A simple colorimetric detection of telomerase exploiting specific cleavage of exonuclease III coupled with telomeric DNA controlled aggregation of nanogold
Huynh Thi
Le Huyen
ab,
Vo Thi
Cam Duyen
ab and
Phuoc Long
Truong
 *ab
*ab
aSchool of Biomedical Engineering, International University, Ho Chi Minh City 700000, Vietnam. E-mail: tplong@hcmiu.edu.vn; Tel: +8428 372 442 70 ext. 3236
bVietnam National University, Ho Chi Minh City 700000, Vietnam
First published on 8th November 2024
Abstract
Telomerase activity has piqued scientists' interest for the reason that it has the potential to be employed for early-stage cancer detection, anticancer therapy and studies related to cancer progression and metastasis. Several approaches have been developed to detect telomerase activity. However, these approaches were lengthy, challenging to quantify, of limited sensitivity and prone to polymerase chain reaction (PCR)-related artefacts. We herein developed a novel nanoplasmonic sensing platform for colorimetric detection of telomerase activity relying on the telomere elongation of telomerase at the 3′ end, structure-specific cleavage activity of exonuclease III that removes mononucleotides from the 3′-hydroxyl termini of double-stranded DNA, and electrostatic interaction of elongated telomeres with plasmonic nanoparticles. Using HeLa cells as a model for colorimetric detection of telomerase activity, this biosensor could detect telomerase activity with a detection limit of ∼100 cells per reaction by visible inspection and ∼5 cells per reaction by spectroscopic measurement and analysis time within about three hours. The proposed sensing method provided a novel tool for simple, rapid, and low-cost detection of telomerase activity, eliminating the necessity for thermal cycling, primers in PCR-based assays, and amplification of telomerase extension products. It exhibits significant potential as a label-free, simple, ultrasensitive strategy for on-site detection of telomerase activity in proteomics and clinical diagnostics.
1. Introduction
Telomerase is an essential eukaryotic ribonucleoprotein enzyme functioning as a telomere terminal transferase, attaching telomeric repeats (TTAGGG)n onto the 3′-end of chromosomes through reverse transcription using its intrinsic RNA as a template in reproductive cells, human stem cells, and cancer cells using reverse transcription with the intrinsic RNA template.1 The RNA component comprises a complementary region to one hexameric unit (TTAGGG) of the telomere DNA repeats. Telomerase attaches to the 3′-end of chromosomal DNA strands and elongates them by replicating its intrinsic RNA template in numerous hexamer repeat sequences.2 This process is responsible for maintaining telomere stability during cell division and the survival of continuously dividing cells, and it has thus been hypothesized to account for the infinite life of cancer cells. Telomeres are progressively shortened with a round of cell division (shortened by 50–200 bp with each cell division), and until the telomeres are short enough, the cells will begin to grow irreversibly, called cell ageing. The majority of human cancer cells have short telomeres and express high levels of telomerase, but telomerase is nearly nonexistent in adjacent normal somatic cells. Furthermore, the catalytic proteins telomerase ribonucleoprotein (RNP) and human telomerase reverse transcriptase (hTERT) are overexpressed in nearly all human cancerous cells but not in cells that are normal. Telomerase has been reported to be overexpressed in more than 85% of all human tumors, indicating a tight association between telomerase, cell immortalization and tumorigenesis.3–5 Recently, research findings from genetic association studies and functional analyses of telomerase have revealed a link between telomerase and disorders, including cancer, age-related disorders, and premature ageing syndromes.6–8 Therefore, telomerase has the potential to be employed as a biomarker in cancer screening, early diagnosis, prognosis, and surveillance to indicate residual illness and age-related disorders.9,10 In order to comprehend the association between telomerase and cancer, significant advances in biomedical telomerase research have necessitated an advancement of analytical techniques for quick, sensitive, and reliable recognition of telomerase in specific cells, clinical tissues, and bodily fluids.11,12Although numerous assays are available for detecting telomerase activity, the telomeric repeat amplification protocol (TRAP) remains the gold standard for evaluating telomerase activity due to its remarkable sensitivity, repeatability, and accuracy.13–15 However, this technique has shortcomings because of polymerase chain reaction (PCR) amplification, which makes it time-consuming, difficult to quantify, and subject to PCR-derived artefacts. Furthermore, the TRAP test requires particular focus since there are impurities in telomerase extracts that could hinder the TRAP reaction.11,13 A variety of PCR-free assays have been proposed to address the challenges of the TRAP approach and to tackle several technological and scientific problems confronted by biologists, including CRISPR-Cas12a-based biosensing,15 colorimetric assay that employs telomeric hemin/G-quadruplex controlled accumulation of gold colloids,16 fluorescence resonance energy transfer (FRET) relying on RNA probes in combination with RNase H-assisted signal cycling amplification,17 and electrochemical recognition for analyzing telomerase activity.18 These tests primarily relied on direct probing or forming a sandwiched structure of elongated strands. Nevertheless, each has limitations, including time-consuming processes, sophisticated manipulation, limited sensitivity, and complicated laboratory equipment.14,15 Thus, scientists encounter several obstacles in improving or developing more robust telomerase assays for fundamental research and clinical diagnostic applications.
In recent years, advances in nanotechnology have made it possible to create new sensing platforms that circumvent the constraints of standard analytical approaches.19 Plasmonic nanoparticles, among nanomaterials, have a wide range of beneficial features for clinical applications, including an impressive surface-to-volume ratio, shape and size-related optoelectronic features, tunable plasmon resonance, and exceptional biocompatibility with low toxicity, making them a promising subject for sensing and biosensing research.20 Noble metal nanoparticles' distinctive optical properties, which result from localized surface plasmon resonance (LSPR) extinction in the visible range, make them suitable materials for colorimetric biosensors that can assess biomolecules with the naked eye or affordable mobile devices.
Colorimetric nanoplasmonic biosensors were invented by exploiting the color transformation corresponding with the aggregates of nanoparticles, and these colorimetric biosensors have been demonstrated to be highly beneficial for both qualitative and quantitative evaluation of a variety of analytes because of the outstanding analytical capabilities of plasmonic nanoparticles such as simplicity, sensitivity, specificity and capability for in situ detection with minimal volumes of reagents, experimental time and cost for analysis. Platform construction for biomolecule colorimetric detection has been designed based on the size-dependent spectral features and high extinction coefficients of noble metallic nanostructures. Nanoparticle-based colorimetric assays have more advantages than instrument analysis, such as simplicity, sensitivity, cost-effectiveness, and practicality.
Among colorimetric nanoplasmonic biosensors, the most common sensing mode is the analyte-induced agglomeration of plasmonic nanoparticles through various interactions due to molecular binding of bio-receptors and analytes near the nanoparticle surface.21,22 Recently, the electrostatic interaction of single- and double-stranded DNA (ssDNA and dsDNA) with metal nanostructures has been employed to deliver innovative sensing technologies for clinical diagnostics. This contact causes ssDNA to be absorbed on the surface of nanoparticles, stabilizing it to prevent agglomeration through the effect of electrostatic repulsion. Furthermore, the visual appearance of colloidal metallic nanoparticles is primarily determined by the resonance phenomena of free electrons on the surface of nanoparticles, which is significantly impacted by nanoparticle aggregating.23,24 As a result, the electrostatic interaction between DNA and metal nanoparticles can be leveraged to create simplified nanoparticle-based colorimetric testing.
This study presents a simple, label-free, highly sensitive, and PCR-free approach for the colorimetric identification of human telomerase activity in cell lysate. The mechanism of the proposed sensing method relies on telomere extension by telomerase at the 3′-OH terminus, specific cleavage activity of exonuclease III and electrostatic characteristics of elongated telomeres when adsorbed on the plasmonic nanoparticle surface. The telomerase substrate (TS primer) was extended in cell extracts containing telomerase at the 3′ terminus. Once elongated, the product of the telomere extension was hybridized with the complementary strand of TS, leading to the formation of dsDNA with blunt-ends and 3′ overhangs containing more than four bases. The dsDNA was then cleaved by exonuclease III (Exo III) to remove nucleotides from the 3′ blunt-end of dsDNA to release single strands of telomere that help stabilize the Au nanoparticles against agglomeration via electrostatic repulsion. In the case of lacking telomerase, the TS primer-TS complementary complexes were totally degraded by Exo III. In this scenario, Exo III identifies the dsDNA with two blunt ends and removes mononucleotides from the blunt 3′ termini in a sequential manner. The product of the degradation inadequately protects Au nanoparticles resulting in agglomeration in the dielectric medium with a high concentration of salt, which can be monitored by unassisted sight or simple spectroscopy. Employing HeLa cells, referred to as a model for identifying telomerase activity in tumor cells, the nanoplasmonic biosensor was able to identify cancer cells with a limit of detection of a few cells. This proposed approach eliminates the requirement for heat cycling and primers set in PCR-based tests for telomerase activity analysis, and it holds great potential as a label-free, simple, ultrasensitive technique for detecting telomerase in circulating tumor cells for cancer point-of-care diagnosis and prophylaxis.
2. Experimental
2.1 Chemicals and reagents
Trisodium citrate, hydrogen tetrachloroaurate(III) trihydrate (HAuCl4 × 3H2O, 99.9%), diethylpyrocarbonate (DEPC), ethylenediaminetetraacetic acid (EDTA), sodium chloride (NaCl), UltraPure™ Tris hydrochloride, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and a penicillin–streptomycin–neomycin (PSN) antibiotic mixture were purchased from Sigma-Aldrich Inc. (USA). CHAPS was provided by Bio Basic Inc. (Canada). The oligonucleotide sequence used for telomerase extension (TS: 5′-AAT CCG TCG AGC AGA GTT-3′), the sequence of TS complementary (TS complementary: 5′-AAC TCT GCT CGA CGG ATT-3′) and dNTPs were ordered from PHUSA Biochem Co. (Vietnam). Normal human fibroblast cells, mouse fibroblast cells (L929), mouse colon carcinoma cells (C26), adipose-derived stem cells (ADSCs) and HeLa cells were purchased from ATCC (USA). Bovine aortic endothelial cells (BAECs) were provided by Cell Applications, Inc. All chemical solutions were prepared using ultra-pure water (18.2 MΩ cm) purified with a reverse-osmosis filtering system. After rinsing with a freshly prepared aqua regia solution, every item of glass used in the experiments was thoroughly washed with distilled water. DEPC was used to process all of the solutions.2.2. Preparing cell extracts from cultured cells
HeLa cells, mouse fibroblast cells (L929), bovine aortic endothelial cells (BAECs), mouse colon carcinoma cells (C26), adipose-derived stem cells (ADSCs) and normal human fibroblast cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and the penicillin-streptomycin-neomycin (PSN) antibiotic mixture at the temperature 37 °C with 5% CO2. Harvested cells were measured, and a sample containing 106 cells was pelletized in culture media. The supernatant was carefully eliminated, and the pellet was kept at −80 °C until use. Telomerase was isolated from cells employing the CHAPS lysis buffer.25,26 Cell pellets (106 cells) were immersed in 200 μL of ice-cold 1× CHAPS lysis buffer (50 mM Tris-HCl pH 7.5, NaCl 110 mM, CHAPS 1%, and EDTA 5 mM) and then preserved on ice for 60 minutes. The lysate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 minutes at 4 °C, and 160 μL of the supernatant was transferred to a new tube. The resultant extract was aliquoted, flash-frozen in liquid nitrogen, and then preserved at −80 °C until usage.
000 rpm for 20 minutes at 4 °C, and 160 μL of the supernatant was transferred to a new tube. The resultant extract was aliquoted, flash-frozen in liquid nitrogen, and then preserved at −80 °C until usage.
2.3 Gold nanoparticle preparation
Spherical Au nanoparticles (AuNPs) are formed by chemically reducing gold ions (Au3+) to gold atoms (Au0) in the presence of citrate.27 Briefly, 10 mL of 1.0 mM HAuCl4 was heated to the boiling point while stirring. After boiling, 1.0 mL of 38.8 mM trisodium citrate was immediately added to the reaction solution. The resulting solution remained boiling for 5 minutes in order to finish the citrate reduction of the gold ions with vigorous stirring. After that, the solution was agitated for approximately 30 minutes until it cooled. The colloidal gold was filtered using a 0.22 μm filter to eliminate aggregating nanoparticles. It was then characterized employing a UV-Vis spectrophotometer (Thermo Scientific™ Varioskan™ LUX Multimode Microplate Reader, Thermo Fisher Scientific). The shape, size, and homogeneity of Au nanoparticles were examined using a Zetasizer Nano-ZS (Malvern Panalytical) and high-resolution transmission microscopy (HR-TEM) on JEM3010 equipment with a 300 kV voltage setting.2.4 Colorimetric nanosensor for analysis of telomerase activity
2.5 Assessment of generality and specificity of the nanoplasmonic biosensor for analysis of telomerase activity
To demonstrate that the proposed nanoplasmonic biosensor is universal and specific for analysis of telomerase activity, various cell types were tested. These cell lines were normal human fibroblast cells, bovine aortic endothelial cells (BAECs), mouse fibroblast cells (L929), HeLa cells, adipose-derived stem cells (ADSCs), and mouse colon cancer cells (C26).2.6. Statistical analysis
To evaluate statistically significant differences between the means of test groups, one-way ANOVA was applied. If the P-value for the group average was less than 0.05, the difference was regarded as statistically significant. The data are displayed as the data set's mean ± standard deviation (SD).3. Results and discussion
In this study, the activity of telomerase in cell lysates was analyzed based on the telomere elongation activity of telomerase at the 3′-OH end, the specific catalytic activity of Exo III that removes mononucleotides from the 3′-hydroxyl termini of the hybrid DNA duplex and the electrostatic interaction between single-stranded DNA and plasmonic Au nanoparticles. The presented assay sensing mechanism is described in Fig. 1.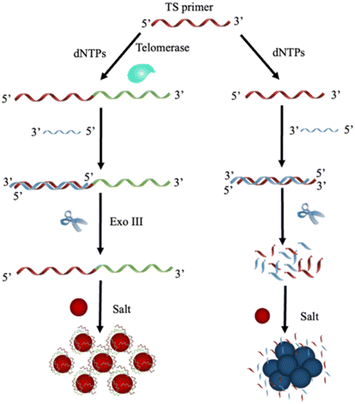 | ||
| Fig. 1 Conceptual schematic representing the experimental procedures for the colorimetric nanoplasmonic biosensor-based telomerase activity recognition in cell lysate. | ||
The first step in the telomerase activity analysis involves the binding between the telomerase substrate (TS primer) and telomerase. The TS primer was extended at the 3′-OH end when telomerase existed in cell extracts. The product of the extension reaction was then hybridized with the complementary strand of the TS primer, resulting in DNA duplex molecules with blunt ends and 3′ overhangs containing more than four bases. In the next step of the assay, exonuclease III (Exo III) was exploited to cleave the hybrid dsDNA from the 3′ blunt-end, resulting in the release of single-stranded DNA (ssDNA) of elongated telomere that helps stabilize Au nanoparticles against salt-induced agglomeration via electrostatic repulsion among the adjacent nanoparticles by adhering the positively charged nucleobases of ssDNA with negatively charged Au nanoparticles while exposing the charged phosphate moieties of ssDNA to the dielectric environment.24,28,33 This type of adsorption results in a charge redistribution that makes the nanoparticle surface appear more negatively charged. Moreover, electrostatic repulsion among nanoparticles helps avoid the strong force of van der Waals attraction, thereby improving colloidal gold stability. As a result of this process, the colloidal gold retains a ruby-red color similar to the original.
Without telomerase in the cell extract, Exo III degraded the TS primer-TS complementary complex. In this scenario, Exo III recognizes dsDNA with two blunt ends before catalytically removing mononucleotides from the blunt 3′ terminals. The product of the degradation process is free dNMPs (deoxyribonucleoside monophosphates), which cannot protect the gold nanostructures, resulting in the agglomeration of AuNPs under the high level of sodium chloride in the reaction. This leads to the characteristic color alteration of colloidal gold and the formation of a new peak in the resonant spectra of the nanoparticles. In this case, the signal response of the nanoplasmonic biosensor corresponds to the stability of colloidal gold nanoparticles, which depends upon the activity of telomerase of cell extracts. Thus, the activity of telomerase in cell extracts can be discriminated by visible inspection and absorbance measurement.
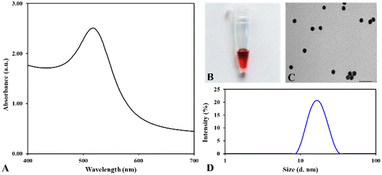
To investigate the colorimetric response of the nanoplasmonic biosensor for telomerase activity detection, Au nanoparticles were chemically synthesized by reducing the hydrogen tetrachloroaurate(III) ion with sodium citrate, employing Turkevich's methodology with minor modifications.34 This approach delivered a spherical shape, homogeneous solution, and ruby-red nanoparticles of ∼15 nm diameter, as disclosed using a multimode microplate reader, transmission electron microscope (TEM), and Zetasizer Nano ZS. As illustrated in Fig. 2, the UV-Vis spectra of the reaction solution after reducing with sodium citrate showed a ruby red color with the plasmon resonance peak at 518 nm, indicating gold nanoparticle formation.29 The excess amount of sodium citrate in the reaction solution offers a charge on the gold nanoparticle's surface and prevents the agglomeration of colloidal gold nanoparticles. The nanoparticle size and zeta potential analyzer revealed that the hydrodynamic size of the gold nanoparticle was 14.70 ± 1.21 nm with a PDI of 0.065 ± 0.01, and the zeta potential of nanoparticles was measured at −36.8 mV ± 7.4. These results demonstrated that the gold nanoparticles are fairly homogeneous and quite stable. These features play an essential role in the analytical performance of gold nanoparticles in designing nanoplasmonic biosensors. Hence, the prepared gold nanoparticles can be exploited for practical applications in clinical diagnostics.
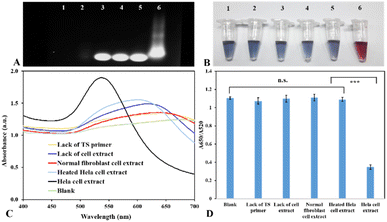
Telomerase is now well-known that it is over-expressed in almost all human cancer cells, but it is not detected in normal cells.3,4 Hence, to verify the feasibility of the presented method for analyzing the activity of telomerase, we tested the analytical performance of nanoplasmonic biosensors by conducting trials with the positive control (HeLa cell extract), blank (extraction buffer), lack of the TS primer, lack of cell extract and negative controls, including normal human fibroblast cell extract and heated HeLa cell extract. Before performing the assay with the nanoplasmonic biosensor, we first checked the presence of elongated primers in the reaction mixtures by agarose gel electrophoresis. In this study, the reaction mixtures were verified by 1% agarose gel electrophoresis at 100 V. As demonstrated in Fig. 3A, in the case of the blank sample and lack of the TS primer, no band showed up at land 1 and land 2 in the gel. It means that there was no elongation in the reaction mixture, and this result indicated that the extraction buffer and cell extract did not contain any primer for elongation. Due to the absence/inactivation of telomerase in the sample without cell extract, the normal cell extract and heated HeLa cell extract, the TS primer was not lengthened in these cases. As a result, the gel only showed the bands of the TS primer (land 3, land 4, and land 5). In the case of HeLa cell extract, the gel showed a bright smear that indicated the extension of the TS primer by the telomerase in HeLa cell extract, and the product of the elongation reaction was telomeres with various lengths (land 6). Therefore, it can be concluded that the TS primer is specific for the reaction of telomerase elongation, and the reaction was not affected by other components in cell extracts and contamination.
After checking the presence of elongated telomeres in the reaction mixture, we checked the feasibility of the presented colorimetric nanosensor for detecting telomerase activity. As illustrated in Fig. 3B and C, the blank sample and the negative controls turned blue color, whereas the positive sample maintained the original ruby red color of Au nanoparticles because of the presence of elongated primers in the solution, and the absorption spectral analysis of colloidal gold showed a spectral transition of Au nanoparticles with a significant wavelength shift from 518 nm to 650 nm. In this scenario, the typical resonant peak of AuNPs at 518 nm was reduced progressively, which demonstrated the agglomeration of AuNPs. This result clearly indicated that the elongated primers in the reaction mixture can prevent the formation of blue aggregates from the colloidal gold in the medium with high salt concentrations. Fig. 3D demonstrates that the positive sample's absorbance ratio (A650/A520) was remarkably lower than that of the control samples. The statistical analysis revealed that the difference between control samples and HeLa cell extract was statistically significant (P < 0.01). These findings show evidence that the proposed nanosensor was capable of being exploited for the detection of the telomerase activity that relies on the telomerase catalytic activity, structure-specific cleavage of exonuclease III, and electrostatic attraction forces between elongated primers and negatively charged gold nanoparticles.
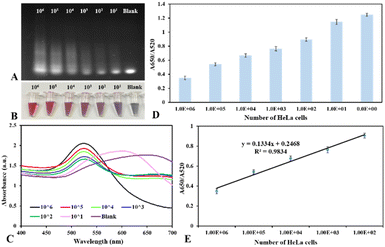
To test the sensitivity of the proposed biosensor, HeLa cell lysates extracted from 0 to 106 cells were subject to incubation with the TS primer in the reaction buffer for detecting telomerase activity. As demonstrated in Fig. 4A, the agarose gel electrophoresis showed that the amount of elongated primers and their length from the telomere extension reaction increases when the concentration of telomerase in HeLa cell extracts increases. In the presence of telomerase in a cell extract, it catalyzes to yield elongated primers that help stabilize colloidal nanogold afterward, resulting in retaining the ruby red color and state of dispersed gold nanoparticles at high salt concentrations (Fig. 4B). In contrast, in the cell extract without the presence of telomerase or a deficient concentration of telomerase in the cell extract, the color of colloidal gold transforms into blue/purple. The UV-Vis spectrum of colloidal nanogold displayed a huge decrease in the resonant peak at 520 nm and a newly emerged peak at ∼650 nm due to the resonant peak's redshift, which originated from the salt-induced cluster appearance of spherical Au nanoparticles (Fig. 4C). In this scenario, the citrate-capped gold nanostructures were not protected because the elongated primers were not enough to cover the nanoparticle surface. When increasing the concentration of telomerase in HeLa cell extract for the proposed assay, the color of the reaction solution slowly changed from blue/purple to ruby red color, and the 520 nm-resonant peak witnessed an increase, whereas the peak at 650 nm diminished gradually, which expressed the state of monodispersion of spherical gold nanostructures. By comparing the color difference between test samples and control samples, the telomerase extracted from 102 HeLa cells could be distinguished from that extracted from 101 HeLa cells, implying that by visible inspection, the detection limit of the colorimetric biosensor is about 100 cells per reaction.
For quantitative analysis of telomerase activity in cell extracts, the UV-Vis spectrum was exploited to measure the signal response of the nanoplasmonic biosensor as a function of concentration of telomerase in HeLa cell lysates. The spectrophotometric analysis indicated that the absorbance ratio (A650/A520) is proportional to the concentration of telomerase in HeLa cell samples (Fig. 4D). Consequently, this ratio was employed to assess the analytical performance of the proposed test for telomerase activity analysis, and by estimating the ratio (A650/A520) through the absorbance measurement, we are able to evaluate telomerase activity in particular cells, tissues, and body fluids that helps diagnose cancers via clinical samples.11,25Fig. 4E illustrates the wide linear relationship between the absorbance ratio (A650/A520) and number of HeLa cells, and this absorption ratio was linear in the range from 102 to 106 cells. Actually, the linear range might be wider, but due to the scarcity of HeLa cell samples with a large number of cells, we are unable to test telomerase samples with higher concentrations until the A650/A520 ratio reaches the plateau. The linear regression equation for detecting telomerase activity with the range from 102 to 106 HeLa cells was y = 0.1334x + 0.2468 with an r-squared value of 0.9834, where x and y are the concentration of HeLa cell extract and the absorbance ratio of A650/A520, respectively. The detection limit (LOD) of the presented nanoplasmonic biosensor can be assessed based on the slope of the linear calibration curve (s) and the standard deviation of the blank sample (α) according to the formula 3α/s. The analysis disclosed that the proposed biosensor has very high sensitivity, and it could be exploited to recognize the telomerase activity in cell lysate with a detection limit of ∼5 cells per reaction within a total analysis time of about three hours.
In comparison with the previous reported assays and biosensors applied for detection of telomerase activity that require complex sample pre-treatments, expensive reagents and costly instrumentation, such as TRAP based assays, PCR based assays, fluorescence-based assays and electrochemical assays, the designed plasmonic nanosensor shows its merits in terms of simplicity, analysis cost, experimental time and ability for POC testing. Moreover, the analytical performance including the detection limit and linear dynamic range is comparable to that of PCR-based assays and better than those of most reported PCR-free assays (Table 1). These results indicated that the presented sensing platform is ultrasensitive and it could be used for early diagnosis of cancers via analysis of telomerase activity in cell lysate.
| Assay formats and/or biosensor formats | Linear dynamic range | Detection limit | Ref. |
|---|---|---|---|
| TRAP based on primer-modified AuNPs | — | 5 cells | 38 |
| Paper-based detection of telomerase activity via enlargement of AuNPs | 6–2.5 × 104 cells | 6 cells | 35 |
| PCR-free detection of telomerase activity via polyvalent oligonucleotide nanoparticle conjugates | — | 10 cells | 14 |
| Single nanosensor for detection of telomerase based on specific interaction between telomeres and telomerase | 102–105 cells | 10 cells | 26 |
| Assay exploiting telomerase activity, enrichment by magnetic separation and a pH meter as readout | 50–104 cells | 20 cells | 36 |
| Fluorescence based assay based on RNA probes and RNase H-assisted signal recycling amplification | 5–80 cells | 5 cells | 17 |
| Electrochemical detection based on SNA AuNPs triggered mimic HCR dual signal amplification | 10–104 cells | 2 cells | 18 |
| Fluorescence based assay using a switchable nanoprobe | 0–4 × 103 cells | 59 cells per mL | 37 |
| Quantum dots – streptavidin conjugate based biosensor | — | 2.94 × 104 cells per mL | 39 |
| Visual detection of telomerase via specific cleavage of Exo III and interaction between the elongated primer and AuNPs | — | ∼5 cells (spectral analysis) ∼100 cells (naked eyes) | Our work |
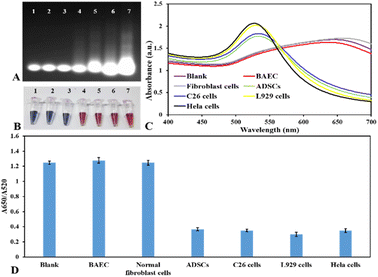
For the purpose of demonstrating the universality and specificity of the presented nanoplasmonic biosensor for analyzing the activity of telomerase, this research scrutinized the telomerase activity of diverse kinds of cells, including HeLa cells, mouse colon carcinoma cells (C26), adipose-derived stem cells (ADSCs), mouse fibroblast cells (L929), bovine aortic endothelial cells (BAECs) and normal human fibroblast cells. As depicted in Fig. 5, the blank sample and normal cell samples, including human fibroblast cells and bovine aortic endothelial cells, did not create any colorimetric and spectral signal because of the scarcity of telomerase activity in normal cells. However, in the case of cancer cell lines and mesenchymal stem cells, these cells demonstrated a positive signal for the activity of telomerase. Regarding L929 cells, the fibroblast cells from subcutaneous connective tissue of mice, the analytical result showed that this cell line demonstrated high telomerase activity. This is because these fibroblast cells transform into the cells that have features of cancerous cells after several passages. These analytical findings were consistent with the expression levels of telomerase in somatic cells, tumor cells, and stem cells,30–32 and they exhibited the excellent specificity of the presented nanoplasmonic biosensors for on-site colorimetric detection of activity of telomerase in various types of cells that show great potential for clinical diagnostic applications.
4. Conclusions
In short, this study proposed a label-free, simple, and ultrasensitive nanosensor for on-site-colorimetric detection of telomerase activity in cell lysates that relies on the telomere elongation activity at the 3′-OH end of telomerase, the structure-specific cleavage activity of exonuclease III that eliminates mononucleotides from the 3′-OH termini of dsDNA, and the electrostatic properties of elongated primers when interacting with plasmonic gold nanoparticles. The proposed nanoplasmonic biosensor is suitable for on-site colorimetric detection of telomerase activity in cell extracts owing to the rapid salt-induced agglomeration of colloidal gold, making it easy to determine the color and spectral shift of gold nanoparticles. Using HeLa cells as a model for detection of cancers via telomerase activity, this nanoplasmonic biosensor could recognize telomerase activity from as few as ∼100 cells per reaction by the naked eye and ∼5 cells per reaction by spectrophotometry with a wide linear dynamic range. Our proposed approach has rejected the demand for thermal cycling and primers in PCR-based tests for the scrutinization of telomerase activity, and it has overcome the limitations of the traditional TRAP approach, which is lengthy, difficult to quantify, and subject to PCR-derived artefacts. Moreover, the proposed sensing platform provides an ultrasensitive method for colorimetric telomerase activity detection, and it holds impressive potential for telomerase detection in circulating tumor cells and on-site diagnosis of cancers.Data availability
Data supporting this study are included within the article and/or supporting materials.Author contributions
Conceptualization, methodology, supervision, preparation of the original draft, and justifying the manuscript are conducted by Dr Phuoc Long Truong. Huynh Thi Le Huyen and Cam-Duyen Thi Vo are responsible for research design, data analysis, data curation, and the compilation of the original manuscript. All authors have reviewed and consented to the manuscript information.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Vietnam National University HoChiMinh City (VNU-HCM) under grant number C2022-28-03.Notes and references
- J. W. Shay, Y. Zou, E. Hiyama and W. E. Wright, Hum. Mol. Genet., 2001, 10, 677–685 CrossRef PubMed.
- K. E. McKenzie, C. B. Umbricht and S. Sukumar, Mol. Med. Today, 1999, 5, 114–122 CrossRef PubMed.
- N. W. Kim, M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich and J. W. Shay, Science, 1994, 266, 2011–2015 CrossRef PubMed.
- J. W. Shay and S. A. Bacchetti, Eur. J. Cancer, 1997, 33, 787–791 CrossRef.
- J. Nandakumar and T. R. Cech, Nat. Rev. Mol. Cell Biol., 2013, 14, 69–82 CrossRef PubMed.
- C. B. Harley, Nat. Rev. Cancer, 2008, 8, 167–179 CrossRef PubMed.
- N. Relitti, A. P. Saraswati, S. Federico, T. Khan, M. Brindisi, D. Zisterer, S. Brogi, S. Gemma, S. Butini and G. Campiani, Curr. Top. Med. Chem., 2020, 20, 433–457 CrossRef.
- M. A. Blasco, Nat. Rev. Genet., 2005, 6, 611–622 CrossRef.
- E. Rampazzo, P. Del Bianco, R. Bertorelle, C. Boso, A. Perin, G. Spiro, F. Bergamo, C. Belluco, A. Buonadonna, E. Palazzari and S. Leonardi, Br. J. Cancer, 2018, 118, 878–886 CrossRef.
- S. Chen, S. Hu, B. Zhou, B. Cheng, H. Tong, D. Su, X. Li, Y. Chen and G. Zhang, Sci. Rep., 2023, 13, 10586 CrossRef.
- X. Zhou and D. Xing, Chem. Soc. Rev., 2012, 41, 4643–4656 RSC.
- D. Wang, W. Xue, X. Ren and Z. Xu, TrAC, Trends Anal. Chem., 2021, 134, 116115 CrossRef.
- B. S. Herbert, A. E. Hochreiter, W. E. Wright and J. W. Shay, Nat. Protoc., 2006, 1, 1583–1590 CrossRef.
- G. Zheng, W. L. Daniel and C. A. Mirkin, J. Am. Chem. Soc., 2008, 130, 9644–9645 CrossRef PubMed.
- H. Wang, S. Wang, H. Wang, Y. Liang and Z. Li, Talanta, 2023, 253, 123999 CrossRef.
- E. Sharon, E. Golub, A. Niazov-Elkan, D. Balogh and I. Willner, Anal. Chem., 2014, 86, 3153–3158 CrossRef PubMed.
- H. Wang, H. Wang, Y. Jia, M. Zhang and Z. Li, RSC Adv., 2019, 9, 14817–14821 RSC.
- W. J. Wang, J. J. Li, K. Rui, P. P. Gai, J. R. Zhang and J. J. Zhu, Anal. Chem., 2015, 87, 3019–3026 CrossRef PubMed.
- G. Aragay, F. Pino and A. Merkoçi, Chem. Rev., 2012, 112, 5317–5338 CrossRef PubMed.
- K. Nejati, M. Dadashpour, T. Gharibi, H. Mellatyar and A. Akbarzadeh, J. Cluster Sci., 2022, 33, 1–16 CrossRef.
- H. Jans and Q. Huo, Chem. Soc. Rev., 2012, 41, 2849–2866 RSC.
- A. Miti, S. Thamm, P. Müller, A. Csáki, W. Fritzsche and G. Zuccheri, Biosens. Bioelectron., 2020, 167, 112465 CrossRef PubMed.
- N. Farkhari, S. Abbasian, A. Moshaii and M. Nikkhah, Colloids Surf., B, 2016, 148, 657–664 CrossRef.
- P. L. Truong, N. T. T. Thao, H. T. Le Huyen and T. H. Nguyen, J. Nanomater., 2022, 2022, 1107081 CrossRef.
- M. A. Piatyszek, N. W. Kim, S. L. Weinrich, K. Hiyama, E. Hiyama, W. E. Wright and J. W. Shay, Methods Cell Sci., 1995, 17, 1–15 CrossRef.
- X. Ma, P. L. Truong, N. H. Anh and S. J. Sim, Biosens. Bioelectron., 2015, 67, 59–65 CrossRef.
- V. T. C. Duyen, V. Van Toi, T. Van Hoi and P. L. Truong, Anal. Methods, 2023, 5, 3991–3999 RSC.
- P. A. Mirau, J. E. Smith, J. L. Chávez, J. A. Hagen, N. Kelley-Loughnane and R. Naik, Langmuir, 2018, 34, 2139–2146 CrossRef.
- B. K. Pong, H. I. Elim, J. X. Chong, W. Ji, B. L. Trout and J. Y. Lee, J. Phys. Chem. C, 2007, 111, 6281–6287 CrossRef.
- E. Hiyama and K. Hiyama, Br. J. Cancer, 2007, 96, 1020–1024 CrossRef PubMed.
- Y. S. Cong, W. E. Wright and J. W. Shay, Microbiol. Mol. Biol. Rev., 2002, 66, 407–425 CrossRef PubMed.
- K. H. Park, S. Y. Rha, C. H. Kim, T. S. Kim, N. C. Yoo, J. H. Kim, J. K. Roh, S. H. Noh, J. S. Min, K. S. Lee and B. S. Kim, Int. J. Oncol., 1998, 13, 489–584 Search PubMed.
- H. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036–14039 CrossRef.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- T. Mahmoudi, A. P. Tazehkand, M. Pourhassan-Moghaddam, M. Alizadeh-Ghodsi, L. Ding, B. Baradaran and M. E. Warkiani, Microchem. J., 2020, 154, 104594 CrossRef.
- L. Wang, C. Chen, H. Huang, D. Huang, F. Luo, B. Qiu and H. Yang, Biosens. Bioelectron., 2018, 121, 153–158 CrossRef PubMed.
- H. Shi, T. Gao, L. Shi, T. Chen, Y. Xiang, Y. Li and G. Li, Sci. Rep., 2018, 8(1), 16341 CrossRef PubMed.
- Y. Xiao, K. Y. Dane, T. Uzawa, A. Csordas, J. Qian, H. T. Soh, P. S. Daugherty, E. T. Lagally, A. J. Heeger and K. W. Plaxco, J. Am. Chem. Soc., 2010, 132, 15299–15307 CrossRef.
- R. Díaz-Ayala, M. López-Nieves, E. S. Colón Berlingeri, C. R. Cabrera, L. Cunci, C. I. González and P. F. Escobar, ACS Omega, 2022, 7(11), 9964–9972 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |