) indicate that the polarization of the B3+ doped SC83 cathodes is lower than that of the bare SC83 cathode, especially when the voltage is 4.2 V. Fig. 5a shows the Li+ diffusivity of the bare and B3+ doped SC83 cathodes during the charge process calculated from the GITT measurements. The 2B-A and 6B-A SC83 cathodes show a little higher Li+ diffusivity than the bare and 12B-A SC83 cathodes below 4.2 V, which is attributed to the higher electronic conductivity for the 2B-A and 6B-A SC83 cathodes. As the voltage increases, all curves show a sharp decline. This is attributed to the H2–H3 phase transition of SC83 around 4.2 V leading to serious shrinkage of the c axis, which reduces the Li+ diffusivity. It is noteworthy that the Li+ diffusivity of the B3+ doped SC83 cathodes is much higher than that of the bare SC83 cathode upon 4.2 V. The Li+ diffusivity of the 6B-A and 12B-A SC83 cathodes is similar, while both are higher than that of the 2B-A SC83 cathode. The similar phenomenon is also observed during the discharge process (Fig. S17, ESI†). The highest Li+ diffusivity of the 6B-A SC83 cathode is responsible for the highest rate capability of it. XRD measurements are performed to measure the c axis length of the SC83 cathodes below and upon 4.2 V (Fig. S18, ESI†). As shown in Fig. 5b, all cathodes show the same position of the (003) peak at 4.1 V, which indicates that the c axis length among them is the same. As the voltage increases to 4.4 V, the (003) peaks of all the SC83 cathodes shift to higher positions at 4.4 V, while the (003) peak of the B3+ doped SC83 cathode shows much fewer shifts than that of the bare SC83 cathode (Fig. 5c). The 6B-A and 12B-A SC83 cathodes show almost the same position of the (003) peaks at 4.4 V, and they show lower position of the (003) peaks than the 2B-A SC83 cathode at 4.4 V. The calculated c axis length of the bare and 6B-A SC83 respectively decrease by 0.2808 Å and 0.1152 Å from 4.1 V to 4.4 V, which indicates that 6B-A SC83 exhibits a 59.0% lower shrinkage of the c axis compared to the bare SC83 during the H2–H3 phase transition. The long c axis is beneficial to the transfer of Li+, which explains the much higher Li+ diffusivity of the 6B-A SC83 cathode than the bare SC83 cathode at 4.4 V. According to the above phenomena, it could be inferred that B3+ exists in the Li layers. B3+ plays the role of the pillar ions to support the Li layers, which alleviates the shrinkage of the c axis for SC83 during the H2–H3 phase transition. Hence, it induces better Li+ kinetics for the SC83 cathode upon 4.2 V. Moreover, the content of B3+ bulk doping influences the support to the lattice of the SC83. As the content of B3+ doping increases, the support to the lattice increases until B3+ is saturated in the bulk of the SC83.
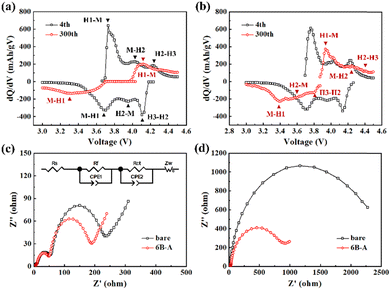
The differential capacity plots are analyzed to understand the electrochemical behavior of the bare and 6B-A SC83 cathodes during the charge–discharge cycling. As shown in Fig. 6a, three redox peaks represent the transition of the bare SC83 cathode from hexagonal to monoclinic phase (H1–M), monoclinic to hexagonal phase (M–H2), and hexagonal to hexagonal phase (H2–H3) at the 4th charge process, respectively. While at the 300th charge process, only one redox peak representing the H1–M phase transition can be distinguished. Moreover, the peak notably shifts to the right, accompanied by a decline in intensity. In contrast, for the 6B-A SC83 cathode, three redox peaks still can be distinguished after cycling (Fig. 6b). Moreover, both the shift of the peak position and the decline of the peak intensity are less, indicating better reversibility. This phenomena can also be observed during the discharge process. It indicates that the 6B-A SC83 cathode suffers from less degradation during the charge–discharge cycling. Fig. 6c shows the EIS of the bare and 6B-A SC83 cathodes after the 4th charge–discharge cycle at the charged state. The surface film impedance (Rf) of the bare and 6B-A SC83 cathodes is similar, while the charge transfer resistance (Rct) of the 6B-A SC83 cathode is lower than the bare SC83 cathode, indicating higher kinetics of Li ions at the interface between the 6B-A SC83 cathode and electrolyte. Moreover, the impedance at low-frequency reflects the Li+ diffusivity in the bulk of the SC83 cathode. The slope of the 6B-A SC83 cathode is lower than that of the bare SC83 cathode, which indicates faster Li+ diffusivity in the bulk of the 6B-A SC83 cathode (Fig. S19, ESI†). This is consistent with the GITT results. As shown in Fig. 6d, the Rct of the 6B-A SC83 cathode is much lower than that of the bare SC83 cathode after cycling.
Furthermore, the increase of Rct for the 6B-A SC83 cathode is also much lower than that of the bare SC83 cathode, which demonstrates a more robust interface between the 6B-A SC83 cathode and the electrolyte.
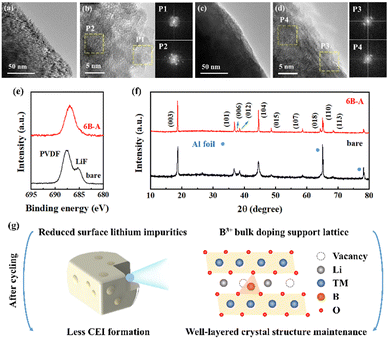
To investigate the degradation mechanisms of the SC83 cathodes at a microscopic level during cycling, the SC83 cathodes recovered from the coin cells are observed by SEM. As shown in Fig. S20 (ESI†), fewer spotty species are observed on the surface of the 6B-A SC83 cathode than the bare SC83 cathode, suggesting less formation of cathode electrolyte interface (CEI) layers on the surface of the 6B-A SC83 cathode.52 Furthermore, TEM is performed to characterize the surface morphology of the bare and 6B-A SC83 cathodes. The smooth edges and well single crystalline layered structure are observed on the surface and interior of the bare and 6B-A SC83 cathodes before cycling (shown in Fig. S21, ESI†), while changes happen after cycling. As shown in Fig. 7a, the edges of the bare SC83 particles are rough. Fig. 7b exhibits the rock salt NiO-like and amorphous structure on the surface of the bare SC83. The amorphous structure may be converted from the rock salt NiO-like structure.53 Besides, polycrystalline domains are observed on the interior of the bare SC83, which may be due to the severe deformation of the layered structure.53 In contrast, the edges of the 6B-A SC83 are smooth (shown in Fig. 7c). The single crystalline layered structure is observed on the surface and interior of the 6B-A SC83, though the lattice distortion still occurs on the surface of the 6B-A SC83 (shown in Fig. 7d). These results indicate that the surface structure stability of 6B-A SC83 is better than that of bare SC83 during cycling. XPS characterization is performed to detect the F containing species on the surface of the SC83 cathode. As shown in Fig. 7e, the F 1s peaks at 685.1 eV and 687.5 eV can be assigned to LiF and PVDF, respectively.54 The intensity of the F 1s peak at 685.1 eV for the 6B-A SC83 cathode is much lower than that of the bare SC83 cathode, which indicates much less LiF formation on the surface of the 6B-A SC83 cathode during cycling. As LiF is detrimental due to the sluggish Li+ transport kinetics, less LiF formation on the surface is beneficial to the capacity retention of the SC83 cathodes. Moreover, XRD characterization is carried out to investigate the crystal structure of the cycled SC83 cathodes. As shown in Fig. 7f, the full width at half-maximum (FWHM) of the peaks for the bare SC83 cathode is much broader than that of the 6B-A cathode, which indicates a lower crystallinity of the bare SC83 cathode after cycling. Moreover, the well-separated (006)/(012) peaks and (108)/(110) peaks can be distinguished in the 6B-A SC83 cathode, while not in the bare SC83 cathode. This indicates that 6B-A SC83 keeps a well-layered crystal structure after cycling, while the bare SC83 does not. The above results show that the B2O3 ALD coupled with post-annealing stabilizes the surface and crystal structure of the SC83. As represented in Fig. 7g, the reduced surface lithium impurities decrease the CEI formation on the SC83 during cycling. The B3+ bulk doping supports the lattice, which better maintains the crystal structure of the SC83 during cycling. This better preserves the Li+ transferring channels and storing sites in the B3+ doped SC83, hence leading to higher capacity retention for the B3+ doped SC83 cathode.
Conclusions
In summary, B3+ doped SC83 is constructed by B2O3 ALD coupled with post-annealing. Surface LiOH can be consumed and transformed into LiBOx during the B2O3 ALD process, whereas surface Li2CO3 can be reduced during the post-annealing treatment. B2O3 coatings can be transformed into B3+ doping after post-annealing, which expands the a and c axes and reduces the Li+/Ni2+ mixing of the SC83. B3+ is found to diffuse into the bulk of the SC83 during the post-annealing. As B3+ is saturated in the bulk, excess B3+ is left on the surface of the SC83. Saturated B3+ bulk doping with virtually no B3+ surface segregation gives the highest improvement in the rate capability of the SC83 cathodes. This is attributed to the reduced surface lithium impurities, decreased Li+/Ni2+ mixing, expanded lattice, and alleviated c axis shrinkage during the H2–H3 phase transition, which improves the electronic conductivity and Li+ diffusivity of the SC83. Besides, the cycling stability of the SC83 cathode is also improved due to the reduced formation of deleterious CEI layers and better crystal structure maintenance. Our study offers ideas for simultaneously stabilizing the surface and crystal structure of NCM.Author contributions
Jiawei Li: conceptualization, methodology, investigation, formal analysis, writing – original draft. Junren Xiang: methodology and investigation. Ge Yi: visualization. Zhijia Hu: investigation. Xiao Liu: writing – review & editing, supervision. Rong Chen: writing – review & editing, supervision.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (2022YFF1500400), the National Natural Science Foundation of China (51835005, 52350349), and the New Cornerstone Science Foundation through the XPLORER PRIZE. We acknowledge the valuable comments and suggestions from Prof. Bin Shan. We would also like to acknowledge the technology support from the Analytic Testing Center and Flexible Electronics Research Center of HUST.Notes and references
- H.-H. Ryu, K.-J. Park, C. S. Yoon and Y.-K. Sun, Chem. Mater., 2018, 30, 1155–1163 CrossRef CAS
.
- J. Zhang, Y. Jin, J. Liu, Q. Zhang and H. Wang, Sustainable Energy Fuels, 2021, 5, 5114–5138 RSC
.
- Y. Huang, Interdiscip. Mater., 2022, 1, 323–329 CrossRef
.
- J. Kim, H. Lee, H. Cha, M. Yoon, M. Park and J. Cho, Adv. Energy Mater., 2018, 8, 1702028 CrossRef
.
- W. Liu, P. Oh, X. Liu, M.-J. Lee, W. Cho, S. Chae, Y. Kim and J. Cho, Angew. Chem., Int. Ed., 2015, 54, 4440–4457 CrossRef CAS PubMed
.
- H.-H. Ryu, B. Namkoong, J.-H. Kim, I. Belharouak, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2021, 6, 2726–2734 CrossRef CAS
.
- H. Gao, Q. Wu, Y. Hu, J. P. Zheng, K. Amine and Z. Chen, J. Phys. Chem. Lett., 2018, 9, 5100–5104 CrossRef CAS PubMed
.
- J. Kim, J. Lee, H. Ma, H. Y. Jeong, H. Cha, H. Lee, Y. Yoo, M. Park and J. Cho, Adv. Mater., 2018, 30, 1704309 CrossRef PubMed
.
- C.-H. Jo, D.-H. Cho, H.-J. Noh, H. Yashiro, Y.-K. Sun and S. T. Myung, Nano Res., 2014, 8, 1464–1479 CrossRef
.
- J. Zhu, Y. Li, L. Xue, Y. Chen, T. Lei, S. Deng and G. Cao, J. Alloys Compd., 2019, 773, 112–120 CrossRef CAS
.
- J. Yan, H. Huang, J. Tong, W. Li, X. Liu, H. Zhang, H. Huang and W. Zhou, Interdiscip. Mater., 2022, 1, 330–353 CrossRef
.
- S. S. Zhang and L. Ma, J. Electrochem. Soc., 2021, 168, 080512 CrossRef CAS
.
- Y. Ding, B. Deng, H. Wang, X. Li, T. Chen, X. Yan, Q. Wan, M. Qu and G. Peng, J. Alloys Compd., 2019, 774, 451–460 CrossRef CAS
.
- H. H. Sun, H.-H. Ryu, U.-H. Kim, J. A. Weeks, A. Heller, Y.-K. Sun and C. B. Mullins, ACS Energy Lett., 2020, 5, 1136–1146 CrossRef CAS
.
- Z. Cui, X. Li, X. Bai, X. Ren and X. Ou, Energy Storage Mater., 2023, 57, 14–43 CrossRef
.
- T. He, L. Chen, Y. Su, Y. Lu, L. Bao, G. Chen, Q. Zhang, S. Chen and F. Wu, J. Power Sources, 2019, 441, 227195 CrossRef CAS
.
- J. Wang, C. Liu, G. Xu, C. Miao, M. Wen, M. Xu, C. Wang and W. Xiao, Chem. Eng. J., 2022, 438, 135537 CrossRef CAS
.
- R. He, A. Wei, L. Zhang, W. Li, X. Bai and Z. Liu, Solid State Ionics, 2019, 337, 56–62 CrossRef CAS
.
- Z. Cui, Q. Xie and A. Manthiram, ACS Appl. Mater. Interfaces, 2021, 13, 15324–15332 CrossRef CAS PubMed
.
- H. Li, P. Zhou, F. Liu, H. Li, F. Cheng and J. Chen, Chem. Sci., 2019, 10, 1374–1379 RSC
.
- Y. Kim, H. Park, J. H. Warner and A. Manthiram, ACS Energy Lett., 2021, 6, 941–948 CrossRef CAS
.
- Y. Kim, H. Park, K. Shin, G. Henkelman, J. H. Warner and A. Manthiram, Adv. Energy Mater., 2021, 2101112 CrossRef CAS
.
- T. Weigel, F. Schipper, E. M. Erickson, F. A. Susai, B. Markovsky and D. Aurbach, ACS Energy Lett., 2019, 4, 508–516 CrossRef CAS
.
- Y. Su, L. Li, L. Chen, L. Wang, Y. Lu, Q. Zhang, L. Bao and F. Wu, ACS Appl. Energy Mater., 2022, 5, 2231–2241 CrossRef CAS
.
- Q. Xie, W. Li, A. Dolocan and A. Manthiram, Chem. Mater., 2019, 31, 8886–8897 CrossRef CAS
.
- Z. Luo, G. Hu, W. Wang, K. Du, Z. Peng, J. Zeng, L. Li and Y. Cao, J. Power Sources, 2022, 548, 232092 CrossRef CAS
.
- W. Yang, W. Xiang, Y.-X. Chen, Z.-G. Wu, W.-B. Hua, L. Qiu, F.-R. He, J. Zhang, B.-H. Zhong and X.-D. Guo, ACS Appl. Mater. Interfaces, 2020, 12, 10240–10251 CrossRef CAS PubMed
.
- S. F. Amalraj, R. Raman, A. Chakraborty, N. Leifer, R. Nanda, S. Kunnikuruvan, T. Kravchuk, J. Grinblat, V. Ezersky, R. Sun, F. L. Deepak, C. Erk, X. Wu, S. Maiti, H. Sclar, G. Goobes, D. T. Major, M. Talianker, B. Markovsky and D. Aurbach, Energy Storage Mater., 2021, 42, 594–607 CrossRef
.
- C. Roitzheim, L.-Y. Kuo, Y. J. Sohn, M. Finsterbusch, S. Möller, D. Sebold, H. Valencia, M. Meledina, J. Mayer, U. Breuer, P. Kaghazchi, O. Guillon and D. Fattakhova-Rohlfing, ACS Appl. Energy Mater., 2021, 5, 524–538 CrossRef
.
- K.-J. Park, H.-G. Jung, L.-Y. Kuo, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2018, 8, 1801202 CrossRef
.
- F. Li, Z. Liu, C. Liao, X. Xu, M. Zhu and J. Liu, ACS Energy Lett., 2023, 8, 4903–4914 CrossRef CAS
.
- S. M. George, Chem. Rev., 2010, 110, 111–131 CrossRef CAS PubMed
.
- Z. Li, J. Li, X. Liu and R. Chen, Chem. Eng. Process., 2021, 159, 108234 CrossRef CAS
.
- X. Liu, Y. Su and R. Chen, Int. J. Extreme Manuf., 2023, 5, 022005 CrossRef
.
- J. Li, J. Xiang, G. Yi, Y. Tang, H. Shao, X. Liu, B. Shan and R. Chen, Coatings, 2022, 12, 84 CrossRef CAS
.
- C. Zhou, C. Dong, W. Wang, Y. Tian, C. Shen, K. Yan, L. Mai and X. Xu, Interdiscip. Mater., 2024, 3, 306–315 CrossRef CAS
.
- F. Mattelaer, M. Van Daele, M. M. Minjauw, M. Nisula, S. D. Elliott, T. Sajavaara, J. Dendooven and C. Detavernier, Chem. Mater., 2020, 32, 4152–4165 CrossRef CAS
.
- L. Chen, J. G. Connell, A. Nie, Z. Huang, K. R. Zavadil, K. C. Klavetter, Y. Yuan, S. Sharifi-Asl, R. Shahbazian-Yassar, J. A. Libera, A. U. Mane and J. W. Elam, J. Mater. Chem. A, 2017, 5, 12297–12309 RSC
.
- Q. Li, W. Zhuang, Z. Li, S. Wu, N. Li, M. Gao, W. Li, J. Wang and S. Lu, ChemElectroChem, 2020, 7, 998–1006 CrossRef CAS
.
- J. Shen, B. Zhang, X. He, B. Xiao, Z. Xiao, X. Li and X. Ou, J. Colloid Interface Sci., 2022, 629, 388–398 CrossRef PubMed
.
- B. Oprea, T. Radu and S. Simon, J. Non-Cryst. Solids, 2013, 379, 35–39 CrossRef CAS
.
- A. Pilli, J. Jones, V. Lee, N. Chugh, J. Kelber, F. Pasquale and A. LaVoie, J. Vac. Sci. Technol., A, 2018, 36, 061503 CrossRef
.
- A. Pilli, V. Lee, J. Jones, N. Chugh, J. Du, F. Pasquale, A. LaVoie and J. Kelber, J. Phys. Chem. C, 2020, 124, 25846–25858 CrossRef CAS
.
- X. Zeng, T. Jian, Y. Lu, L. Yang, W. Ma, Y. Yang, J. Zhu, C. Huang, S. Dai and X. Xi, ACS Sustainable Chem. Eng., 2020, 8, 6293–6304 CrossRef CAS
.
- D. A. Hensley and S. H. Garofalini, Appl. Surf. Sci., 1994, 81, 331–339 CrossRef CAS
.
- K. Nagao, M. Suyama, A. Kato, C. Hotehama, M. Deguchi, A. Sakuda, A. Hayashi and M. Tatsumisago, ACS Appl. Energy Mater., 2019, 2, 3042–3048 CrossRef CAS
.
- Y. Liu, X. Wang, J. Cai, X. Han, D. Geng, J. Li and X. Meng, J. Mater. Sci. Technol., 2020, 54, 77–86 CrossRef CAS
.
- N. Schulz, R. Hausbrand, C. Wittich, L. Dimesso and W. Jaegermann, J. Electrochem. Soc., 2018, 165, A833–A846 CrossRef CAS
.
- L. Pan, Y. Xia, B. Qiu, H. Zhao, H. Guo, K. Jia, Q. Gu and Z. Liu, J. Power Sources, 2016, 327, 273–280 CrossRef CAS
.
- Y. Liu, X. Fan, B. Luo, Z. Zhao, J. Shen, Z. Liu, Z. Xiao, B. Zhang, J. Zhang, L. Ming and X. Ou, J. Colloid Interface Sci., 2021, 604, 776–784 CrossRef CAS PubMed
.
- Y. Kim, D. Kim and S. Kang, Chem. Mater., 2011, 23, 5388–5397 CrossRef CAS
.
- X. Li, J. Liu, M. N. Banis, A. Lushington, R. Li, M. Cai and X. Sun, Energy Environ. Sci., 2014, 7, 768–778 RSC
.
- H.-H. Sun and A. Manthiram, Chem. Mater., 2017, 29, 8486 CrossRef CAS
.
- W. Bao, G. Qian, L. Zhao, Y. Yu, L. Su, X. Cai, H. Zhao, Y. Zuo, Y. Zhang, H. Li, Z. Peng, L. Li and J. Xie, Nano Lett., 2020, 20, 8832–8840 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ya00206g |
| This journal is © The Royal Society of Chemistry 2024 |