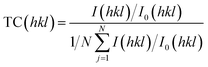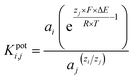A new compact potentiometric electrode for pH monitoring built upon a glass substrate with a Ce-doped SnO2 layer
Ayman H.
Kamel
 *ab,
Noor H. A.
Al-Sabbagh
b,
Ibrahim
Moussa
c,
M.
Obaida
c,
Hisham S. M.
Abd-Rabboh
*ab,
Noor H. A.
Al-Sabbagh
b,
Ibrahim
Moussa
c,
M.
Obaida
c,
Hisham S. M.
Abd-Rabboh
 d and
Waleed E.
Boraie
e
d and
Waleed E.
Boraie
e
aDepartment of Chemistry, Faculty of Science, Ain Shams University, Abbasia, Cairo 11566, Egypt. E-mail: ahkamel76@sci.asu.edu.eg
bDepartment of Chemistry, College of Science, Sokheer 32038, Kingdom of Bahrain. E-mail: ahmohamed@uob.edu.bh
cSolid State Physics Department, Physics Research Institute, National Research Centre, Dokki, Giza 12622, Egypt
dChemistry Department, College of Science, King Khalid University, P. O. Box 9004, Abha 62223, Saudi Arabia
eDepartment of Chemistry, College of Science, King Faisal University, P. O. Box 400, Al-Ahsa 31982, Saudi Arabia
First published on 21st November 2024
Abstract
A novel compact potentiometric electrode specifically designed for pH monitoring, featuring a good construction on a glass substrate coated with a cerium-doped tin oxide (Ce-doped SnO2) layer. The Ce-doped SnO2 thin film was created by spray-pyrolysis it on a glass substrate. Field emission scanning electron microscopy (FESEM) and X-ray diffraction (XRD) were used to characterize the deposited metal oxide coating. As a miniature potentiometric electrode, the synthesized Ce-doped SnO2/glass substrate was utilized to measure a broad pH range (pH 2–12) in aqueous solutions. The electrode had a perfect near-Nernstian response (slope of −58.6 ± 0.7 mV per decade), high potential stability, mechanical durability, and great selectivity towards some common interfering cations and anions. These characteristics made it ideal for quality control and assurance purposes. The electrode's performance parameters and validation measurements were assessed using established procedures. The Ce-doped SnO2-based electrode satisfactorily monitored the pH of several genuine water, drink, and fruit juice samples, and the data compared well with those obtained using a traditional pH glass electrode. The integration of Ce-doped SnO2 as the active material marks a significant advancement, providing enhanced electrochemical stability, improved sensitivity, and a wider pH detection range compared to conventional electrodes. The compact design not only reduces the sensor's footprint but also facilitates its application in miniaturized and portable pH monitoring devices, making it highly suitable for advanced analytical and environmental sensing applications.
1 Introduction
Electrochemical pH sensors are popular because they respond quickly (less than 10 seconds), can measure pH levels from 2 to 12, are sensitive (near the Nernstian response of 59.12 mV pH−1), are easy to attach to wearable or flexible materials, are biocompatible, and don't cost much to make. They are used in many areas, such as food processing, health monitoring, agriculture, and nuclear power. Ion-sensitive metal oxides (MOx) with micro- and nanostructured shapes have received a lot of attention because they could be used in many scientific fields, such as electrochemistry and biomedical sciences. Their unique electrical, electrochemical, and biocompatible qualities demonstrate their value in the creation of sensors for use in industrial settings, wearable medical devices, and food and water quality monitoring, among other uses. MOx increases catalytic activity, and sensitivity due to its high surface-to-volume ratio. Furthermore, MOx is an excellent choice for the upcoming wave of online monitoring and wireless sensing applications because of their quick response time and extended lifespan in a variety of environmental situations. One of the main uses of MOx among the different types of sensors in these applications is the creation of electrochemical pH sensors. MOx is at the forefront of materials research due to the importance of pH in a variety of sectors, such as chemistry, agriculture, food processing, pharmaceuticals, environmental science, and chemical and biomedical engineering applications.1–8 The measurement and regulation of hydrogen ion concentration—expressed in terms of the pH value-is crucial for many chemical and biological interactions. The pH level can significantly impact a person's physiological, biochemical, and medical conditions.9–14Advancements in pH sensing technology, using appropriate sensitive materials, have primarily focused on improving sensitivity, operational life, biocompatibility, rapid response in wider pH ranges, cost-effectiveness, and mobility. Recently, researchers have described several MOx materials for the development of pH sensors in expanding fields such as flexible and wearable systems,1,3,5,15–20 biological and food quality applications,10,21 and pollution monitoring.22–24Fig. 1 shows a few instances of recently created pH sensors based on MOx that are essential for our everyday lives. Given that several factors, including the rise of MOx as a crucial component for the development of these sensors, determine a pH sensor's performance, this comprehensive evaluation of the MOx-based pH sensor will be beneficial for future advancements in both fabrication and application. Examining the ionic exchange between sensitive nanomaterials and electrolytes is essential. The electrochemical characteristics of an aqueous solution, such as its pH level and ionic content, can drastically alter the electrodes' capacitance, impedance, and electrochemical potential.
 | ||
| Fig. 1 Different metal oxide-based pH sensors including essential techniques for fabricating sensors and preparing electrode materials, types of sensors, parameters for analysis, and applications. | ||
Conventional glass-based sensors have been widely used to monitor pH. However, their deployment in new fields like wearable systems is hampered by their mechanical fragility and lack of flexibility and bendability. Moreover, their employment in industrial applications such as pollution monitoring is restricted due to their instability in performance at high temperatures and pressures and their durability. The limitations of glass-based sensors have prompted researchers to investigate pH-sensing methods that are not glass-based. Of these, MOx-based pH sensors have garnered a lot of interest because of their near-Nernstian sensitivity, quick response time (<10 s), extended lifespan (>1 year), ease of miniaturization, and stability in a variety of environments, and the biocompatibility of most MOx.
The choice of material composition and the deposition technique have a significant impact on the MOx pH sensor's sensitivity because they can affect the material's microstructure, porosity, surface uniformity, and crystalline structure. This causes the electrodes' sensitivity to vary. Typically, we use the Nernstian response to express the sensitivity of pH sensors. If the sensitivity factor approaches the optimal Nernstian response of 59.14 mV pH−1 at 25 °C, as calculated from the Nernst equation, the sensor should perform exceptionally well.
Ruthenium oxide (RuO2) and iridium oxide (IrO2), distinguished by their exceptional sensitivity to hydrogen ions and great accuracy, are the most well-known and frequently used oxides for the creation of pH sensors. For use as pH sensors, we can also tell the difference between these oxides: tantalum(V) oxide Ta2O5, titanium dioxide TiO2, SnO2, CeO2, WO3, and PbO2, and mixed oxides Bi2O3–Nb2O5, IrO2–TiO2, and RuO2–TiO2. Furthermore, bismuth and antimony-based metal/MOx-based pH sensors have been the subject of some research. The redox equilibrium between the metal/MOx phases (e.g., Sb–Sb2O3) is believed to be the source of the pH sensitivity in these couples.25,26 A comparison was made between the observed and theoretical sensitivity of several MOx, namely IrO2,27,28 RuO2,23,29–33 TiO2,31,33,34 SnO2,29,35 Ta2O5,23,36 WO3,37,38 CeO2,39 PbO2,40 MnO2,41 and CoO2.42Table 1 compares the analytical properties of MOx-based sensors, such as the pH measurement range, calibration curve slope, and response time.
| Sensing material | Slope [mV pH−1] | pH range | Time response, s | Ref. |
|---|---|---|---|---|
| RuO2 | 55.64 | 1–13 | — | 30 |
| RuO2–TiO2 | 56.03 | 2–12 | — | 34 |
| RuO2–Ni | 52 | 1.5–12.5 | — | 31 |
| RuO2–nanotubes | 55 | 2–12 | <40 | 32 |
| RuO2–Ta2O5 | 56 | 2–12 | <8 | 23 |
| RuO2–SnO2 | 56.5 | 2–12 | <5 | 29 |
| IrO2 | 63.5 | 2–10 | 60 | 27 |
| IrO2–TiO2 | 59.1 | 1–13 | 120 | 28 |
| Ta2O5 | 58–59 | 2–12 | 0.3 | 36 |
| SnO2 | 58.1 | 2–12 | — | 35 |
| TiO2 | 58.73 | 1–11 | — | 33 |
| WO3 | 44.85 | 1–7 | — | 37 |
| WO3–nanotubes | 41 | 2–12 | <90 | 38 |
| CeO2 | 38 | 7.2–10.8 | — | 39 |
| PbO2 | 64.82 | 1.5–12.5 | <1 | 40 |
| MnO2 | 78.3 | 2–12 | 5 | 41 |
| CoO2 | 54.9 | 1–12 | <60 | 42 |
This work introduces a novel compact potentiometric electrode specifically designed for pH monitoring, featuring a groundbreaking construction on a glass substrate coated with a cerium-doped tin oxide (Ce-doped SnO2) layer. The integration of Ce-doped SnO2 as the active material marks a significant advancement, providing enhanced electrochemical stability, improved sensitivity, and a wider pH detection range compared to conventional electrodes. The compact design not only reduces the sensor's footprint but also facilitates its application in miniaturized and portable pH monitoring devices, making it highly suitable for advanced analytical and environmental sensing applications. This study represents a critical step forward in the development of next-generation pH sensors, combining innovative materials science with practical engineering solutions.
2 Experimental
2.1 Chemicals and reagents
Tin(IV) chloride (SnCl4), cerium(III) chloride heptahydrate (CeCl3·7H2O) and all other chemicals were of the highest purity, purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received. Deionized distilled water was used throughout the present work. Britton–Robinson (BR) buffer solutions were prepared with pH ranging from 2 to 12. The buffer was prepared by dissolving 0.04 mol of H3PO4, H3BO3 and CH3COOH in one liter of deionized water. The pH of the buffer solution was adjusted to 4.0 using 1.0 M NaOH, resulting in an ionic strength of approximately 0.044 M, which was counterbalanced by the concentrations of [H2PO4−], [CH3COO−], and [H+]. This consideration is crucial for accurate determination of the selectivity coefficients.2.2 Apparatus
All electrochemical measurements were carried out using a PalmSens4 (Bi)Potentiostat/Galvanostat/Impedance Analyzer [PalmSens BV, Houten, Netherland]. The data are collected using PSTrace 5 software (version 5.10). The electrochemical cell volume in all experiments was 10 mL, and all measurements were performed at room temperature. The pH of the solutions was measured using an Orion (Cambridge, MA, USA) Model STAR A211 pH mV−1 m−1. X-ray diffraction (XRD) (Bruker AXS-D8, Germany) and CuKα radiation (λ = 1.5406 Å) were used to study the synthesized undoped and Ce-doped SnO2 thin films in the 2θ range of 10° to 90°. In addition, the surface morphology of the fabricated thin films was characterized, and the elemental composition was measured using an energy dispersive X-ray spectroscopy (EDS) instrument (QUANTA FEG 250 FE-SEM with an EDAX system, Netherlands) at 450 °C. The thickness of the undoped and Ce-doped SnO2 thin films was measured using a UVISEL Plus ellipsometer (HORIBA Scientific, Japan).2.3 Electrode fabrication
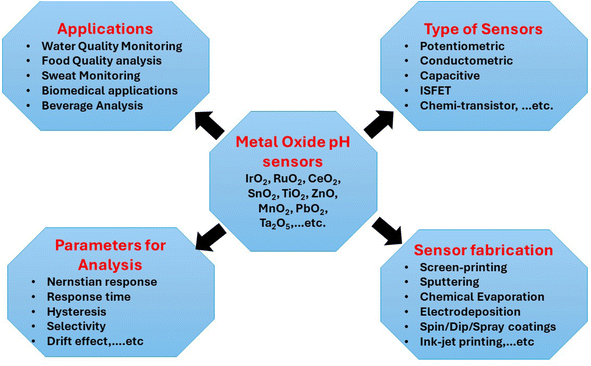
Non-phosphate detergents, deionized water, and isopropanol were used under sonication to clean the glass substrates and to make sure that no unwanted absorption—such as lipids—had taken place. Using a homemade pulsed spray pyrolysis (PSP) apparatus with a revolving heater, Ce-doped SnO2 films are formed on glass substrates.43,44 The Ce-doped SnO2 layer was deposited after heating the substrate to a constant temperature of 450 °C. The distance between the substrate and nozzle is set at 30 cm. The system operates in an ON/OFF mode for 5 seconds at a time—1 second of ON and 4 seconds of OFF—with a total deposition duration of 20 min. To atomize the solution across the heated glass substrates at a flow rate of 2.5 mL min−1, compressed dry air is delivered at a pressure of 2.5 bars. Fig. 2 illustrates the method of applying a handmade pulsed spray pyrolysis (PSP) apparatus to deposit the Ce-doped SnO2 coating on the glass substrate. | ||
| Fig. 2 An illustration shows the steps involved in applying a homemade pulsed spray pyrolysis (PSP) system to deposit a Ce-doped SnO2 coating on a glass substrate. | ||
2.4 Sensor assembly
After the spraying of the tin and cerium chloride solution onto the glass substrate (aluminosilicate glass), the coated glass was subjected to a thermal annealing process at 450 °C for 20 min, facilitating the conversion of the metal chlorides into their respective oxides. This process ensured the formation of a uniform Ce-doped SnO2 layer on the glass substrate. Post-annealing, the glass disk with the oxide coating was cut into smaller pieces of 10 mm × 10 mm, ensuring that each piece had a uniform oxide coating on one face. To create a low-resistance contact, the aluminosilicate glass was coated with polyaniline (PANI), a conductive polymer with significantly lower resistivity than glass. Polyaniline provides a stable, conductive surface that reduces the overall resistance between the coated and uncoated faces of the glass substrate, which is crucial for potentiometric and impedance measurements. With PANI's resistivity in the range of 100 to 10−1 Ω cm, it ensures that the total resistance is indeed significantly lower than a gigaohm. A thin layer of silver epoxy paste, applied to the uncoated side of the glass slide, served to securely attach a thin copper wire. To minimize resistance, the epoxy was applied uniformly, and the connection was thoroughly tested for electrical continuity. We encapsulated the entire assembly to protect it from contact with the solution during measurements. Preliminary tests indicated that the electrical resistance of the connection was significantly lower than a gigaohm, ensuring that potentiometric and impedance measurements remain feasible. To prevent direct exposure of the electrical connections to the solution, the sensor was coated with a thin, chemically inert protective layer, leaving only the Ce-doped SnO2 surface exposed to the solution.2.5 Potential measurements
Using conventional potentiometric techniques, the potential response of the Ce-doped SnO2/glass electrodes was measured. In conjunction with an Ag/AgCl/KCl 3.5 M double-junction reference electrode that was loaded with 10% (w/v) KNO3, the Ce-doped SnO2/glass electrode was used as an indicator electrode. The pH range of the Britton–Robinson buffer solutions was used to generate the calibration curve. After stabilizing to approximately ±1 mV, we took the potential readings and plotted the electromotive force (EMF) against the pH of the solutions.For optimal performance, the Ce-doped SnO2 sensors were soaked in deionized water for approximately 24 hours to ensure proper hydration of the surface and stabilization of the sensor's response. This soaking process allows the sensor to reach a steady-state potential, which is crucial for accurate pH measurements. After the initial conditioning, the sensors can be dried and reused without significant degradation in performance.
The fabricated pH electrode was subjected to a range of pH solutions, from 2 to 12, to investigate the response time. The potential corresponding to each pH was monitored for two minutes. Utilizing the two-solution method (TSM),45 the pH electrode's selectivity coefficients against various interfering ions were determined. The glass substrate was subjected to electrochemical impedance spectroscopy (EIS) experiments in both undoped SnO2 and Ce-doped SnO2 membrane-based sensors. Using a pH 6 Britton–Robinson buffer, the Nyquist plot (−Z′′vs. Z′) was obtained under the open circuit potential with a frequency range of 100 kHz to 1.0 Hz.
3 Results and discussion
3.1 XRD analysis
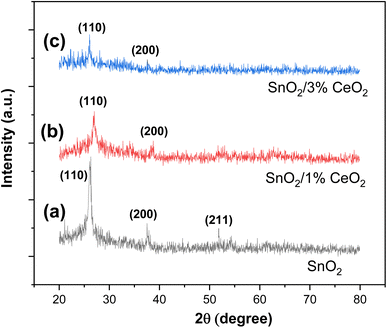
The XRD patterns of undoped and Ce-doped SnO2 thin films are displayed in Fig. 3. The SnO2 film that is not doped has a polycrystalline structure. The diffraction peaks at Θ = 26.12°, 37.69°, and 51.88° are in the (110), (200), and (211) planes, according to JCPDS card 41-1445. They are linked to the tetragonal phase of SnO2 (space group P42/mnm). This finding indicates that a tetragonal phase is present in the undoped SnO2 thin film. Fig. 3b and c show how the XRD patterns of undoped and Ce-doped thin films change structurally as the concentration of Ce varies. Peak intensity varies in response to an increase in Ce concentration. Ce concentrations up to 3 wt% still exhibit the tetragonal phase. Another important finding from the XRD study is that there are no peaks for other phases, like Ce2O3. This means that Ce3+ ions have replaced Sn4+ ions in the SnO2 lattice.The XRD results (Fig. 3) indicate that the production of distortions and dislocations in the SnO2 lattice most likely causes the decrease in peak intensity of the tetragonal phase with increasing doping concentration. These limitations are caused by the different ionic radii of Sn4+ (0.069 nm) and Ce4+ (0.088 nm), as well as the influence of doping during the nucleation process, which can either accelerate or impede the formation of SnO2 crystallites.
Since the intensity of the (110) plane is higher, the (110) peak for the tetragonal phase has the strongest and greatest intensity. As a result, we can acknowledge that there may have been a preferred growth along this direction (110). Using the following formula to calculate the texture coefficient TC (hkl), a quantitative investigation of variations in preference orientation was carried out.46
Table 2 summarizes the computed TC (hkl) for the elaborated samples for the (110), (200), and (211) planes. The texture coefficient must be greater than unity to define the desired growth orientation. For all SnO2 tetragonal phase samples, the (110) plane dominates in intensity. Another noteworthy observation pertains to the differences detected between the TC (200) and TC (211) of the tetragonal phase and the TC (110) of the same phase. These data suggest that the axis (110) is the preferred growth direction for SnO2 crystallites in the tetragonal phase.
| Ce (wt%) | 0% | 1% | 3% |
|---|---|---|---|
| Average crystallite size (nm) | 21.43 | 24.62 | 27.52 |
| Lattice parameters (Å) | a = 4.7339 | a = 4.7356 | a = 4.7321 |
| c = 3.1974 | c = 3.1961 | c = 3.1924 | |
| TC (hkl) | (110) 1.80 | (110) 1.85 | (110) 1.41 |
| (200) 0.55 | (200) 0.69 | (200) 0.65 | |
| (211) 0.63 | (211) 0.44 | (211) 0.0.42 | |
| Microstrain (ε × 10−5) | 166 | 135 | 123 |
| Cell volume (Å3) (at2 × ct) | 71, 65 | 71, 67 | 71, 48 |
Using the following formulae, the lattice parameters of elaborated SnO2 thin films were computed from the XRD pattern data to assess the impact of Ce doping on the volume of the crystalline SnO2 cell.47,48
2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ θ = nλ |
| 1/dhkl2 = h2/a2 + k2/bc2 + l2/c2 |
The average sizes of crystallites were calculated using the Scherrer formula49
D = 0.9λβ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
The results found are listed in Table 2. For the undoped sample, the calculated lattice parameters of the tetragonal phase are a = b = 4.7339 Å and c = 3.1974 Å, whereas the standard ones are a = b = 4.7382 Å, and c = 3.1871 Å for the tetragonal phase. It was noted that the tetragonal phase's lattice parameters slightly increase as the Ce concentration increases from 0 to 3 wt%. Given the large difference in ionic radius between Ce3+ and Sn4+, we hypothesize that the lattice distortions of SnO2 caused by Ce doping are the reason for this shift in lattice parameters.
Table 2 shows the microstrains for the tetragonal phase, as well as the average size of SnO2 crystallites. The estimated crystallite size and the microstrains in the tetragonal phase agree. It is evident that films with a larger crystallite size and reduced stress result from doping with 3 wt% Ce. The inverse relationship between microstrains and average crystallite sizes supports the generation of larger SnO2 crystallites as the defect amount decreases, ultimately leading to the production of high-quality films with an average thickness of 986 nm which was obtained using ellipsometry.
The scanning electron microscopy (SEM) images depict the surface morphology of pure SnO2 and Ce-doped SnO2 thin films deposited on glass substrates via the spray pyrolysis technique (Fig. 4). The SEM image of the pure SnO2 film shows a relatively smooth and uniform surface, with some agglomeration of particles, characteristic of nanostructured films. The grain size appears to be consistent across the surface, which is typical for films deposited using spray pyrolysis, indicating a controlled growth process. In contrast, the Ce-doped SnO2 film exhibits a more heterogeneous structure with a slight increase in surface roughness. The incorporation of Ce into the SnO2 matrix seems to have influenced the film's morphology by promoting the formation of larger and more defined grains, which can be attributed to the effect of Ce ions on the nucleation and growth dynamics during deposition. This modified surface morphology is likely to affect the electrical and sensing properties of the doped films, making them potentially more responsive in sensor applications.
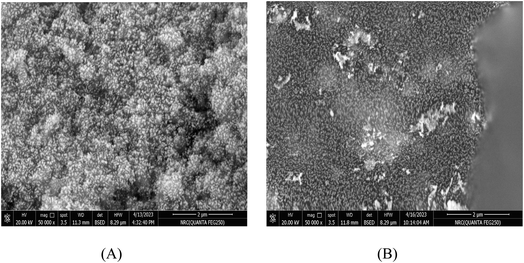
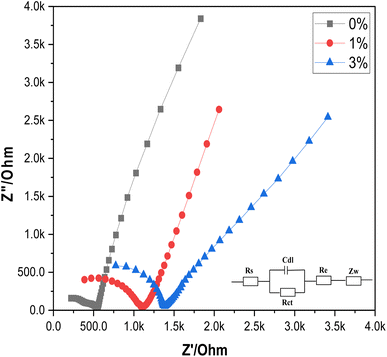
3.2 Electrochemical impedance spectroscopy (EIS) measurements
EIS is employed to study the impedance characteristics of both undoped and Ce-doped SnO2 thin films as shown in Fig. 5. The impedimetric responses of these films provide insights into the underlying electrochemical processes, including charge transfer resistance and diffusion. The measurements reveal that both diffusion and charge transfer processes dominate the impedance behavior, which is consistent with the expectation for materials used in electrochemical applications. The experimental data were fitted using the traditional Randles' circuit model, a well-established approach for analyzing impedance data. In this model, Rs represents the solution resistance, Rct is the charge transfer resistance, Cdl denotes the double layer capacitance, Re represents the added high-resistance component, and Zw represents the Warburg impedance, which is associated with the diffusion of electroactive species. The fitting of the data to this model allows for the quantification of these key electrochemical parameters. The analysis shows a significant difference in the charge transfer resistance between the undoped and Ce-doped SnO2 thin films. The undoped SnO2 exhibits a relatively low Rct value of 349.8 Ω, indicating efficient charge transfer. However, when SnO2 is doped with cerium, the Rct values increase to 714.7 Ω and 578.8 Ω for 1% and 3% doping levels, respectively. This increase suggests that the introduction of cerium atoms into the SnO2 lattice affects the charge transfer process, potentially due to changes in the electronic structure or surface chemistry of the doped films, which correlates with better electrochemical reactivity of the surface. This allows for more efficient electron exchange during protonation/deprotonation at the surface, a fundamental process for potentiometric pH sensing. In addition, the introduction of Ce into the SnO2 matrix enhances surface activity by increasing the density of oxygen vacancies, which acts as active sites for the adsorption of H+ ions, essential for the potentiometric response. The reduction in Rct combined with increased surface capacitance (Cdl) from the EIS data highlights enhanced surface charging behavior, crucial for a quick response to pH changes. This could be due to the increased surface area or more active sites provided by the doped SnO2 films, which facilitate the accumulation of charge carriers. This suggests a trade-off between charge transfer efficiency and charge storage capability. This dual effect of doping is crucial for optimizing the performance of SnO2-based electrodes in their potentiometric response. In conclusion, Ce-doping is introduced into the SnO2 matrix to address specific limitations of pure SnO2 in pH sensing. SnO2 alone suffers from lower surface conductivity and limited sensitivity to pH changes due to fewer available surface sites for proton exchange. As evidenced by the EIS results, Ce doping reduces the overall impedance and enhances the conductive pathways for charge transport across the electrode surface. In addition, Ce-doped SnO2 exhibits higher resistance to corrosion and chemical degradation under varying pH conditions, ensuring a longer sensor lifetime and consistent performance. The data presented in Table 3 further corroborate these observations and provide a quantitative basis for evaluating the impact of cerium doping on the electrochemical performance of SnO2 thin films.| Electrode | R s (Ω) | R ct (Ω) | C dl (μF) |
|---|---|---|---|
| Undoped SnO2 | 187.2 | 349.8 | 41.4 |
| Ce-doped SnO2 (1%) | 379.7 | 714.7 | 60.2 |
| Ce-doped SnO2 (3%) | 772.9 | 578.8 | 62.6 |
The EIS results show that the Ce-doped SnO2 sensor exhibits a significant change in charge transfer resistance (Rct) and double-layer capacitance (Cdl) compared to undoped SnO2. These impedance characteristics directly influence the potentiometric response of the sensor. Specifically, the increased Rct observed in Ce-doped SnO2 correlates with a slower charge transfer process, which impacts the sensor's sensitivity and response time. Conversely, the higher Cdl values are associated with improved capacitive effects, enhancing the sensor's ability to detect pH changes more accurately.
3.3 Potentiometric measurements
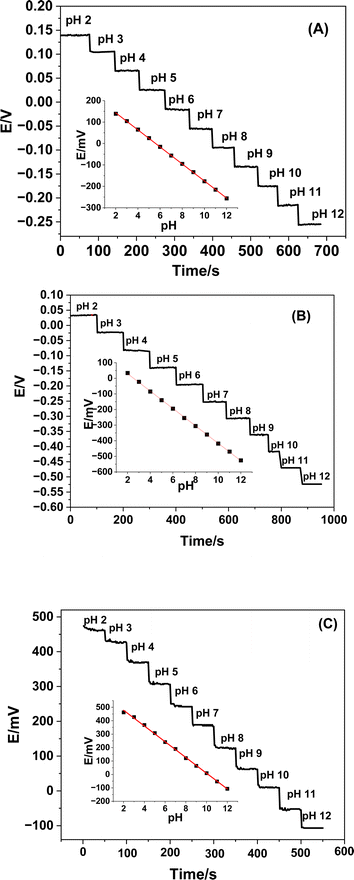
At ambient temperature, the potentiometric responses of both undoped and Ce-doped SnO2 thin film-based sensors were assessed and tested in relation to pH changes. The fabricated sensors were submerged in Britton–Robinson buffer solutions in each scenario, which ranged in pH from 2 to 12, from the most acidic to the most alkaline. The pH of the solution under examination determines each sensor's potential, as Fig. 6 illustrates. Table 4 confirms that, despite differences in each plot's Nernstian slope, all plots are linear.| Parameter | Undoped SnO2 | Ce-doped SnO2 (1%) | Ce-doped SnO2 (3%) |
|---|---|---|---|
| Slope, mV per decade | −39.8 ± 0.2 | −55.8 ± 0.3 | −58.6 ± 0.7 |
| Linear response range, pH | 2–12 | 2–12 | 2–12 |
| Correlation coefficient, r2 | 0.9998 | 0.9997 | 0.9986 |
| Average response time, s | ∼15 | ∼13 | ∼10 |
| Precision, (%) | 1.1 | 0.9 | 0.7 |
| Accuracy (recovery), (%) | 99.2 | 99.4 | 99.2 |
| Trueness, (%) | 99.1 | 99.3 | 99.2 |
By immersing the electrodes in buffer solutions with pH ranges of 2 to 12 and monitoring the potential corresponding to each pH for two minutes, the response times of the manufactured sensors were assessed. Compared to un-doped Ce–SnO2 based sensors, it was observed that Ce-doped SnO2-based sensors require less time to reach the equilibrium state (Fig. 5). This is most likely the result of the sensors' distinct response mechanisms. The electrodes manufactured at doping percentages of 0.0, 1.0, and 3.0% exhibited average reaction times of 15, 13, and 10 s, respectively.
The near-Nernstian slopes observed for both the RuO2–SnO2 (ref. 29) and bare SnO2 (ref. 35) sensors, as well as for the Ce-doped SnO2 sensors, indicate that these materials are effective in providing a predictable and reliable potentiometric response over a wide pH range (pH 2 to 12). The fact that the 1% and 3% Ce-doped SnO2 sensors exhibit near-Nernstian slopes (−55.8 mV pH−1 and −58.6 mV pH−1, respectively) similar to RuO2–SnO2 and bare SnO2 highlights their potential as robust pH sensors. Although the Ce-doped sensors show similar pH ranges and slopes to the other sensors listed in Table 1, one of the unique aspects of the Ce-doped SnO2 sensors is their improved sensitivity at lower doping levels. The 1% Ce-doped sensor, with a slope of −55.8 mV pH−1, maintains high sensitivity while potentially offering advantages in stability and selectivity due to the incorporation of cerium. The 3% Ce-doped sensor further enhances this sensitivity, achieving a slope of −58.6 mV pH−1, which is very close to the ideal Nernstian response. In contrast, the undoped SnO2 sensor exhibits a significantly lower slope (−39.8 mV pH−1), indicating a less efficient response in terms of sensitivity. This improvement in sensitivity with Ce doping suggests that cerium incorporation enhances the electrochemical properties of SnO2, possibly by increasing the density of active sites or improving the electron transfer characteristics of the material.
Another critical attribute of the Ce-doped sensors is the potential improvement in stability and selectivity, which is not solely reflected in the calibration slopes but also in their operational robustness. Cerium doping is known to stabilize the SnO2 structure and reduce the impact of interfering ions, which could explain the stable performance of the Ce-doped sensors across a wide pH range. While RuO2–SnO2 (ref. 29) and bare SnO2 (ref. 35) sensors perform well, the introduction of cerium offers an additional layer of material stability and potentially enhances the long-term operational stability of the sensor.
3.4 Sensors' selectivity
To demonstrate the selective efficiency of the pH sensors in various matrices, the selectivity of the manufactured sensors towards some of the common interfering ions has been investigated. The selectivity coefficient values (Kpot) were computed using the following formula and assessed using the two solution method (TSM):50where ai, zi, aj and zj are the activities and the charges of the primary and the interfering ions, respectively. ΔE is the difference between the electrode potentials in a solution which contains both the primary (ai) and the interfering (aj) ions and solution which contains only the primary ion (ai), knowing that (ai) is the same in both solutions. Potentiometric measurements were conducted at a concentration of 0.1 M for the interfering ions, with an H+ ion activity of approximately 1.0 × 10−4 M at pH 4.0. The concentrations of all interfering ions are adjusted according to the appropriate activity coefficients (gamma) as per the Davies equation [the gamma value of 0.76 is correct for monovalent and of 0.6 for divalent interfering ions]. This activity reflects the reduced effective concentration of ions, which is critical for accurate KA,Bpot calculations, as outlined in IUPAC guidelines. Table 5 contains a tabulation of the selectivity coefficients. The product aj × Kij ≪ ai indicates that the pH sensors exhibit good selectivity for H+ ions over the interfering ions, while accuracy is influenced by other factors such as drift and hysteresis. The determined constants reflect both the effects of the counterions and any resulting pH shifts, as well as the interference from the targeted ions.
| Interfering ion | Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kpotij Kpotij |
||
|---|---|---|---|
| Undoped SnO2 | Ce-doped SnO2 (1%) | Ce-doped SnO2 (3%) | |
| a All anions and cations were in the form of sodium and chloride salts, respectively. | |||
| Na+ | −3.01 | −3.24 | −3.43 |
| K+ | −3.13 | −3.22 | −3.31 |
| Li+ | −3.43 | −3.62 | −3.87 |
| NH4+ | −3.56 | −3.74 | −3.91 |
| Ca2+ | −4.97 | −5.02 | −5.11 |
| Mg2+ | −4.77 | −5.00 | −5.01 |
| Ba2+ | −4.73 | −4.87 | −5.02 |
| Cl− | −3.02 | −3.16 | −3.19 |
| NO3− | −3.40 | −3.51 | −3.57 |
| I− | −3.40 | −3.48 | −3.67 |
| SCN− | −3.66 | −3.72 | −3.81 |
| CH3COO− | −4.12 | −4.36 | −4.44 |
| PO43− | −5.32 | −5.39 | −5.43 |
| NO2− | −3.78 | −3.88 | −4.01 |
The impedance measurements provide insight into the sensor's selectivity towards different ions. The higher Rct observed in Ce-doped SnO2 films suggests a reduced interaction with interfering ions, enhancing the sensor's selectivity. The impedance data support the observed selectivity by indicating that the doped films have a more stable response to pH changes while minimizing interference from ions such as Na+ and K+.
3.5 Potential drift
Potential drift refers to the gradual change in the electrode's potential over time when it is immersed in a solution of constant pH. It reflects the stability of the electrode's response. To evaluate it, the electrode is immersed in a buffer solution of pH 7. The potential (mV) was recorded over a full day. The potential at regular intervals (e.g., every 15 minutes) was measured. From the slope of the potential versus time plot, it was found that the drift of the undoped SnO2, Ce-doped SnO2 (1%) and Ce-doped SnO2 (3%) based sensors was 0.32, 0.12, and 0.08 mV h−1, respectively. The drift rate of a standard glass pH electrode was 0.3 mV h−1.3.6 Sensing mechanism
The sensing mechanism of pH sensors based on MOx has been the subject of extensive research. (i) Simple ion exchange in a surface layer containing –OH groups; (ii) a redox equilibrium between two different solid phases; (iii) a redox equilibrium involving only one solid phase whose hydrogen content can be continuously varied by passing current through the electrode; (iv) single phase “oxygen intercalation” of the electrode; and (v) steady-state corrosion of the electrode material are the five different mechanisms that Fog and Buck51 considered to explain the pH sensitivity of MOx. Researchers have discovered that “oxygen intercalation” provides the most plausible explanation for the mechanism behind pH sensitivity.52 According to Fog and Buck's51 explanation, Mihell and Atkinson,53,54 have also suggested that ion exchange in the surface layer containing eOH groups could potentially trigger the pH response. Dissociative adsorption of water causes hydroxide groups to generally blanket the MOx surface when the sensor comes into contact with a solution. A potential difference between the sensing electrode (SE) and reference electrode (RE) may arise from the oxide sites created by proton release, which may lead to the production of a few higher- and lower-valency MOx. Site-binding theory33,55,56 says that when most oxides are placed in water, they form the surface groups –OH−, –OH, and –OH3+, as seen in Fig. 7a. Protons and hydroxide ions from the solution attract oxygen ions from the MOx crystal lattice and the surface cations, respectively. As a result of this, hydroxide groups cover MOx. The formed metal hydroxide groups can either absorb a proton from the solution, transforming into a positive surface group, or donate a proton to the solution, producing a negative surface group.33,55,57,58 As seen in Fig. 6b, the charged surface groups at the MOx-solution interface form an electrical double layer (edl) structure. The edl is composed of the diffuse layer (Stern layer) and the Helmholtz layer (compact layer). As seen in Fig. 7b, the Helmholtz layer consists of the inner Helmholtz plane (IHP) and the outer Helmholtz plane (OHP). The IHP contains the charged surface groups, which chemically link to the MOx surface to form a strong adhesion. In the OHP, coulombic contact draws the ions from the solution to the surface charge. Ions in the solution flow freely into the diffuse layer as a result of thermal or electrical forces.29,55,56 Ions can cause these reactions by diffusing, adhering, or combining with ion adsorption at the MOx surface.29,57 Changes in the pH of a solution can affect the MOx surface's equilibrium state, which could lead to changes in the SE's electrical properties and surface potential (Ψ).29,33,59–61 The kind of electrode material and its chemical reactivity at the solution interface determine how the electrical characteristics of the edl vary. In order to look into how pH ISFETs work, Liao et al.33 did a lot of experiments and theoretical work based on site binding theory at the point of zero charge pHPZC and the surface potential of a tin oxide gate ISFET. The alterations of MOx33 are described in the following way:| 2.303(pHPZC − pH) = qϕ/kT + sinh−1[qϕ/kTβ] |
| β = 2q2Ns(KaKb)12/kTCdl |
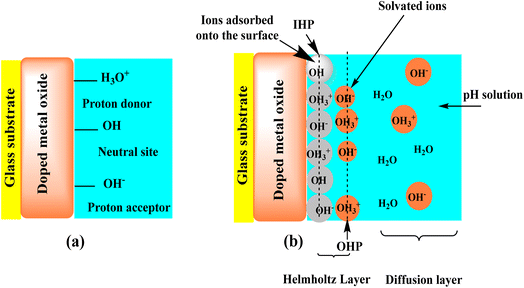 | ||
| Fig. 7 Schematic of the site-binding theory of a MOx-based pH sensor (a) and the adsorption of potential determining ions on the surface and the resulting edl structure (b). | ||
The EIS analysis reveals key aspects of the sensing mechanism. The observed increase in Rct in Ce-doped SnO2 is a consequence of the more complex charge transfer mechanism induced by the incorporation of cerium ions into the SnO2 lattice. The Ce doping introduces oxygen vacancies, which significantly influence both electronic and ionic conductivity. Oxygen vacancies act as shallow donors, contributing to the electron density in the conduction band of SnO2. However, they also introduce localized states that can trap charge carriers, leading to an increase in Rct. The oxygen vacancies increase the availability of active sites for H+ ion adsorption, which is essential for the pH response. The charge transfer mechanism at the sensor-electrolyte interface is dominated by protonation and deprotonation reactions involving these vacancies. However, the presence of Ce introduces a second effect: the cerium ions can exist in mixed oxidation states (Ce3+/Ce4+), which participate in reversible redox reactions. This redox behavior creates an additional pathway for electron exchange, complicating the charge transfer process at the electrode surface. The involvement of the Ce3+/Ce4+ couple adds a layer of complexity to the electron transfer, contributing to the observed higher Rct. Several studies have demonstrated that dopants, such as Ce, can modify the defect chemistry of metal oxides, leading to altered charge transport mechanisms. According to Xu et al. (2024),62 Ce doping can lead to the formation of deep-level traps, which act as recombination centers for charge carriers, thus increasing the charge transfer resistance in electrochemical measurements. This is consistent with the increase in Rct observed in our EIS results, indicating that the charge transfer mechanism is no longer a simple electron conduction process, but involves additional ionic and redox pathways. Furthermore, the literature on Ce-doped oxides63,64 suggests that the interaction between Ce and oxygen vacancies enhances the material's chemical stability and catalytic activity, which is beneficial for pH sensing. However, this also leads to more complex surface chemistry, as the cerium ions contribute to both the generation of oxygen vacancies and the electron trapping processes that increase Rct.
The increased Rct does not negatively affect the sensor's pH sensitivity. In contrast, the oxygen vacancies and the Ce redox couple contribute to enhanced surface reactivity. The increased number of active sites for H+ adsorption improves the sensor's ability to rapidly respond to pH changes, even though the overall charge transfer mechanism becomes more complex. In fact, the higher Rct reflects the enhanced surface interactions between the electrode and the electrolyte, which are crucial for accurate and stable pH measurements.
3.7 Analytical applications
The pH of tap water, bottled drinking water, seawater, beverages, and fruit juices was measured using the developed pH sensors. Three replicates' averages were compared to those obtained from using the commercial glass electrode. The results are summarized in Table 6. The doped Ce–SnO2-based sensors displayed pH values that were comparable to or close to those found in the traditional glass electrode, whereas the undoped SnO2-based sensor had the least sensitivity to pH. The pH unit variations ranged from 0.06 to 0.3. The outcomes show how well the doped Ce–SnO2-based sensors work to measure the pH of various environmental, clinical, and industrial samples.| Sample type | Orion™ based glass membrane electrode | The fabricated sensors | ||
|---|---|---|---|---|
| Undoped SnO2 | Ce-doped SnO2 (1%) | Ce-doped SnO2 (3%) | ||
| a Calibration buffers used: – pH 4 (Britton–Robinson buffer, with NaCl added to simulate seawater conditions) – pH 7 (Britton–Robinson buffer) – pH 10 (Britton–Robinson buffer). | ||||
| Sea water, Zallaq, Bahrain | 8.04 ± 0.07 | 7.98 ± 0.05 | 8.02 ± 0.02 | 8.05 ± 0.01 |
| Tap water, University of Bahrain | 8.38 ± 0.01 | 8.12 ± 0.1 | 8.27 ± 0.07 | 8.31 ± 0.03 |
| Drinking bottled water (Areef) | 7.79 ± 0.05 | 7.45 ± 0.3 | 7.81 ± 0.04 | 7.83 ± 0.02 |
| Drinking bottled water (Adhari) | 7.81 ± 0.07 | 7.52 ± 0.2 | 7.75 ± 0.06 | 7.76 ± 0.1 |
| Soft drink, citrus | 3.01 ± 0.02 | 2.86 ± 0.1 | 3.07 ± 0.04 | 3.02 ± 0.06 |
| Soft drink, pomegranate | 2.51 | 2.76 ± 0.07 | 2.67 ± 0.02 | 2.62 ± 0.07 |
| Mix fruit juice, Suntop, Binzagr Limited Co., KSA | 3.18 | 3.33 ± 0.09 | 3.09 ± 0.06 | 3.07 ± 0.03 |
| Peach juice (Awal Dairy Co., Bahrain) | 3.64 | 3.42 ± 0.04 | 3.60 ± 0.03 | 3.55 ± 0.04 |
| Mango juice (Awal Dairy Co., Bahrain) | 3.96 | 4.16 ± 0.06 | 3.99 ± 0.02 | 3.87 ± 0.03 |
| Orange juice (Sunkist, Food Refreshments Co., Kuwait) | 3.07 | 3.24 ± 0.07 | 3.17 ± 0.05 | 3.09 ± 0.02 |
4 Conclusion
Using the spray pyrolysis method, undoped and Ce-doped SnO2 thin films were deposited on a glass substrate for 20 min. at 450 °C. The deposited films' surface shape and crystalline structure were described. The synthesized undoped and Ce-doped SnO2 based membrane was utilized to create a compact, selective, and sensitive planar electrode for measuring pH across a broad range (2–12). The electrode demonstrated a fast reaction time, steady readings, high selectivity, and a near-Nernstian slope of −58.6 ± 0.7 mV per decade. The electrode was effectively employed to measure the pH of a few natural samples, and the findings were comparable to those of the conventional glass electrode. This study successfully demonstrates the design and fabrication of a compact potentiometric electrode for pH monitoring, utilizing a glass substrate and a cerium-doped tin oxide (Ce-doped SnO2) layer. The incorporation of Ce-doped SnO2 has been shown to significantly enhance the electrode's performance, offering superior sensitivity, stability, and a broad pH detection range compared to traditional pH sensors. The compact form factor, coupled with the robust electrochemical properties of Ce-doped SnO2, makes this electrode a promising candidate for integration into portable and miniaturized pH monitoring systems. This work not only contributes to the advancement of pH sensor technology but also opens new avenues for the development of high-performance sensors in various analytical and environmental applications.Data availability
Data will be made available on request.Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through the Large Groups Project under grant number (RGP.2/282/45). The potentiometric measurements carried out using a PalmSens4 potentiostat were supported by grant number (2/2023) funded by the Deanship of Scientific Research, University of Bahrain.References
- T. Guinovart, G. Valdés-Ramírez, J. R. Windmiller, F. J. Andrade and J. Wang, Electroanalysis, 2014, 26, 1345–1353 CrossRef.
- S. Zhuiykov, Sens. Actuators, B, 2012, 161, 1–20 CrossRef.
- L. Manjakkal, C. G. Núñez, W. Dang and R. Dahiya, Nano Energy, 2018, 51, 604–612 CrossRef.
- A. J. Bandodkar, V. W. S. Hung, W. Jia, G. Valdés-Ramírez, J. R. Windmiller, A. G. Martinez, J. Ramírez, G. Chan, K. Kerman and J. Wang, Analyst, 2013, 138, 123–128 RSC.
- W. Dang, L. Manjakkal, W. T. Navaraj, L. Lorenzelli, V. Vinciguerra and R. Dahiya, Biosens. Bioelectron., 2018, 107, 192–202 CrossRef PubMed.
- J. Kim, T. N. Cho, G. Valdés-Ramírez and J. Wang, Talanta, 2016, 150, 622–628 CrossRef PubMed.
- K. Paek, H. Yang, J. Lee, J. Park and B. J. Kim, ACS Nano, 2014, 8, 2848–2856 CrossRef PubMed.
- H. Cao, V. Landge, U. Tata, Y.-S. Seo, S. Rao, S.-J. Tang, H. F. Tibbals, S. Spechler and J.-C. Chiao, IEEE Trans. Biomed. Eng., 2012, 59, 3131–3139 Search PubMed.
- P. P. Ravi, J. Lindner, H. Oechsner and A. Lemmer, Bioresour. Technol., 2018, 247, 96–102 CrossRef PubMed.
- W. Lonsdale, M. Wajrak and K. Alameh, Talanta, 2018, 180, 277–281 CrossRef PubMed.
- Y. Lian, W. Zhang, L. Ding, X. Zhang, Y. Zhang and X. Wang, in Novel Nanomaterials for Biomedical, Environmental and Energy Applications, Elsevier, 2019, pp. 241–273 Search PubMed.
- J. Han, D. Cui, Y. Li, H. Zhang, Y. Huang, Z. Zheng, Y. Zhu and X. Li, Sens. Actuators, B, 2000, 66, 203–204 CrossRef CAS.
- D. H. Kohn, M. Sarmadi, J. I. Helman and P. H. Krebsbach, J. Biomed. Mater. Res., 2002, 60, 292–299 CrossRef CAS PubMed.
- J. Srivastava, D. L. Barber and M. P. Jacobson, Physiology, 2007, 22, 30–39 CrossRef CAS PubMed.
- L. Manjakkal, B. Sakthivel, N. Gopalakrishnan and R. Dahiya, Sens. Actuators, B, 2018, 263, 50–58 CrossRef CAS.
- W. Dang, L. Manjakkal, L. Lorenzelli, V. Vinciguerra and R. Dahiya, in 2017 IEEE SENSORS, IEEE, 2017, pp. 1–3 Search PubMed.
- L. Manjakkal, W. Dang, N. Yogeswaran and R. Dahiya, Biosensors, 2019, 9, 14 CrossRef PubMed.
- S. Nakata, T. Arie, S. Akita and K. Takei, ACS Sens., 2017, 2, 443–448 CrossRef.
- S. Anastasova, B. Crewther, P. Bembnowicz, V. Curto, H. M. D. Ip, B. Rosa and G.-Z. Yang, Biosens. Bioelectron., 2017, 93, 139–145 CrossRef.
- H. Lee, T. K. Choi, Y. B. Lee, H. R. Cho, R. Ghaffari, L. Wang, H. J. Choi, T. D. Chung, N. Lu and T. Hyeon, Nat. Nanotechnol., 2016, 11, 566–572 CrossRef PubMed.
- A. M. Gibson, N. Bratchell and T. A. Roberts, Int. J. Food Microbiol., 1988, 6, 155–178 CrossRef.
- R. Martínez-Máñez, J. Soto, E. García-Breijo, L. Gil, J. Ibáñez and E. Gadea, Sens. Actuators, A, 2005, 120, 589–595 CrossRef.
- L. Manjakkal, K. Zaraska, K. Cvejin, J. Kulawik and D. Szwagierczak, Talanta, 2016, 147, 233–240 CrossRef PubMed.
- M. Simić, G. M. Stojanović, L. Manjakkal and K. Zaraska, in 2016 24th Telecommunications Forum (TELFOR), IEEE, 2016, pp. 1–4 Search PubMed.
- L. Manjakkal, D. Szwagierczak and R. Dahiya, Prog. Mater. Sci., 2020, 109, 100635 CrossRef.
- N. Lenar, R. Piech, C. Wardak and B. Paczosa-Bator, Membranes, 2023, 13, 876 CrossRef PubMed.
- S. A. M. Marzouk, S. Ufer, R. P. Buck, T. A. Johnson, L. A. Dunlap and W. E. Cascio, Anal. Chem., 1998, 70, 5054–5061 CrossRef PubMed.
- R. Karimi Shervedani, H. R. Zare Mehrdjardi and S. H. Kazemi Ghahfarokhi, J. Iran. Chem. Soc., 2007, 4, 221–228 CrossRef.
- L. Manjakkal, K. Cvejin, J. Kulawik, K. Zaraska, D. Szwagierczak and G. Stojanovic, J. Electroanal. Chem., 2015, 759, 82–90 CrossRef.
- Y.-H. Liao and J.-C. Chou, Sens. Actuators, B, 2008, 128, 603–612 CrossRef.
- G. M. Da Silva, S. G. Lemos, L. A. Pocrifka, P. D. Marreto, A. V Rosario and E. C. Pereira, Anal. Chim. Acta, 2008, 616, 36–41 CrossRef PubMed.
- B. Xu and W.-D. Zhang, Electrochim. Acta, 2010, 55, 2859–2864 CrossRef.
- Y.-H. Liao and J.-C. Chou, Mater. Chem. Phys., 2009, 114, 542–548 CrossRef.
- L. A. Pocrifka, C. Gonçalves, P. Grossi, P. C. Colpa and E. C. Pereira, Sens. Actuators, B, 2006, 113, 1012–1016 CrossRef.
- C.-N. Tsai, J.-C. Chou, T.-P. Sun and S.-K. Hsiung, IEEE Sens. J., 2006, 6, 1243–1249 Search PubMed.
- D.-H. Kwon, B.-W. Cho, C.-S. Kim and B.-K. Sohn, Sens. Actuators, B, 1996, 34, 441–445 CrossRef.
- J.-L. Chiang, S.-S. Jan, J.-C. Chou and Y.-C. Chen, Sens. Actuators, B, 2001, 76, 624–628 CrossRef.
- W.-D. Zhang and B. Xu, Electrochem. Commun., 2009, 11, 1038–1041 CrossRef.
- S. Betelu, K. Polychronopoulou, C. Rebholz and I. Ignatiadis, Talanta, 2011, 87, 126–135 CrossRef.
- H. Razmi, H. Heidari and E. Habibi, J. Solid State Electrochem., 2008, 12, 1579–1587 CrossRef.
- L. Telli, B. Brahimi and A. Hammouche, Solid State Ionics, 2000, 128, 255–259 CrossRef.
- L. Qingwen, L. Guoan and S. Youqin, Anal. Chim. Acta, 2000, 409, 137–142 CrossRef.
- S. S. M. Hassan, M. A. Fathy, I. Moussa, M. Obaida and A. H. Kamel, Sens. Actuators, B, 2023, 380, 133397 CrossRef.
- M. Obaida, I. Moussa, S. Hassan, H. Afify and A. Abouelsayed, Egypt. J. Chem., 2020, 63, 2331–2344 Search PubMed.
- C. Maccà, Electroanalysis, 2003, 15, 997–1010 CrossRef.
- Dislocations in Crystals, Dislocations in Solids, ed. P. Paufler and F. R. N. Nabarro, North-Holland Publ. Co., Amsterdam, 1979, vol. 2, p. 94 Search PubMed.
- R. J. D. Tilley, Perovskites: Structure-Property Relationships, John Wiley & Sons, 2016 Search PubMed.
- A. S. Reddy, N. M. Figueiredo and A. Cavaleiro, Appl. Surf. Sci., 2012, 258, 8902–8907 CrossRef.
- B. D. Cullity, Adv. X-Ray Anal., 1976, 20, 259–271 CrossRef.
- Y. Umezawa, P. Bühlmann, K. Umezawa, K. Tohda and S. Amemiya, Pure Appl. Chem., 2000, 72, 1851–2082 CrossRef.
- A. Fog and R. P. Buck, Sens. Actuators, 1984, 5, 137–146 CrossRef.
- H. N. McMurray, P. Douglas and D. Abbot, Sens. Actuators, B, 1995, 28, 9–15 CrossRef.
- J. A. Mihell and J. K. Atkinson, Sens. Actuators, B, 1998, 48, 505–511 CrossRef.
- S. Trasatti, Electrochim. Acta, 1991, 36, 225–241 CrossRef.
- S. Al-Hilli and M. Willander, Sensors, 2009, 9, 7445–7480 CrossRef.
- D. E. Yates, S. Levine and T. W. Healy, J. Chem. Soc., Faraday Trans. 1, 1974, 70, 1807–1818 RSC.
- M. Chen, Y. Jin, X. Qu, Q. Jin and J. Zhao, Sens. Actuators, B, 2014, 192, 399–405 CrossRef.
- P. Kurzweil, J. Power Sources, 2009, 190, 189–200 CrossRef.
- E. Gill, K. Arshak, A. Arshak and O. Korostynska, Microsyst. Technol., 2008, 14, 499–507 CrossRef.
- M. Kosmulski, J. Colloid Interface Sci., 2002, 253, 77–87 CrossRef PubMed.
- E. Tombácz, Period. Polytech., Chem. Eng., 2009, 53, 77–86 CrossRef.
- T. Xu, R. Chu, X. Chen, Y. Wei, G. Li and Z. Xu, J. Mater. Sci.: Mater. Electron., 2024, 35, 446 CrossRef CAS.
- L. Yang and Y. Guo, Separations, 2023, 10, 560 CrossRef CAS.
- R. Patel, A. K. Mishra, J. Singh and I. Mukhopadhyay, J. Solid State Electrochem., 2024, 1–12 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |