Integration of paper-based analytical devices with digital microfluidics for colorimetric detection of creatinine†
Larissa G.
Velasco
a,
Danielly S.
Rocha
a,
Richard P. S.
de Campos
 b and
Wendell K. T.
Coltro
b and
Wendell K. T.
Coltro
 *ac
*ac
aInstituto de Química, Universidade Federal de Goiás – UFG, 74690-900, Goiânia, GO, Brazil
bNanotechnology Research Centre, National Research Council of Canada, Edmonton, AB, Canada
cInstituto Nacional de Ciência e Tecnologia de Bioanalítica, 13084-971, Campinas, SP, Brazil. E-mail: wendell@ufg.br
First published on 8th October 2024
Abstract
Digital microfluidics (DMF) is a platform that enables the automated manipulation of individual droplets of sizes ranging from nanoliter to microliter and can be coupled with numerous techniques, including colorimetry. However, although the DMF electrode architecture is highly versatile, its integration with different analytical methods often requires either changes in sample access, top plate design, or the integration of supplementary equipment into the system. As an alternative to overcome these challenges, this study proposes a simple integration between paper-based analytical devices (PADs) and DMF for automated and eco-friendly sample processing aiming at the colorimetric detection of creatinine (CR, an important biomarker for kidney disease) in artificial urine. An optimized and selective Jaffé reaction was performed on the device, and the reaction products were delivered to the PAD, which was subsequently analyzed with a bench scanner. The optimal operational parameters on the DMF platform were a reaction time of 45 s with circular mixing and image capture after 5 min. Under optimized conditions, a linear behavior was obtained for creatinine concentrations ranging from 2 to 32 mg dL−1, with limits of detection and quantitation equal to 1.4 mg dL−1 and 2.0 mg dL−1, respectively. For the concentration range tested, the relative standard deviation varied from 2.5 to 11.0%, considering four measurements per concentration. CR-spiked synthetic urine samples were subjected to analysis via DMF-PAD and the spectrophotometric reference method. The concentrations of CR determined using both analytical techniques were close to the theoretical values, with the resultant standard deviations of 2–9% and 1–4% for DMF-PADs and spectrophotometry, respectively. Furthermore, the recovery values were within the acceptable range, with DMF-PADs yielding 96–108% and spectrophotometry producing 95–102%. Finally, the greenness of the DMF-PAD and spectrophotometry methods was evaluated using the Analytical Greenness (AGREE) metric software, in which 0.71 and 0.51 scores were obtained, respectively. This indicates that the proposed method presents a higher greenness level, mainly due to its miniaturized characteristics using a smaller volume of reagent and sample and the possibility of automation, thus reducing user exposure to potentially toxic substances. Therefore, the DMF-PADs demonstrated great potential for application in the clinical analysis of creatinine, aiding in routine tests by introducing an automated, simple, and environmentally friendly process.
1. Introduction
Digital microfluidics (DMF) is a powerful technology for manipulating individual droplets of sizes ranging from nanoliter to microliter on an array of isolated electrodes.1 DMF enables various operations such as dispensing from a reservoir, merging, mixing (circular, linear, or cross), and splitting multiple droplets in parallel on-chip. The literature reports using DMF devices in numerous applications, including chemical and biological analysis, clinical diagnosis, sample preparation, synthesis, and cell culture.2–10 Moreover, the electrode design versatility of this analytical tool allows for its application in decentralized field tests and point-of-need analysis.10,11 The operations performed on DMF devices can be programmed and adjusted according to the demands of the experiments and applications without changing the design of the actuation electrode array. Another important aspect of this technology is its huge potential for automating these procedures and the possibility of using the same DMF device for simultaneous analyses.12,13 Moreover, DMF has been successfully integrated with various analytical techniques, such as electrochemistry,13,14 electrochemiluminescence,15 electrophoresis,16 mass spectrometry,17 Raman spectroscopy,18 nuclear magnetic resonance (NMR) spectroscopy,19 and colorimetry.12,20,21 However, the integration with the DMF platform commonly requires changes in the device layout or removal of the device top plate for sample access4,14,17,22,23 and the incorporation of auxiliary equipment,12,13,15,24–26 among other complex and study-specific modifications. As such, there is a clear need for a new form of integration that is simple and cost-effective and requires minimal modifications to the commercial chip.Digital image colorimetry (DIC) has been successfully demonstrated as a colorimetric technique that stands out for its ability to perform qualitative and quantitative analyses without requiring sophisticated instrumentation. The DIC approach is based on pixel intensity analysis of a region of interest from digital images, which can be captured using a variety of detectors (such as webcams, smartphones, cameras, and scanners) and analyzed via specialized software including Adobe Photoshop, ImageJ, MATLAB, Pantone Studio, Color Photo-Paint, Digital Colorimeter, Photometrix®, and ColorMeter27–30 in different color spaces.28
Paper-based analytical devices (PADs) have emerged as powerful tools for colorimetric assays due to their ability to promote a high-contrast, colorless, and stable background for color change analysis.31 In addition, PADs offer additional advantages like simple handling, global affordability, ease of manufacturing, self-pumping capabilities through capillarity action, portability, and versatility.32,33 A variety of manufacturing techniques involving different types of paper substrates have been demonstrated in the literature.33–37 Among these, wax printing has been the most popular fabrication technique for manufacturing paper-based devices due to its instrumental simplicity, short fabrication time, and low cost.38 One of the main challenges often reported in colorimetric assays performed on PADs is the washing effect, which may compromise the analytical performance due to poor color distribution.39 The integration of DMF and PADs can be an alternative strategy for minimizing this phenomenon and enhancing the analytical reliability of colorimetric measurements.
Considering the frequent need for modifications in the top plate and equipment when integrating DMF with other analytical techniques, it becomes evident that a more cost-effective and straightforward alternative form of integration would be beneficial. Campos and colleagues reported a method for exchanging embedded sensors integrated with the DMF using a customized DMF top plate with a paper wick, which transported the analytes from the droplet to the sensors.14 Abadian and colleagues reported the manufacturing of a combined μPAD and paper-based DMF on a single substrate. The authors demonstrated the proof of concept through glucose colorimetric sensing.40 Komatsu and colleagues performed colorimetric detection of lithium ions in whole blood samples by adding paper sensors between the plates of the DMF device during its manufacturing process.20 Ho and colleagues integrated DMF with distance-based detection in the paper substrate to perform a loop-mediated isothermal amplification test (LAMP).21
Therefore, this study proposes an integration approach between DMF and PADs (DMF-PADs) aiming to detect creatinine (CR). In brief, the reaction was performed on the DMF platform through an automated process that delivered the resulting product into a wax-printed PAD. For colorimetric measurements, the image capture was conducted using a desktop scanner followed by software analysis. This strategy aims to streamline the application of this platform across various analytical techniques by facilitating the transport of solutions from DMF to other platforms. As it is well-known, CR is a valuable biomarker for assessing kidney function, and its quantitation is often performed using colorimetric techniques.41–45 The proposed integration of DMF-PADs was successfully demonstrated through the colorimetric detection of CR in synthetic urine samples using the Jaffé reaction under optimized experimental and operational parameters and compared with a spectrophotometric method.
2. Materials and methods
2.1. Chemicals and samples
All reagents were of analytical grade and used without purification. Picric acid (PA), creatinine (CR), sodium hydroxide (NaOH), sodium bicarbonate, ammonium chloride, calcium chloride dihydrate, potassium chloride, sodium chloride, D(+)glucose, potassium phosphate monobasic, dibasic sodium phosphate, magnesium sulfate heptahydrate, sodium sulfate, urea, and ethylenediamine tetrakis (ethoxylate-block-propoxylate) tetrol (Tetronic 90R4) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Ascorbic acid, citric acid, and lactic acid were purchased from Synth (Diadema, SP, Brazil), Neon (Suzano, SP, Brazil), and Cromoline Química Fina (Diadema, SP, Brazil), respectively. The ultrapure water used for preparing analytical solutions was processed through a Direct-Q®3 water purification system (Millipore, Darmstadt, Germany) with a resistivity of 18.2 MΩ cm at 25 °C.Artificial urine was prepared following the procedure described by Brooks and Keevil,46 with some modifications (Table S1†). For analytical purposes, the artificial urine was spiked with CR standard solutions prepared in the concentration range between 0 and 300 mg dL−1.
2.2. Optimization of reaction conditions
The optimization of PA and NaOH concentrations was realized in a polyester microwell plate, containing 96 square zones distributed in 8 rows and 12 columns. Each zone has a side of 3 mm and a maximum volume of approximately 8 μL. The plate was manufactured according to the protocol described elsewhere.47 The experiments were performed by adding 4 μL of a mixture containing PA (1 to 10 mmol L−1) in a 250 mmol L−1 NaOH solution, and 4 μL of the CR solution prepared in concentrations between 8 and 32 mg dL−1. The analysis was carried out in quadruplicate using a scanner. For the alkaline conditions, the NaOH concentration was investigated ranging from 50 to 450 mmol L−1 using 5 mmol L−1 PA.Potential interfering compounds in the Jaffé reaction such as ascorbic acid, magnesium sulfate, sodium chloride, glucose, urea, ammonium chloride, and potassium chloride at a concentration of 500 mg dL−1 were analyzed with 5 mmol L−1 PA prepared in 300 mmol L−1 NaOH. Regarding the CR solution, the used concentration was 32 mg dL−1. These tests were carried out using a polyester microwell plate composed of 96 square zones (4 mm × 4 mm), which can store 10 μL each. The plate was manufactured as described elsewhere.47
2.3. Fabrication of paper-based analytical devices
The PADs were manufactured using the wax printing method described by Carrilho and coworkers.38 Briefly, a “U”-shaped design with a 4 mm opening and a 1.5 mm line width (Fig. S1†) was created using CorelDRAW version 8 software and printed on Whatman type 1 chromatography paper, from GR Healthcare (Buckinghamshire, UK) using a ColorQube 8570 wax printer (XEROX, Wilsonville, USA). After printing, the paper was heated in an oven at 150 °C for 3 min. Finally, the PADs were manually cut using scissors.2.4. DMF operations
For experiments, portable DMF equipment obtained from Sci-Bots (Kitchener, ON, Canada) was used. Operations on the 90-pin array v3 model DMF devices were controlled using a DropBot system model DB3-120 and the open-source MicroDrop software (version 3.1.3). A Logitech c920 webcam, assembled on a removable holder printed with a 3D printer, was used to visualize the operations, as seen in Fig. S2.† The device was operated at 10 kHz, and the device's automated protocol used an actuation potential of 90 VRMS for reservoir filling and 100 to 110 VRMS for other types of droplet operations. The duration of the applied potentials ranged between 1 and 12 s, depending on the operation of droplets. All solutions used with DMF were supplemented with 0.1% Tetronic 90R4.2.5. Colorimetric detection of creatinine
To perform colorimetric reactions, four PADs were inserted between the top and the bottom plates into four reservoir electrodes on the DMF device (Fig. 1A). Subsequently, the DMF device was placed on the DropBot platform, and 6 μL aliquots of the chromogenic reagent containing 5 mmol L−1 PA prepared in 300 mmol L−1 NaOH and CR in different concentrations (2, 4, 8, 16 and 32 mg dL−1 of CR), prepared in ultrapure water, were added to two reservoir electrodes (Fig. 1B). For the blank experiment, ultrapure water was used instead of CR, with all other steps remaining identical.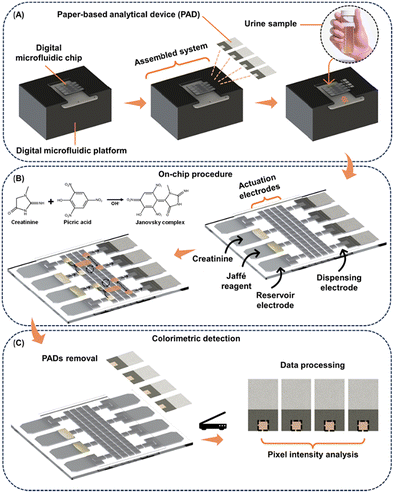 | ||
| Fig. 1 Schematic representation of (A) the assembly of DMF-PADs, (B) the automated Jaffé DMF reaction, and (C) the CR colorimetric detection process. | ||
After sample and reagent loading, the DMF automated Jaffé reaction protocol was executed, which consisted of the following steps. (1) Single-unit droplets (covering the volume of one DMF electrode, approximately 0.9 μL) of reagent and CR solutions were dispensed from each respective reservoir and then moved to the designated electrodes in the DMF array. This process was repeated for a second set of droplets. (2) Reagent and analyte droplets were then merged, resulting in two double-unit droplets (∼1.8 μL). (3) The double-unit droplets were mixed for 45 s in a circular pattern by programming a routine, identified as optimal during the optimization step. (4) After the mixing stage, the double-unit droplets were split to form four single-unit droplets, constituting the Janovsky complex. (5) Single-unit droplets were then delivered to the PADs. Additional operation information can be seen in Video S1† and Fig. S3.†
The PADs were subsequently removed from the device (Fig. 1C) using tweezers and scanned using a desktop scanner model G405 from Hewlett-Packard (Palo Alto, CA, USA) for analysis in Corel Photo-Paint software using the CMYK color system, focusing on the yellow channel.
2.6. Creatinine analysis in synthetic urine
To analyze synthetic urine on the DMF-PAD, it was necessary to add sample dilution cycles before performing the Jaffé reaction described in section 2.5. Initially, single-unit droplets of artificial urine and ultrapure water were dispensed from the DMF reservoirs and merged. The resulting double-unit droplet was mixed for 30 s in a circular movement and was then split into two single-unit droplets. One of these droplets (waste droplets) was discarded onto a waste reservoir, while the other was advanced to a subsequent dilution cycle. The serial dilution process was performed for a total of three cycles, with the waste droplet being discarded after the two initial cycles but not after the last cycle. Finally, both resulting single-unit droplets were used in the automated Jaffé reaction protocol detailed in section 2.5. Additional information can be visualized in Video S2 and Fig. S4.† For the movement of the samples and diluent (ultrapure water) on the DMF device, a preliminary dilution was performed. This involved a mixture of 75% sample or ultrapure water and 25% surfactant (Tetronic 90R4). This resulted in a final concentration of 0.1% of Tetronic 90R4.2.7. Comparative spectrophotometric analysis
Synthetic urine spiked with CR concentrations between 30 and 300 mg dL−1 was analyzed using spectrophotometric detection, as a comparative assay to the DMF-PAD method. Measurements were performed using a UV–visible spectrophotometer model UV-M51 purchased from Bel Photonics (Piracicaba, SP, Brazil), using a quartz cuvette with an optical path length of 1 cm, and wavelength fixed at 500 nm. The Jaffé reaction was performed directly in the cuvette using a commercial creatinine assay kit, acquired from Wiener Lab (São Luiz, SP, Brazil).2.8. Green analytical chemistry metric for creatinine analysis
To assess the greenness metric of the proposed DMF analysis in comparison with spectrophotometry, we utilized the free software AGREE. The evaluation within this software was conducted concerning the 12 principles of green analytical chemistry, including sample treatment (P1), sample amount (P2), device positioning (P3), sample preparation stages (P4), automation, miniaturization (P5), derivatization (P6), waste (P7), analysis throughput (P8), energy consumption (P9), source of reagents (P10), toxicity (P11) and operator safety (P12).48The analysis generates a color-coded pictogram on a semaphoric scale, ranging from 0 to 1, representing the scores for each principle. Additionally, the width of each band corresponds to the weight of the respective principle. The overall score for the evaluated method is determined by the weighted average of the individual principle scores. While adjusting the weights of the principles based on their significance to the analyzed method is possible, equal weights have been maintained for all principles during method comparison.48
3. Results and discussion
3.1. Optimization of reaction conditions
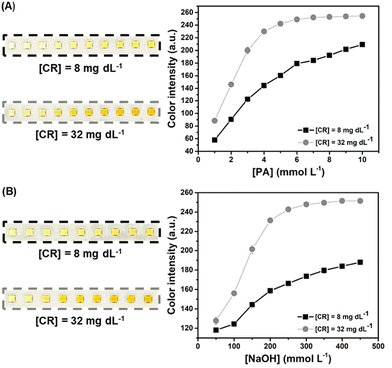
The reaction between CR and PA under alkaline conditions (in the presence of NaOH) yields the Janovsky complex (Fig. S5†), changing the observed color of reaction media from yellow to orange. This reaction is known as the Jaffé reaction.49 Therefore, the yellow channel in the CMYK color system is indicative of the reaction completion, and the color intensity was obtained by using image analysis in Corel Photo-Paint software.Aiming to obtain a high color intensity for the colorimetric reaction, the optimization of PA (Fig. 2A) and NaOH (Fig. 2B) concentrations was investigated. The concentrations of 5 mmol L−1 for PA and 300 mmol L−1 for NaOH revealed greater color intensity before saturation of the color channel at the concentration of 32 mg dL−1 of CR. Therefore, these values were selected for the subsequent experiments.
The potential interference of different compounds in the Jaffé reaction under the optimized conditions was also evaluated. Urine excreted by the human body contains several potential interference substances, such as glucose, metabolites, and drug residues, depending on the health condition and dietary habits of individuals.50–52 Thus, the possible interferences were evaluated (Fig. S6†). An increase in color intensity was exclusively observed in the microwell containing CR. Furthermore, the difference in color intensity between the blank and the wells containing potential interfering compounds did not differ statistically at a 95% confidence level. The achieved results indicate that the method based on the Jaffé reaction possesses a high selectivity towards the detection of CR, as also described in other reports.41,49,53–55
3.2. Optimization of operational parameters
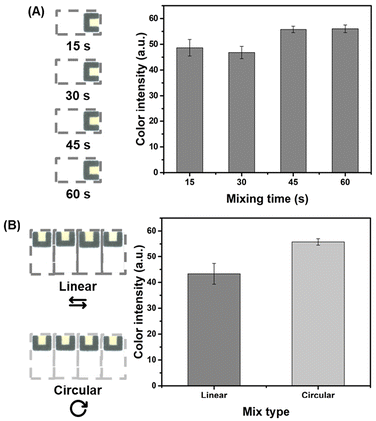
Once the reaction parameters and Jaffé reaction selectivity were successfully evaluated, operational parameters of DMF, such as mixing type and mixing time were investigated. On the DMF platform, the process of mixing two droplets requires consideration of the aspects inherent to their laminar flow behavior. To optimize the efficiency of the mixing process and minimize the time required, various mixing formats can be explored.56 The linear type of mixture (subsequent activation of a linear matrix of electrodes) does not yield sufficient mixing in the bulk of the droplet for efficient mixing to occur quickly because it is mainly governed by slow diffusion. On the other hand, a mixture made in a circular pattern (loop motion, subsequent activation of a square matrix of electrodes), with the same number of electrodes in the matrix, induces folding and stretching–folding patterns to the droplet corner, dramatically increasing mixing efficiency.56 This efficiency has led to prevalence of circular mixing patterns as the most employed mixing format in DMF research.Thus, the mixing duration in circular mode between PA and CR droplets was evaluated under alkaline conditions, employing intervals between 15 and 60 s (15 s increments) for quadruplicate analyses (Fig. 3A). Based on the data shown in Fig. 3A, the color intensity did not present a noticeable difference when the mixing time increased from 15 to 30 s and from 45 to 60 s. In both cases, the intensity values were not statistically different from each other (tcalculated < tcritical) at a 95% confidence level. On the other hand, when the mixing time varied from 30 to 45 s, a remarkable enhancement was seen for the measured color intensity, thus suggesting that a better mixing efficiency was achieved. Therefore, the mixing time of 45 s was selected as the optimal condition. A comparative analysis was also conducted between the circular and linear mixing models, each utilizing four active actuation electrodes (Fig. 3B). As anticipated, a duration of 45 s was insufficient for efficient mixing in the linear model, as evidenced by the lower color intensity when compared to that following circular mixing with the same duration. Consequently, to streamline the protocol, a circular mixing duration of 45 s was retained for subsequent experiments.
Additionally, quadruplicate analyses were conducted to determine the time for image acquisition of PADs following the colorimetric reaction. The colorimetric reaction for the blank and at the concentration of 8 mg dL−1 CR was performed using the DMF automated Jaffé reaction protocol, with PAD images captured from 5 to 30 min at intervals of 5 min (Fig. S7†). Data analysis revealed that the complex formed during the Jaffé reaction demonstrated stability within the tested time interval. This was supported by a relative standard deviation (RSD) of 4.2% between color intensities for all image acquisition times. To expedite the overall process, a standard time of 5 min post-droplet delivery on the PAD was established for image acquisition in all subsequent experiments.
3.3. Analytical performance of the integrated protocol
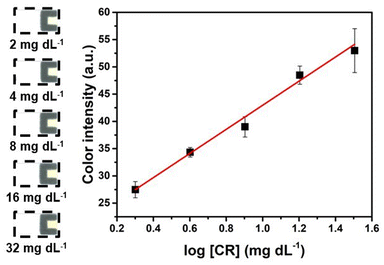
Upon determining the optimal conditions, a reaction protocol was programmed and employed to conduct quantitative analyses of CR on DMF-PADs. An analytical curve (Fig. 4) was constructed by evaluating the yellow color intensity generated on PADs after performing the automated Jaffé reaction protocol for CR detection. The color intensities of four replicates for each concentration tested were obtained using a single DMF chip, and the relationship between the color intensity and the analyzed CR concentrations revealed a logarithmic behavior, similar to that of other reports found in the literature.41,44,57 The linear regression equation of the linearized curve was y = (22.1 ± 1.1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[CR] + (20.8 ± 1.4), with R2 = 0.983.
log[CR] + (20.8 ± 1.4), with R2 = 0.983.
The limits of detection (LOD) and quantification (LOQ) for CR were calculated based on the ratio between three and ten times the standard deviation for the blank and the slope of the analytical curve, respectively.58,59 The LOD and LOQ values were 1.4 mg dL−1 and 3.1 mg dL−1, respectively. Furthermore, when detecting a CR concentration of 2 mg dL−1 (the lowest concentration on the analytical curve), the results demonstrated an accuracy of 0.7% among quadruplicates and a precision of 1.5%. The accuracy was quantified as the relative error by comparing the obtained value with the theoretical value, while precision was expressed as the RSD. The obtained LOD and LOQ are comparable to those of other studies that employ Jaffé's colorimetric reaction in PADs for CR detection, as shown in Table 1. However, it is important to highlight that the proposed method boasts the benefit of necessitating merely small volumes of reagents and samples. Considering the volume used (∼1.0 μL), the LOD achieved by employing DMF-PADs can be expressed as 14 ng of CR per PAD, which is significantly lower than that in other studies listed in Table 1. In addition to being automated and portable, this method minimizes operator-induced random errors usually present in entirely manual methods.
| Method | Volume of sample | Limit of detection | Limit of quantification | Ref. |
|---|---|---|---|---|
| PAD – spot test | 2 μL | 5.4 mg dL−1 | 16.3 mg dL−1 | 41 |
| μPADs | 10 μL | 1.57 mg dL−1 | 5.24 mg dL−1 | 50 |
| μPADs | 50 μL | 0.08 mmol L−1 (0.9 mg dL−1) | 0.26 mmol L−1 (2.92 mg dL−1) | 55 |
| μPADs | 55 μL | 1.69 mg dL−1 | 1.69 mg dL−1 | 61 |
| μPADs | Until filled | 0.35 mmol L−1 (3.95 mg dL−1) | 0.82 mmol L−1 (9.25 mg dL−1) | 44 |
| PAD – spot test | 4 μL | 5.3 mg dL−1 | Not reported | 43 |
| DMF-PADs | ≅1 μL | 1.4 mg dL−1 | 2 mg dL−1 | This study |
The repeatability of on-chip colorimetric assays on DMF devices was also evaluated. Repeatability tests for analytical methods are crucial for producing consistent, adequate, reliable, and reproducible results. Thus, intraday precision was determined from eight CR analyses at 8 mg dL−1 using the same device and operator. The mean of color intensities was calculated, yielding an RSD value of 11.9%, lower than the maximum value of 15% required by Resolution of the Collegiate Board (RDC) number 27, which deals with the validation of bioanalytical methods. Therefore, the analytical performance results indicate that the automated method proposed here can be a promising tool for CR analysis.60
3.4. Synthetic sample analysis and reference analysis
Artificial urine samples were spiked with creatinine (CR) at concentrations of 30 mg dL−1, 75 mg dL−1, 150 mg dL−1, 225 mg dL−1, and 300 mg dL−1. These concentrations span the range of values considered normal for both men and women, which are 41 to 305 mg dL−1 and 37 to 255 mg dL−1, respectively.49 Before starting the DMF automated Jaffé reaction protocol, a three-step dilution process for the CR sample was programmed on-chip to ensure that the color intensity fell within the linear range of the analytical curve. Each dilution step on-chip reduced the analyte concentration by half. Considering the execution time for the automated Jaffé dilution and reaction protocol within the DMF, along with the time required to remove the PADs from the device and analyze them using the scanner, the total time for analysis was 15 min per quadruplicate.All analyses of the artificial urine were conducted on a single DMF device, thereby highlighting its reusability and demonstrating the benefit of performing multiple analyses using a single device. The color intensities obtained were applied to the linear equation of the analytical curve, and the logarithm values of CR concentrations were converted back to CR concentration to obtain the sample concentration in the performed dilutions. A comparative study with a spectrophotometric method (the reference technique for colorimetric detection) was also performed with the same samples tested with the DMF-PAD protocol. Table 2 shows the final calculated concentrations for each sample using both DMF-PADs and spectrophotometry.
| Spiked (mg dL−1) | DMF-PAD | Spectrophotometry | ||
|---|---|---|---|---|
| (mg dL−1) | Recovery (%) | (mg dL−1) | Recovery (%) | |
| 30 | 32 ± 4 | 105 | 29 ± 2 | 98 |
| 75 | 76 ± 2 | 102 | 74 ± 4 | 99 |
| 150 | 162 ± 9 | 108 | 153 ± 4 | 102 |
| 225 | 242 ± 4 | 107 | 222 ± 1 | 99 |
| 300 | 287 ± 6 | 96 | 286 ± 2 | 95 |
The accuracy of the proposed protocol was evaluated via recovery assays. The recovery values using DMF-PADs ranged from 96 to 108%, indicating that the proposed method yielded concentration quantification of CR added to synthetic urine within 10% of the nominal added concentration, as required by RDC number 27.60 In comparison, the spectrophotometric method yielded recoveries ranging from 95 to 102%.
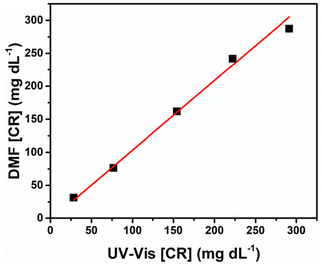
Using the results presented in Table 2, a fitting curve was constructed to establish a comparison between the CR concentrations obtained using DMF-PADs and spectrophotometry (Fig. 5). In a perfect linear regression, it is expected that values for R2, angular coefficient a, and linear coefficient b equal to 1, 1, and 0, respectively, are obtained.62
Comparatively, in the correlation curve plotted in Fig. 5, the values obtained were R2 = 0.995, a = 1.1 ± 0.1, and b = −2.2 ± 4.7. Since R2 is comparable to the ideal value and the confidence intervals calculated for the angular and linear coefficients include the values 1 and 0, respectively, it can be concluded that the proposed method for CR detection using DMF-PADs does not differ systematically from the conventional spectrophotometric method at a 95% confidence level.
3.5. Analytical Greenness (AGREE) metric
Upon investigation of the 12 principles of green chemistry via the AGREE software, the obtained values were 0.71 and 0.51 for DMF-PADs and UV–visible, respectively, as depicted in the pictograms and reports in Fig. S8.† The automation capacity and miniaturized feature of the proposed methodology are the main contributors to its superior value when compared to spectrophotometry. These characteristics confer an advantage in terms of reducing the sample amount, waste generation, and toxic reagent volume, which is particularly significant given that both methodologies employ PA, which is explosive and toxic, as well as NaOH, known for its corrosive properties. Furthermore, DMF-PADs can conduct quadruplicate analyses with single automated sample preparation, thereby reducing the analysis time. Consequently, the proposed methodology exhibits greater alignment with the principles of green chemistry.4. Conclusion
The integration of DMF and PADs for the colorimetric detection of CR in synthetic urine samples has been successfully demonstrated. The procedure involved automated on-chip execution of the Jaffé reaction, followed by image acquisition using a scanner and subsequent analysis via Corel Photo-Paint. The obtained results demonstrated that DMF streamlines manual steps and holds the potential to facilitate routine analysis that requires dilutions, reagent mixings, and aliquoting samples for testing by employing droplet handling automated technology and delivering the reaction product to desired PADs. In addition, DMF allowed for simultaneous analyses, providing a significant advantage in obtaining rapid results. Since the DMF-PADs present minimal sample and reagent consumption, reduced waste generation, and are miniaturized, this technique aligns with the principles of green chemistry. Consequently, DMF-PADs may emerge as a promising alternative for multiple clinical applications, as exemplified by the CR analysis reported in this study. Moreover, its portability enables point-of-care and point-of-need analysis, benefiting not only clinical diagnostics but also environmental, forensic, and food fields, for example. Future investigations will be focused on the application of DMF-PADs to detect CR and other relevant biomarkers in biological fluids aiming to explore the sample-in–answer-out capabilities of these integrated platforms for rapid and portable diagnostics.Author contributions
Larissa G. Velasco: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, and writing – original draft; Danielly S. Rocha: conceptualization, investigation, methodology, and writing – review & editing; Richard P. S. de Campos: conceptualization, investigation, methodology, and writing – review & editing; and Wendell K. T. Coltro: conceptualization, funding acquisition, project administration, resources, supervision, and writing – review & editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
The authors would like to thank INCTBio (grant 465389/2014–7), CNPq (grants 307554/2020–1 and 405620/2021–7), CAPES (code 001), and FAPEG (grant 202310267000258) for the financial support and granted scholarships and researcher fellowship. They also recognize Fabrício R. de Souza and Daniel S. de Paula for their technical support during the experimental measurements.References
- K. Choi, A. H. C. Ng, R. Fobel and A. R. Wheeler, Annu. Rev. Anal. Chem., 2012, 5, 413–440 CrossRef PubMed.
- H. Cheng, H. Liu, W. Li and M. Li, Electrophoresis, 2021, 42, 2329–2346 CrossRef PubMed.
- L. Pang, J. Ding, X. X. Liu and S. K. Fan, TrAC, Trends Anal. Chem., 2019, 117, 291–299 CrossRef.
- J. Peng, C. Chan, S. Zhang, A. A. Sklavounos, M. E. Olson, E. Y. Scott, Y. Hu, V. Rajesh, B. B. Li, M. D. Chamberlain, S. Zhang, H. Peng and A. R. Wheeler, Chem. Sci., 2023, 14, 2887–2900 RSC.
- A. A. Sklavounos, J. Lamanna, D. Modi, S. Gupta, A. Mariakakis, J. Callum and A. R. Wheeler, Clin. Chem., 2021, 67, 1699–1708 CrossRef PubMed.
- M. Torabinia, U. S. Dakarapu, P. Asgari, J. Jeon and H. Moon, Sens. Actuators, B, 2021, 330, 129252 CrossRef CAS.
- B. Wu, S. von der Ecken, I. Swyer, C. Li, A. Jenne, F. Vincent, D. Schmidig, T. Kuehn, A. Beck, F. Busse, H. Stronks, R. Soong, A. R. Wheeler and A. Simpson, Angew. Chem., 2019, 131, 15516–15520 CrossRef.
- X. Xu, Q. Zhang, J. Song, Q. Ruan, W. Ruan, Y. Chen, J. Yang, X. Zhang, Y. Song, Z. Zhu and C. Yang, Anal. Chem., 2020, 92, 8599–8606 CrossRef CAS PubMed.
- D. S. Rocha, R. P. S. de Campos, H. A. Silva-Neto, G. F. Duarte-Junior, F. Bedioui and W. K. T. Coltro, Anal. Chim. Acta, 2023, 1254, 341077 CrossRef CAS PubMed.
- A. K. Knipes, A. Summers, A. A. Sklavounos, J. Lamanna, R. P. S. de Campos, T. Narahari, C. Dixon, R. Fobel, Y. D. Ndjakani, L. Lubula, A. Magazani, J. J. Muyembe, Y. Lay, E. Pukuta, D. Waku-Kouomou, L. Hao, J. K. Kayembe, C. Fobel, J. Dahmer, A. Lee, M. Ho, J. G. C. Valenzuela, D. G. Rackus, R. Shih, B. Seale, A. Chang, G. Paluku, P. A. Rota, A. R. Wheeler and H. M. Scobie, PLoS One, 2022, 17, e0278749 CrossRef CAS PubMed.
- A. H. C. Ng, R. Fobel, C. Fobel, J. Lamanna, D. G. Rackus, A. Summers, C. Dixon, M. D. M. Dryden, C. Lam, M. Ho, N. S. Mufti, V. Lee, M. A. M. Asri, E. A. Sykes, M. D. Chamberlain, R. Joseph, M. Ope, H. M. Scobie, A. Knipes, P. A. Rota, N. Marano, P. M. Chege, M. Njuguna, R. Nzunza, N. Kisangau, J. Kiogora, M. Karuingi, J. W. Burton, P. Borus, E. Lam and A. R. Wheeler, Sci. Transl. Med., 2018, 10, eaar6076 CrossRef PubMed.
- Z. Gu, M. L. Wu, B. Y. Yan, H. F. Wang and C. Kong, ACS Omega, 2020, 5, 11196–11201 CrossRef CAS PubMed.
- D. G. Rackus, M. D. M. Dryden, J. Lamanna, A. Zaragoza, B. Lam, S. O. Kelley and A. R. Wheeler, Lab Chip, 2015, 15, 3776–3784 RSC.
- R. P. S. de Campos, D. G. Rackus, R. Shih, C. Zhao, X. Liu and A. R. Wheeler, Anal. Chem., 2019, 91, 2506–2515 CrossRef CAS PubMed.
- M. H. Shamsi, K. Choi, A. H. C. Ng, M. D. Chamberlain and A. R. Wheeler, Biosens. Bioelectron., 2016, 77, 845–852 CrossRef CAS PubMed.
- T. Liénard-Mayor, M. Taverna, S. Descroix and T. D. Mai, Anal. Chim. Acta, 2021, 1143, 281–297 CrossRef PubMed.
- S. C. C. Shih, H. Yang, M. J. Jebrail, R. Fobel, N. McIntosh, O. Y. Al-Dirbashi, P. Chakraborty and A. R. Wheeler, Anal. Chem., 2012, 84, 3731–3738 CrossRef CAS PubMed.
- Y. Wang, Q. Ruan, Z.-C. Lei, S.-C. Lin, Z. Zhu, L. Zhou and C. Yang, Anal. Chem., 2018, 90, 5224–5231 CrossRef CAS PubMed.
- I. Swyer, R. Soong, M. D. M. Dryden, M. Fey, W. E. Maas, A. Simpson and A. R. Wheeler, Lab Chip, 2016, 16, 4424–4435 RSC.
- T. Komatsu, M. Tokeshi and S.-K. Fan, Biosens. Bioelectron., 2022, 195, 113631 CrossRef CAS PubMed.
- M. Ho, N. Sathishkumar, A. A. Sklavounos, J. Sun, I. Yang, K. P. Nichols and A. R. Wheeler, Lab Chip, 2024, 24, 63–73 RSC.
- M. H. Shamsi, K. Choi, A. H. C. Ng and A. R. Wheeler, Lab Chip, 2014, 14, 547–554 RSC.
- G. Sathyanarayanan, M. Haapala, C. Dixon, A. R. Wheeler and T. M. Sikanen, Adv. Mater. Technol., 2020, 5, 2000451 CrossRef.
- C. Liu, K. Choi, Y. Kang, J. Kim, C. Fobel, B. Seale, J. L. Campbell, T. R. Covey and A. R. Wheeler, Anal. Chem., 2015, 87, 11967–11972 CrossRef PubMed.
- K. Choi, E. Boyaci, J. Kim, B. Seale, L. Barrera-Arbelaez, J. Pawliszyn and A. R. Wheeler, J. Chromatogr. A, 2016, 1444, 1–7 CrossRef PubMed.
- C. R. Nemr, A. A. Sklavounos, A. R. Wheeler and S. O. Kelley, SLAS Technol., 2023, 28, 2–15 CrossRef PubMed.
- F. R. De Souza, G. F. Duarte-Junior, P. T. Garcia and W. K. T. Coltro, Quim. Nova, 2014, 37, 1171–1176 CrossRef.
- Y. Fan, J. Li, Y. Guo, L. Xie and G. Zhang, Measurement, 2021, 171, 108829 CrossRef.
- G. M. Fernandes, W. R. Silva, D. N. Barreto, R. S. Lamarca, P. C. F. L. Gomes, J. F. da S Petruci and A. D. Batista, Anal. Chim. Acta, 2020, 1135, 187–203 CrossRef PubMed.
- S. Patel, R. Jamunkar, D. Sinha, Monisha, T. K. Patle, T. Kant, K. Dewangan and K. Shrivas, Trends Environ. Anal. Chem., 2021, 31, e00136 CrossRef.
- G. Alberti, C. Zanoni, L. R. Magnaghi and R. Biesuz, Int. J. Environ. Res. Public Health, 2020, 17, 1–23 Search PubMed.
- E. Noviana, D. B. Carrão, R. Pratiwi and C. S. Henry, Anal. Chim. Acta, 2020, 1116, 70–90 CrossRef PubMed.
- H. A. Silva-Neto, I. V. S. Arantes, A. L. Ferreira, G. H. M. do Nascimento, G. N. Meloni, W. R. de Araujo, T. R. L. C. Paixão and W. K. T. Coltro, TrAC, Trends Anal. Chem., 2023, 158, 116893 CrossRef CAS.
- T. Ozer, C. McMahon and C. S. Henry, Annu. Rev. Anal. Chem., 2020, 13, 85–109 CrossRef PubMed.
- L. R. Sousa, H. A. Silva-Neto, L. F. Castro, K. A. Oliveira, F. Figueredo, E. Cortón and W. K. T. Coltro, Anal. Bioanal. Chem., 2023, 415, 4391–4400 CrossRef CAS PubMed.
- E. Noviana, T. Ozer, C. S. Carrell, J. S. Link, C. McMahon, I. Jang and C. S. Henry, Chem. Rev., 2021, 121, 11835–11885 CrossRef CAS PubMed.
- R. A. Ruiz, J. L. Gonzalez, M. Vazquez-Alvarado, N. W. Martinez and A. W. Martinez, Anal. Chem., 2022, 94, 8833–8837 CrossRef CAS PubMed.
- E. Carrilho, A. W. Martinez and G. M. Whitesides, Anal. Chem., 2009, 81, 7091–7095 CrossRef CAS PubMed.
- S. V. De Freitas, F. R. De Souza, J. C. Rodrigues Neto, G. A. Vasconcelos, P. V. Abdelnur, B. G. Vaz, C. S. Henry and W. K. T. Coltro, Anal. Chem., 2018, 90, 11949–11954 CrossRef CAS PubMed.
- A. Abadian, S. S. Manesh and S. J. Ashtiani, Microfluid. Nanofluid., 2017, 21, 65 CrossRef.
- S. Chaiyo, K. Kalcher, A. Apilux, O. Chailapakul and W. Siangproh, Analyst, 2018, 143, 5453–5460 RSC.
- R. Hiraoka, K. Kuwahara, Y.-C. Wen, T.-H. Yen, Y. Hiruta, C.-M. Cheng and D. Citterio, ACS Sens., 2020, 5, 1110–1118 CrossRef CAS PubMed.
- N. Nurrahmah, K. T. Amalia, S. Hermin and S. Akhmad, J. Appl. Pharm. Sci., 2022, 12, 140–148 CAS.
- I. Lewińska, M. Speichert, M. Granica and Ł. Tymecki, Sens. Actuators, B, 2021, 340, 129915 CrossRef.
- R. Cánovas, M. Cuartero and G. A. Crespo, Biosens. Bioelectron., 2019, 130, 110–124 CrossRef PubMed.
- T. Brooks and C. W. Keevil, Lett. Appl. Microbiol., 1997, 24, 203–206 CrossRef CAS PubMed.
- N. S. Moreira, C. L. S. Chagas, K. A. Oliveira, G. F. Duarte-Junior, F. R. de Souza, M. Santhiago, C. D. Garcia, L. T. Kubota and W. K. T. Coltro, Anal. Chim. Acta, 2020, 1119, 1–10 CrossRef CAS PubMed.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed.
- B. Debus, D. Kirsanov, I. Yaroshenko, A. Sidorova, A. Piven and A. Legin, Anal. Chim. Acta, 2015, 895, 71–79 CrossRef CAS PubMed.
- E. L. Rossini, M. I. Milani, E. Carrilho, L. Pezza and H. R. Pezza, Anal. Chim. Acta, 2018, 997, 16–23 CrossRef CAS PubMed.
- N. Mudgal, A. Saharia, A. Agarwal, J. Ali, P. Yupapin and G. Singh, Opt. Quantum Electron., 2020, 52, 307 CrossRef CAS.
- N. N. Gabaj, M. Miler, A. Unic, L. M. Kopcinovic, A. Vrtaric and J. Culej, Ann. Clin. Biochem., 2020, 57, 64–68 CrossRef PubMed.
- S. Chattopadhyay, R. Ram, A. Sarkar and S. Chakraborty, Measurement, 2022, 199, 111492 CrossRef.
- J. Sittiwong and F. Unob, Anal. Sci., 2016, 32, 639–643 CrossRef CAS PubMed.
- S. Sununta, P. Rattanarat, O. Chailapakul and N. Praphairaksit, Anal. Sci., 2018, 34, 109–113 CrossRef CAS PubMed.
- J. Berthier, Micro-Drops and Digital Microfluidics, William Andrew, 2008.
- S. Feng, R. Shi, P. Xu, J. R. Bhamore, J. Bal, S. H. Baek, C. Y. Park, J. P. Park and T. J. Park, New J. Chem., 2020, 44, 15828–15835 RSC.
- Ministério da Saúde Agência Nacional de Vigilância Sanitária, Resolução Da Diretoria Colegiada – RDC No. 166, DE 24 DE JULHO DE 2017, Brazil, 2017 Search PubMed.
- S. A. Gegenschatz, F. A. Chiappini, C. M. Teglia, A. Muñoz de la Peña and H. C. Goicoechea, Anal. Chim. Acta, 2022, 1209, 339342 CrossRef CAS PubMed.
- A. Mathaweesansurn, S. Thongrod, P. Khongkaew, C. M. Phechkrajang, P. Wilairat and N. Choengchan, Talanta, 2020, 210, 120675 CrossRef CAS PubMed.
- Ministério da Saúde Agência Nacional de Vigilância Sanitária, Resolução – RDC No 27, DE 17 DE MAIO DE 2012, Brazil, 2012 Search PubMed.
- J. N. Miller and J. C. Miller, Statistics and chemometrics for analytical chemistry, Pearson/Prentice Hall, 2005 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4an00688g |
| This journal is © The Royal Society of Chemistry 2025 |