Ring expansion of 3-hydroxyoxindoles to 4-quinolones via palladium-catalyzed C–C(acyl) bond cleavage†
Zhi-Cong
Huang
a,
Zhi-Ling
Ruan
c,
Hui
Xu
a and
Hui-Xiong
Dai
 *abc
*abc
aState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China. E-mail: hxdai@simm.ac.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
cSchool of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, Jiangsu 210023, China
First published on 21st November 2024
Abstract
We report herein the construction of 4-quinolones via palladium-catalyzed regioselective β-acyl elimination of 3-hydroxyoxindoles and a subsequent Camps cyclization process. This protocol is highly efficient and various 4-quinolone derivatives are obtained in high yields. The construction of the core skeleton of the 4-quinolone antibiotics demonstrated the synthetic utility of this method.
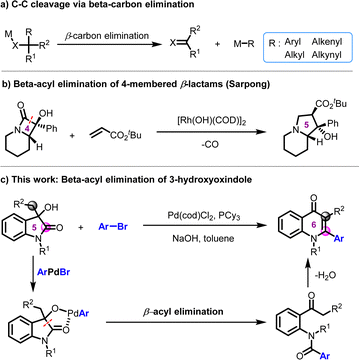
Transition metal-catalyzed C–C bond cleavage has emerged as a transformative platform for the skeletal modification of core scaffolds and late-stage functionalizations (LSF) of natural products and drugs.1 Oxidative addition of the transition metal catalysts into the strained or unstrained C–C bond is an important strategy to generate the C–M–C species.2 β-Carbon elimination is another main pathway to cleave the C–C bond, which does not involve the changes in the oxidation state of the metal catalyst. To date, transition metal-catalyzed C–C bond cleavage via β-aryl, alkyl, alkenyl, and alkynyl eliminations have been well documented (Scheme 1a).3 However, β-acyl elimination to generate acyl–metal species is less explored. In 2020, Sarpong and coworkers reported the synthesis of substituted indolizidines from α-hydroxy-β-lactams (Scheme 1b).4 The reaction proceeded via a Rh-catalyzed β-acyl elimination, decarbonylation, 1,4-addition, and aldol cyclization process. Using a palladium catalyst, oxidative addition of Pd(0) with ArBr and subsequent β-alkyl elimination occurred to form α-arylated piperidine products.5 Furthermore, Chen and coworkers reported the synthesis of ynamides, ynoates, and ynones from β-carbonyl alcohols by photoredox catalysis.6 Carbamoyl, alkoxylcarbonyl, or acyl radicals were generated via the β-carbonyl alkoxyl radical-enabled carbonyl–C(sp3) bond cleavage.
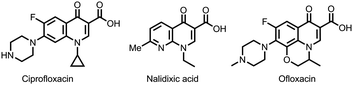
4-Quinolone derivatives are important nitrogen-containing heterocycles and are widely found in bioactive molecules.7 Given the ubiquity and structural significance, quinolone-containing compounds have exhibited a spectrum of bioactivities, including antibacterial,8 antimalarial,9 anti-HIV,10 antiviral,11 and cathepsin inhibitory effects.12 For example, 4-quinolones and their analogs have been extensively applied in antibiotic medications, such as ciprofloxacin, ofloxacin, nalidixic acid, etc. (Fig. 1). The allure of their biological properties has promoted chemists to devise an array of synthetic pathways to access these scaffolds, such as Conrad Limpach reaction, Niementowski reaction, Camps cyclization reaction, etc.13 In recent years, transition metal-catalyzed tandem cyclization has paved new avenues to construct 4-quinolone derivatives.13a,14
Isatin and its derivatives are often employed as the precursors for drug synthesis.15 Dong and coworkers achieved the Rh-catalyzed decarbonylative coupling of isatine with alkyne to construct 2-quinolinone derivatives.16a The ortho-directing group is essential for the C–C cleavage. Wu employed TBHP as the oxidant and elegantly synthesized the 3-carboxylate-4-quinolone skeleton via oxidative cyclization of isatins and alkynes.16b Considering the particular pharmaceutical value of 4-quinolone scaffolds and our continued interest in regioselective C–C bond cleavage,17 we report herein the palladium-catalyzed ring expansion of isatin-derived 3-hydroxyoxindoles to 4-quinolones (Scheme 1c). The reaction proceeded via a β-acyl elimination and subsequent Camps cyclization process. The core skeleton of the 4-quinolone antibiotics was synthesized using this protocol.
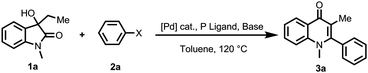
Initially, we started our investigation using 3-hydroxyoxindole (1a) and bromobenzene (2a) as the model substrates to optimize the reaction conditions (Table 1). The model reaction proceeded smoothly to give the desired product 3a in 77% yield when Pd2dba3 was employed as the catalyst in the presence of Cs2CO3 (entry 1, Table 1). The C–C bond cleavage occurred regioselectively, possibly because the coordination between the palladium catalyst and the acyl group facilitated the C(O)–C bond cleavage.4 After screening various palladium catalysts, the reaction gave 80% yield of 3a when Pd(cod)Cl2 was employed as the catalyst (entries 2–4). Next, different phosphine ligands were optimized and the PCy3 afforded 86% yield of 3a (entries 5–7). NaOH could further improve the yield to 88% (entries 8–11). Control experiments indicated that no desired product was observed in the absence of a palladium catalyst, phosphine ligand, and base under the standard conditions (entries 12–14). When PhCl and PhI were employed, the target product 3a was obtained in 40% and 17% yields (entries 15 and 16).
| Entry | [Pd] | Ligands | Bases | X | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (1.5 equiv.), [Pd]. (10 mol%), ligand (20 mol%), base (2.0 equiv.), toluene (2.0 mL), 120 °C, 12 h, N2. Yields were determined by 1H NMR of crude products using 1,3,5-trioxane as the internal standard. b Without Pd(cod)Cl2. c Without PCy3. d Without NaOH. e TFP = Tri(2-furyl)phosphine. f Isolated yield. | |||||
| 1 | Pd2dba3 | PPh3 | Cs2CO3 | Br | 77 |
| 2 | Pd(OAc)2 | PPh3 | Cs2CO3 | Br | 74 |
| 3 | Pd(cod)Cl2 | PPh3 | Cs2CO3 | Br | 80 |
| 4 | Pd(MeCN)2Cl2 | PPh3 | Cs2CO3 | Br | 67 |
| 5 | Pd(cod)Cl2 | TFPe | Cs2CO3 | Br | 16 |
| 6 | Pd(cod)Cl2 | PCy3 | Cs2CO3 | Br | 86 |
| 7 | Pd(cod)Cl2 | dppb | Cs2CO3 | Br | 10 |
| 8 | Pd(cod)Cl2 | PCy3 | Na2CO3 | Br | 0 |
| 9 | Pd(cod)Cl2 | PCy3 | MeONa | Br | 0 |
| 10 | Pd(cod)Cl2 | PCy3 | K3PO4 | Br | 21 |
| 11 | Pd(cod)Cl2 | PCy3 | NaOH | Br | 88, 86f |
| 12b | None | PCy3 | NaOH | Br | 0 |
| 13c | Pd(cod)Cl2 | None | NaOH | Br | 0 |
| 14d | Pd(cod)Cl2 | PCy3 | None | Br | 0 |
| 15 | Pd(cod)Cl2 | PCy3 | NaOH | Cl | 40 |
| 16 | Pd(cod)Cl2 | PCy3 | NaOH | I | 17 |
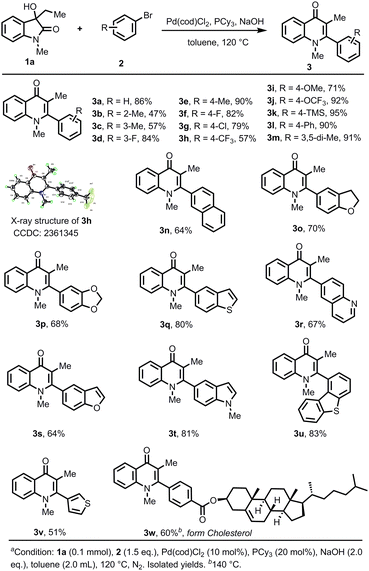
With the optimized conditions in hand, the substrate scope of the aryl bromides was investigated (Scheme 2). Various aryl bromides bearing both electron-withdrawing and electron-donating substituents at the 2, 3, and 4-positions were tolerated, giving the corresponding 4-quinolones in very good to excellent yields (3a–3m). 2-Bromotoluene gave a lower yield, possibly due to the steric effect (3b). Substrates bearing TMS could be well tolerated, and could be employed for further functionalization (3k). The structure of 4-CF3-substituted product 3h was characterized by X-ray diffraction. 2-Naphthyl bromide gave the product 3n in 64% yield. Notably, heteroaromatic substrates were suitable for the transformation (3o–3v). Substrates containing 1,3-benzodioxole (3p), quinoline (3r), benzofuran (3s), indole (3t), thiophene (3v) and benzothiophene (3q, 3u) proceeded smoothly to give the products in 51–83% yields. To demonstrate the synthetic utilities, the introduction of a cholesterol fragment into the 4-quinolone core was showcased in 60% yield (3w).
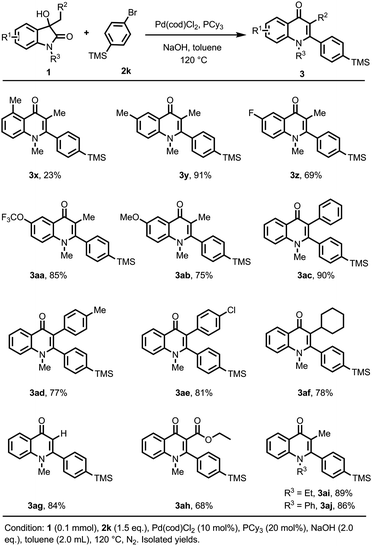
Next, the scope of 3-hydroxyoxindole was studied using aryl bromide 2k as the coupling partner (Scheme 3). It was found that the steric effect of the substituent on the aryl ring led to a sharp decrease in the yield. C4-methyl substituted 3-hydroxyoxindole gave only 23% yield of 3x. However, the substrates bearing –Me, –F, –OCF3, and –OMe at the C5 position showed high reactivities (3y–3ab). C3-aryl, cyclohexyl, and hydrogen-substituted 4-quinolone products 3ac–3ag were obtained in 77–90% yields. 4-Quinolone-3-carboxylic acid ester product 3ah, the core skeleton of the 4-quinolone antibiotics, was obtained in 68% yield. N-Ethyl and aryl-substituted substrates gave the corresponding products 3ai and 3aj in excellent yields.
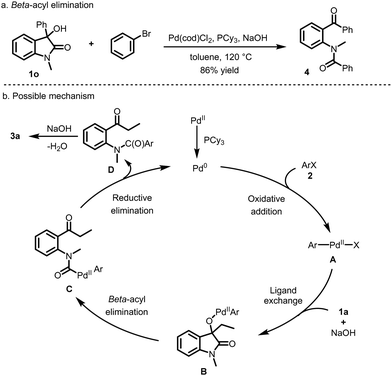
To probe the reaction mechanism, 3-aryl-3-hydroxyoxindole 1o was employed as the substrate. The ring-opening arylation product 4 was obtained in 86% yield under the standard reaction conditions, indicating that β-acyl elimination occurred. On the basis of the control experiment and previous reports,3,13 a possible reaction mechanism was proposed, as shown in Scheme 4. Oxidative addition of the aryl halide to Pd(0) afforded the aryl palladium intermediate A. Base-assisted ligand exchange of A with 3-hydroxyoxindole 1 yielded the intermediate B. Subsequently, C–C bond cleavage of intermediate Bvia β-acyl elimination generated the acyl-Pd species C, which underwent reductive elimination to release compound D with the regeneration of the Pd(0) catalyst. In the presence of base, Camps cyclocondensation reaction of D afforded the final product 3.
In summary, we have developed an efficient protocol for the synthesis of 4-quinolones via palladium-catalyzed regioselective β-acyl elimination and subsequent Camps cyclization of 3-hydroxyoxindoles. This protocol tolerates various functional groups and heterocycles. The core skeleton of the 4-quinolone antibiotics could be efficiently constructed.
We gratefully acknowledge the Shanghai Institute of Materia Medica, the Chinese Academy of Sciences, the National Natural Science Foundation of China (22171276, 21920102003), the Science and Technology Commission of Shanghai Municipality (17JC1405000, 21ZR1475400, 23ZR1474400 and 18431907100), and the Program of Shanghai Academic Research Leader (19XD1424600) for financial support.
Data availability
The data supporting this study are available in the published article and the ESI.† Crystallographic data for compound 3h (CCDC: 2361345†) can be obtained from the CCDC viahttps://www.ccdc.cam.ac.uk/structures/.Conflicts of interest
There are no conflicts to declare.Notes and references
- For reviews, see: (a) Y. F. Liang, M. Bilal, L. Y. Tang, T. Z. Wang, Y. Q. Guan, Z. R. Cheng, M. H. Zhu, J. L. Wei and N. Jiao, Chem. Rev., 2023, 123, 12313 CrossRef CAS PubMed; (b) C.-H. Jun, Chem. Soc. Rev., 2004, 33, 610 RSC; (c) M. Murakami and N. Ishida, J. Am. Chem. Soc., 2016, 138, 13759 CrossRef CAS PubMed; (d) F. Chen, T. Wang and N. Jiao, Chem. Rev., 2014, 114, 8613 CrossRef CAS PubMed; (e) D.-S. Kim, W.-J. Park and C.-H. Jun, Chem. Rev., 2017, 117, 8977 CrossRef CAS PubMed; (f) I. Marek, A. Masarwa, P.-O. Delaye and M. Leibeling, Angew. Chem., Int. Ed., 2015, 54, 414 CrossRef CAS; (g) Y. Xia and G. Dong, Nat. Rev. Chem., 2020, 4, 600 CrossRef CAS PubMed; (h) L. Deng and G. Dong, Trends Chem., 2020, 2, 183 CrossRef CAS; (i) X.-Y. Yu, J.-R. Chen and W.-J. Xiao, Chem. Rev., 2021, 121, 506 CrossRef CAS PubMed.
- (a) L. Souillart and N. Cramer, Chem. Rev., 2015, 115, 9410 CrossRef CAS PubMed; (b) B. Rybtchinski and D. Milstein, Angew. Chem., Int. Ed., 1999, 38, 870 CrossRef.
- (a) M. E. O’Reilly, S. Dutta and A. S. Veige, Chem. Rev., 2016, 116, 8105 CrossRef; (b) N. Cramer and T. Seiser, Synlett, 2011, 449 CrossRef CAS; (c) M. Murakami, M. Makino, S. Ashida and T. Matsuda, Bull. Chem. Soc. Jpn., 2006, 79, 1315 CrossRef CAS.
- J. S. Ham, B. Park, M. Son, J. B. Roque, J. Jurczyk, C. S. Yeung, M. H. Baik and R. Sarpong, J. Am. Chem. Soc., 2020, 142, 13041 CrossRef CAS PubMed.
- J. B. Roque, Y. Kuroda, J. Jurczyk, L.-P. Xu, J. S. Ham, L. T. Göttemann, C. A. Roberts, D. Adpressa, J. Saurí, L. A. Joyce, D. G. Musaev, C. S. Yeung and R. Sarpong, ACS Catal., 2020, 10, 2929 CrossRef CAS PubMed.
- K. Jia, Y. Pan and Y. Chen, Angew. Chem., Int. Ed., 2017, 56, 2478 CrossRef CAS.
- (a) G. Y. Lesher, E. J. Froelich, M. D. Gruett, J. H. Bailey and R. P. Brundage, J. Med. Chem., 1962, 5, 1063 CrossRef CAS PubMed; (b) H. Husen and M. Whiteley, Chem. Rev., 2011, 111, 152 CrossRef PubMed; (c) D. Fort, J. Litvak, J. Chen and Q. Lu, J. Nat. Prod., 1998, 61, 1528 CrossRef CAS; (d) J. A. Wiles, B. J. Bradbury and M. J. Pucci, Expert Opin. Ther. Pat., 2010, 20, 1295 CrossRef CAS PubMed.
- Y. L. Chen, K. C. Fang, J. Y. Sheu, S. L. Hsu and C. C. Tzeng, J. Med. Chem., 2001, 44, 2374 CrossRef CAS PubMed.
- (a) R. M. Cross, A. Monastyrskyi, T. S. Mutka, J. N. Burrows, D. E. Kyle and R. Manetsch, J. Med. Chem., 2010, 53, 7076 CrossRef CAS PubMed; (b) A. Nilsen, G. P. Miley, I. P. Forquer, M. W. Mather, K. Katneni, Y. Li, S. Pou, A. M. Pershing, A. M. Stickles and E. Ryan, J. Med. Chem., 2014, 57, 3818 CrossRef CAS.
- (a) M. Sato, T. Motomura, H. Aramaki, T. Matsuda, M. Yamashita, Y. Ito, H. Kawakami, Y. Matsuzaki, W. Watanabe and K. Yamataka, J. Med. Chem., 2006, 49, 1506 CrossRef CAS PubMed; (b) V. Cecchetti, C. Parolin, S. Moro, T. Pecere, E. Filipponi, A. Calistri, O. Tabarrini, B. Gatto, M. Palumbo and A. Fravolini, J. Med. Chem., 2000, 43, 3799 CrossRef CAS.
- (a) B. D. A. Lucero, C. R. B. Gomes, I. C. P. P. Frugulhetti, L. V. Faro, L. Alvarenga, M. C. B. De Souza, T. M. De Souza and V. F. Ferreira, Bioorg. Med. Chem. Lett., 2006, 16, 1010 CrossRef CAS; (b) A. Baxter, M. Chambers, F. Edfeldt, K. Edman, A. Freeman, C. Johansson, S. King, A. Morley, J. Petersen and P. Rawlins, Bioorg. Med. Chem. Lett., 2011, 21, 777 CrossRef CAS.
- E. F. Marques, M. A. Bueno, P. D. Duarte, L. R. Silva, A. M. Martinelli, C. Y. dos Santos, R. P. Severino, D. Brömme, P. C. Vieira and A. G. Corrêa, Eur. J. Med. Chem., 2012, 54, 10 CrossRef CAS PubMed.
- (a) C. Shen, A. Wang, J. Xu, Z. An, K. Y. Loh, P. Zhang and X. Liu, Chem., 2019, 5, 1059 CrossRef CAS; (b) V. V. Kouznetsov, L. Y. V. Méndez and C. M. M. Gómez, Curr. Org. Chem., 2005, 9, 141 CrossRef CAS; (c) A. Weyesa and E. Mulugeta, RSC Adv., 2020, 10, 20784 RSC; (d) R. H. Reitsema, Chem. Rev., 1948, 43, 43 CrossRef CAS PubMed; (e) G. A. Ramann and B. J. Cowen, Tetrahedron Lett., 2015, 56, 6436 CrossRef CAS; (f) S. Niementowski, Ber. Dtsch. Chem. Ges., 1894, 27, 1394 CrossRef; (g) J. F. Meyer and E. C. Wagner, J. Org. Chem., 1943, 08, 239 CrossRef CAS; (h) R. Camps, Chem. Ber., 1899, 32, 3228 CrossRef CAS.
- (a) C. P. Jones, K. W. Anderson and S. L. Buchwald, J. Org. Chem., 2007, 72, 7968 CrossRef CAS PubMed; (b) F. Wang, L. Jin, L. Kong and X. Li, Org. Lett., 2017, 19, 1812 CrossRef CAS PubMed; (c) J. Wu, Y. Zhou, T. Wu, Y. Zhou, C. Y. Chiang and A. Lei, Org. Lett., 2017, 19, 6432 CrossRef CAS; (d) S. Torii, H. Okumoto and L. H. Xu, Tetrahedron Lett., 1991, 32, 237 CrossRef CAS; (e) Y. Yoshino, T. Kurahashi and S. Matsubara, J. Am. Chem. Soc., 2009, 131, 7494 CrossRef CAS PubMed.
- V. Sonam and R. Kakkar, Med. Chem. Commun., 2019, 10, 351 RSC.
- (a) R. Zeng and G. Dong, J. Am. Chem. Soc., 2015, 137, 1408 CrossRef CAS PubMed; (b) S.-F. Jiang, C. Xu, Z.-W. Zhou, Q. Zhang, X.-H. Wen, F.-C. Jia and A.-X. Wu, Org. Lett., 2018, 20, 4231 CrossRef CAS PubMed.
- (a) H.-Y. Li, B. Ma, Q.-S. Liu, M.-L. Wang, Z.-Y. Wang, H. Xu, L.-J. Li, X. Wang and H.-X. Dai, Angew. Chem., Int. Ed., 2020, 59, 14388 CrossRef CAS; (b) H. Xu, B. Ma, Z.-Y. Fu, H.-Y. Li, X. Wang, Z.-Y. Wang, L.-J. Li, T.-J. Cheng, M.-Y. Zheng and H.-X. Dai, ACS Catal., 1758, 2021, 11 Search PubMed; (c) M.-L. Wang, H. Xu, H.-Y. Li, B. Ma, Z.-Y. Wang, X. Wang and H.-X. Dai, Org. Lett., 2021, 23, 2147 CrossRef CAS; (d) Z.-Y. Wang, B. Ma, H. Xu, X. Wang, X. Zhang and H.-X. Dai, Org. Lett., 2021, 23, 8291 CrossRef CAS; (e) X. Wang, L.-J. Li, Z.-Y. Wang, H. Xu and H.-X. Dai, iScience, 2022, 25, 104505 CrossRef CAS PubMed; (f) L. J. Li, X. Wang, H. Xu and H. X. Dai, Chem. Commun., 2023, 59, 3269 RSC; (g) Z. Y. Wang, X. Zhang, W. Q. Chen, G. D. Sun, X. Wang, L. Tan, H. Xu and H. X. Dai, Angew. Chem., Int. Ed., 2024, 63, e202319773 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2361345. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc05369a |
| This journal is © The Royal Society of Chemistry 2025 |