A sensitive electrochemical sensor based on CoWO4/multi-walled carbon nanotubes for the selective determination of chlorpromazine hydrochloride†
Si
Zeng
a,
Peiyao
Zhu
a,
Deyu
Liu
a,
Yongmei
Hu
a,
Qitong
Huang
*ab and
Haiping
Huang
 *a
*a
aKey Laboratory of Testing and Tracing of Rare Earth Products for State Market Regulation, School of Chemistry and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou, 341000, China. E-mail: hqt@gmu.edu.cn; huanghp@jxust.edu.cn
bSchool of Medical and Information Engineering, The Science Research Center, Gannan Medical University, Ganzhou, China
First published on 15th November 2024
Abstract
In this work, a novel electrochemical sensor based on cobalt tungstate/multi-walled carbon nanotube (CoWO4/MWCNT) nanocomposites has been used to detect chlorpromazine hydrochloride (CPZ). The CoWO4/MWCNT nanocomposite was obtained by solvothermal technology and ultrasonic method and analyzed using different characterization techniques such as scanning electron microscopy (SEM), X-ray diffractometry (XRD), energy-dispersive spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS). The electrochemical behavior of CoWO4/MWCNT/GCE was explored using cyclic voltammetry (CV). Electrochemical experiments confirm that CoWO4/MWCNT/GCE exhibits excellent electrocatalytic activity towards CPZ with good selectivity, reproducibility and stability. The linear dynamic range of CPZ was observed to be 1–2000 μM with a detection limit of 0.33 μM. Moreover, the actual sample was analyzed using lake water with satisfactory results, portraying the sensor as a potential candidate for the detection of CPZ.
Introduction
Chlorpromazine hydrochloride (CPZ, C17H19ClN2S-HCl), which belongs to the phenothiazine class of drugs, is typically used as an antipsychotic drug to treat major depressive illness, schizophrenia, and acute and chronic mania.1 In addition, CPZ can be used as an antiemetic during palliative treatment.2 However, overdosage of CPZ may cause some diseases such as cataracts, interpalpebral conjunctiva, visual problems, and contact dermatitis.3,4 Therefore, fast and accurate detection of CPZ has attracted more attention. Up to now, CPZ can be sensitively measured by various analytical techniques, which include chemiluminescence,5 high-performance liquid chromatography,6 gas chromatography,7 electrochemical potentiometry,8 and spectrophotometry.9 According to previous literature surveys, the above analysis methods exhibit some drawbacks, including long duration, instrument complexity and high costs.10 It is necessary to identify an alternative technique to overcome these disadvantages.1 In comparison to the above methods, electrochemical technique has advantages of low cost, high accuracy, fast response, excellent reliability, good efficiency, and ultra-selectivity.11 Therefore, the electrochemical based technique plays a more significant role in electrocatalytic determination of CPZ.In recent years, the metal tungsten oxides (AWO4, A = Co, Fe, Cd, Cu, Ni, etc.) have captured the interest of researchers because of their wide range of applications in multiple fields, including photocatalytic degradation,12 supercapacitors,13 Li-ion batteries,14 and photoelectrochemical water oxidation.15 Due to its lower cost and superior catalytic activity over other metal tungsten oxides, cobalt tungstate (CoWO4) nanomaterial is considered as a strong candidate for catalytic use.16 For example, Alagumalai et al. synthesized a unique cashew-structured CoWO4 through co-precipitation method and demonstrated its promising prospects in the electrochemical detection of promethazine hydrochloride.17 Through a simple solvothermal technology, micro-ring structured CoWO4 was prepared and utilized for electrochemical non-enzymatic detection of glucose.18 Moreover, with the addition of different quantities of surfactant sodium dodecyl sulphonate, the morphology of CoWO4 nanospheres could be controlled.19 These reports powerfully prove that CoWO4 has great potential in the field of electrochemical sensors.20,21
Carbon-based nanotubes, including porous carbon, carbon quantum dots, carbon black, and graphene oxide, have been employed extensively in electrochemical research owing to their outstanding electrocatalytic characteristics.22 Among them, MWCNTs have the characteristics of high surface area, excellent structure, low cost, ease of preparation, and the mechanical properties required to make them electrode materials for constructing electrochemical sensors.23,24 Herein, based on the above statement, a novel composite was formed by combining CoWO4 with MWCNTs and employed for the electrochemical oxidation of CPZ. Excellent electrocatalytic activity was demonstrated with a wider linear range and good selectivity, reproducibility, and stability. Moreover, the CoWO4/MWCNT/GCE sensor exhibited excellent electrocatalytic activity in real lake water samples.
Experimental
All chemicals were of analytical grade and were used without further purification. The CoWO4 nano-materials were synthesized following directions from previous literature.25 The pristine MWCNTs were functionalized by the traditional/acidification route.26 The as-prepared nanocomposite was characterized by scanning electron microscopy (SEM), X-ray diffractometry (XRD), energy-dispersive spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS). The detailed process of nanomaterial synthesis and electrochemical sensor fabrication can be found in the ESI.†Results and discussion
SEM, XRD and XPS analysis of CoWO4/MWCNTs
The microstructures of the prepared CoWO4 and CoWO4/MWCNT nanomaterials were investigated by SEM (Fig. 1). In Fig. 1A, it can be observed that CoWO4 is an irregular nanoparticle. For CoWO4/MWCNTs (Fig. 1B), it can be seen that CoWO4 and MWCNTs are uniformly mixed with each other, indicating that the CoWO4/MWCNT nanocomposites have been successfully fabricated. Additionally, the presence of C, Co, W and O elements has been confirmed via EDS analysis (Fig. 1C). | ||
| Fig. 1 SEM images of CoWO4 (A) and CoWO4/MWCNT composite (B). EDS analysis of CoWO4/MWCNT composite (C). | ||
For the analysis of the phase purity of nanomaterials, the XRD patterns of CoWO4, MWCNTs and CoWO4/MWCNT nanocomposite are presented in Fig. 2A. The XRD pattern of MWCNTs shows two peaks at 2θ of 25.91° and 44.22°, which are attributed to the crystal planes of (002) and (100), respectively. For CoWO4, the major characteristic peaks occur at 23.83°, 30.68°, 36.43°, 53.88°, 61.70° and 64.84°, corresponding to the (−110), (−111), (120), (−202), (−311), (−113) crystal planes. The observed diffraction pattern agrees with the standard card of CoWO4 (JCPDS No. 15-0867). The absence of impurity peaks indicates that the prepared monoclinic structure phase of CoWO4 has high purity. For the CoWO4/MWCNT nanocomposite, all the diffraction peaks are similar to CoWO4. Due to the strong diffraction peaks of CoWO4, the diffraction peaks of MWCNTs are occulted and disappeared in the spectrum of the CoWO4/MWCNT nanocomposite.
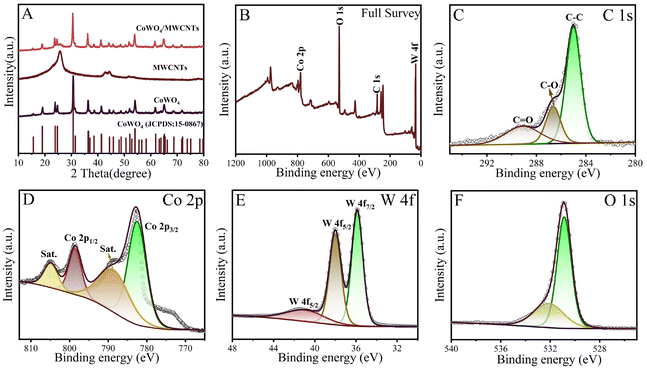 | ||
| Fig. 2 XRD patterns (A) of CoWO4, MWCNTs and CoWO4/MWCNT composite. Full XPS survey spectrum of CoWO4/MWCNTs (B) and high resolution XPS spectra of C 1s (C), Co 2p (D), W 4f (E) and O 1s (F) regions. | ||
To further demonstrate the successful synthesis of the nanocomposites, their chemical bonding state and elemental composition were assessed by XPS. The spectra of the CoWO4/MWCNT nanomaterial are displayed in Fig. 2B–F. In the XPS full spectrum, the characteristic peaks of elements C, Co, W, and O are observed (Fig. 2B). The XPS spectrum of C 1s is given in Fig. 2C. Three characteristic peaks are observed with binding energies of 285.0, 286.64 and 288.91 eV, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O and O–C
C, C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Fig. 2D displays the core level spectrum of Co 2p, with the binding energies of 780.87 and 796.99 eV accounted for by Co 2p3/2 and Co 2p1/2 of the Co2+ oxidation state, respectively. The satellite (sat) peaks at 786.05 and 802.86 eV further prove that the oxidation state of cobalt is +2.27,28 As shown in the W 4f XPS spectrum (Fig. 2E), there are three characteristic peaks with binding energies at 35.34, 37.39 and 40.33 eV, which are associated with the W 4f7/2 and 4f5/2 levels, indicating the W6+ oxidation state in the sample.15 In the O 1s core level spectrum (Fig. 2F), the peaks with binding energies of 531.64 eV and 530.31 eV are assigned to the lattice oxygen and surface adsorbed hydroxyl groups, respectively.29
O. Fig. 2D displays the core level spectrum of Co 2p, with the binding energies of 780.87 and 796.99 eV accounted for by Co 2p3/2 and Co 2p1/2 of the Co2+ oxidation state, respectively. The satellite (sat) peaks at 786.05 and 802.86 eV further prove that the oxidation state of cobalt is +2.27,28 As shown in the W 4f XPS spectrum (Fig. 2E), there are three characteristic peaks with binding energies at 35.34, 37.39 and 40.33 eV, which are associated with the W 4f7/2 and 4f5/2 levels, indicating the W6+ oxidation state in the sample.15 In the O 1s core level spectrum (Fig. 2F), the peaks with binding energies of 531.64 eV and 530.31 eV are assigned to the lattice oxygen and surface adsorbed hydroxyl groups, respectively.29
Electrochemical response of CPZ on different electrodes
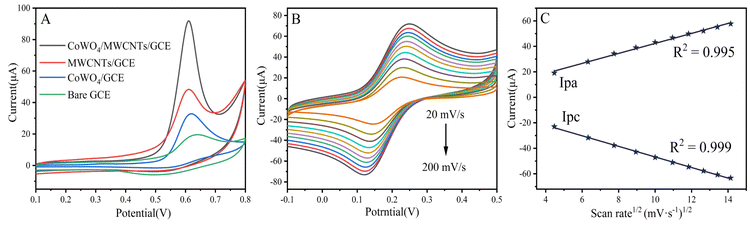
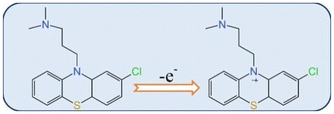
The electrocatalytic activities of CPZ at bare GCE, CoWO4/GCE, MWCNTs/GCE, and CoWO4/MWCNTs/GCE were investigated through cyclic voltammetry. Fig. 3A shows the oxidation peak current response of CPZ at different electrodes, in which the bare GCE shows a weak anodic peak (16.58 μA). The oxidation peak currents for CoWO4/GCE and MWCNTs/GCE are 25.20 and 36.60 μA, respectively, and their current values are higher than those of the unmodified electrode, which indicates that both CoWO4 and MWCNTs are able to promote the electro-oxidation reaction of CPZ. Finally, compared with CoWO4/GCE and MWCNTs/GCE, CoWO4/MWCNTs/GCE resulted in the highest oxidation peak current (85.09 μA), which was about 5-fold that of bare GCE. This may be explained by the synergistic effect of CoWO4 and MWCNTs, which significantly enhances the electrocatalytic capability of CPZ. Fig. 3B displays the CV results of CoWO4/MWCNTs/GCE at different scan rates from 20 to 200 mV s−1 in 2 mM [Fe(CN)6]3−/4− with 0.1 M KCl. As the scanning rate increases, there is an apparent rise in both the anodic peak current (Ipa) and the cathodic peak current (Ipc). As shown in Fig. 3C, the linear calibration plot is plotted for oxidation/reduction currents vs. square root of scan rate. The calculated equations are Ipa (μA) = 4.016v1/2 + 3.998 (R2 = 0.995) and Ipc (μA) = −4.094v1/2 – 5.856 (R2 = 0.999), indicating that the CoWO4/MWNTs nanocomposite results from a diffusion-controlled process. Based on the above scan rates, the electrochemical active surface area (ECSA) of CoWO4/MWCNTs/GCE is calculated to be 0.125 cm2 with reference to the Randles–Sevick equation.A strong oxidation peak is observed when adding CPZ, but there is no corresponding reduction peak during the reverse scanning process, indicating that the electro oxidation of CPZ is an irreversible process. The entire process is caused by the electrochemical oxidation of nitrogen atoms which are present in the CPZ. The overall electrochemical oxidation mechanism is displayed in Scheme 1.30
The electrochemical behaviors of CPZ in different electrolyte acidity (pH) were studied by CV. Fig. 4A represents the CV response of CoWO4/MWCNTs/GCE with the addition of CPZ concentration (400 μM) at different electrolyte pH values (5.0 to 9.0). As shown in Fig. 4A, the highest oxidation peak current with a sharp peak was obtained at pH 7.0. For Fig. 4B of relationship between the anodic peak current and the pH value, the anodic peak current increased progressively with variation of pH from 5.0 to 7.0. With rising pH from 7.0 to 9.0, the peak current decreases. This indicates that the maximum electro-oxidation current is reached at pH 7.0. Consequently, pH 7.0 was selected as optimum pH for further experiments.
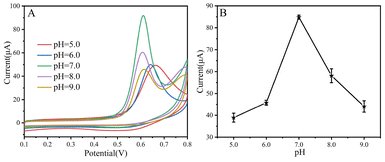 | ||
| Fig. 4 CV curves of CoWO4/MWCNTs/GCE in different electrolyte pH values from 5.0 to 9.0 at fixed concentration of CPZ (400 μM) with 50 mV s−1 (A); the relationship between peak current vs. pH (B). | ||
Determination of CPZ at CoWO4/MWCNTs/GCE
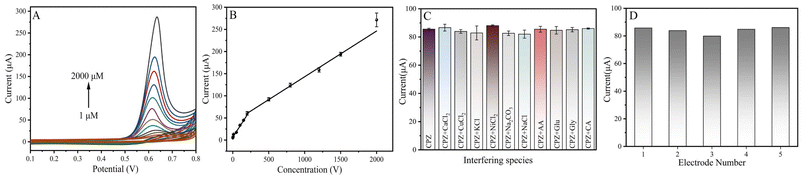
To further explore the electro-oxidation behavior of CPZ, the relationship between various concentrations of CPZ (1 to 2000 μM) and the oxidation peak current was recorded through CV. In Fig. 5A, with the increase of the CPZ concentrations from 1 to 2000 μM, the corresponding oxidation peak current also increased. This demonstrates that the fabricated electrochemical sensor has the potential for the detection of CPZ with different concentrations. The bilinear relationship of CPZ in the range from 1 to 200 μM and 200 to 2000 μM was plotted in Fig. 5B. The two linear regression equations and their correlated coefficients are as follows: Ipa (μA) = 0.2637c (μM) + 6.5894 with R2 = 0.990 for 1–200 μM and Ipa (μA) = 0.1134c (μM) + 32.918 with R2 = 0.987 for 200–2000 μM. The limit of detection (LOD) of CoWO4/MWCNTs/GCE was calculated to be approximately 0.33 μM (S/N = 3). Additionally, Table 1 compares the calculated results of CoWO4/MWCNTs/GCE with previously published literature on CPZ detection. The results show that the CoWO4/MWCNTs/GCE sensor has a relatively wider linear dynamic range in comparison to WS2/GCE, PTN/GCE, and CdO NPs/IL/CPE, and a lower detection limit in comparison to Alizarin Red S/GCE and NiFe LDH/GCE. The above comparisons confirm that the fabricated CoWO4/MWCNTs/GCE has outstanding electrocatalytic activity and can be employed as a novel electrochemical sensor to detect CPZ.| Electrode material | Techniques | Linear range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| a NiFe-layered double hydroxide. b CdO/nanoparticles (NPs) ionic liquid. c Preparation of polythiophene nanowires. | ||||
| Alizarin red S/GCE | LSV | 10–500 | 5.16 | 31 |
| WS2/GCE | DPV | 0–689 | 4.0 | 32 |
| NiFe LDHa/GCE | CV | 75–1000 | 1.23 | 33 |
| CdO NPs/ILb/CPE | SWV | 0.1–350 | 0.07 | 34 |
| PTNc/GCE | DPV | 0.1–130 | 0.03 | 35 |
| MIP/pTHi/Ni-MOF/Fe-MOF-5/AuNPs/GCE | SWV | 0.1–900 | 0.025 | 36 |
| CoWO4/MWNTs/GCE | CV | 1–2000 | 0.33 | This work |
Anti-interfering property, reproducibility, and stability investigation
The anti-interference, reproducibility, and stability of electrochemical sensor are important factors in the real sample analysis of detection performance. To evaluate the anti-interference capability of the sensor, the effect of various interfering compounds on CPZ (400 μM) was determined by CV in 0.2 M PBS (pH 7.0). From Fig. 5C, the peak current of CPZ was recorded by adding 25-fold concentration of inorganic substances (CaCl2, CuCl2, KCl, NiCl2, Na2CO3, NaCl) and 2-fold concentration of organic substances (ascorbic acid (AA), glucose (Glu), glycine (Gly) and citric acid (CA)) separately. It is evident that the peak current response of CPZ remains almost unchanged after the addition of interfering molecules. This outcome means that the sensor has good selectivity; hence it can be used for real time applications. To verify the reproducibility of the sensor, five modified electrodes were assayed via CV in 0.2 M PBS (pH 7.0) with 400 μM CPZ. The corresponding CV data could be found in Fig. 5D. The RSD of the five electrodes were calculated to be 3.24% from the data in Fig. 5D, indicating a good level of reproducibility. For the stability investigation, the modified electrode was placed in the dark at room temperature for 14 days. The sensor still retained 97.12% of the initial electrochemical signal response, suggesting excellent stability.Practical feasibility experiments
To investigate the practical feasibility of the sensor, real sample analysis was conducted with local lake water. Before conducting electrochemical experiments, the lake water was diluted with 0.2 M PBS and added to a known amount of CPZ. The anodic peak current of the actual sample solution was then measured by CV under optimum conditions. From Table 2, the sensor shows good recovery rates of 99.09% to 104.51% and an RSD of 2.1 to 5.1% for lake water analysis. This confirms that the sensor is effective for the detection of CPZ in real samples.| Samples | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Lake water | 10 | 10.45 | 104.51 | 2.1 |
| 30 | 29.79 | 99.31 | 5.1 | |
| 50 | 49.54 | 99.09 | 3.3 | |
| 80 | 79.84 | 99.80 | 3.7 |
Conclusions
The nanomaterial CoWO4 was prepared by a simple solvothermal technique and utilized to form CoWO4/MWCNT nanocomposite with pre-acidulated MWCNT. The CoWO4/MWCNTs modified electrode was used to detect the antipsychotic drug CPZ by CV method. The results revealed that the CPZ oxidation peak current of the sensor increased approximately five-fold that of bare GCE. As for the determination of CPZ, the comparison of the CoWO4/MWCNT/GCE with other reported modified electrodes confirmed that the as-prepared electrochemical sensor had the better linear range (1 to 2000 μM) and detection limit (0.33 μM). It also had excellent performance with regard to anti-interference, reproducibility, and stability. Moreover, the excellent recovery rate for lake water analysis means that the composite has reliable potential for real sample analysis.Author contributions
Si Zeng: conceptualization, methodology, formal analysis, investigation, writing – original draft. Peiyao Zhu: formal analysis, investigation, resources. Deyu Liu: methodology, formal analysis, investigation. Yongmei Hu: investigation, resources, visualization. Qitong Huang: writing – review & editing, supervision, funding acquisition. Haiping Huang: conceptualization, writing – review & editing, supervision, project administration, funding acquisition.Data availability
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Natural Science Foundation of China (22364015), Natural Science Foundation of Jiangxi Province (20242BAB26032 and 20202ACBL214012), Jiangxi Provincial Key Laboratory of Functional Molecular Materials Chemistry (20212BCD42018), and the Youth Jinggang Scholars Program in Jiangxi Province.References
- R. Shanmugam, J. Ganesamurthi, T. W. Chen, S. M. Chen, M. Balamurugan, M. A. Ali, A. M. Al-Mohaimeed, W. A. Al-onazi and K. Alagumalai, Mater. Today Chem., 2022, 25, 100982 CrossRef.
- A. Motaharian, M. R. M. Hosseinic and K. Naseri, Sens. Actuators, B, 2019, 288, 356–362 CrossRef.
- B. B. Petkovic, D. Kuzmanovic, T. Dimitrijevic, M. P. Krstic and D. M. Stankovic, Int. J. Electrochem. Sci., 2017, 12, 3709–3720 CrossRef.
- S. Ahmadzadeh, F. Karimi, N. Atar, E. R. Sartori, E. Faghih-Mirzaei and E. Afsharmanesh, Inorg. Nano-Met. Chem., 2017, 47, 347–353 CrossRef.
- A. Mokhtari and B. Rezaei, Anal. Methods, 2011, 4, 996–1002 RSC.
- Y. Yamini and M. Faraji, J. Pharm. Anal., 2014, 4, 279–285 CrossRef.
- L. Q. Zhang, P. G. Wu, Y. M. Zhang, Q. Jin, D. J. Yang, L. Y. Wang and J. Zhang, Anal. Methods, 2014, 2, 503–508 RSC.
- E. Y. Z. Frag, M. A. Zayed, M. M. Omar, S. E. A. Elashery and G. G. Mohamed, Int. J. Electrochem Sci., 2012, 7, 650–662 CrossRef.
- A. M. El-Didamony and S. M. Hafeez, Main Group Chem., 2012, 11, 113–124 Search PubMed.
- L. Y. Sheng, Z. J. Li, A. L. Meng and Q. H. Xu, Sens. Actuators, B, 2018, 254, 1206–1215 CrossRef.
- B. Muthukutty, J. Ganesamurthi, S. M. Chen, B. Arumugam, F. M. Chang, S. M. Wabaidur, Z. A. ALOthman, T. Altalhi and M. A. Ali, Electrochim. Acta, 2021, 386, 138482 CrossRef CAS.
- S. Mgidlana and T. Nyokong, J. Photochem. Photobiol., A, 2021, 418, 113421 CrossRef CAS.
- T. Nandagopal and G. Balaji, Energy Technol., 2024, 12, 2301625 CrossRef CAS.
- P. R. Ilango, K. Prasanna, Y. N. Jo, P. Santhoshkumar and C. W. Lee, Mater. Chem. Phys, 2018, 207, 367–372 CrossRef CAS.
- M. I. Ahmed, A. Adam, A. Khan, M. N. Siddiqui, Z. H. Yamani and M. Qamar, Mater. Lett., 2016, 177, 135–138 CrossRef CAS.
- C. Bhuvaneswari, A. Elangovan, C. Sharmila, K. Sudha and G. Arivazhagan, Colloids Surf., A, 2023, 656, 130299 CrossRef CAS.
- K. Alagumalai, M. Balamurugan, S. M. Chen and M. Selvaganapathy, Microchem. J., 2020, 159, 105381 CrossRef.
- J. C. Zhang, C. Y. Xu, R. C. Zhang, X. Y. Guo, J. Wang, X. H. Zhang, D. J. Zhang and B. Q. Yuan, Mater. Lett., 2018, 210, 291–294 CrossRef.
- L. Xu, S. H. Wang, Y. Jin, N. P. Liu, X. Q. Wu and X. Wang, Sep. Purif. Technol., 2021, 276, 119405 CrossRef.
- G. A. Meenakshi, S. Sakthinathan and T. W. Chiu, J. Electrochem. Soc., 2024, 170, 127508 CrossRef.
- X. Zeng, X. H. Li, Y. Zhang, C. C. Wang, Y. Y. Liu, C. J. Hou and D. Q. Huo, Anal. Methods, 2024, 16, 5902–5908 RSC.
- G. J. Feng, Y. Yang, J. T. Zeng, J. Zhu, J. J. Liu, L. Wu, Z. M. Yang, G. Y. Yang, Q. X. Mei, Q. H. Chen and F. Y. Ran, J. Pharm. Anal., 2022, 12, 453–459 CrossRef PubMed.
- W. Huang, F. P. Liu, G. Xiang, Z. F. Zhang, Q. Huang, Z. J. Pan, W. F. Zhuge and J. Y. Peng, Analyst, 2023, 148, 3524–3530 RSC.
- Y. Shu, Q. Lu, F. Yuan, Q. Tao, D. Q. Jin, H. Yao, Q. Xu and X. Y. Hu, ACS Appl. Mater. Interfaces, 2021, 12, 49480–49488 CrossRef PubMed.
- V. P. Singh, G. Singh, R. Patel, U. K. Gaur and M. Sharma, J. Environ. Chem. Eng., 2023, 11, 111208 CrossRef.
- S. Abedini, A. A. Rafati and A. Ghaffarinejad, New J. Chem., 2022, 46, 20403–22041 RSC.
- S. M. Chen, G. Yang, Y. Jia and H. J. Zheng, ChemElectroChem, 2016, 3, 1490–1149 CrossRef.
- V. K. V. P. Srirapu, A. Kumar, P. Srivastava, R. N. Singh and A. S. K. Sinha, Electrochim. Acta, 2016, 209, 75–84 CrossRef.
- X. J. Liu, M. Y. Dou, G. Yang, E. K. Liu, Z. M. Li, B. C. Han, H. Yang, D. C. Li and J. M. Dou, Sep. Purif. Technol., 2024, 354, 128680 CrossRef.
- S. Kubendhiran, R. Sakthivel, S. M. Chen, R. Anbazhagan and H. C. Tsai, Composites, Part B, 2019, 168, 282–290 CrossRef CAS.
- M. A. Karimi, A. Hatefi-Mehrjardi, M. M. Ardakani, R. B. Ardakani, M. H. Mashhadizadeh and S. Sargazi, Russ. J. Electrochem., 2011, 47, 34–41 CrossRef CAS.
- N. Subramanian, K. M. Priya, M. O. Valappil, N. Nesakumar, S. Kesavan and S. Alwarappan, J. Electrochem. Soc., 2019, 166, 749–755 CrossRef.
- N. Nesakumar, S. Anantharaj, N. Subramanian, S. Kundu and S. Alwarappan, J. Electrochem. Soc., 2018, 165, 536–542 CrossRef.
- S. Ahmadzadeh, F. Karimi, N. Atar, E. R. Sartori, E. Faghih-Mirzaei and E. Afsharmanesh, Inorg. Nano-Met. Chem., 2017, 47, 347–353 CrossRef.
- A. Hajian, A. A. Rafati, A. Afraz and M. Najafi, J. Electrochem. Soc, 2014, 161, 196–200 CrossRef.
- Z. W. Lu, K. Wei, H. Ma, Q. Q. Xiong, Y. B. Li, M. M. Sun, X. X. Wang and Y. Y. Wang, Sep. Purif. Technol., 2023, 327, 124858 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4an01298d |
| This journal is © The Royal Society of Chemistry 2025 |