Stabilizing 4π electron pyrrolyl cations by inducing aromaticity†‡
Astha
Gupta§
 a,
Mohammad Ovais
Dar§
a,
Mohammad Ovais
Dar§
 ac,
Tejender
Singh
ac,
Tejender
Singh
 a,
Gurudutt
Dubey
a,
Gurudutt
Dubey
 a,
Subash C.
Sahoo
a,
Subash C.
Sahoo
 b and
Prasad V.
Bharatam
b and
Prasad V.
Bharatam
 *a
*a
aNational Institute of Pharmaceutical Education and Research (NIPER), Sector 67, S.A.S. Nagar-160062, Punjab, India. E-mail: pvbharatam@niper.ac.in
bDepartment of Chemistry, Panjab University, Sector 14, Chandigarh-160014, India
cM. M. College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana, Ambala-133207, Haryana, India
First published on 26th November 2024
Abstract
The pyrrolyl cation is a 4π electron ring system which is anti-aromatic and unstable. This work reports an experimental procedure to obtain stable pyrrolyl cations. Electron donation from N-heterocyclic carbenes makes the ring stable by converting a 4π electron ring system into a 6π electron ring system.
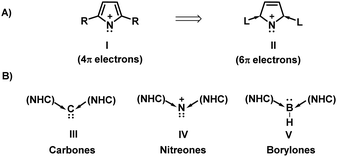
Pyrrolyl cations (I) are rarely reported in chemistry because they are anti-aromatic (4π electron), unstable systems. Falvey and coworkers unequivocally established that the pyrrolyl cation I (R = H) is anti-aromatic and unstable.1 Furthermore, they also established that even when the pyrrolyl cation ring is fused as in indolyl and carbazolyl cations, the rings remain anti-aromatic and unstable (using laser flash photolysis and computational studies).1
N-Heterocyclic carbenes (NHCs) are excellent electron donors, and they make excess electrons available at the receiving centre. For example, in the case of carbones (III),2–6 nitreones (IV),7–9 borylones (V),10 (Fig. 1) and related species,11–14 the two NHC ligands facilitate the availability of two extra electrons at the receiving end. Thus, NHCs can significantly modify the character of the electron acceptor centre/unit in organic molecules. This concept can be extrapolated to 4π electron ring systems when they are at the acceptor end.
Many attempts have been made to stabilize anti-aromatic (4π electron) cyclobutadienes and cyclopentadienyl cations. Fig. 2 shows a few examples in which anti-aromatic rings were stabilized with the help of electron-donating ligands. However, to the best of our knowledge, no attempts have been made to stabilize the equally important anti-aromatic pyrrolyl cations. The challenge is to introduce aromatic character in the pyrrolyl cation ring. The examples given in Fig. 2 provide a few clues, in which case, the ylide stabilized ring chemistry was highlighted and, the corresponding increase in π electrons helped in stabilizing anti-aromatic rings.
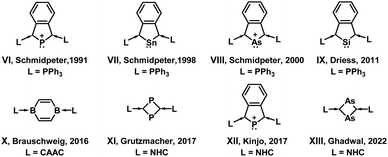 | ||
| Fig. 2 A few ring systems stabilized by coordination bonds from two ligands, and in many cases a dative bond was not highlighted but shown in this work.15–25 | ||
These examples helped in envisaging that the coordination bonds between the NHC ligands and the pyrrolyl cation ring would make two more electrons available to the central ring such that the 4π electron ring (I) would become a 6π electron ring system (II). To explore this hypothesis, synthesis of 4a–4d has been carried out and quantum chemical analysis has been performed.
Using a retrosynthetic approach, pyrrole-2,5-dicarboxaldehyde 1 was found to be a good starting material for the generation of pyrrolyl cations. Compound 1 was obtained from pyrrole-2-carboxaldehyde in a three-step reaction (Scheme S1, ESI‡): (i) protection with ethyl cyanoacetate, (ii) formylation via Vilsmeier–Haack reaction and subsequent (iii) deprotection via alkaline hydrolysis using the reported procedures.26 The reaction of 1 with 2a in acetonitrile in the presence of the oxidizing agent urea-hydrogen peroxide (UHP) at 70 °C for 4–5 hours yielded the neutral pyrrole derivative 3a with 71% yield (Scheme 1).27 The methylation of 3a using DMS (dimethyl sulphate) at 90 °C followed by base treatment, afforded the designed pyrrolyl cation 4a, which was isolated using preparative TLC, characterized using 1H NMR, 13C NMR, HRMS techniques and X-ray diffraction (see ESI‡). Compounds 4b–4d were also synthesized using a similar route as in Scheme 1.
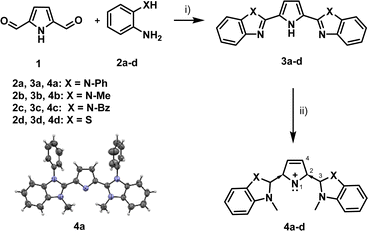 | ||
| Scheme 1 Synthetic scheme for NHC-stabilized pyrrolyl cations 4a–4d. Conditions: (i) UHP, I2, acetonitrile, 70 °C, 4–5 h, yield 42–65%; (ii) DMS or MeOTf, base (see ESI‡), yield 23–64%. ORTEP plot of the molecular structure of 4a. Thermal ellipsoids are drawn at the 50% probability level. The CCDC number for 4a is 2299060.‡ | ||
From 1H NMR, it is clear that there is no peak of –NH proton in the spectrum of 4a, and the appearance of six more protons (in comparison to that of 3a) of two methyl groups confirmed the formation of compound 4a. In the 1H NMR spectra, the proton at the C4 center appears at 5.79, 7.39, 7.13 and 7.56 ppm for compounds 4a–4d, respectively (Tables S1 and S2, ESI‡). All these values lie in the aromatic region. The upfield shift in 4a (also in 3a) is because of the direct exposure of C4 to the aryl group π-electron cloud of the phenyl substitution on the imidazole nitrogen. In 4a, the 13C NMR peak of C3 (electron donating centre) appears at 147 ppm and C2 (electron accepting centre) appears at 111 ppm; similar 13C peaks were observed in carbones.28
In order to study the optical properties of the compound 4a, absorption (UV-Vis) and emission (fluorescence) spectra of compound 4a (Fig. S1–S3, ESI‡) were recorded and compared with the results from TD-DFT calculations. The absorption band with λmax at 344 nm (calculated λmax = 347 nm, f = 1.46) is attributable to the π → π* (HOMO → LUMO) transition, confirming the aromatic character of the molecule. Upon excitation at λex = 280 nm, it displays a broad emission band with λmax at 418 nm (calculated λmax = 428 nm, f = 1.84) (Fig. 3). It is interesting to note that the work done by Augusti and co-workers established the unstable state of compound I (R = H);29 the current modification made the compounds 4a–d bench-stable (mp >200 °C).
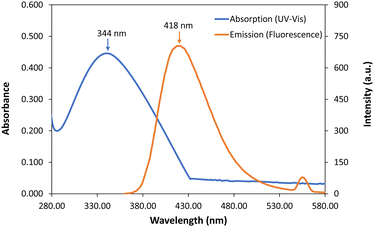 | ||
| Fig. 3 Absorption (UV-Vis) and emission (fluorescence) spectra of compound 4a (λex = 280 nm) in MeCN solution (see Fig. S1–S3, ESI‡ for further details). | ||
The crystals of compound 4a were obtained by recrystallizing in the DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN (1
MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture by slow evaporation for about 2–3 days. Bright yellow needle-shaped crystals were obtained and subjected to single-crystal X-ray diffraction. Scheme 1 includes the ORTEP diagram of 4a. The three main rings in compound 4a are almost coplanar with each other. The unit cell consists of two molecules of 4a separated by two anionic counterparts. No π-stacking between molecules in the crystal lattice was observed because the phenyl groups in 4a are orthogonal to the principal plane of the molecule (see ESI‡). The N+ atom of the pyrrolyl cation ring does not participate in any inter or intramolecular interactions. The C2–C3 bond length in 4a is 1.43 Å (Fig. S4, ESI‡), much shorter than the C–C single bond length (1.54 Å) but much longer than the C
1) mixture by slow evaporation for about 2–3 days. Bright yellow needle-shaped crystals were obtained and subjected to single-crystal X-ray diffraction. Scheme 1 includes the ORTEP diagram of 4a. The three main rings in compound 4a are almost coplanar with each other. The unit cell consists of two molecules of 4a separated by two anionic counterparts. No π-stacking between molecules in the crystal lattice was observed because the phenyl groups in 4a are orthogonal to the principal plane of the molecule (see ESI‡). The N+ atom of the pyrrolyl cation ring does not participate in any inter or intramolecular interactions. The C2–C3 bond length in 4a is 1.43 Å (Fig. S4, ESI‡), much shorter than the C–C single bond length (1.54 Å) but much longer than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond length (1.34 Å). Thus, the coordination character between the benzimidazole and the pyrrolyl cation ring is confirmed; furthermore, the C2–C3 rotation barrier in 4a is only ∼3.4 kcal mol−1, indicating the absence of π-character between the ligand and the pyrrolyl cation ring.
C double bond length (1.34 Å). Thus, the coordination character between the benzimidazole and the pyrrolyl cation ring is confirmed; furthermore, the C2–C3 rotation barrier in 4a is only ∼3.4 kcal mol−1, indicating the absence of π-character between the ligand and the pyrrolyl cation ring.
Aromaticity in NHC coordinated pyrrolyl cation: Aromaticity and anti-aromaticity are topics of renewed interest in chemical research.30–32 It is worth exploring the modification of aromatic character in 4e and 4f. The electronic structures of 4e (simplest model of I) and 4f (simplest model of II) have been evaluated first to explore their aromatic character and to compare it with that of related cyclic nitrenium ions 5–7 (for which analogs are experimentally known) (Fig. 4). Density functional theory provided the structural, energetic and electronic characteristics of these designed compounds (Tables 1 and 2).
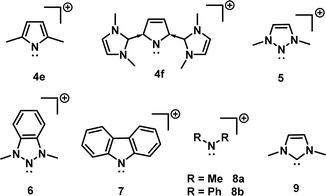 | ||
| Fig. 4 2D structures of 4e (model of IV), 4f (model of 4a–4d), 5,6 (models of experimentally known triazolium ions), 7 (carbazolyl nitrenium ion), 8 (open chain nitrenium ions), and 9 (standard NHC). | ||
| 4e (4π e) | 4f (6π e) | |
|---|---|---|
| NICS is in ppm, ASE is in kcal mol−1. | ||
| NICS(0) | +24.2 | −10.5 |
| NICS(1) | +9.9 | −10.1 |
| HOMA | −0.95 | +0.95 |
| FLU | 0.055 | 0.008 |
| ASE | −35.90 | +27.09 |
| EDDBP | 0.858 | 3.904 |
| ΔEST | HIA | PA | ΔEAuCl | NICS(1) | ASE | |
|---|---|---|---|---|---|---|
| Where ΔEST is the singlet–triplet gap (positive values represent singlet stability), HIA = hydride ion affinity, PA = proton affinity, ΔEAuCl is the complexation energy with AuCl, and ASE = aromatic stabilization energy. All energy values are in kcal mol−1. NICS is nucleus-independent chemical shift (in ppm). | ||||||
| 4a | 58.9 | 126.8 | 185.7 | −30.3 | −9.9 | 21.61 |
| 4b | 62.8 | 127.2 | 168.9 | −30.5 | −9.7 | 25.26 |
| 4c | 65.5 | 120.9 | 185.5 | −29.3 | −9.3 | 23.68 |
| 4d | 48.1 | 152.9 | 172.4 | −24.5 | −8.3 | 15.31 |
| 4e | 23.5 | 263.1 | 107.1 | −8.9 | 9.9 | −35.9 |
| 4f | 62.9 | 109.1 | 178.7 | −28.4 | −10.1 | 27.09 |
| 5 | 87.8 | 153.7 | 82 | −1.6 | −11.6 | 21.01 |
| 6 | 67.4 | 164.3 | 95.4 | −2.0 | −11.8 | — |
| 7 | 8.8 | 265.6 | 122.2 | −18.8 | 32.1 | — |
| 8a | −3.1 | 291.1 | 45.6 | −16.4 | — | — |
| 8b | 8.2 | 248.7 | 123.7 | −11.6 | — | — |
The extent of aromaticity of a given ring system can be evaluated using quantum chemical methods in many ways such as geometric (HOMA, harmonic oscillator measure of aromaticity),33 electronic (FLU, aromatic FLUctuation index34 and EDDB, electron density of delocalized bonds),35 energetic (ASE, aromatic stabilization energy)1 and magnetic (NICS, nucleus independent chemical shift,36 and ACID, anisotropy of the current (induced) density)37 parameters. Several theoretical studies on aromaticity were carried out to understand the differences in the aromaticity parameters between 4e and 4f (Table 1).
NICS values are important characteristic features in distinguishing aromatic vs. anti-aromatic species. The NICS(1) value of the pyrrole cation ring in 4e is +9.9 ppm, implying that it is anti-aromatic. When the methyl group in 4e is replaced with an NHC ligand in 4f, the NICS(1) value becomes negative −10.1 ppm in the central ring of 4f (Table 1). Compounds 4a–4d carry NICS(1) values close to −10 ppm in the central ring (Table 2). Similarly, a comparison of the FLU, HOMA, and ASE values also indicates that 4e is anti-aromatic and 4f is aromatic, and all these parameters indicate that the 4e to 4f modulation leads to the introduction of aromatic character in the pyrrolyl cation ring.
Falvey and coworkers1 employed isodesmic equations to estimate aromatic stabilization energy (ASE) and established the anti-aromatic character of pyrrolyl cation I (R = H) and carbazolyl cation 7. In this work, ASE values were estimated for the cyclic nitrenium ions (4e and 4f) using isodesmic equations (as shown in Fig. S5, ESI‡), and the results are listed in Table 1. The ASE for 4e is negative (−35.90 kcal mol−1) indicating anti-aromaticity, whereas the ASE for 4f is positive (+27.09 kcal mol−1) indicating aromaticity. Similarly, the compounds 4a–4d also carry positive ASE values (Table 2), indicating strong aromatic character in these species.
ACID analysis is a very useful method to illustrate aromaticity pictorially.37 ACID plots use surfaces (yellow) and vectors (green) to show the current density and direction of the ring current. The ACID plot of compound 4e displays the ring current in an anti-clockwise direction, confirming its anti-aromatic character. At the same time, distinct clockwise circulations were observed in the central pyrrolyl cation ring of 4f (Fig. 5). The ACID analysis of compounds 4a–4d also confirms the aromatic character of the pyrrolyl cation ring (Fig. S6, ESI‡). EDDB analysis provides quantitative (Table 1) and visual information (Fig. S7, ESI‡) on the aromatic and non-aromatic interactions in the ring systems.35 The continuum in the contour map of 4f indicates aromatic character while the discontinuous electron distribution in 4e establishes the anti-aromatic character. This comparison indicates that the anti-aromatic pyrrolyl cation ring is becoming an aromatic ring with structural modification i.e., replacing an R group (as in 4e) with a ligand L (as in 4f). Fig. S8 (ESI‡) shows the π molecular orbitals of compounds 4e and 4f, along with the molecular orbital energies and their electron occupancy details. In 4e, only two π MOs are occupied, and in 4f, three π MOs are occupied (in the pyrrolyl cation ring), supporting the argument regarding the aromaticity of compound 4f, which is absent in compound 4e.
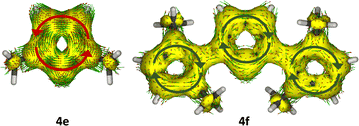 | ||
| Fig. 5 The ACID plots (with an isosurface value of 0.050 a.u.) of 4e and 4f. The magnetic field vector is orthogonal to the ring plane (the green and red arrows represent the corresponding diatropic (aromatic) and paratropic (anti-aromatic) ring currents, respectively). Details are provided in the ESI.‡ | ||
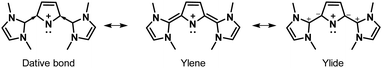
The bonding situation in 4a–4d (and 4f) may be represented as a resonance hybrid of three alternatives for 4f (dative, ylene, ylide), as shown in Fig. 6. EDA-NOCV analysis38–40 was carried out to understand the nature of the bonding situation between the carbene moieties and the central pyrrolyl cation ring. Comparing the ΔEOrb, it became clear that the donor–acceptor bonding situation is preferred over the electron-sharing bonding situation. The major contribution to the dative bond originates from the sigma donation from the carbene moieties to the pyrrole ring and a small contribution of 5% from the π-back-donation from pyrrole to the carbene moieties (see ESI‡). In addition to the compounds 4a–4d, the ligand-stabilized ring systems (VI–XIII) were also evaluated to understand the gain in aromaticity using the model systems (VI–XIII). The results support the hypothesis proposed in this work (Fig. S9 and S10, ESI‡).
Apart from the aromaticity features, compounds 4a–4d carry many interesting electronic characteristics. For example, the ΔEST values of the pyrrolyl cations 4a–4d are in the range of 45–65 kcal mol−1 (due to π–π* interaction) (Table 2), indicating singlet stability similar to that of imidazol-2-ylidene (9).41 The hydride ion affinity (HIA) values of 4a–4d are in the range of 110–150 kcal mol−1, quite different from that of 4e and 7 (only marginally less than that of 5 and 6). The proton affinity (PA) values of 4a–4d are in the range of 172–185 kcal mol−1. These values lie between the PA of water (168 kcal mol−1)42 and the PA of ammonia (203 kcal mol−1),43 indicating that 4a–4d may exhibit sufficient Lewis basic character. These nitrenium ions (4a–4d) carry significantly different characteristics than open-chain nitrenium ions (8a and 8b). Compounds generated in this work 4a–4d can be considered as cyclic nitrenium ions, as they possess many properties comparable to the experimentally known cyclic nitrenium ions44 (as shown in Fig. S11, ESI‡). Compounds 4a–4d also exhibit a few unique electronic characteristics; thus, this work led to the generation of new examples of cyclic nitrenium ions.
In conclusion, pyrrolyl cations could not be studied earlier thoroughly because they are anti-aromatic and unstable. The electron donation from two exocyclic NHC groups to an anti-aromatic system through the coordination interactions can be the most plausible opportunity for transforming a 4π electron ring system into a 6π electron ring system. This work provides direct evidence by stabilizing the pyrrolyl cation, which is hitherto considered to be impossible. Four novel pyrrolyl cations were generated experimentally and one of them was characterized using X-ray diffraction. This innovative approach led to the generation of a novel class of stable cyclic nitrenium ions.
We thank Prof. Rainer Herges, Kiel University, for providing us with the ACID software. The authors would like to acknowledge the Department of Biotechnology (DBT), Government of India, New Delhi, for financial support (grant number: BT/PR140164).
Data availability
The data that support the findings of this study are available in the ESI‡ of this article. Crystallographic data of 4a have been uploaded to the Cambridge Crystallographic Data Centre under CCDC number 2299060.‡Conflicts of interest
There are no conflicts to declare.Notes and references
- A. H. Winter, H. H. Gibson and D. E. Falvey, J. Org. Chem., 2007, 72, 8186–8195 CrossRef CAS.
- M. A. L. Johansen and A. Ghosh, Nat. Chem., 2023, 15, 1042 CrossRef CAS.
- R. Tonner and G. Frenking, Chem. – Eur. J., 2008, 14, 3260–3272 CrossRef CAS PubMed.
- R. Tonner and G. Frenking, Angew. Chem., Int. Ed., 2007, 46, 8695–8698 CrossRef CAS.
- G. Frenking and R. Tonner, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 869–878 CAS.
- W. Petz, Coord. Chem. Rev., 2015, 291, 1–27 CrossRef CAS.
- G. Dubey, N. Mahawar, T. Singh, N. Saha, S. C. Sahoo and P. V. Bharatam, Phys. Chem. Chem. Phys., 2022, 24, 629–633 RSC.
- D. S. Patel and P. V. Bharatam, Chem. Commun., 2009, 1064–1066 RSC.
- N. Patel, R. Sood and P. V. Bharatam, Chem. Rev., 2018, 118, 8770–8785 CrossRef CAS PubMed.
- R. Kinjo, B. Donnadieu, M. A. Celik, G. Frenking and G. Bertrand, Science, 2011, 333, 610–613 CrossRef CAS PubMed.
- Y. Xiong, S. Yao, S. Inoue, J. D. Epping and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 7147–7150 CrossRef CAS PubMed.
- E. M. Dionisi, J. F. Binder, J. H. LaFortune and C. L. Macdonald, Dalton Trans., 2020, 49, 12115–12127 RSC.
- L. Zhao, S. Pan, N. Holzmann, P. Schwerdtfeger and G. Frenking, Chem. Rev., 2019, 119, 8781–8845 CrossRef CAS.
- W. Petz and G. Frenking, in Advances in Inorganic Chemistry, ed. R. Van Eldik, and C. D. Hubbard, Elsevier, 2022, vol. 79, pp. 247–299 Search PubMed.
- T. Soos, G. Hajós and A. Messmer, J. Org. Chem., 1997, 62, 1136–1138 CrossRef CAS.
- A. Fürstner, M. Alcarazo, K. Radkowski and C. W. Lehmann, Angew. Chem., Int. Ed., 2008, 47, 8302–8306 CrossRef PubMed.
- S.-y. Nakafuji, J. Kobayashi and T. Kawashima, Angew. Chem., Int. Ed., 2008, 47, 1141–1144 CrossRef CAS PubMed.
- M. Asay, S. Inoue and M. Driess, Angew. Chem., Int. Ed., 2011, 50, 9589–9592 CrossRef CAS.
- M. Arrowsmith, J. Böhnke, H. Braunschweig, M. A. Celik, C. Claes, W. C. Ewing, I. Krummenacher, K. Lubitz and C. Schneider, Angew. Chem., Int. Ed., 2016, 55, 11271–11275 CrossRef CAS PubMed.
- Z. Li, X. Chen, D. M. Andrada, G. Frenking, Z. Benkö, Y. Li, J. R. Harmer, C.-Y. Su and H. Grützmacher, Angew. Chem., Int. Ed., 2017, 56, 5744–5749 CrossRef CAS PubMed.
- C. C. Chong, B. Rao, R. Ganguly, Y. Li and R. Kinjo, Inorg. Chem., 2017, 56, 8608–8614 CrossRef CAS PubMed.
- H. Steffenfauseweh, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, D. M. Andrada and R. S. Ghadwal, Angew. Chem., Int. Ed., 2022, 61, e202207415 CrossRef CAS PubMed.
- A. Schmidpeter and M. Thiele, Angew. Chem., Int. Ed. Engl., 1991, 30, 308–310 CrossRef.
- A. Schmidpeter, H. P. Schrödel and J. Knizek, Heteroat. Chem., 1998, 9, 103–108 CrossRef CAS.
- F. Breitsameter, A. Schmidpeter and H. Nöth, Chem. – Eur. J., 2000, 6, 3531–3539 CrossRef CAS.
- G. M. Peters, J. B. Winegrad, M. R. Gau, G. H. Imler, B. Xu, S. Ren, B. B. Wayland and M. J. Zdilla, Inorg. Chem., 2017, 56, 3377–3385 CrossRef CAS PubMed.
- M. L. P. R. Alapati, S. R. Abburi, S. B. Mukkamala and R. M. Krishnaji, Syn. Commun., 2015, 45, 2436–2443 CrossRef CAS.
- C. A. Dyker, V. Lavallo, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2008, 47, 3206–3209 CrossRef CAS.
- R. Sparrapan, M. N. Eberlin and R. Augusti, Rapid Commun. Mass Spectrom., 2005, 19, 1775–1778 CrossRef CAS.
- G. Merino, M. Solà, I. Fernández, C. Foroutan-Nejad, P. Lazzeretti, G. Frenking, H. L. Anderson, D. Sundholm, F. P. Cossío, M. A. Petrukhina, J. Wu, J. I. Wu and A. Restrepo, Chem. Sci., 2023, 14, 5569–5576 RSC.
- H. Ottosson, Chem. Sci., 2023, 14, 5542–5544 RSC.
- Y. Schulte, C. Wölper, S. M. Rupf, M. Malischewski, D. J. SantaLucia, F. Neese, G. Haberhauer and S. Schulz, Nat. Chem., 2024, 16, 651–657 CrossRef CAS PubMed.
- T. M. Krygowski, H. Szatylowicz, O. A. Stasyuk, J. Dominikowska and M. Palusiak, Chem. Rev., 2014, 114, 6383–6422 CrossRef CAS PubMed.
- E. Matito, M. Duran and M. Sola, J. Chem. Phys., 2005, 122, 014109 CrossRef PubMed.
- D. W. Szczepanik, M. Andrzejak, J. Dominikowska, B. Pawełek, T. M. Krygowski, H. Szatylowicz and M. Sola, Phys. Chem. Chem. Phys., 2017, 19, 28970–28981 RSC.
- P. V. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. van Eikema Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS PubMed.
- R. Herges and D. Geuenich, J. Phys. Chem. A, 2001, 105, 3214–3220 CrossRef CAS.
- T. Ziegler and A. Rauk, Theor. Chim. Acta, 1977, 46, 1–10 CrossRef CAS.
- M. Mitoraj and A. Michalak, Organometallics, 2007, 26, 6576–6580 CrossRef CAS.
- ADF2020.102, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, see https://www.scm.com, (accessed October 5, 2024).
- H. V. Huynh, Chem. Rev., 2018, 118, 9457–9492 CrossRef CAS.
- M. Munson and J. L. Franklin, J. Phys. Chem., 1964, 68, 3191–3196 CrossRef CAS.
- K. A. Peterson, S. S. Xantheas, D. A. Dixon and T. H. Dunning, J. Phys. Chem. A, 1998, 102, 2449–2454 CrossRef CAS.
- I. Avigdori, K. Singh, A. Pogoreltsev, A. Kaushansky, N. Fridman and M. Gandelman, Z. Anorg. Allg. Chem., 2023, 649, e202200326 CrossRef CAS.
Footnotes |
| † Dedicated to Professor E. D. Jemmis on the occasion of his 73rd birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2299060. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc04560b |
| § These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |