Controlling chemical interface damping by removing aromatic monothiol and dithiol groups from gold nanorods using sodium borohydride solution†
Ji Min
Kim
a and
Ji Won
Ha
 *ab
*ab
aDepartment of Chemistry, University of Ulsan, 93 Daehak-ro, Nam-gu, Ulsan 44610, Republic of Korea. E-mail: jwha77@ulsan.ac.kr; Fax: +8252 712 8002; Tel: +82 52 712 8012
bEnergy Harvest-Storage Research Center (EHSRC), University of Ulsan, 93 Daehak-ro, Nam-gu, Ulsan 44610, South Korea
First published on 2nd December 2024
Abstract
Chemical interface damping (CID) in gold nanorods (AuNRs) significantly influences their optical properties due to the direct transfer of hot electrons from the AuNRs to adsorbed molecules. Despite ongoing research on CID, reversible tuning of CID at the single particle level remains a challenging task. In this study, we investigated the adsorption and removal of thiol-functionalized aromatic molecules, specifically thiophenol (TP) and benzene-1,2-dithiol (BDT), using sodium borohydride (NaBH4) solution as a reagent, with confirmation through surface-enhanced Raman scattering (SERS) measurements. We further examined the effect of NaBH4 solution pH, immersion time in solution, and the number of thiol groups in the adsorbate (TP and BDT) on removal efficiency from the AuNR surfaces. Additionally, we extended this approach to directly control CID in single AuNRs via the adsorption and desorption of TP and BDT molecules under dark-field microscopy and spectroscopy. Therefore, this study provides insights into the removal of aromatic thiol molecules using NaBH4, as well as the direct control of CID in individual AuNRs.
The functionalization of gold nanoparticles with thiol-containing molecules has garnered significant interest in nanotechnology and surface chemistry due to its wide-ranging applications, including catalysis, biosensing, and drug delivery.1–4 Gold nanorods (AuNRs), in particular, are a popular choice for such functionalization because of their unique optical properties, governed by localized surface plasmon resonance (LSPR).5,6 The adsorption of thiol-functionalized molecules onto AuNR surfaces is primarily driven by the strong affinity between the sulfur atoms in thiol groups and the Au surface, forming robust Au–S bonds.7–9 This interaction has facilitated the development of various surface-enhanced Raman scattering (SERS)-based sensing platforms, where AuNRs serve both as the sensing substrate and as the plasmonic enhancer.10–12
Despite the strength and utility of Au–S bonds, there is growing interest in controlling the reversibility of thiol adsorption on Au surfaces.13 Reversible adsorption–desorption processes offer the potential to reuse functionalized surfaces and dynamically regulate molecular interactions at the nanoscale. Several methods have been reported for removing thiol groups from Au surfaces, including sodium borohydride (NaBH4) treatment, plasma cleaning, thermal desorption, ozone and UV radiation, a laser-beam irradiation, etc.14–21 Among these, NaBH4, a widely used reducing agent, has emerged as a simple and effective method for mediating the desorption of thiols from Au surfaces. NaBH4 displaces thiol molecules by generating hydride ions (H−), which have a stronger binding affinity to AuNR surfaces than thiol groups.17 This process enables the removal of adsorbed thiols, regenerating bare AuNRs for subsequent functionalization.
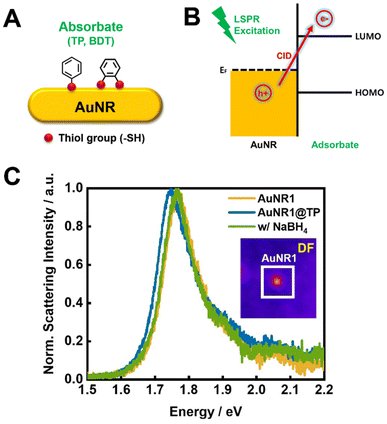
In addition to controlling thiol adsorption and desorption, the interaction between AuNRs and adsorbed thiol molecules can induce significant changes in the electronic and optical properties of the nanorods. A phenomenon known as chemical interface damping (CID)—one of the plasmon damping pathways in AuNRs—occurs when adsorbate molecules are strongly bound to AuNR surfaces.22–30 This mechanism involves the transfer of hot electrons, generated by surface plasmons in the Au, directly to the lowest unoccupied molecular orbital (LUMO) of the adsorbate. The CID effect is characterized by a redshift and broadening of the LSPR peak, along with a decrease in scattering intensity.31–34 Although recent studies have explored CID in single AuNRs, the effects of NaBH4 solution in controlling the adsorption and desorption of thiol-functionalized aromatic molecules, and its application in tuning CID, have not been reported. Thus, to our knowledge, this is the first report to use NaBH4 for achieving reversible tuning of CID using thiol-functionalized aromatic molecules in single AuNRs.
In this study, we employed SERS to investigate the adsorption and desorption of thiol-functionalized aromatic molecules—specifically thiophenol (TP) and benzene-1,2-dithiol (BDT)—on AuNR surfaces using NaBH4 as a desorption agent. We investigated how varying the pH of the NaBH4 solution affects the rate and efficiency of thiol removal and compared the desorption behavior of monothiol TP and dithiol BDT molecules. Additionally, we examined the tunability of CID in single AuNRs induced by the adsorption and desorption of these thiols, utilizing dark-field (DF) microscopy and spectroscopy.
The AuNRs employed in this study were purchased from Nanopartz (Loveland, CO, USA) and characterized using scanning electron microscopy (SEM). Fig. 1A shows an SEM image of AuNRs stabilized by a cetyl trimethyl ammonium bromide (CTAB) layer. The average lengths of the AuNRs were measured to be 26.23(±4.93) nm along the vertical axis and 74.29(±8.73) nm along the horizontal axis (Fig. S1†), yielding an aspect ratio of 2.85(±0.61) (Fig. 1B). The UV-Vis extinction spectrum of AuNRs dispersed in distilled water revealed two characteristic LSPR peaks: a transverse peak at 516 nm and a longitudinal peak at 718 nm (Fig. S2†).
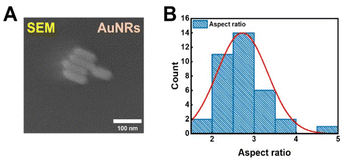 | ||
| Fig. 1 (A) SEM image of the CTAB-stabilized AuNRs used in this study. (B) Histogram showing the average aspect ratio, determined to be 2.85(±0.60). | ||
In this study, we first investigated the adsorption and subsequent desorption of thiol-functionalized aromatic molecules, specifically monothiol TP and dithiol BDT, using sodium borohydride (NaBH4) as a reducing agent. SERS measurements were utilized to confirm these processes. TP and BDT were selected due to their strong soft–soft interactions between the sulfur atoms in the thiol group and the AuNR surface.9,35 Fig. S3 and S4† illustrate the experimental setup and schematic representation of the SERS technique, respectively. A 785 nm laser wavelength was used as the excitation source, maximizing resonance effects and enhancing Raman signal intensity.28 The procedure for surface thiolation and desorption on AuNRs is depicted in Fig. 2. First, aromatic thiols (TP and BDT) were introduced to thiolate the AuNR surfaces (Step 1). Following this, the thiolated AuNRs were immersed in a NaBH4 solution at pH 12 to remove the thiols, restoring the AuNR surface to its bare state (Step 2). This removal is possible because the hydride ion (H−) generated by NaBH4 has a high binding energy of 118 kcal mol−1, exhibiting a stronger affinity for the AuNR surface than thiol groups, thereby enabling efficient desorption.17 We first verified the thiolation (Step 1) and desorption (Step 2) of TP and BDT on the AuNR surfaces using UV-Vis extinction spectroscopy (Fig. S5†). The LSPR peak of AuNRs was observed to redshift upon TP introduction, followed by a blueshift after the addition of NaBH4 (pH 12), indicating the removal of TP. Afterward, to monitor the adsorption process in real time, we recorded SERS spectra during the attachment of 1 mM TP and 1 mM BDT to AuNRs dispersed in ethanol (Fig. S6 and S7†). The prominent Raman peak observed at ∼1089 cm−1 corresponds to the Au–S covalent bond and is indicative of the benzene ring present in the thiol molecules.28 After 1 h of attachment, the intensity of the C–S vibration peak increased, signifying the formation of a strong Au–S bond between the molecules and the AuNR surface. This confirmed the successful adsorption of both TP and BDT onto the AuNRs.
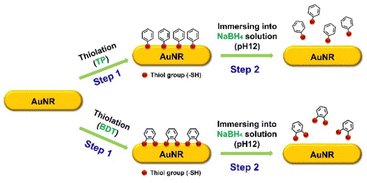 | ||
| Fig. 2 Schematic illustration of the sequential process of thiolation and desorption of thiol functional groups with aromaticity (TP and BDT). | ||
We then proceeded to the desorption phase by adding a 0.5 M NaBH4 solution to the thiolated AuNRs, monitoring the C–S vibration peak as a marker for desorption in real-time (Fig. 3A and B, and S8†). As desorption occurred, the intensity of the C–S peak gradually decreased, confirming that aromatic thiols (TP and BDT) were effectively removed from the AuNR surface. A comparison of the desorption rates between TP and BDT revealed distinct differences (Fig. 3D). The intensity of the TP peak decreased sharply after 9 min, whereas the BDT peak decreased more gradually, becoming notable only after 48 min. This suggests that the presence of dithiol BDT strengthens the Au–S bond, making desorption more challenging. More specifically, the monothiol molecules with an –SH reactive group (here TP) having a firm affinity for the Au surface has a free energy of adsorption on Au by Au–S bond is −14.7 kJ mol−1, thus forming strong covalent bonds between Au and S atoms on AuNRs. Since the BDT molecules have two –SH reactive groups, the free energy of adsorption of BDT on Au is approximated at −27.3 kJ mol−1, thus form a twice stronger adsorption to Au atoms.36,37 Therefore, having a higher free energy of adsorption, BDT molecules exhibit a slower desorption kinetics as evident form our single-particle LSPR study. In addition, control experiments, where AuNRs were immersed in ethanol and NaBH4 without thiol molecules, showed no significant change in the Raman peak intensity, confirming that NaBH4 selectively removes thiols (Fig. S9†).
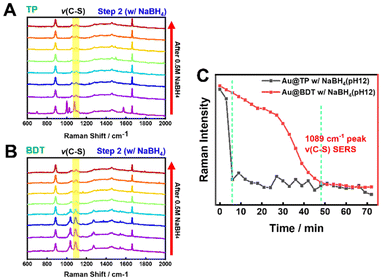 | ||
| Fig. 3 SERS spectra showing the removal of each molecule (TP, BDT) from AuNRs using NaBH4 solution prepared at pH 12. (A) A rapid decrease in peak intensity corresponding to the C–S vibration is observed 9 min after attachment of 1 mM TP to Au (Fig. S8†). (B) A decrease in peak intensity corresponding to the C–S vibration is observed 48 min after attachment of 1 mM BDT to Au (Fig. S8†). (C) Comparison of intensity changes at the 1089 cm−1 peak, corresponding to the C–S vibration, when thiolated AuNRs with TP and BDT are immersed in NaBH4 solution. | ||
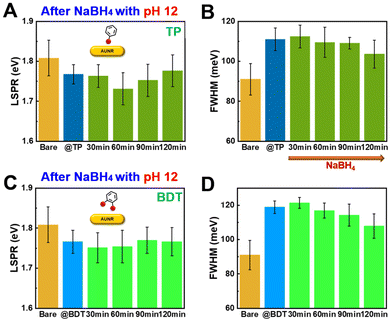
Next, we investigated the influence of NaBH4 solution pH on the desorption process. The chemical stability of NaBH4, and consequently its efficiency in removing thiols, can be adjusted by varying the pH of the solution. We compared the desorption of TP-thiolated AuNRs using NaBH4 solutions at pH 8 and pH 12. The real-time SERS spectra (Fig. 4A and B, and S10†) revealed that at pH 8, desorption of TP occurred after 51 min, significantly slower than the 9 min observed at pH 12. Similarly, for BDT, desorption occurred after 75 min at pH 8, compared to 48 min at pH 12. This suggests that higher pH enhances the stability of NaBH4, facilitating more efficient desorption. These findings demonstrate that the pH of the NaBH4 solution plays a critical role in regulating the desorption rate and that thiol desorption is more effective in alkaline conditions.
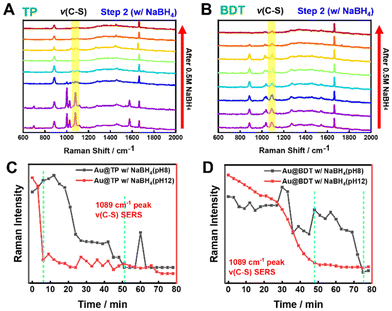 | ||
| Fig. 4 SERS spectra showing the removal of each molecule (TP, BDT) from AuNRs using NaBH4 solution prepared at pH 8. (A) Time-dependent variation of the C–S vibration Raman peak for AuNRs with 1 mM TP attached. A decrease in the intensity of the C–S vibration peak is observed after 24 min (Fig. S10†). (B) Time-dependent variation of the C–S vibration Raman peak for AuNRs with 1 mM BDT attached. A decrease in the intensity of the C–S vibration peak is observed after 72 min (Fig. S10†). (C and D) The intensity changes of the C–S vibration peak at 1089 cm−1 were compared for AuNRs thiolated with (C) TP and (D) BDT immersed in NaBH4 solutions at pH 8 and pH 12. | ||
To further explore the molecular interactions and distinct optical properties of AuNRs, we conducted single-particle scattering measurements using dark-field (DF) microscopy and spectroscopy (Fig. S11†). The experimental approach followed was similar to the SERS experiments (Fig. 2). As shown in Fig. 5A and B, the TP and BDT molecules, strongly interacting with the AuNR surface, induce interfacial hot electron transfer, a phenomenon known as CID. This occurs when energetic hot electrons generated in Au transfer to the empty molecular orbital (LUMO) of the adsorbed molecules (Fig. 5B), leading to changes in the LSPR spectrum.25,31,32 Specifically, CID results in a broadening of the full width at half maximum (FWHM) and a redshift in the LSPR spectrum, along with a reduction in scattering intensity. We first tried to confirm the adsorption and subsequent removal of TP on single AuNRs. The inset of Fig. 5C shows a DF scattering image of an individual AuNR deposited on a glass slide. The LSPR scattering spectra for a single AuNR were measured before and after the adsorption of TP in ethanol, and again after thiol removal using NaBH4 (Fig. 5C). TP adsorption resulted in a redshift in the LSPR peak, indicating successful surface functionalization. Following the addition of NaBH4, the LSPR peak shifted back to its original position, confirming effective thiol removal. Additionally, the FWHM increased after TP adsorption, suggesting the occurrence of CID in single AuNR1. Moreover, control experiments in which AuNRs were exposed to ethanol or NaBH4 without thiol molecules showed no significant spectral changes (Fig. S12†).
To further validate these observations, we performed a comprehensive statistical analysis of the LSPR peak energy and FWHM across multiple AuNRs (Fig. 6 and S13 and S14†). Consistent with the single AuNR results shown in Fig. 5, TP thiolation caused a redshift in the LSPR peak, while desorption led to a gradual blueshift over time (Fig. 6A). The FWHM increased after TP adsorption, indicating stronger molecular interaction and the occurrence of CID, and subsequently decreased after the thiol was removed with NaBH4 (Fig. 6B). These trends were also observed for BDT (Fig. 6C and D, and S14†), confirming that both TP and BDT can be effectively desorbed from the AuNR surface using NaBH4 solution. Moreover, the results demonstrate that CID can be tuned by controlling the adsorption and desorption of aromatic thiols on AuNR surfaces, highlighting the potential for reversible modulation of the optical properties.
In summary, this study provides a comprehensive examination of the adsorption and desorption of thiol-functionalized aromatic molecules (monothiol TP and dithiol BDT) on AuNR surfaces using NaBH4 solution as a reagent. We demonstrated that the desorption efficiency is strongly influenced by the pH of the NaBH4 solution and the number of thiol groups (monothiol or dithiol) in the adsorbate molecules. Additionally, we used DF microscopy and spectroscopy to reveal the CID effect induced by TP and BDT on individual AuNRs and showed how CID can be controlled through the reversible adsorption and desorption of these molecules. Our findings offer new insights into the manipulation of thiol desorption and CID at the nanoscale, with potential applications in nanotechnology and surface chemistry.
Author contributions
J. M. Kim performed all the experiments. J. M. Kim and J. W. Ha analyzed the data and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by two National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIP) (No. RS-2023-00208346, RS-2024-00407859, and 2019R1A6A1A11053838). DF spectroscopic analysis was done by using microscope camera system at total-period analysis center for Ulsan chemical industry. SDG.References
- A. Karnwal, R. S. Kumar Sachan, I. Devgon, J. Devgon, G. Pant, M. Panchpuri, A. Ahmad, M. B. Alshammari, K. Hossain and G. Kumar, ACS Omega, 2024, 9, 29966–29982 CrossRef CAS PubMed.
- S. A. Bansal, V. Kumar, J. Karimi, A. P. Singh and S. Kumar, Nanoscale Adv., 2020, 2, 3764–3787 RSC.
- P. M. Tiwari, K. Vig, V. A. Dennis and S. R. Singh, Nanomaterials, 2011, 1, 31–63 CrossRef CAS PubMed.
- K. Nejati, M. Dadashpour, T. Gharibi, H. Mellatyar and A. Akbarzadeh, J. Cluster Sci., 2022, 33, 1–16 CrossRef CAS.
- H. Hou, L. Chen, H. He, L. Chen, Z. Zhao and Y. Jin, J. Mater. Chem. B, 2015, 3, 5189–5196 RSC.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 8410–8426 CrossRef CAS.
- Y. Xue, X. Li, H. Li and W. Zhang, Nat. Commun., 2014, 5, 4348 CrossRef CAS PubMed.
- T. Bürgi, Nanoscale, 2015, 7, 15553–15567 RSC.
- S. W. Moon, P. V. Tsalu and J. W. Ha, Phys. Chem. Chem. Phys., 2018, 20, 22197–22202 RSC.
- B. M. DeVetter, P. Mukherjee, C. J. Murphy and R. Bhargava, Nanoscale, 2015, 7, 8766–8775 RSC.
- J. Ahn, S. Shi, B. Vannatter and D. Qin, J. Phys. Chem. C, 2019, 123, 21571–21580 CrossRef CAS.
- Y. Hong, R. Wang, Z. Jiang, Z. Cong and H. Song, Int. J. Anal. Chem., 2020, 2020, 9271236 Search PubMed.
- Y. A. Hong and J. W. Ha, Analyst, 2023, 148, 3719–3723 RSC.
- M. Ramasamy and J. W. Ha, J. Chem. Phys., 2022, 157, 014702 CrossRef CAS PubMed.
- J. Lee and J. W. Ha, Analyst, 2021, 146, 4125–4129 RSC.
- E. Ito, H. Kang, D. Lee, J. B. Park, M. Hara and J. Noh, J. Colloid Interface Sci., 2013, 394, 522–529 CrossRef CAS PubMed.
- S. M. Ansar, F. S. Arneer, W. F. Hu, S. L. Zou, C. U. Pittman and D. M. Zhang, Nano Lett., 2013, 13, 1226–1229 CrossRef CAS PubMed.
- H. T. N. Le, L. M. T. Phan and S. Cho, Materials, 2022, 15, 2218 CrossRef CAS PubMed.
- M. R. Shadnam, S. E. Kirkwood, R. Fedosejevs and A. Amirfazli, J. Phys. Chem. B, 2005, 109, 11996–12002 CrossRef CAS PubMed.
- M. R. Shadnam and A. Amirfazli, Chem. Commun., 2005, 41, 4869–4871 RSC.
- C. G. Worley and R. W. Linton, J. Vac. Sci. Technol., A, 1995, 13, 2281–2284 CrossRef CAS.
- B. Foerster, A. Joplin, K. Kaefer, S. Celiksoy, S. Link and C. Sönnichsen, ACS Nano, 2017, 11, 2886–2893 CrossRef CAS PubMed.
- J. W. Ha, Chem. Phys. Lett., 2017, 676, 65–69 CrossRef CAS.
- S. E. Heo and J. W. Ha, Bull. Korean Chem. Soc., 2022, 43, 797–800 CrossRef CAS.
- J. M. Kim and J. W. Ha, Chem. Commun., 2024, 60, 6312–6315 RSC.
- S. W. Moon and J. W. Ha, Phys. Chem. Chem. Phys., 2019, 21, 7061–7066 RSC.
- M. Ramasamy and J. W. Ha, J. Phys. Chem. Lett., 2023, 14, 5768–5775 CrossRef CAS PubMed.
- M. J. Seo, G. W. Kim, P. V. Tsalu, S. W. Moon and J. W. Ha, Nanoscale Horiz., 2020, 5, 345–349 RSC.
- A. J. Therrien, M. J. Kale, L. Yuan, C. Zhang, N. J. Halas and P. Christopher, Faraday Discuss., 2019, 214, 59–72 RSC.
- J. Lee and J. W. Ha, Anal. Chem., 2022, 94, 7100–7106 CrossRef CAS PubMed.
- J. Lee and J. W. Ha, ACS Appl. Mater. Interfaces, 2024, 16, 45763–45770 CrossRef CAS PubMed.
- S. Y. Lee, P. V. Tsalu, G. W. Kim, M. J. Seo, J. W. Hong and J. W. Ha, Nano Lett., 2019, 19, 2568–2574 CrossRef CAS PubMed.
- Y. Y. Alizar, M. Ramasamy, G. W. Kim and J. W. Ha, JACS Au, 2023, 3, 3247–3258 CrossRef CAS PubMed.
- H. B. Jeon, S. Park, K. R. Ryu, S. K. Ghosh, J. Jung, K. M. Park and J. W. Ha, Chem. Sci., 2021, 12, 7115–7124 RSC.
- S. E. Heo and J. W. Ha, Bull. Korean Chem. Soc., 2023, 44, 916–920 CrossRef CAS.
- M. Ramasamy and J. W. Ha, Anal. Chem., 2024, 96, 18043–18051 CrossRef CAS PubMed.
- Y. J. Lee, I. C. Jeon, W.-K. Paik and K. Kim, Langmuir, 1996, 12, 5830–5837 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary figures. See DOI: https://doi.org/10.1039/d4an01187b |
| This journal is © The Royal Society of Chemistry 2025 |