Catalyst to cure: applications of a new copper-based nanocatalyst in organic synthesis and cancer treatment†
Harini G.
Sampatkumar
 a,
Srushti S.
Gundakanal
a,
Byresh
Gowda
a,
Srushti S.
Gundakanal
a,
Byresh
Gowda
 b,
Sudhanva
M. S.
b,
Siddalingeshwar V.
Doddamani
c,
B. S.
Sasidhar
b,
Sudhanva
M. S.
b,
Siddalingeshwar V.
Doddamani
c,
B. S.
Sasidhar
 c,
Alejandro
Bugarin
c,
Alejandro
Bugarin
 *d and
Siddappa A.
Patil
*d and
Siddappa A.
Patil
 *a
*a
aCentre for Nano and Material Sciences, Jain (Deemed-to-be University), Jain Global Campus, Kanakapura, Ramanagaram, Bangalore 562112, India. E-mail: p.siddappa@jainuniversity.ac.in
bAdichunchanagiri Institute for Molecular Medicine, Adichunchanagiri Institute of Medical Sciences, Adichunchanagiri University, BG Nagara-571448, Karnataka, India
cChemical Sciences and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology (NIIST), Thiruvananthapuram 695019, India
dDepartment of Chemistry & Physics, Florida Gulf University, Fort Myers, Florida 33965, USA. E-mail: abugarin@fgcu.edu
First published on 25th November 2024
Abstract
This research explores the diverse applications of copper(0) nanoparticles grafted onto boron carbon nitride nanosheets, using dill leaf extract as a natural reducing and stabilizing agent. This nanocatalyst efficiently catalyzes the synthesis of tetrazole and aniline derivatives, demonstrating good recyclability and promising potential in cancer therapy. By merging sustainability with innovation, this nanocatalyst offers transformative solutions in both synthesis and medical fields.
Nanocatalysis is an exciting and rapidly evolving field within chemistry that involves the use of nanometer-sized catalysts to accelerate chemical reactions.1 Nanocatalysts are unique compared to bulk materials due to their high surface area-to-volume ratio and the ability to tailor their surface properties. In addition, it effectively combines high activity, selectivity and tunability of homogeneous catalysts with stability, recoverability and reusability of heterogeneous catalysts.2 This unique position allows the nanocatalyst to serve as a bridge between the two traditional catalysis systems, paving the way for more efficient and sustainable chemical processes.
In the realm of nanotechnology, two-dimensional (2D) support materials have become the avant-garde platform for nanocatalyst synthesis.3 This integration marks a paradigm shift towards more sustainable and efficient catalytic processes. Materials like clays, carbon-based compounds and silica are utilized for their ability to stabilize metal nanoparticles (MNPs)4,5 and prevent agglomeration, maintaining high catalytic activity over time. Among these, boron carbon nitride (BCN) stands out, combining the exceptional properties of boron nitride and carbon. Its better surface area, tunable electronic properties and excellent thermal stability make it an ideal support material for dispersing catalytic NPs.6 These attributes ensure strong adhesion and enhanced stability, maximizing exposure to reactants and boosting overall catalytic performance.
Subsequently, biogenic synthesis of MNPs is revolutionizing the field by harmonizing technological advancement with environmental sustainability. This method uses natural entities like plants, fungi, bacteria and algae to synthesize MNPs, offering a greener alternative to traditional methods. By minimizing hazardous chemicals, reducing energy consumption, and generating less waste, it aligns perfectly with green chemistry principles. These biological entities act as reducing and stabilizing agents, simplifying and making their syntheses more sustainable. The resulting MNPs exhibit enhanced properties such as high catalytic activity and biocompatibility, with applications in catalysis, medicine and environmental remediation.7 For instance, plant extracts rich in phytochemicals like flavonoids and terpenoids can reduce and stabilize metal ions. Dill (Anethum graveolens), an aromatic plant from the Apiaceae family, is a potent source of these compounds and has been used in traditional medicine for over 2000 years,8 thus making it an excellent candidate to synthesize eco-friendly MNPs. Many successful syntheses of MNPs using plant extracts have been achieved, especially with noble metals,9 though costly. To cut expenses, researchers are now using first-row transition metals like copper (Cu), which is widely valued for its conductivity, malleability and corrosion resistance. The green synthesis of Cu NPs is particularly attractive due to lower costs, efficient treatment and simpler isolation.10 Its multiple valence states [Cu(0), Cu(I) and Cu(II)] enable complex electron transfers, making it ideal for diverse applications.
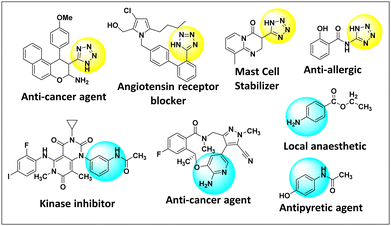
Among many biologically active compounds, tetrazoles stand out for their myriad biological roles in combatting viruses, bacteria and cancers alike paving indispensable paths in pharmaceutical innovation (Fig. 1).11 Equally pivotal is the transformation of nitroarenes into amines, crucial for the synthesis of numerous pharmaceuticals, dyes, agrochemicals and polymers.12 Usually, these transformations rely on harsh conditions, high-energy processes, toxic reagents and lengthy reaction times.13,14 However, the advent of nanotechnology has introduced a groundbreaking approach: the use of biogenically synthesised Cu NPs as catalysts, offers a more efficient, environmentally friendly and versatile method. The process often involves milder reaction conditions, moderate energy consumption and reduced waste generation.
 | ||
| Fig. 1 Some examples of pharmaceutically important tetrazole (yellow) and aniline (blue) derivatives. | ||
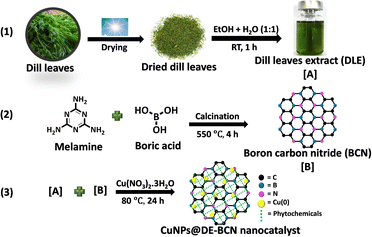
In the ongoing quest to conquer cancer, biogenically synthesized Cu NPs have emerged as a promising frontier. Harnessing the power of nature and cutting-edge technology, these NPs represent a novel approach in the fight against cancer. With their precise targeting capabilities and potent therapeutic effects, they hold the potential to redefine treatment strategies, offering new hope in the battle against this devastating disease. This work focuses on a clean, non-toxic and eco-friendly method for producing Cu(0) NP supported BCN, utilizing dill leaf extract as a reducing and stabilizing agent (Scheme 1). It explores their catalytic prowess in synthesizing tetrazoles and anilines, with potential applications as potent anticancer agents.
The synthesis of the CuNPs@DE-BCN nanocatalyst was performed by using BCN and aqueous extract of dill leaves without using any harmful chemicals, as shown in Scheme 1. Dill leaves were chosen for the presence of phytochemicals, carbohydrates, phenolic acid and aromatic compounds. These phytochemicals help in the reduction of Cu(II) to Cu(0). Initially, BCN was prepared from melamine as a carbon and nitrogen source and boric acid as the boron source by calcination at 550 °C for 4 h, and a pale-yellow product was obtained, which differs to the dark yellow colour of bulk graphitic carbon nitride. Then, the synthesized BCN was dispersed with the aqueous dill leaf extract (DLE) and sonicated for 30 min. Furthermore, it is dispersed with dissolved Cu(NO3)2·3H2O by continuous stirring for 24 h at 80 °C. The phytochemicals from the DLE show important roles of reducing as well as stabilizing agents. In the final stage, the desired CuNPs@DE-BCN nanocatalyst was obtained as an olive green coloured solid. Following the synthesis of the CuNPs@DE-BCN nanocatalyst, an array of microscopic and spectroscopic analyses was conducted to meticulously examine its structure and composition. Detailed findings of these analyses are thoroughly discussed in the ESI.†
The surface chemical composition of the synthesized CuNPs@DE-BCN was analysed with XPS and its high-resolution elemental spectrum is shown in Fig. 2a–e. The deconvoluted spectrum of Cu in Fig. 2(a) shows two major peaks at 932.6 (Cu 2p1/2) and 952.4 eV (Cu 2p3/2) confirming the zero state of Cu NPs. Meanwhile, a negligible peak at 934.6 eV could be attributed to Cu–(OH)2 due to the phytochemical interactions with zerovalent Cu.15 This can be further confirmed with the deconvoluted spectrum of oxygen 1s (Fig. 2b) at 531.6 eV. A further peak at 530.4 eV was attributed to oxygen interaction with the carbon network of BCN from phytochemicals.16 The N 1s deconvoluted spectrum from the support material is depicted in Fig. 2c. The two peaks at 398.6 and 397.5 are mainly attributed to nitrogen with boron and pyridinic N-bonding.17 From the peak intensity (Fig. 2d), it was observed that C–N bonding is more dominant than C–B bonding in BCN. This could be attributed to the favourable binding energy and bond length of C–N, which resembles the C–C bond length, while C–B possesses longer bond length. This can further be confirmed in the C 1s and B 1s deconvoluted spectrum of Fig. 2d and e. For instance, the peak intensity of C–N (286.8 eV) and B–N (190.5 eV) is significantly higher compared to C–B (283.4 eV) and B–C (188.3 eV and 189.4 eV).18 It is worth mentioning that the two different interactions of B–C are mainly attributed to B4C and BC3.19
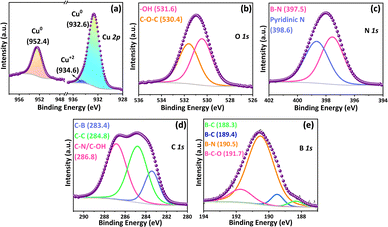 | ||
| Fig. 2 High resolution XPS spectra of (a) Cu 2p, (b) O 1s, (c) N 1s, (d) C 1s and (e) B 1s of the CuNPs@DE-BCN nanocatalyst. | ||
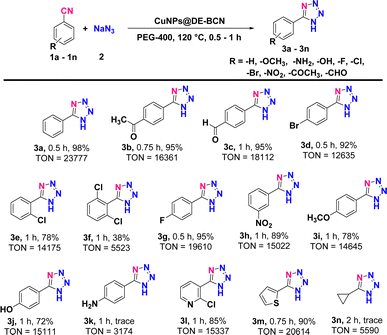
Furthermore, to uncover the catalytic potential of our CuNPs@DE-BCN nanocatalyst and advance our efforts in synthesizing 5-phenyl-1H-tetrazole and its derivatives, we investigated its ability to drive [3+2] cycloaddition reactions of nitriles with sodium azide (NaN3). To our delight, the catalyst was highly efficient in delivering the expected tetrazole products (Table 1). To optimize the reaction conditions, NaN3 and benzonitrile were selected as the model reaction. Subsequently, the effects of various parameters and conditions were systematically examined to determine the optimal reaction conditions and the results are summarized in Table S2 (ESI†).
A wide variety of structurally diverse nitriles were employed. The results summarized in Table 1 demonstrated that although there is an impact on reaction times and product yields, the catalysis performed remarkably well across a broad spectrum of aryl nitriles, consistently yielding the corresponding tetrazoles in high yields. There was a notable difference in reaction times and yields between aromatic nitriles with electron-withdrawing groups (EWGs) and electron-donating groups (EDGs), attributable to the nitriles' electrophilic characteristics. Unsubstituted aromatic nitriles and those with EWGs such as –COMe, –CHO, –Br, –Cl, and –F at para and –NO2 (Table 1, entries 3b–3d, 3g and 3h) and ortho positions (Table 1, entries 3e and 3f) required shorter reaction times and produced higher yields. On the other hand, nitriles with EDGs took longer and produced lower yields (Table 1, entries 3i–3k). This is because the cycloaddition reaction proceeds more rapidly with aromatic nitriles containing EWGs, which increase the polarity (inductive effect) of the cyanide group, compared to those with EDGs. Likewise, heteroaryl nitriles proved to be highly reactive substrates, quickly yielding the corresponding tetrazoles in the presence of the nanocatalyst (Table 1, entries 3l and 3m). However, attempts to react aliphatic nitriles under the optimized conditions were not so successful (Table 1, entry 3n). These findings highlight that CuNPs@DE-BCN is an exceptional nanocatalyst for synthesizing substituted 5-phenyl-1H-tetrazole, capable of tolerating a wide variety of substituents regardless of their electronic nature or position on the aromatic ring. Additionally, it showed recyclability up to five cycles (Fig. S3, ESI†) without the significant loss of its catalytic activity.
A plausible reaction mechanism, suggested by previous studies,20 is depicted in Scheme S1 in the ESI.† The proposed mechanism begins with the coordination of the nitrogen atom in the nitrile group to CuNPs@DE-BCN, forming complex (I). This complex acts as an ideal template for the [3+2]-cycloaddition reaction between the activated nitrile and azide. Notably, the cycloaddition does not occur in the absence of the catalyst (Table S2, entry 1, ESI†), underscoring the role of Cu(0) as a Lewis acid in nitrile activation. Following the [3+2]-cycloaddition between the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond of nitrile and azide, a five-membered heterocyclic ring (II) is formed. Subsequent protonolysis of intermediate (II) with 4 N HCl produces 5-phenyl-1H-tetrazole (III).
N bond of nitrile and azide, a five-membered heterocyclic ring (II) is formed. Subsequent protonolysis of intermediate (II) with 4 N HCl produces 5-phenyl-1H-tetrazole (III).
To delve deeper into the catalytic activity of the CuNPs@DE-BCN nanocatalyst for synthesizing pharmaceutically significant molecules, we embarked on the synthesis of anilines by reducing a wide range of nitroarenes. This process was carried out under optimized reaction conditions (Table S3, ESI†). As shown in Table S4 (ESI†), our approach is fairly accurate and applicable to a wide range of nitroarenes. The nitroarenes containing both EWGs like –CHO, –COMe, and –I (Table S4, entries 5b, 5g and 5d, ESI†) and EDGs like –OME, and –Me (Table S4, entries 5i and 5j, ESI†) are successfully reduced to aryl amines with a good yield. Bromo (Table S4, entries 5e and 5h, ESI†) and chloro (Table S4, entry 5f, ESI†) substituted nitroarenes were also evaluated affording their adducts with modest yields. In addition, the ability to selectively reduce nitro groups on fused bicyclic ring systems such as indole, makes this approach highly promising for drugs that contain indole rings (Table S4, entry 5k, ESI†). Similarly, the reduction of dinitro substrates could occur under the identical optimized conditions with a 90% yield (Table S4, entry 5l, ESI†). Furthermore, a scale up experiment (1 g) using our optimized conditions for the model nitrobenzene reduction reaction was performed and a 92% yield was achieved in 15 min. Hence, these results show that our nanocatalyst is capable of reducing various substituted nitroarenes. To our delight, our catalytic system was very selective toward nitro groups, while other reducible functional groups remained unaffected (Table S4, entries 5m and 5n, ESI†). To expand the use of our nanocatalyst, a local anaesthetic benzocaine (4-aminobenzoic acid ethyl ester) was synthesized (Table S4, entry 5o, ESI†) under our green conditions in just 35 minutes at room temperature by reducing ethyl-4-nitrobenzoate with the CuNPs@DE-BCN nanocatalyst, achieving a 96% yield. The synthesized nanocatalyst demonstrated high selectivity towards the tetrazole and aniline derivatives with high turnover number (TON), thus indicating the CuNPs@DE-BCN nanocatalyst to be very selective.
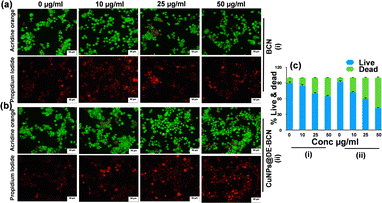
Addressing the fundamental issue of cancer, uncontrolled cell division is a hallmark of cancer cells, leading to the formation of tumour masses. To tackle this, we evaluated our synthesized BCN and CuNPs@DE-BCN nanocatalysts as anticancer agents, which demonstrated significant anti-proliferative potential by inducing cell death in colorectal cancer cells. The effect on cell proliferation was evaluated by an acridine orange/propidium iodide dual staining assay. Acridine orange is a fluorescent cationic dye that can penetrate the cell membrane of both live and dead cells, while propidium iodide is a fluorescent dye impermeant to live cells but penetrates and stains dead cells. The results demonstrate the induction of apoptosis in a concentration dependent manner by the nanocatalyst. Interestingly, the CuNPs@DE-BCN nanocatalyst induced significant cell death compared to BCN (Fig. 3a–c). The results demonstrate the potent anti-proliferative effect of the synthesised nanocatalyst against colorectal cancer.
The anti-proliferative propensity of the nanocatalyst was evaluated by Hoechst/PI dual staining. Hoechst is a fluorescent dye that can penetrate the membrane of both live and dead cells and binds specifically to the minor grove of double strand DNA. Hoechst stains the nuclear architecture of the cells and emits intense blue fluorescence upon emission. The results clearly unveil altered nuclear membrane in both BCN and CuNPs@DE-BCN in a concentration dependent manner. The results substantiate the data obtained by the AO/PI assay (Fig. 4a–c). In addition, metastasis plays a crucial role in spreading primary tumours into distant sites in the body. The effect of BCN and CuNPs@DE-BCN on migration was evaluated by scratch assay. The scratch was treated with the nanocatalysts and allowed to recover; the results demonstrate significant reduction of migration in both treatments compared to the control treated well (Fig. S4a and b, ESI†). This clearly unveils the antimetastatic propensity of the synthesized nanocatalyst against colorectal cancer cells.
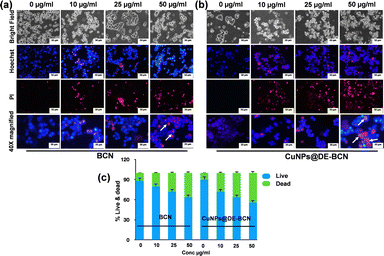 | ||
| Fig. 4 Effect of nanocatalyst on viability: (a) and (b) Hoechst/PI images, (c) histogram analysis of live and dead cells. All experiments were repeated thrice and scale bars are depicted. | ||
In summary, we developed a green, in situ biogenic method for the synthesis of copper(0) nanoparticles on dill leaf extract-modified boron carbon nitride (CuNPs@DE-BCN), resulting in an efficient and eco-friendly nanocatalyst. Characterization confirmed its robust structure and stability, along with its exceptional activity and selectivity for producing 5-phenyl-1H-tetrazoles and anilines, achieving yields of 72–98% and 52–98%, respectively. The catalyst showed excellent reusability, maintaining its performance over multiple cycles, and also enabled the green synthesis of the benzocaine drug molecule with notable anticancer activity. This methodology is expected to have a significant impact in both academic and industrial applications, particularly in medicinal chemistry.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Nasrollahzadeh, S. M. Sajadi, M. Sajjadi and Z. Issaabadi, Interface Sci. Technol., 2019, 28, 1–27 CrossRef CAS.
- F. Zaera, Chem. Rev., 2022, 122(9), 8594–8757 CrossRef CAS.
- W. Qian, S. Xu, X. Zhang, C. Li, W. Yang, C. R. Bowen and Y. Yang, Nanomicro Lett., 2021, 13, 1–38 Search PubMed.
- H. Ghafuri, M. Ghafori Gorab and H. Dogari, Sci. Rep., 2022, 12(1), 4221 CrossRef CAS PubMed.
- H. Veisi, T. Tamoradi, B. Karmakar, P. Mohammadi and S. Hemmati, Mater. Sci. Eng., C, 2019, 104, 109919 CrossRef CAS.
- B. Chandrashekharan, H. G. Sampatkumar, D. George, A. M. Antony, S. V. Doddamani, B. S. Sasidhar, R. G. Balakrishna and S. A. Patil, Diam. Relat. Mater., 2024, 111261 CrossRef CAS.
- A. Rana, K. Yadav and S. Jagadevan, J. Cleaner Prod., 2020, 272, 122880 CrossRef CAS.
- S. Selen Isbilir and A. Sagiroglu, Int. J. Food Prop., 2011, 14(4), 894–902 CrossRef.
- H. G. Sampatkumar, A. M. Antony, M. Trivedi, M. Sharma, M. Ghate, M. Baidya, R. B. Dateer and S. A. Patil, Biomass Convers. Biorefin., 2024, 14(9), 10045–10066 CrossRef CAS.
- M. Rafique, A. J. Shaikh, R. Rasheed, M. B. Tahir, H. F. Bakhat, M. S. Rafique and F. Rabbani, Nano, 2017, 12(04), 1750043 CrossRef CAS.
- R. Vishwakarma, C. Gadipelly and L. K. Mannepalli, ChemistrySelect, 2022, 7(29), e202200706 CrossRef CAS.
- A. R. Patel, I. Patel and S. Banerjee, Curr. Org. Chem., 2024, 28(5), 375–389 CrossRef.
- V. Rama, K. Kanagaraj and K. Pitchumani, J. Org. Chem., 2011, 76(21), 9090–9095 CrossRef CAS PubMed.
- A. Lucchesi Schio, M. R. Farias Soares, G. Machado and T. Barcellos, ACS Sustainable Chem. Eng., 2021, 9(29), 9661–9670 CrossRef CAS.
- P. V. F. de Sousa, A. F. de Oliveira, A. A. da Silva and R. P. Lopes, Environ. Sci. Pollut. Res., 2019, 26, 14883–14903 CrossRef CAS PubMed.
- H. Idriss, Surf. Sci., 2021, 712, 121894 CrossRef CAS.
- L. Ci, L. Song, C. Jin, D. Jariwala, D. Wu, Y. Li, A. Srivastava, Z. F. Wang, K. Storr, L. Balicas, F. Liu and P. M. Ajayan, Nat. Mater., 2010, 9(5), 430–435 CrossRef CAS PubMed.
- S. Angizi, M. A. Akbar, M. Darestani-Farahani and P. Kruse, ECS J. Solid State Sci. Technol., 2020, 9(8), 083004 CrossRef CAS.
- H. Künzli, P. Gantenbein, R. Steiner and P. Oelhafen, Fresenius’ J. Anal. Chem., 1993, 346, 41–44 CrossRef.
- C. Tao, B. Wang, L. Sun, J. Yi, D. Shi, J. Wang and W. Liu, J. Chem. Res., 2017, 41(1), 25–29 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc04025b |
| This journal is © The Royal Society of Chemistry 2025 |