Nanocarbon eco-hydrogel kit: on-site visual metal ion sensing and dye cleanup, advancing the circular economy in environmental remediation†
Omkar S.
Nille
 a,
Akanksha G.
Kolekar
a,
Pooja V.
Devre
b,
Sneha V.
Koparde
a,
Aniket H.
Sawat
ab,
Daewon
Sohn
c,
Shashikant P.
Patole
a,
Akanksha G.
Kolekar
a,
Pooja V.
Devre
b,
Sneha V.
Koparde
a,
Aniket H.
Sawat
ab,
Daewon
Sohn
c,
Shashikant P.
Patole
 *d,
Prashant V.
Anbhule
*d,
Prashant V.
Anbhule
 a,
Anil H.
Gore
a,
Anil H.
Gore
 *b and
Govind B.
Kolekar
*b and
Govind B.
Kolekar
 *a
*a
aFluorescence Spectroscopy Research Laboratory, Department of Chemistry, Shivaji University, Kolhapur-416004, Maharashtra, India. E-mail: gbk_chem@unishivaji.ac.in
bTarsadia Institute of Chemical Science, Uka Tarsadia University, Maliba Campus, Bardoli, Tarsadi-394350, Surat, Gujarat, India. E-mail: anilanachem@gmail.com
cDepartment of Chemistry and Research Institute for Convergence of Basic Science, Hanyang University, Seoul Campus, Seoul, South Korea
dKhalifa University of Science and Technology, Abu Dhabi 127788, United Arab Emirates. E-mail: shashikant.patole@ku.ac.ae
First published on 26th November 2024
Abstract
The naked-eye detection of hazardous pollutants through simple and cost-effective techniques is of great interest to the scientific community and related stakeholders in analytical science. The present study emphases the development of a stimuli-responsive probe by encountering sophisticated techniques for the detection of environmental pollutants. Herein, highly swellable and fluorescent-WTR-CDs-loaded HB-Alg/Gel@WTR-CDs was fabricated through a simple extrusion dripping method. The fluorescent WTR-CDs-loaded composite hydrogel showed rapid (within 10–15 min) naked-eye detection with high selectivity and sensitivity towards Cr6+ and Mn7+ ions over other metal ions. The developed probe had a linear detection range of 0–10 μg mL−1 with a detection limit of 0.28 μg mL−1 and 0.30 μg mL−1 for Cr6+ and Mn7+ ions, respectively. Interestingly, the hydrogel-based fluorescent sensor enabled on-site naked-eye detection of real water samples with good recovery. Additionally, the recyclability and reusability approach were employed for the removal of model pollutant dyes with Alg/Gel-carbon, which was synthesized using spent hydrogel beads after sensing. The present study demonstrates the tremendous potential applications of HB-Alg/Gel@WTR-CDs for simple, low-cost and fast visual detection of environmental pollutants. According to the analytical greenness evaluation (AGREE), the developed analytical method is green with an AGREE score of 0.81 and an ecofriendly circular-economy approach.
1. Introduction
The recent scientific development of innovative and sustainable approaches with advanced materials has opened a window for a wide field of applications. The combination of two or more materials improves the physico-chemical properties compared to the pristine materials, resulting in high demand for such materials. Three-dimensional (3D) networked polymeric hydrogels are one such class of materials and have attracted great attention.1 The impregnation of nanomaterials (NMs) into a hydrogel matrix integrates the effectiveness of composite hydrogels.2 Nanocomposite hydrogels possess fascinating properties viz. enhanced mechanical strength, good water/analyte holding capacity, biocompatibility, swellability, and controlled/ stimuli (pH, temperature, etc.)-responsive release behavior, making them remarkable candidates for multifaceted applications.2,3 Hydrogels have been used as supporting materials for various drugs, carbon dots (CDs), NMs, and various analytes, etc., making them good carrier materials for immobilized materials.4–6 Nanocomposite hydrogels integrate the properties of both the NMs as well as polymers.In the last few decades, anthropogenic activities and growing industrialization have given rise to the destruction of environmental and natural resources. The toxic byproducts and wastes generated from various industries, viz. the textile, leather, printing, dyeing, agrochemical, electronics, chemical processing, mining, and metallurgy industries, etc., have increased tremendously.7–10 During these processes, the wastewater produced is inevitably contaminated with heavy metals.5,11–14 Inappropriate waste disposal and direct contamination of the environment as well as bodies of water have caused serious health issues.15–18 Chromium (Cr) and manganese (Mn) are used in various processes and are often discharged into wastewater without proper treatment. In nature, chromium exists in the Cr6+ and Cr3+ oxidation states, of which Cr3+ is found to be less toxic and stable.19–21 Cr3+ is considered to be an essential nutrient that plays a vital role in the regulation of glucose hemostasis.22 Similarly, potassium permanganate (KMnO4) in the typical Mn7+ oxidation state is commonly used as a disinfectant and antiseptic material, as well as for treating polluted water; it is also an essential trace nutrient in various enzymatic and biological processes.23,24 According to the standards set by the World Health Organization (WHO) and the United States Environmental Protection Agency (US EPA) for water quality, the permissible range of Cr6+ in drinking water is 0.05 μg L−1 and 0.10 μg L−1, respectively.25–27 Whereas manganese acts as a nutrient at low concentrations and continuous exposure to Mn7+ leads to serious health threats. According to the WHO and US EPA, the permissible limit for Mn7+ in drinking water is 80.00 μg L−1 and 50.00 μg L−1, respectively.28–30 Continuous exposure to very low doses of Cr6+ and Mn7+ may cause serious health related issues such as vomiting, diarrhea, cytotoxicity, renal damage, damage to the skin and respiratory tract, disorders of the central nervous system, severely damaging fetal malformations, and cancer.22,31,32 Therefore, it is of prime importance to develop a highly selective and sensitive tool for the recognition of the hazardous pollutants like Cr6+ and Mn7+ in contaminated bodies of water.
Carbon dots (CDs) are new age materials from the carbon family. CDs are tiny materials having a size of less than 10 nm with superior properties, making them a rising star in diverse fields.33–35 The fascinating and unique properties of fluorescent CDs include tunable optical and surface properties, low toxicity, high biocompatibility, and high chemical stability.36–38 CDs provide promising wide range applicability in many research areas viz. bio-imaging,39 drug delivery,40 angiogenesis,41–43 fluorescent ink, photocatalysis,44 optoelectronic devices,45 hazardous pollutant sensors,46–48 wastewater treatment49–51 and environmental remediation.52 CDs with desired surface functional groups (–COOH, –OH, –NH2) can be synthesized, which can be easily embedded into hydrogel matrices via weak van der Waals forces, π–π, and ionic interactions with the functional groups of the polymers.52
The fascinating applicability of nanocomposite hydrogels is greatly influencing researchers around the globe. In particular, the nanocomposite hydrogels have been developed with great interest for environmental remediation as well as sensing and detection of hazardous pollutants. Interestingly, solid-state nanocomposite hydrogel probes have been introduced by various research groups for diverse applications. Prominently, the research group of Devasish Chowdhury has published some interesting reports on CDs-based hydrogel systems for various applications such as smart stimuli-responsive drug delivery systems,53 as well as hazardous metal ion detection (Cr6+, Cu2+, Fe3+, Pb2+, Mn2+).54 In addition, Vaibhav Naik from our research group has developed NCDs-loaded hydrogel strips for the naked-eye detection of dopamine and gold ions.55,56 Also, CDs-loaded alginate–gelatin-based nanocomposite hydrogels have previously been explored for tissue engineering as well as sustainable drug delivery applications.57–59 Carbon-based composite hydrogel beads have specifically been used for applications in drug delivery and the adsorption of environmental pollutants.4,60–63 The swellable nature, stimuli-responsiveness and mechanical properties of carbon-based composite hydrogels have increasing demand for targeted applications. To date, film/strip-based sensors that lack mechanical strength and reusability have been developed. Hence, in the present study, we have developed stimuli-responsive hydrogel beads for the naked-eye detection of hazardous pollutants.
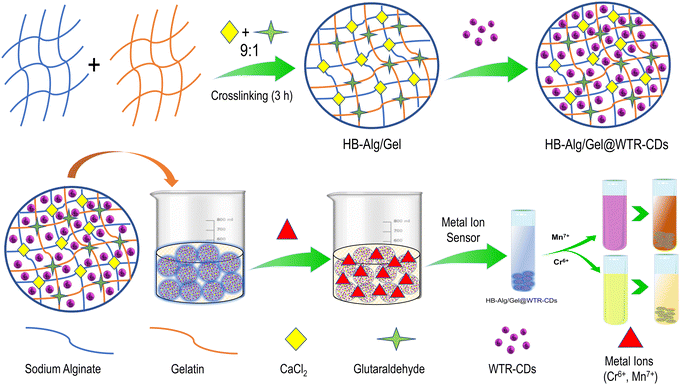
In the present study, a simple, sustainable, low-cost and waste-valorizing approach was applied for the synthesis of fluorescent waste tea residue carbon dots (WTR-CDs). Additionally, a biocompatible and biopolymeric sodium-alginate- and gelatin-based hydrogel system was developed by a simple extrusion dripping method. Further, the alginate/gelatin hydrogel (HB-Alg/Gel) beads were applied for the incorporation of WTR-CDs into the crosslinked polymer network. The WTR-CDs loaded stimuli-responsive composite hydrogel (HB-Alg/Gel@WTR-CDs) beads were applied for the optical detection of hazardous metal ions. The developed probe releases WTR-CDs in a stimuli-responsive manner, which in turn selectively and visually detects Cr6+ and Mn7+ ions. The probe shows consistent results with real water samples. The applied beads were further carbonized and reused as an adsorbent material for the removal of dyes. Also, the greenness of the developed system as per the 12 principles of green analytical chemistry (GAC) is one of the essential parameters. The greenness of the HB-Alg/Gel@WTR-CDs was evaluated using AGREE software. Thus, stimuli-responsive HB-Alg/Gel@WTR-CDs have been successfully synthesized and developed, and have potential applications in hazardous metal ion detection, forensics, wastewater treatment and environmental remediation.
2. Materials and methods
2.1 Equipment
Detailed information regarding the equipment used is given in Text S1 of the ESI.†2.2 Materials
Detailed information regarding the materials and reagents used is given in Text S2 of the ESI.†2.3 Synthesis of waste tea residue (WTR-CDs) carbon dots
The waste tea residue (WTR) was collected from a kitchen and then shade-dried at room temperature. After that, the dried WTR powder was ground into a fine powder. Then, 5 g of fine WTR powder was weighed out and kept in a furnace maintained at a temperature of 200 °C for 5 h.46 After completion of the process, the resultant carbonized WTR powder was ground into a fine powder. The fine carbonized WTR powder was placed in a beaker containing 100 mL of distilled water and kept this beaker on a magnetic stirrer for 5 h. Subsequently, the golden-brownish-colored supernatant solution of WTR-CDs was filtered through the Whatman filter paper 41. The collected WTR-CDs solution was purified by centrifugation at 8000 RPM for 20 min and again passed through a 0.22 μm syringe filter. Fig. S1† displays a schematic representation of the synthesis of waste tea residue carbon dots (WTR-CDs).2.4 Preparation of alginate/gelatin (HB-Alg/Gel) hydrogel beads
The hydrogel beads of alginate and gelatin were prepared using a simple and one-step drop-casting (extrusion dripping) method.40,64 In this method, the calculated/required amounts of the sodium alginate and gelatin polymers were added to two beakers separately and then dissolved in 100 mL of distilled water using a magnetic stirrer for 2 h; the temperature was maintained at 50–60 °C. Finally, a homogeneous solution of 5% sodium alginate (w/v) and 5% gelatin (w/v) was prepared. Subsequently, the freshly prepared 5% sodium alginate and 5% gelatin were combined in different ratios (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5
3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3
5, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 2
7, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, and 1
8, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). From among these ratios, we selected 3
9). From among these ratios, we selected 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 as the optimum ratio of sodium alginate and gelatin, because this ratio gives better size, shape, mechanical strength, and better swelling properties than the other ratios. The homogeneous mixture of sodium alginate and gelatin (3
7 as the optimum ratio of sodium alginate and gelatin, because this ratio gives better size, shape, mechanical strength, and better swelling properties than the other ratios. The homogeneous mixture of sodium alginate and gelatin (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) was added dropwise to a beaker containing a CaCl2 (5%) and glutaraldehyde (2.5%) solution (9
7) was added dropwise to a beaker containing a CaCl2 (5%) and glutaraldehyde (2.5%) solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The spherical beads were allowed to crosslink for 3 h and then washed several times with distilled water to remove excess and unreacted CaCl2 and glutaraldehyde. The prepared HB-Alg/Gel beads were finally dried in an oven at 65 °C for 12 h and stored in air pack container for further use (Fig. S2†).
1). The spherical beads were allowed to crosslink for 3 h and then washed several times with distilled water to remove excess and unreacted CaCl2 and glutaraldehyde. The prepared HB-Alg/Gel beads were finally dried in an oven at 65 °C for 12 h and stored in air pack container for further use (Fig. S2†).
2.5 Preparation of carbon-dot-loaded alginate/gelatin hydrogel beads (HB-Alg/Gel@WTR-CDs)
The completely dried HB-Alg/Gel hydrogel beads were placed in a beaker, and 20 mL of fluorescent WTR-CDs were added to the same beaker, which was then placed on a magnetic stirrer for the homogeneous absorption of the WTR-CDs into the HB-Alg/Gel hydrogel matrix for 12 h (Fig. S2†). The HB-Alg/Gel@WTR-CDs beads were washed with distilled water to remove surface-adhered and loosely bound WTR-CDs, and then dried in an oven at 65 °C for 12 h and stored for further metal ion sensing applications.2.6 Swelling ratio study
Detailed information regarding the swelling ratio study is given in Text S3 of the ESI.†2.7 Interaction of metal ions with HB-Alg/Gel@WTR-CDs: selectivity study
Various metal ions (cations and anions) namely Co2+, Ca2+, Na2+, Zn2+, Fe3+, Hg2+, Sn2+, Mg2+, Fe2+, Mn7+, Li+, Cr6+, Cu2+, So32−, IO3−, I−, SO42−, Cd2+, Ag+, Cr3+, Mn2+, and Pb2+ were used for the sensing study. First, 1 mL of each metal ion solution was diluted to 10 mL and added to a test-tube containing HB-Alg/Gel@WTR-CDs hydrogel beads, and the solution was analyzed after 15 min under ambient conditions. The present study was carried out in triplicate to validate the reproducibility of the developed probe (n = 3). Further, the fluorescence emission spectra of the above samples were recorded at an excitation wavelength of 380 nm. The selectivity of the highly fluorescent HB-Alg/Gel@WTR-CDs towards Cr6+ and Mn7+ ions was investigated, and linearity studies were performed by reacting different concentrations of metal ions with HB-Alg/Gel@WTR-CDs.2.8 Recycling of used beads for synthesis of adsorbent (Alg/Gel-carbon)
The reuse and recycling of used materials is one of the best approaches for cost effectiveness and waste management, as well as valorization of materials. Simple treatments (physical/chemical) of the used materials can result in value-added products. In the present study, the used HB-Alg/Gel@WTR-CDs hydrogel beads were collected and washed with hot distilled water several times for the removal of surface-adhered metal ions. Alginate and gelatin are biopolymers consisting of carbohydrates, which can be used as a precursor of carbon. The washed beads were dried in an oven at 65 °C for 5 h. The dried beads were then placed in a silica crucible and carbonized in a furnace at 400 °C for 3 h. The resultant carbon from the hydrogel beads (Alg/Gel-carbon) was ground using a mortar and pestle and further used as an adsorbent for the removal of model pollutants.2.9 Quantum yield measurement
Detailed information regarding the quantum yield is given in Text S4 of the ESI.†2.10 Measurement of greenness of developed method using AGREE
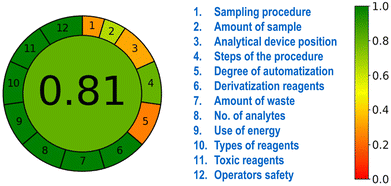
The green and sustainable approach of the developed HB-Alg/Gel@WTR-CDs-based protocol is one of the important parameters. The greenness of the developed method was evaluated using the 12 principles of green analytical chemistry (GAC), and the results were calculated using the open-source Analytical Greenness Calculator software (AGREE).65 Each one of the input variables was converted to a 0 to 1 scale, and the sum of all twelve inputs results in a green, yellow and red scale. The final assessment result is the product of the results for each principle. The dark green colour suggests the developed protocol is more environmentally friendly.3. Results and discussion
The kitchen waste tea residue-derived WTR-CDs, HB-Alg/Gel and their composite HB-Alg/Gel@WTR-CDs hydrogel beads were characterized to investigate both their physico-chemical and morphological properties.3.1 Optical properties of WTR-CDs
The optical properties of the WTR-CDs were investigated using UV-Vis absorption and fluorescence emission spectra. The UV-Vis absorption of the as-prepared WTR-CDs showed a strong peak at 250 nm and a short hump-like absorption peak at 320 nm owing to the n–π* and π–π* transitions due to the sp2 hybridization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities, respectively.35 These were assigned to the typical characteristics of CDs. The WTR-CDs showed excitation-dependent emission behaviour with maximum emission at 450 nm when excited at 380 nm. The fluorescence quantum yield in comparison with quinine sulphate (QS) as a reference was found to be 18.45%. The normalized UV-Vis absorption, as well as the excitation and emission spectra and fluorescence emission spectra of the WTR-CDs at different excitation wavelengths, are shown in Fig. 1.
O functionalities, respectively.35 These were assigned to the typical characteristics of CDs. The WTR-CDs showed excitation-dependent emission behaviour with maximum emission at 450 nm when excited at 380 nm. The fluorescence quantum yield in comparison with quinine sulphate (QS) as a reference was found to be 18.45%. The normalized UV-Vis absorption, as well as the excitation and emission spectra and fluorescence emission spectra of the WTR-CDs at different excitation wavelengths, are shown in Fig. 1.
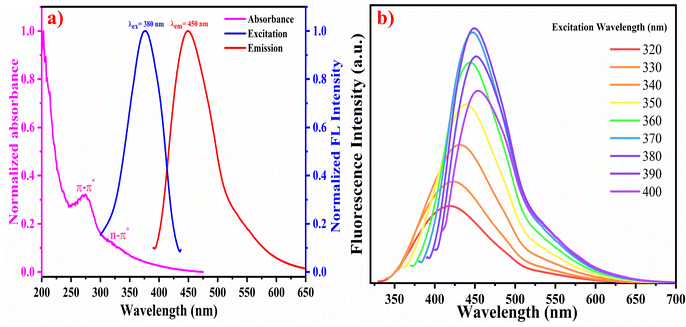 | ||
| Fig. 1 (a) Normalized absorbance, excitation, and emission spectra of the WTR-CDs, (b) Emission spectra of the WTR-CDs at different excitation wavelengths. | ||
3.2 TEM analysis
The TEM analysis showed uniformly shaped WTR-CDs (Fig. S3a–c†). The morphology and size of the WTR-CDs were found to be quasi-spherical shaped with an average particle size in the range of 2–6 nm (Fig. S3d†). The SAED pattern of the WTR-CDs displayed faded circles indicating its amorphous nature along with an interplanar spacing of 0.25 nm corresponding to the (100) plane. The calculated (100) and (002) planes of the SAED pattern match well with the HR-TEM results and planes from XRD analysis, respectively (Fig. S3c and 3e†). The EDX and elemental mapping results showed the prominent presence of the elements C, N, and O in the carbon core (Fig. S3f–g†).3.3 XPS analysis
The WTR-CDs were further characterized using X-ray photoelectron spectroscopy (XPS) to confirm their elemental composition. The survey spectrum of the WTR-CDs was recorded from 0 to 800 eV. The three significant peaks at binding energies of 284 eV, 399 eV and 531 eV correspond to the presence of C 1s, N 1s and O 1s, respectively (Fig. 2). The high-resolution deconvoluted spectra were well fitted with gaussian fittings. The high-resolution deconvoluted spectra of C 1s had four peaks, viz. 283.60 eV, 284.37 eV, 285.49 eV and, 287.25 eV, corresponding to C–C/C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, and C
C, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, respectively (Fig. 2b).66 Further, in Fig. 2c, the N 1s peak is deconvoluted into four peaks attributed to the C–N, N–H, C
N, respectively (Fig. 2b).66 Further, in Fig. 2c, the N 1s peak is deconvoluted into four peaks attributed to the C–N, N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and N–O peaks with respective binding energies of 397.91 eV, 398.77 eV, 399.51 eV and 400.63 eV.67,68 The O 1s spectrum contains two deconvoluted peaks at 530.45 eV and 532.01 eV for C
N, and N–O peaks with respective binding energies of 397.91 eV, 398.77 eV, 399.51 eV and 400.63 eV.67,68 The O 1s spectrum contains two deconvoluted peaks at 530.45 eV and 532.01 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and, C–O respectively.44,55 The XPS and FTIR spectra of the WTR-CDs are highly consistent, confirming the presence of different hydrophilic functionalities leading to interactions with the hydrogel network and with analytes.
O and, C–O respectively.44,55 The XPS and FTIR spectra of the WTR-CDs are highly consistent, confirming the presence of different hydrophilic functionalities leading to interactions with the hydrogel network and with analytes.
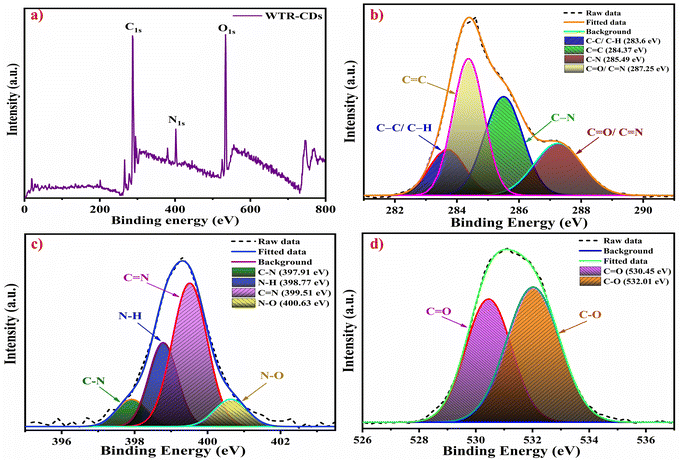 | ||
| Fig. 2 (a) Full-scan XPS survey spectrum of the WTR-CDs. High resolution and deconvoluted (fitted) spectra of (b) C 1s, (c) N 1s, and (d) O 1s. | ||
3.4 FTIR and XRD analysis
Detailed information regarding the FTIR and XRD is given in Text S5 of the ESI.†3.5 FE-SEM and EDX analysis
Detailed information regarding the FE-SEM and EDX analysis is given in Text S6 of the ESI.†3.6 pH effect
Detailed information regarding the pH effect is given in Text S7 of the ESI.†3.7 Swelling study
Detailed information regarding the swelling study is given in Text S8 of the ESI.†3.8 Selectivity of the sensing system
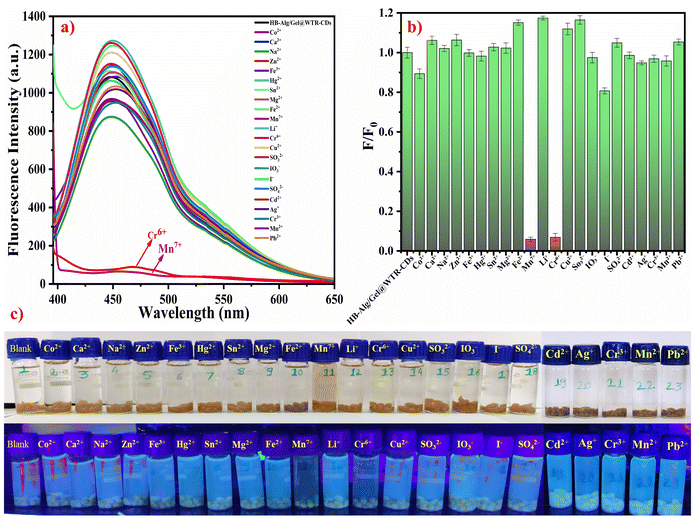
Interestingly, the stimuli-responsiveness of the developed HB-Alg/Gel@WTR-CDs systems could be applied for the release of fluorescent WTR-CDs in aqueous media. The obtained results indicate that only Cr6+ and Mn7+ ions showed a decrease in fluorescence intensity. i.e., they showed strong and highly selective quenching over the other metal ions (Fig. 3a). More importantly, the solution of WTR-CDs released from the hydrogel matrix gradually changed colour in the presence of Cr6+ and Mn7+ metal ions selectively, while no colour change was observed for the other metal ions. Therefore, the fluorescent sensor based on HB-Alg/Gel@WTR-CDs showed selective and rapid fluorescence quenching and optical detection of the metal ions Cr6+ and Mn7+ within 10–15 min. The F/F0 bar graph of the fluorescence intensities for the various metal ions is presented in Fig. 3b, showing that Cr6+ and Mn7+ have strongly diminished values. In addition, photographic images taken in daylight and under UV light strongly support the results and prove that there is a naked-eye-observable change for the Cr6+ and Mn7+ metal ions selectively (Fig. 3c). The developed HB-Alg/Gel@WTR-CDs proved to be applicable as a stimuli-responsive and fluorescent probe for the highly selective and sensitive recognition of Cr6+ and Mn7+ ions. From the characterization and analysis of the results, it was seen that Alg/Gel@WTR-CDs are enriched in hydroxyl, amine, and carboxylic functionalities. These functional groups selectively interact with the Cr6+ and Mn7+ metal ions, and the complex formation between the respective metal ions and Alg/Gel@WTR-CDs results in diminished fluorescence of the CDs.3.9 Optical detection of Cr6+ and Mn7+ ions
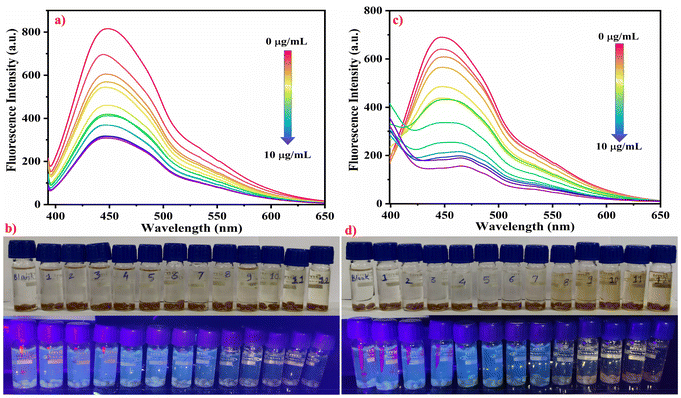
The sensitivity of the developed stimuli-responsive fluorescent HB-Alg/Gel@WTR-CDs probe was verified using different concentrations of Cr6+ and Mn7+ metal ions (Fig. 4). For the study of the linearity, different concentrations (0 to 10 μg mL−1) of both Cr6+ and Mn7+ metal ions were used. The results obtained show that as the concentration of both Cr6+ and Mn7+ metal ions increases, the fluorescence intensity of WTR-CDs decreases gradually, i.e., quenching is observed. Fig. 4b and d depict the visual change in the colour of Cr6+ and Mn7+ as well as the gradual and linear decrease in the fluorescence emission colour of the WTR-CDs with increasing concentration. The photographic images in daylight and under UV light strongly evidence that the developed HB-Alg/Gel@WTR-CDs show sensitive detection of Cr6+ and Mn7+ ions.The fitting of the above linear relationship between F0/F and the concentration of Cr6+ and Mn7+ in the range of 0 to 10 μg mL−1 was well described by the Stern–Volmer equation (eqn (1)).
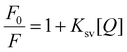 | (1) |
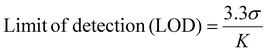 | (2) |
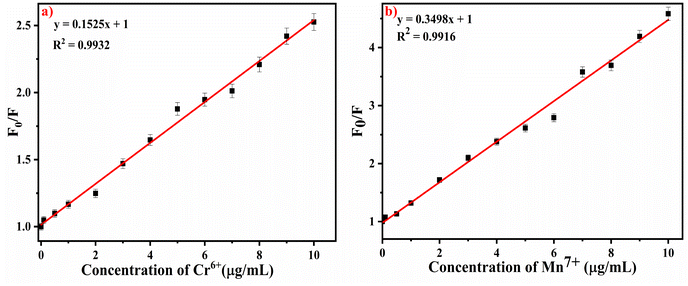 | ||
| Fig. 5 Stern–Volmer plot of the effects of the concentration of (a) Cr6+ and (b) Mn7+ ions on the WTR-CDs. | ||
The LOD of the proposed method was found to be 0.28 μg mL−1 and 0.30 μg mL−1 for Cr6+ and Mn7+, respectively. The sensing performance of the proposed method was compared with previously reported methods and found to be superior to the other methods (Table S1†).
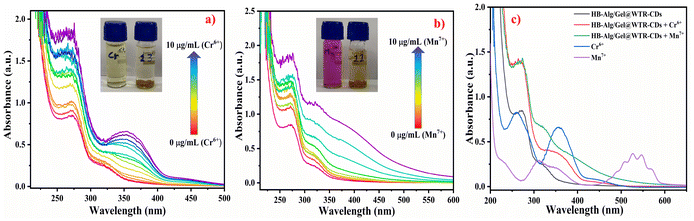
Fig. 6a and b demonstrates the effect of increasing concentrations of Cr6+ and Mn7+ on the absorbance of WTR-CDs; their concentrations were varied from 0 to 10 μg mL−1. The UV-Vis absorbance study suggests that there was an increase in absorbance of the WTR-CDs with the gradual increase of the concentration of the respective metal ions. The colour of the resultant solution changes from a faint-yellow to a brownish-yellow and from a purple to a dark-brown colour for Cr6+ and Mn7+, respectively. Further, the sensing mechanism can be determined from Fig. 6c, which clearly confirms the change in peak wavelength and diminishment of the peaks of pristine Cr6+ and Mn7+ in the presence of HB-Alg/Gel@WTR-CDs. This suggests that there was interaction of the surface functional groups of WTR-CDs and metal ions and that complex formation took place.69 As per a literature survey and to the best of our knowledge, there are very few reports of the use of CDs-loaded hydrogel probes as a fluorescent sensor for the detection of Cr6+ and Mn7+, and the results are summarized in Table S1.† The assessment of the hydrogel-based fluorescent probe, linear range and LOD of different methods are summarized in Table S1.†
3.10 Mechanism for optical detection of hazardous Cr6+ and Mn7+ metal ions
The sensing mechanism of Cr6+ and Mn7+ metal ions was well supported with a time correlated single photon count (TCSPC) study, i.e., fluorescence lifetime measurement, in addition to the UV-Vis absorbance and fluorescence study. Usually, the quenching behavior of the fluorophore can be well illustrated with different sensing mechanisms based on the generation of a non-radiative ground state complex (static quenching), excited state interaction of the fluorophore and analyte, i.e., dynamic quenching, Förster resonance energy transfer (FRET), inner filter effect (IFE), and photoinduced electron transfer (PET).23,70Fig. 7 depicts the fluorescence decay profile of the HB-Alg/Gel@WTR-CDs in the presence of different concentrations of Cr6+ and Mn7+. The results demonstrate that as the concentration of Cr6+ was increased from 2.50 to 5.00 μg mL−1, the average lifetime remained constant (τ = 4.37 ns). This lack of change in the fluorescence lifetime proves that there may be complex formation at the ground state (i.e., static type of quenching). In addition, the Fig. S7† depicts the excitation and emission spectra of the HB-Alg/Gel@WTR-CDs and absorption spectra of Cr6+. The UV-Vis absorption peak of Cr6+, i.e., the quencher, demonstrates the maximum overlap with the excitation spectra of HB-Alg/Gel@WTR-CDs. The condition for the IFE mechanism requires good spectral overlap of the absorption peak of the quencher with the excitation and emission spectra of the fluorophore.71,72 Hence, the fluorescence quenching of Cr6+ follows the conditions of IFE, and the mechanism can be attributed to IFE.73–75 The mechanism for Cr6+ detection follows the static type of quenching associated with IFE.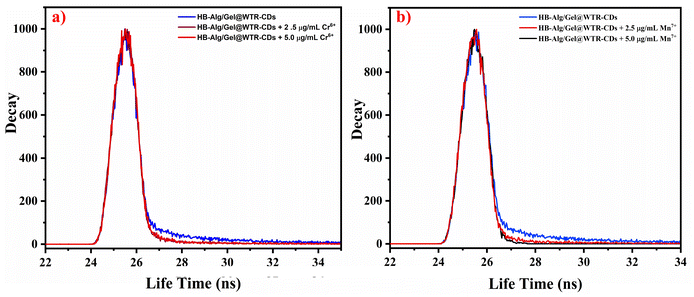 | ||
| Fig. 7 Graph of the fluorescence decay profile of the HB-Alg/Gel@WTR-CDs in the presence of different concentrations of (a) Cr6+ and (b) Mn7+ ions. | ||
In the case of Mn7+, the increase in metal ion concentration affects the fluorescence lifetime. Fig. 7b demonstrates that there was a gradual decrease in the fluorescence lifetime with increasing concentration of the analyte. The change in fluorescence lifetime indicates that, the fluorescence of the Alg/Gel@WTR-CDs was quenched by excited state interaction, confirming the dynamic type of quenching.76 Mn7+ ions can form multisite coordination with the electron-rich moieties present on the Alg/Gel@WTR-CDs. Mn7+ may interact with the Alg/Gel@WTR-CDs through chelation with oxygen-containing functionalities as well as with the π bonds of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C domains of the CDs.77,78 Interestingly, Wenjing Chen et al. found a strong absorption peak in the range of 450 to 600 nm for Mn7+, which is due to the formation of a static complex or chelation.79 On the contrary, in our case, the complete diminishment of the absorption peak in the same region is the main cause for the formation of the coloured complex. Also, upon photoexcitation, there is the possibility of excited-state electron transfer with a redox reaction followed by the reduction of Mn7+ to Mn4+. The change from purple-coloured Mn7+ to a brown-coloured CD–Mn complex results from the reduced Mn4+.80 The results suggest that a quenching mechanism involving electron transfer between Alg/Gel@WTR-CDs and Mn7+ takes place along with the dynamic type of quenching.81
C domains of the CDs.77,78 Interestingly, Wenjing Chen et al. found a strong absorption peak in the range of 450 to 600 nm for Mn7+, which is due to the formation of a static complex or chelation.79 On the contrary, in our case, the complete diminishment of the absorption peak in the same region is the main cause for the formation of the coloured complex. Also, upon photoexcitation, there is the possibility of excited-state electron transfer with a redox reaction followed by the reduction of Mn7+ to Mn4+. The change from purple-coloured Mn7+ to a brown-coloured CD–Mn complex results from the reduced Mn4+.80 The results suggest that a quenching mechanism involving electron transfer between Alg/Gel@WTR-CDs and Mn7+ takes place along with the dynamic type of quenching.81
In addition, Fig. 8 depicts a mechanistic representation of the sensing of the analytes. The scheme shows the crosslinking of the polymer functional groups of alginate and gelatin and further embedding of WTR-CDs into the hydrogel matrix and their interaction with the functional groups of polymers to form the HB-Alg/Gel@WTR-CDs composite. The developed probe showed stimuli-responsive release of CDs from the hydrogel network. The released fluorescent CDs exhibited high stability, selectivity and sensitivity towards the Cr6+ and Mn7+ ions over other metal ions. Interestingly, the developed probe featured naked-eye-observable detection of the respective ions. Hence, as per the results, the HB-Alg/Gel@WTR-CDs hold good promise for the naked-eye as well as fluorometric detection of hazardous metal ions.
3.11 Real application of HB-Alg/Gel@WTR-CDs on various water sources
The practical and real-life applicability of the proposed HB-Alg/Gel@WTR-CDs as a stimuli-responsive fluorescent probe was validated for the determination of Cr6+ and Mn7+ ions in different water samples. The different water samples, viz. tap water and lake water from Shivaji University and a Panchaganga river, were collected. The above samples were first filtered through filter paper to remove suspended particulates and then boiled for 5 min to remove dissolved gases. Afterwards, known concentrations of metal ions were spiked into the samples separately. The results were recorded and compared with a standard system and are summarized in Tables 1 and 2. The results proved that the HB-Alg/Gel@WTR-CDs performed well and showed significant recovery of the spiked samples with acceptable average recoveries above 90%. The relative standard deviation (RSD) is also below 2%, indicating good precision and excellent reproducibility.| Sr. no. | Source of water sample | Amount of standard Cr6+ spiked (μg mL−1) | Total Cr6+ found (μg mL−1) (n = 3) | Recovery of Cr6+ added (%) (n = 3) | RSD (%) |
|---|---|---|---|---|---|
| 1 | Shivaji University Tap Water | 2 | 1.96 | 98.12 | 0.94 |
| 4 | 3.89 | 97.25 | 1.29 | ||
| 6 | 5.47 | 94.28 | 1.60 | ||
| 2 | Shivaji University Lake | 2 | 1.84 | 94.01 | 0.60 |
| 4 | 3.51 | 91.79 | 1.68 | ||
| 6 | 5.28 | 93.07 | 1.53 | ||
| 3 | Panchganga River | 2 | 1.82 | 95.40 | 0.76 |
| 4 | 4.05 | 101.32 | 1.05 | ||
| 6 | 5.56 | 96.78 | 1.23 |
| Sr. No. | Source of water sample | Amount of standard Mn7+ spiked (μg mL−1) | Total Mn7+ found (μg mL−1) (n = 3) | Recovery of Mn7+ added (%) (n = 3) | RSD (%) |
|---|---|---|---|---|---|
| 1 | Shivaji University Tap Water | 2 | 1.84 | 94.05 | 0.43 |
| 4 | 3.80 | 95.02 | 0.74 | ||
| 6 | 5.53 | 92.17 | 1.10 | ||
| 2 | Shivaji University Lake | 2 | 1.82 | 96.13 | 0.92 |
| 4 | 3.75 | 93.92 | 1.02 | ||
| 6 | 5.80 | 96.81 | 0.88 | ||
| 3 | Panchganga River | 2 | 1.97 | 98.92 | 0.62 |
| 4 | 3.61 | 91.26 | 0.35 | ||
| 6 | 5.37 | 90.53 | 0.44 |
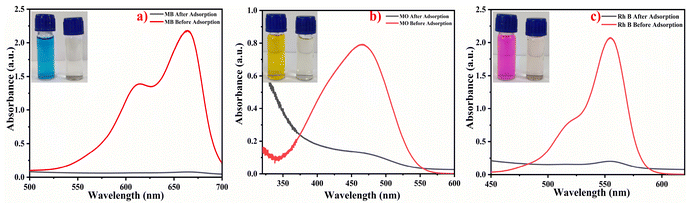
3.12 Application of Alg/Gel-carbon
At present, reusable and recyclable materials are of great interest to the scientific community as well as industries, as they not only reduce costs but also increase the efficiency of the material and allow a waste management approach to be achieved. In the present study, after the successful application of the HB-Alg/Gel@WTR-CDs for hazardous metal ion detection, a valorized Alg/Gel-carbon is prepared as an adsorbent material through a simple process. The adsorption study was conducted using 10 mL (10 mg L−1) solutions of the widely used model pollutants Methylene Blue (MB), Methyl Orange (MO), and Rhodamine B (Rh B) with a pH of 7.0; the dose of the adsorbent i.e., Alg/Gel-carbon, was 10 mg (Fig. 9) (Table 3). The percentage removal efficiency of the model pollutants was calculated using the equation given below (eqn (3)).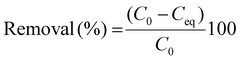 | (3) |
| Sr. No. | Model pollutant used | Removal (%) |
|---|---|---|
| 1 | Methylene blue (MB) | 96.47 |
| 2 | Methyl orange (MO) | 83.73 |
| 3 | Rhodamine B (Rh B) | 90.28 |
The versatility of adsorbent was confirmed by its successful application for the effective removal of hazardous, toxic, and carcinogenic pollutants. The Alg/Gel-carbon as a reused material can be effectively applied for water purification and wastewater treatment.
3.13 Greenness determination
The development of ecofriendly and harmless methods is essential for sustainability applications. The AGREE-software-based approach is one of the several techniques applied for green analytical assessment based on the 12 GAC principles.82 An AGREE score in the range of 0.3 to 0.5 was derived for principles 1, 3 and 5. Principles 2 and 4 had scores in the range of 0.6 to 0.8. The GAC principles 6 to 12 had AGREE scores in the range of 0.9 to 1.0. The overall average AGREE score of our developed HB-Alg/Gel@WTR-CDs-based sensor and environmental remediation approach was 0.81, which is greater than 0.75, indicating excellent greenness and environmental friendliness (Fig. 10).4. Conclusions
The rapidly increasing industrialization and anthropogenic activities have led to the destruction of environment through contamination with hazardous pollutants (metal ions and dyes). These hazardous toxic contaminants have severe health and environmental impacts. To overcome these important problems, sensitive, economical and green approaches need to be investigated. Herein, we have developed stimuli-responsive fluorescent-CDs-loaded functional HB-Alg/Gel@WTR-CDs hydrogel probes for rapid, on-site naked-eye detection of hazardous environmental pollutants with a green and circular economy approach. The developed HB-Alg/Gel@WTR-CDs hydrogel probe showed excellent selective and optical detection of emerging environmental pollutants, i.e., Cr6+ and Mn7+, with detection limits of 0.28 μg mL−1 and 0.30 μg mL−1 respectively. The mechanism for Cr6+ detection follows the static type of quenching involving IFE, whereas electron transfer between the Alg/Gel@WTR-CDs and Mn7+ takes place along with dynamic-type quenching. In addition, the pristine colour of Cr6+ (faint-yellow) and Mn7+ (purple) changes to a brownish-yellow and dark-brown colour, respectively. The developed method performs well for the onsite detection of analytes in real water samples. Further, the circular economy approach Alg/Gel-carbon derived from spent beads showed potential dye remediation applications. In conclusion, the simple, cost-effective, environmentally benign HB-Alg/Gel@WTR-CDs is a stimuli-responsive and portable hydrogel kit with potential applications in the field of forensics, wastewater treatment and environmental remediation.Data availability
All data are included in the paper and the ESI.† The related data will be made available on request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors gratefully acknowledge the Department of Science and Technology (DST), New Delhi (DST/PURSE-Phase-II/2022), Shivaji University, Kolhapur (MS), India, and UKA Tarsadia University, Surat, Gujrat for providing facilities in department. SPP would like to thank Khalifa University for its financial support for high quality publications. Daewon Sohn thanks to the National Research Foundation of Korea (NRF) 2020R1A6A1A06046728, 2022R1A2C1010580 and 2022R1A6C101A779-23.References
- J. Zhang, J. Jin, J. Wan, S. Jiang, Y. Wu, W. Wang, X. Gong and H. Wang, Chem. Eng. J., 2021, 408, 127351 CrossRef CAS
.
- A. Bin Imran, T. Seki and Y. Takeoka, Polym. J., 2010, 42, 839–851 CrossRef CAS
.
- P. V. Devre and A. H. Gore, Langmuir, 2023, 40(1), 141–158 CrossRef PubMed
.
- O. Sreekanth Reddy, M. C. S. Subha, T. Jithendra, C. Madhavi and K. Chowdoji Rao, J. Pharm. Anal., 2021, 11, 191–199 CrossRef CAS PubMed
.
- X. Chen, Z. Song, B. Yuan, X. Li, S. Li, T. Thang, M. Guo and Z. Guo, Chem. Eng. J., 2022, 430, 133154 CrossRef CAS
.
- T. Wu, S. Yu, D. Lin, Z. Wu, J. Xu, J. Zhang, Z. Ding, Y. Miao, T. Liu, T. Chen and X. Cai, ACS Appl. Bio Mater., 2020, 3, 3057–3065 CrossRef CAS PubMed
.
- P. Samaddar, S. Kumar and K. H. Kim, Polym. Rev., 2019, 59, 418–464 CrossRef CAS
.
- M. Jawaid, A. Ahmad, N. Ismail and M. Rafatullah, Environmental Remediation Through Carbon Based Nano Composites, 2021 Search PubMed
.
- A. Pal and N. Dey, Soft Matter, 2024, 20, 3044–3052 RSC
.
- R. S. Fernandes and N. Dey, Ind. Eng. Chem. Res., 2023, 62, 21536–21545 CrossRef CAS
.
- H. V. Barkale and N. Dey, Asian J. Org. Chem., 2024, 13, e202300657 CrossRef CAS
.
- A. Pal and N. Dey, J. Fluoresc., 2024 DOI:10.1007/s10895-023-03393-y
.
- H. V. Barkale and N. Dey, Chem. – Asian J., 2024, 19, e202400058 CrossRef CAS PubMed
.
- B. Chettri, R. S. Fernandes, S. Jha and N. Dey, Spectrochim. Acta, Part A, 2024, 308, 123620 CrossRef CAS PubMed
.
- A. Pal and N. Dey, Colloids Surf., A, 2024, 687, 133397 CrossRef CAS
.
- R. S. Fernandes and N. Dey, J. Mol. Liq., 2022, 367, 120369 CrossRef CAS
.
- R. S. Fernandes and N. Dey, Mater. Chem. Phys., 2023, 302, 127637 CrossRef CAS
.
- S. Mondal and N. Dey, Eur. J. Inorg. Chem., 2023, 26, e202300147 CrossRef CAS
.
- V. K. Gupta, S. Agarwal and T. A. Saleh, Water Res., 2011, 45, 2207–2212 CrossRef CAS PubMed
.
- A. G. Kolekar, S. P. Pawar, D. B. Gunjal, O. S. Nille, P. V. Anbhule, S. V. Koparde, N. Q. Nguyen, D. Sohn, G. B. Kolekar, G. S. Gokavi and V. R. More, RSC Adv., 2024, 14, 3473–3479 RSC
.
- S. V. Koparde, O. S. Nille, A. G. Kolekar, P. P. Bote, K. V. Gaikwad, P. V. Anbhule, S. P. Pawar and G. B. Kolekar, Spectrochim. Acta, Part A, 2024, 321, 124659 CrossRef CAS PubMed
.
- J. S. Marciano, R. R. Ferreira, A. G. de Souza, R. F. S. Barbosa, A. J. de Moura Junior and D. S. Rosa, Int. J. Biol. Macromol., 2021, 181, 112–124 CrossRef CAS PubMed
.
- S. B. Wakshe, P. R. Dongare, A. H. Gore, G. V. Mote, S. Y. Salunkhe, S. T. Mahanwar, P. V. Anbhule and G. B. Kolekar, Inorg. Chim. Acta, 2021, 526, 120534 CrossRef CAS
.
- W. Chen, H. Lin, Y. Wu, M. Yang, X. Zhang, S. Zhu, M. He, J. Xie and Z. Shi, Adv. Compos. Hybrid Mater., 2022, 5, 2378–2386 CrossRef CAS
.
- Chromium in drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality, World Health Organization, 1996 Search PubMed.
-
Guidelines for Guidelines for drinking-water quality, World Health Organization, 4th edn, 2022 Search PubMed
.
- Z. Bahadir, V. N. Bulut, M. Hidalgo, M. Soylak and E. Marguí, Spectrochim. Acta, Part B, 2015, 107, 170–177 CrossRef CAS
.
-
U.S. Environ. Prot. Agency Off. WaterWashington, DC EPA-822-R-04-003, 2004, pp. 1–49 Search PubMed
.
- US EPA, Drinking Water Health Advisory for Manganese, U.S. Environmental Protection Agency Office of Water (4304T) Health and Ecological Criteria Division Washington, DC 20460, 2004, https://www.epa.gov/sites/default/files/2014-09/documents/support_cc1_magnese_dwreport_0.pdf.
- D. Citak, M. Tuzen and M. Soylak, J. Hazard. Mater., 2010, 173, 773–777 CrossRef CAS PubMed
.
- A. Truskewycz, S. A. Beker, A. S. Ball, B. Murdoch and I. Cole, Anal. Chim. Acta, 2020, 1099, 126–135 CrossRef CAS PubMed
.
- W. Yang, S. Niu, Y. Wang, L. Huang, S. Wang, K. C. Popat, M. J. Kipper, L. A. Belfiore and J. Tang, Nanomaterials, 2021, 11, 3283 CrossRef CAS PubMed
.
- H. Wang, Q. Chen and S. Zhou, Chem. Soc. Rev., 2018, 47, 4198–4232 RSC
.
-
K. K. Chan, S. H. K. Yap and K. T. Yong, Biogreen Synthesis of Carbon Dots for Biotechnology and Nanomedicine Applications, SpringerBerlinHeidelberg, 2018, vol. 10 Search PubMed
.
- A. S. Patil, R. D. Waghmare, S. P. Pawar, S. T. Salunkhe, G. B. Kolekar, D. Sohn and A. H. Gore, J. Photochem. Photobiol., A, 2020, 400, 112647 CrossRef CAS
.
- S. A. Shaik, S. Sengupta, R. S. Varma, M. B. Gawande and A. Goswami, ACS Sustainable Chem. Eng., 2021, 9, 3–49 CrossRef CAS
.
- D. Kim, Y. Choi, E. Shin, Y. K. Jung and B. S. Kim, RSC Adv., 2014, 4, 23210–23213 RSC
.
- F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang and Z. Bai, Microchemica Acta, 2019, 186, 583 CrossRef PubMed
.
- A. Sachdev, I. Matai and P. Gopinath, Colloids Surf., B, 2016, 141, 242–252 CrossRef CAS PubMed
.
- N. Gogoi and D. Chowdhury, J. Mater. Chem. B, 2014, 2, 4089–4099 RSC
.
- A. Vibhute, O. Nille, G. Kolekar, S. Rohiwal, S. Patil and S. Lee, J. Fluoresc., 2022, 32, 1789–1800 CrossRef CAS PubMed
.
- S. S. Shendage, V. H. Ingole, A. Rasal, J.-Y. Chang, N. B. Birajdar, A. P. Tiwari, S. Kashte, T. Vuherer, G. Schmitt, M. Cucchiarini and A. V. Ghule, ACS Sustainable Chem. Eng., 2024, 12, 2598–2610 CrossRef CAS
.
- O. S. Nille, A. S. Patil, A. A. Vibhute, S. S. Shendage, A. P. Tiwari, P. V. Anbhule, D. Sohn, A. H. Gore and G. B. Kolekar, Int. J. Biol. Macromol., 2024, 257(Part 1), 128126 CrossRef CAS PubMed
.
- V. K. Jothi, K. Ganesan, A. Natarajan and A. Rajaram, J. Fluoresc., 2021, 31, 427–436 CrossRef CAS PubMed
.
- J. Zhang and S. H. Yu, Mater. Today, 2016, 19, 382–393 CrossRef CAS
.
- D. B. Gunjal, V. M. Naik, R. D. Waghmare, C. S. Patil, R. V. Shejwal, A. H. Gore and G. B. Kolekar, J. Taiwan Inst. Chem. Eng., 2019, 95, 147–154 CrossRef CAS
.
- H. Lu, C. Li, H. Wang, X. Wang and S. Xu, ACS Omega, 2019, 4, 21500–21508 CrossRef CAS PubMed
.
- S. Mondal, H. V. Barkale and N. Dey, Colloids Surf., A, 2023, 677, 132322 CrossRef CAS
.
- U. A. Rani, L. Y. Ng, C. Y. Ng and E. Mahmoudi, Adv. Colloid Interface Sci., 2020, 278, 102124 CrossRef PubMed
.
- P. V. Devre, A. S. Patil, D. Sohn and A. H. Gore, J. Environ. Chem. Eng., 2023, 11, 109368 CrossRef CAS
.
- O. S. Nille, R. S. Patel, B. Y. Borate, S. S. Babar, G. B. Kolekar and A. H. Gore, Environ. Sci. Pollut. Res., 2023, 30, 38425–38442 CrossRef CAS PubMed
.
-
O. S. Nille, A. S. Patil, G. B. Kolekar and A. H. Gore, Carbon-based composite hydrogels for environmental remediation, in Environmental Remediation Through Carbon Based Nano Composites, ed. M. Jawaid, A. Ahmad, N. Ismail and M. Rafatullah, Springer, Singapore, 2021, pp. 427–443 Search PubMed
.
- S. Majumdar, G. Krishnatreya, N. Gogoi, D. Thakur and D. Chowdhury, ACS Appl. Mater. Interfaces, 2016, 8, 34179–34184 CrossRef CAS PubMed
.
- N. Gogoi, M. Barooah, G. Majumdar and D. Chowdhury, ACS Appl. Mater. Interfaces, 2015, 7, 3058–3067 CrossRef CAS PubMed
.
- V. Naik, P. Zantye, D. Gunjal, A. Gore, P. Anbhule, M. Kowshik, S. V. Bhosale and G. Kolekar, ACS Appl. Bio Mater., 2019, 2, 2069–2077 CrossRef CAS PubMed
.
- V. M. Naik, D. B. Gunjal, A. H. Gore, P. V. Anbhule, D. Sohn, S. V. Bhosale and G. B. Kolekar, Anal. Bioanal. Chem., 2020, 412, 2993–3003 CrossRef CAS PubMed
.
- M. Ghanbari, M. Salavati-Niasari and F. Mohandes, RSC Adv., 2021, 11, 18423–18431 RSC
.
- S. Kanungo, N. Gupta, R. Rawat, B. Jain, A. Solanki, A. Panday, P. Das and S. Ganguly, J. Funct. Biomater., 2023, 14, 166 CrossRef CAS PubMed
.
- M. Ghanbari, M. Salavati-Niasari and F. Mohandes, Int. J. Pharm., 2021, 602, 120660 CrossRef CAS PubMed
.
- B. Wang, B. Gao and Y. Wan, J. Ind. Eng. Chem., 2018, 61, 161–168 CrossRef CAS PubMed
.
- X. Yang, T. Zhou, B. Ren, A. Hursthouse and Y. Zhang, Sci. Rep., 2018, 8, 1–16 Search PubMed
.
- W. Zhang, X. Shi, Y. Zhang, W. Gu, B. Li and Y. Xian, J. Mater. Chem. A, 2013, 1, 1745–1753 RSC
.
- H. Guo, T. Jiao, Q. Zhang, W. Guo, Q. Peng and X. Yan, Nanoscale Res. Lett., 2015, 10, 0–9 Search PubMed
.
- V. Mokale, N. Jitendra, S. Yogesh and K. Gokul, J. Pharm. Invest., 2014, 44, 243–252 CrossRef CAS
.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed
.
- F. Arcudi, L. Dordevic and M. Prato, Angew. Chem., Int. Ed., 2016, 55, 2107–2112 CrossRef CAS PubMed
.
- H. Ding, S. B. Yu, J. S. Wei and H. M. Xiong, ACS Nano, 2016, 10, 484–491 CrossRef CAS PubMed
.
- A. Majumdar, S. C. Das, T. Shripathi and R. Hippler, DOI:10.1080/15685543.2012.699751.
- J. Xu, Y. Guo, T. Gong, K. Cui, L. Hou and C. Yuan, Inorg. Chem. Commun., 2022, 145, 110047 CrossRef CAS
.
- D. B. Gunjal, A. H. Gore, A. R. Bhosale, V. M. Naik, P. V. Anbhule, R. V. Shejwal and G. B. Kolekar, J. Photochem. Photobiol., A, 2019, 376, 54–62 CrossRef CAS
.
- L. Yu, L. Zhang, G. Ren, S. Li, B. Zhu, F. Chai, F. Qu, C. Wang and Z. Su, Sens. Actuators, B, 2018, 262, 678–686 CrossRef CAS
.
- S. Chen, Y. Yu and J. Wang, Anal. Chim. Acta, 2018, 999, 13–26 CrossRef CAS PubMed
.
- A. Kundu, B. Maity and S. Basu, ACS Omega, 2023, 8, 22178–22189 CrossRef CAS PubMed
.
- H. Zeng, Z. Hu, C. Peng, L. Deng and S. Liu, Polymers, 2021, 13, 3788 CrossRef CAS PubMed
.
- J. Gogoi and D. Chowdhury, J. Mater. Sci., 2020, 55, 11597–11608 CrossRef CAS
.
- D. B. Gunjal, L. S. Walekar, S. P. Pawar, P. V. Anbhule, M. G. Mali, V. P. Dhulap, D. Sohn, P. G. Mahajan, K. H. Lee, R. V. Shejwal and G. B. Kolekar, Sci. Rep., 2021, 11, 1–9 CrossRef PubMed
.
- A. Kundu, B. Maity and S. Basu, New J. Chem., 2022, 7545–7556 RSC
.
- D. G. Lee and T. Chen, J. Am. Chem. Soc., 1989, 111, 1534–1538 Search PubMed
.
- W. Chen, Adv. Compos. Hybrid Mater., 2022, 5, 2378–2386 CrossRef CAS
.
- S. Jayaweera, K. Yin, X. Hu and W. J. Ng, J. Fluoresc., 2019, 29, 1291–1300 CrossRef CAS PubMed
.
- V. M. Naik, D. B. Gunjal, A. H. Gore, P. V. Anbhule, D. Sohn and S. V. Bhosale, Anal. Bioanal. Chem., 2020, 412, 2993–3003 CrossRef CAS PubMed
.
- F. Shakeel, P. Alam, N. Haq and M. H. Alqarni, ACS Omega, 2023, 8, 39936–39944 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Details related to the equipment, materials, swelling ratio study, quantum yield measurement, results and discussion, FTIR and XRD analysis, FE-SEM and EDX analysis, pH effect, swelling study. Fig S1: Schematic for the synthesis of waste tea residue carbon dots (WTR-CDs). Fig S2: Schematic diagram showing the preparation of HB-Alg/Gel@WTR-CDs. Fig S3: (a–c) HR-TEM analysis, (d) particle size distribution, (e) SAED pattern, (f) TEM-EDX, and (g) elemental mapping of WTR-CDs. Fig S4: (a) FTIR and (b) XRD spectra of (i) WTR-CDs, (ii) HB-Alg/Gel and (iii) HB-Alg/Gel@WTR-CDs. Fig S5: The morphology and EDX of (a), (b) HB-Alg/Gel, and (c), (d) HB-Alg/Gel@WTR-CDs. Fig S6: (a) The effect of pH (2–12) on the fluorescence intensity of the WTR-CDs, and (b) swelling behavior of HB-Alg/Gel and HB-Alg/Gel@WTR-CDs hydrogel beads. (pH = 2–12, V = 10 mL, WHB-Alg/Gel@WTR-CDs = 50 mg) (n = 3). Fig S7: Excitation and emission spectra of HB-Alg/Gel@WTR-CDs and absorbance spectra of Cr6+. Table S1: Comparison of different CDs-based hydrogels for chromium and manganese ion determination. See DOI: https://doi.org/10.1039/d4an00914b |
| This journal is © The Royal Society of Chemistry 2025 |