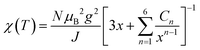Two new tellurite compounds ACu3Te2O8 (A = Ca, Cd) with ferromagnetic spin-1/2 kagomé layers†
Guozhao
Wang
 abc,
Wenya
Xiang
abc,
Zhiying
Zhao
abc,
Wenya
Xiang
abc,
Zhiying
Zhao
 a,
Meiyan
Cui
a,
Meiyan
Cui
 a and
Zhangzhen
He
a and
Zhangzhen
He
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: hezz@fjirsm.ac.cn; hcz1988@hotmail.com
bCollege of Chemistry and Materials Science, Fujian Normal University, Fuzhou 350117, China
cFujian College, University of Chinese Academy of Sciences, Fuzhou 350002, China
First published on 26th November 2024
Abstract
Two new tellurite compounds ACu3Te2O8 (A = Ca, Cd) have been synthesized by a typical hydrothermal method, exhibiting a similar 2D layered structure in the trigonal system of space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, where Cu2+ ions construct a spin-1/2 regular kagomé lattice via corner-sharing. Magnetic measurements confirmed that ACu3Te2O8 (A = Ca, Cd) possesses antiferromagnetic ordering at low temperature, while exchange interactions between magnetic ions within layers are ferromagnetic.
m, where Cu2+ ions construct a spin-1/2 regular kagomé lattice via corner-sharing. Magnetic measurements confirmed that ACu3Te2O8 (A = Ca, Cd) possesses antiferromagnetic ordering at low temperature, while exchange interactions between magnetic ions within layers are ferromagnetic.
Two-dimensional (2D) kagomé compounds composed of 3d transition-metal ions have been a subject of intense research in material sciences and condensed matter physics due to their unique structural features and exotic magnetic phenomena.1–6 For kagomé antiferromagnets, many compounds such as ZnCu3(OH)6Cl2,7 Cu3V2O7(OH)2·2H2O,8 CdCu3(OH)6(NO3)2·H2O,9 BaNi3(VO4)2(OH)2,10 and KFe3(SO4)2(OH)611 with a 2D kagomé structure have been found to show a possible spin-liquid state,12 orbital switching,13 fractional magnetization plateaus,9 spin-glass,10 and spin-chirality behaviors,11 respectively. However, the current interest is mainly focused on the exploration of spin-1/2 perfect kagomé antiferromagnets, which are considered as the most ideal candidates for the realization of a quantum spin-liquid state, because a synergistic effect of strong spin-fluctuation and geometrical-frustration could prevent the appearance of a long-range magnetic order at low temperature.14
On the other hand, kagomé ferromagnets are also found to exhibit various interesting physical properties. For example, Fe3Sn2,15 Co3Sn2S2,16 and LiMn6Sn617 were confirmed to show an anomalous Hall effect, while UCo0.8Ru0.2Al was observed to have a colossal anomalous Nernst effect.18 Moreover, Li9Cr3(P2O7)3(PO4)2 was found to have a large magnetocaloric effect at low temperature.19 Furthermore, kagomé metal CsV3Sb5 was also found to exhibit a superconducting ground state.20,21 Generally, both kagomé antiferromagnetic and kagomé ferromagnetic compounds have given many exciting topics, and the exploration of kagomé compounds is still a great challenge.
It is well-known that the Se4+ and Te4+ ions with stereochemically active lone-pair electrons can usually form SeO3 and TeO3 trigonal pyramids in the oxygen-coordination environments, which often act as “chemical scissors” to introduce a low-dimensional structural framework, resulting in the formation of many novel materials. In our previous works, we have successfully synthesized many kagomé-related selenite or tellurite compounds, including M3(TeO3)(SO4)(OH)2·2H2O (M = Ni, Co) with a distorted striped kagomé lattice,22 A2Cu5(TeO3)(SO4)3(OH)4 (A = Na, K) with a 1D kagomé strip lattice,23 BiOCu2(XO3)(SO4)(OH)·H2O (X = Te, Se) with an octa-kagomé lattice,24 Co3(SeO3)(SO4)(OH)2 with a distorted kagomé lattice,25 K2Ni9(TeO3)4(MoO4)4(OH)4 with a capped-kagomé lattice,26 and Pb(OF)Cu3(SeO3)2(NO3) with a breathing-kagomé lattice.27 In this work, we have recently obtained two new Cu2+-based tellurite compounds of ACu3Te2O8 (A = Ca, Cd) with a trigonal system of space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, exhibiting a regular kagomé lattice built by magnetic Cu2+ ions. Surprisingly, magnetic measurements indicate that ACu3Te2O8 (A = Ca, Cd) both possess an antiferromagnetic (AFM) ordering at low temperature with ferromagnetic (FM) interactions in the kagomé layers.
m, exhibiting a regular kagomé lattice built by magnetic Cu2+ ions. Surprisingly, magnetic measurements indicate that ACu3Te2O8 (A = Ca, Cd) both possess an antiferromagnetic (AFM) ordering at low temperature with ferromagnetic (FM) interactions in the kagomé layers.
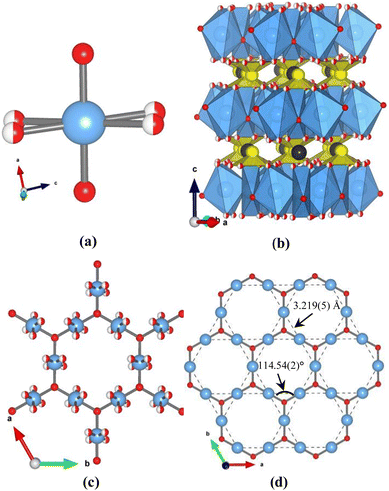
Single crystals of ACu3Te2O8 (A = Ca, Cd) were synthesized by a conventional hydrothermal reaction. The experimental details can be seen in the ESI.† Both compounds are found to crystallize in the trigonal system with a space group of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. Since they are isostructural, CaCu3Te2O8 is selected as a representative for the descriptions of their structural features. There is one Cu site, one Te site, one Ca site, and two O sites (O1 and O2) in an asymmetric unit. The O2 atoms occupy two crystallographically equivalent sites with 1/2 atom occupancy for each site (Fig. 1a). Such cases of disordered oxygen atoms are also seen in many kagomé compounds such as β-BaCu3V2O8(OH)2,28 CdCu3(OH)6(NO3)2·H2O,9 YCu3(OH)6Cl3,29 KMn3O2(Ge2O7),30 and YCa3(MnO)3(BO3)4 series.31,32 Moreover, all Cu2+ ions are equivalent and coordinated by four O atoms, forming a CuO4 square plaquette (Fig. S8, ESI†). The Cu–O bond lengths of the CuO4 square plaquettes range from 1.913(2) to 1.991(8) Å. Te atoms are coordinated by three oxygen atoms, forming TeO3 trigonal pyramids with bond lengths of 1.890(8) Å.
m. Since they are isostructural, CaCu3Te2O8 is selected as a representative for the descriptions of their structural features. There is one Cu site, one Te site, one Ca site, and two O sites (O1 and O2) in an asymmetric unit. The O2 atoms occupy two crystallographically equivalent sites with 1/2 atom occupancy for each site (Fig. 1a). Such cases of disordered oxygen atoms are also seen in many kagomé compounds such as β-BaCu3V2O8(OH)2,28 CdCu3(OH)6(NO3)2·H2O,9 YCu3(OH)6Cl3,29 KMn3O2(Ge2O7),30 and YCa3(MnO)3(BO3)4 series.31,32 Moreover, all Cu2+ ions are equivalent and coordinated by four O atoms, forming a CuO4 square plaquette (Fig. S8, ESI†). The Cu–O bond lengths of the CuO4 square plaquettes range from 1.913(2) to 1.991(8) Å. Te atoms are coordinated by three oxygen atoms, forming TeO3 trigonal pyramids with bond lengths of 1.890(8) Å.
Fig. 1b shows a typical layer-structure of CaCu3Te2O8 running along the c-axis, in which the layers are separated by TeO32− groups and Ca2+ cations. The shortest distance of Cu–Cu in neighbouring layers is 5.879(3) Å. The CuO4 square plaquettes are interconnected via corner-sharing (O1) to form the layers (Fig. 1c), and each TeO32− group connects with three CuO4 square plaquettes via corner-sharing (O2) (Fig. S4, ESI†). It is worth noting that a typical kagomé lattice with a unique planar arrangement of corner-sharing triangles constructed by magnetic Cu2+ ions can be seen on the a–b plane, when nonmagnetic Ca2+ and TeO32− groups of CaCu3Te2O8 are removed from the structural framework. Fig. 1d clearly shows the ideal kagomé topological structure of Cu2+ ions, in which the distance between the Cu2+ ions is 3.219(5) Å and the angle of Cu–O1–Cu is 114.54(2)°. It is also noted that the Cu–Cu distances and the angles of the Cu–O1–Cu pathways in CdCu3Te2O8 are close to those in CaCu3Te2O8, due to quite similar radii of Ca2+ and Cd2+ ions situated between the layers. As a result, the replacement of Ca2+ by Cd2+ shows a slight impact on the kagomé layers.
It is instructive to compare the structure of ACu3Te2O8 (A = Ca, Cd) with regular kagomé compound ZnxCu4−x(OH)6Cl2 (0 ≤ x ≤ 1) (Fig. S8 and Table S6, ESI†).33,34 Although all these compounds are superexchanged by equatorial Cu–O bonds, there are significant differences between them. ZnxCu4−x(OH)6Cl2 (0 ≤ x ≤ 1) is coordinated by four equatorial O atoms and two axial Cl atoms, with all four O atoms in the facet involved in superexchange interactions. However, in ACu3Te2O8 (A = Ca, Cd) since each Cu ion is surrounded by two close equatorial O1 ligands (1.913(2) Å) and two distant equatorial O2 ligands (1.991(8) Å) (Fig. S8, ESI†), only O1 is involved in the superexchange interactions between Cu ions. It is noted that O2 is situated outside the kagomé layers without participating in the superexchange interaction (Fig. S8, ESI†), and thus the disordered O2 sites seem to have a negligible effect on the kagomé layers.28 Moreover, it must be noted that the shortest distance (∼4.7 Å) between the Cu2+ ions and the angle (∼180°) of Cu–O–Cu for kagomé compound Cu3(CO3)2(x)3·2ClO4 (MOF-x) are much larger than those (∼3.2 Å and ∼114°) in ACu3Te2O8 (A = Ca, Cd), where the exchange interactions within the kagomé layers are also ferromagnetic in nature.35
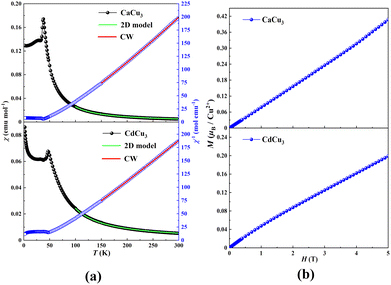
Fig. 2a shows the temperature dependence of magnetic susceptibility (χ) and the corresponding reciprocal one (χ−1) of ACu3Te2O8 (A = Ca, Cd) measured at 0.1 T. The susceptibilities increase with decreasing temperature, while a sharp peak is observed at ∼38 K for CaCu3Te2O8 and ∼47 K for CdCu3Te2O8, confirming the onset of antiferromagnetic (AFM) ordering. Such AFM transition was further confirmed by the specific heat data with zero field (Fig. S5, ESI†), showing a λ-type peak around 38 K for CaCu3Te2O8 and 47 K for CdCu3Te2O8. These are in good agreement with the interlayer interactions of ACu3Te2O8 (A = Ca, Cd), where the interlayer distance of CdCu3Te2O8 is shorter than that of CaCu3Te2O8 (Table S6, ESI†). Moreover, the Curie upturn below 25 K in CdCu3Te2O8 may be attributed to the contribution from free spins due to the crystal defect. Also, it is noted that the plot of χ−1versus T can be fitted well to the Curie–Weiss law above 150 K, giving the Curie constant C = 1.423 emu K mol−1 and Weiss temperature θ = 49.6 K for CaCu3Te2O8 and C = 1.442 emu K mol−1 and θ = 45.1 K for CdCu3Te2O8. The calculated effective magnetic moment of Cu2+ for CaCu3Te2O8 is 1.947μB and for CdCu3Te2O8 is 1.961μB, slightly larger than the theoretical spin value of 1.732μB for Cu2+ (S = 1/2, g = 2). The positive Weiss temperature suggests a dominant ferromagnetic (FM) interaction exchange between neighbouring Cu2+ ions in the layers. Fig. 2b displays the isothermal magnetization (M) as a function of the applied field (H) at 2 K. The magnetization increases linearly with increasing field, which is consistent with an AFM ground state.
The mean field theory θCW = −zJS(S + 1)/3kB (z is the number of neighbouring magnetic atoms around a magnetic atom) can be used to estimate the intralayer exchange coupling |J| = ∼49.6 K for CaCu3Te2O8, and |J| = ∼45.1 K for CdCu3Te2O8. Furthermore, the χ(T) from 100–300 K (Fig. 2a) can be well-fitted to the two-dimensional (2D) Heisenberg model, which is expressed as follows:36
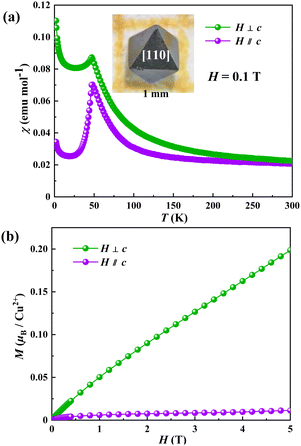
Since CdCu3Te2O8 single crystals obtained here are large enough to further investigate the anisotropic behaviors, we measured magnetic susceptibilities (χ‖ and χ⊥) of CdCu3Te2O8 under an applied field H = 0.1 T parallel and perpendicular to the c-axis using a single crystal sample (Fig. 3a). We note that an obvious divergence between χ‖ and χ⊥ reveals magnetic anisotropy in the system. The rapid decrease of χ‖ below 47 K is indicative of the easy magnetic c-axis. As shown in Fig. S6 (ESI†), the reciprocal susceptibilities (H‖c and H⊥c) at high temperature can be fitted by the Curie–Weiss law with the Weiss temperature θ‖ = −39.4 K and θ⊥ = 27.3 K, showing an AFM interaction between the Cu2+ ions along the c-axis, while a FM interaction is seen between the Cu2+ ions perpendicular to the c-axis. Fig. 3b illustrates the magnetization curves (M‖ and M⊥) at 2 K with applied magnetic fields parallel and perpendicular to the c-axis, both showing an almost linear increase with increasing magnetic field. The differences between M‖ and M⊥ also confirmed strong magnetic anisotropy in the system. The above findings support FM interactions inside the layers and AFM interactions between the neighbouring layers.
It is well known that the Hamiltonian for exchange interaction between any two spins (S1 and S2) can be expressed as H = −2JS1S2, where the magnitude and the sign of the exchange constant J are determined by the bonding geometry according to the Goodenough rules.37–39 Although the superexchange pathways of Cu–O1–Cu in the kagomé layers of ACu3Te2O8 (A = Ca, Cd) with the coupling distance of ∼3.2 Å and the coupling angle of ∼114° (Fig. 1d) look to be an antiferromagnetic interaction according to the Goodenough rules,37–39 the microscopic structure with Cu–O–Te–O–Cu pathways may strongly affect the formation of Cu-centered polyhedra and further change the coupling exchange, leading to FM interactions in the kagomé layers.40 A similar behavior is also observed in breathing kagomé compound Pb(OF)Cu3(SeO3)2(NO3) with coupling angles of Cu–O1–Cu (∼113°) and Cu–F1–Cu (∼116°), showing FM interactions in the kagomé layers. Furthermore, the exchange pathways of the Cu–O–Cu bonds with the coupling angles (>110°) in Cu3Bi(SeO3)2O2X (X = Cl, Br) (CBSX) have been confirmed to be FM interactions using neutron experiments.41,42 More surprisingly, the exchange pathways with the coupling angle of ∼180° in Cu3(CO3)2(x)3·2ClO4 (MOF-x) are also FM interactions.35 Also, it must be noted that ACu3Te2O8 (A = Ca, Cd) and CBSX have a similar structural feature, in which the Cu atoms are coordinated to four O atoms to form CuO4 square plaquettes and such plaquettes are connected to each other via corner-sharing. To further understand the nature of the magnetic behaviors of ACu3Te2O8 (A = Ca, Cd), neutron experiments and theoretical calculations may be required to provide a deeper explanation.
In summary, two new tellurite compounds ACu3Te2O8 (A = Ca, Cd) were successfully synthesized by means of a conventional hydrothermal method, exhibiting a similar 2D layered structure in the trigonal system of space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, where magnetic Cu2+ ions construct a spin-1/2 regular kagomé lattice via corner-sharing. Our results of magnetic and specific heat measurements confirmed the appearance of an AFM ordering at ∼38 K for CaCu3Te2O8 and ∼47 K for CdCu3Te2O8, respectively, where the positive Weiss temperatures suggest the FM exchange interactions of the Cu–O1–Cu pathways in the kagomé layers. Also, magnetic anisotropy was observed using a single crystal sample of CdCu3Te2O8, showing the easy magnetic c-axis. The results further support FM interactions inside the layers and AFM interactions between the neighbouring layers. We envisage that the present study will further enrich the chemical and physical contents of kagomé systems, which may stimulate the exploration of various interesting kagomé compounds.
m, where magnetic Cu2+ ions construct a spin-1/2 regular kagomé lattice via corner-sharing. Our results of magnetic and specific heat measurements confirmed the appearance of an AFM ordering at ∼38 K for CaCu3Te2O8 and ∼47 K for CdCu3Te2O8, respectively, where the positive Weiss temperatures suggest the FM exchange interactions of the Cu–O1–Cu pathways in the kagomé layers. Also, magnetic anisotropy was observed using a single crystal sample of CdCu3Te2O8, showing the easy magnetic c-axis. The results further support FM interactions inside the layers and AFM interactions between the neighbouring layers. We envisage that the present study will further enrich the chemical and physical contents of kagomé systems, which may stimulate the exploration of various interesting kagomé compounds.
This work was financially supported by the National Natural Science Foundation of China (NSFC) (no. 22175173).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Balents, Nature, 2010, 464, 199–208 CrossRef CAS PubMed.
- C. Broholm, R. J. Cava, S. A. Kivelson, D. G. Nocera, M. R. Norman and T. Senthil, Science, 2020, 367, 263 CrossRef PubMed.
- J. R. Chamorro, T. M. McQueen and T. T. Tran, Chem. Rev., 2021, 121, 2898–2934 CrossRef CAS PubMed.
- M. R. Norman, Rev. Mod. Phys., 2016, 88, 041002 CrossRef.
- D. G. Nocera, B. M. Bartlett, D. Grohol, D. Papoutsakis and M. P. Shores, Chem. – Eur. J., 2004, 10, 3850–3859 CrossRef PubMed.
- A. Harrison, J. Phys.: Condens. Matter, 2004, 16, S553–S572 CrossRef CAS.
- M. P. Shores, E. A. Nytko, B. M. Bartlett and D. G. Nocera, J. Am. Chem. Soc., 2005, 127, 13462–13463 CrossRef CAS PubMed.
- Z. Hiroi, M. Hanawa, N. Kobayashi, M. Nohara, H. Takagi, Y. Kato and M. Takigawa, J. Phys. Soc. Jpn., 2001, 70, 3377 CrossRef CAS.
- R. Okuma, D. Nakamura, T. Okubo, A. Miyake, A. Matsuo, K. Kindo, M. Tokunaga, N. Kawashima, S. Takeyama and Z. Hiroi, Nat. Commun., 2019, 10, 1229 CrossRef CAS PubMed.
- D. E. Freedman, R. Chisnell, T. M. McQueen, Y. S. Lee, C. Payen and D. G. Nocera, Chem. Commun., 2012, 48, 64–66 RSC.
- D. Grohol, K. matan, J.-H. Cho, S.-H. Lee, J. W. Lynn, D. G. Nocera and Y. S. Lee, Nat. Mater., 2005, 4, 323–327 CrossRef CAS PubMed.
- T.-H. Han, J. S. Helton, S. Chu, D. G. Nocera, J. A. Rodriguez-Rivera, C. Broholm and Y. S. Lee, Nature, 2012, 492, 406–420 CrossRef CAS PubMed.
- H. Yoshida, J. Yamaura, M. Isobe, Y. Okamoto, G. J. Nilsen and Z. Hiroi, Nat. Commun., 2012, 3, 860 CrossRef PubMed.
- S. M. Yan, D. A. Huse and S. R. White, Science, 2011, 332, 1173–1176 CrossRef CAS PubMed.
- K. I. A. Khan, R. S. Yadav, H. Bangar, A. Kumar, N. Chowdhury, P. K. Muduli and P. K. Muduli, Nanoscale, 2022, 14, 8484–8492 RSC.
- Q. Wang, Y. Xu, R. Lou, Z. Liu, M. Li, Y. Huang, D. Shen, H. Weng, S. Wang and H. Lei, Nat. Commun., 2018, 9, 3681 CrossRef PubMed.
- D. Chen, C. Le, C. Fu, H. Lin, W. Schnelle, Y. Sun and C. Felser, Phys. Rev. B, 2021, 103, 144410 CrossRef CAS.
- T. Asaba, V. Ivanov, S. M. Thomas, S. Y. Savrasov, J. D. Thompson, E. D. Bauer and F. Ronning, Sci. Adv., 2021, 7, eabf1467 CrossRef CAS PubMed.
- A. Magar, V. Singh, J. J. Abraham, Y. Senyk, A. Alfonsov, B. Büchner, V. Kataev, A. A. Tsirlin and R. Nath, Phys. Rev. Appl., 2022, 18, 054076 CrossRef CAS.
- B. R. Ortiz, S. M. L. Teicher, Y. Hu, J. L. Zuo, P. M. Sarte, E. C. Schueller, A. M. M. Abeykoon, M. J. Krogstad, S. Rosenkranz, R. Osborn, R. Seshadri, L. Balents, J. He and S. D. Wilson, Phys. Rev. Lett., 2020, 125, 247002 CrossRef CAS PubMed.
- K. Jiang, T. Wu, J.-X. Yin, Z. Wang, M. Z. Hasan, S. D. Wilson, X. Chen and J. Hu, Natl. Sci. Rev., 2023, 10, 199 CrossRef PubMed.
- Y. Tang, W. Guo, S. Zhang, M. Yang and Z. He, Cryst. Growth Des., 2014, 14, 5206–5211 CrossRef CAS.
- Y. Tang, W. Guo, H. Xiang, S. Zhang, M. Yang, M. Cui, N. Wang and Z. He, Inorg. Chem., 2016, 55, 644–648 CrossRef CAS PubMed.
- Y. Tang, C. Peng, W. Guo, J. F. Wang, G. Su and Z. He, J. Am. Chem. Soc., 2017, 139, 14057–14060 CrossRef CAS PubMed.
- M. Zhang, Y. Song, Z. Zhao, J. Li, W. Zhang, Y. Xie, X. Huang, X. Huang and Z. He, Inorg. Chem., 2020, 59, 8054–8060 CrossRef CAS PubMed.
- W. Zhang, Z. He, Y. Xie, M. Cui, S. Zhang, S. Chen, Z. Zhao, M. Zhang and X. Huang, Inorg. Chem., 2020, 59, 2299–2307 CrossRef CAS PubMed.
- M. Zhang, Z. Zhao, W. Zhang, J. Li, X. Huang and Z. He, Chem. Commun., 2020, 56, 11965–11968 RSC.
- H. Yoshida, Y. Michiue, E. Takayama-Muromachi and M. Isobe, J. Mater. Chem., 2012, 22, 18793–18796 RSC.
- A. Zorko, M. Pregelj, M. Gomilšek, M. Klanjšek, O. Zaharko, W. Sun and J. X. Mi, Phys. Rev. B, 2019, 100, 144420 CrossRef CAS.
- M. S. Williams, J. P. West and S.-J. Hwu, Chem. Mater., 2014, 26, 1502–1504 CrossRef CAS.
- R. Li and C. Greaves, Phys. Rev. B:Condens. Matter Mater. Phys., 2003, 68, 172403 CrossRef.
- C.-H. Wang, M. Avdeev, B. J. Kennedy, M. Küpers and C. D. Ling, Inorg. Chem., 2016, 55, 7535–7541 CrossRef CAS PubMed.
- Z. Hiroi, H. Yoshida, Y. Okamoto and M. Takigawa, J. Phys. Conf. Ser., 2009, 145, 012002 CrossRef.
- R. H. Colman, A. Sinclair and A. S. Wills, Chem. Mater., 2010, 22, 5774–5779 CrossRef CAS.
- S. A. Ivko, K. Tustain, T. Dolling, A. Abdeldaim, O. H. J. Mustonen, P. Manuel, C. Wang, H. Luetkens and L. Clark, Chem. Mater., 2022, 34, 5409–5421 CrossRef CAS PubMed.
- M. E. Lines, J. Phys. Chem. Solids, 1970, 31, 101–116 CrossRef CAS.
- J. B. Goodenough, John Wiley and Sons, Magnetism and the Chemical Bond, New York, p. 1963 Search PubMed.
- J. B. Goodenough, Phys. Rev., 1955, 100, 564–573 CrossRef CAS.
- J. Kanamori, J. Phys. Chem. Solids, 1959, 10, 87–98 CrossRef CAS.
- J. Deisenhofer, R. M. Eremina, A. Pimenov, T. Gavrilova, H. Berger, M. Johnsson, P. Lemmens, H. A. Krug von Nidda, A. Loidl, K. S. Lee and M. H. Whangbo, Phys. Rev. B:Condens. Matter Mater. Phys., 2006, 74, 174421 CrossRef.
- M. Pregelj, O. Zaharko, A. Günther, A. Loidl, V. Tsurkan and S. Guerrero, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 144409 CrossRef.
- H. C. Wu, K. D. Chandrasekhar, J. K. Yuan, J. R. Huang, J. Y. Lin, H. Berger and H. D. Yang, Phys. Rev. B, 2017, 95, 125121 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystal pictures, PXRD patterns, EDS, and additional magnetic data. CCDC 2371102 and 2370923. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc03756a |
| This journal is © The Royal Society of Chemistry 2025 |