A new microporous organic–inorganic hybrid titanium phosphate for selective acetalization of glycerol†
Bhabani
Malakar
a,
Sudip
Bhattacharjee
 a,
Nhat Quang Minh
Tran
a,
Nhat Quang Minh
Tran
 bc,
Tan
Le Hoang Doan
bc,
Tan
Le Hoang Doan
 bc,
Thang Bach
Phan
bc,
Thang Bach
Phan
 bc,
Sayantan
Chongdar
bc,
Sayantan
Chongdar
 a and
Asim
Bhaumik
a and
Asim
Bhaumik
 *a
*a
aSchool of Materials Sciences, Indian Association for the Cultivation of Science, Jadavpur, Kolkata–700032, India. E-mail: msab@iacs.res.in
bCenter for Innovative Materials and Architectures (INOMAR), Ho Chi Minh City, Vietnam
cVietnam National University-Ho Chi Minh City, Ho Chi Minh City, Vietnam
First published on 4th November 2024
Abstract
We developed a novel strategy for synthesizing a highly acidic microporous hybrid titanium phosphate material (H-TiPOx) by incorporating 5-aminosalicylic acid (5-ASA) into the titanium phosphate framework. This new H-TiPOx serves as a Brønsted acid catalyst, exhibiting remarkable total surface acidity of 5.9 mmol g−1 and it efficiently catalyzes the acetalization of abundant biomass derived glycerol to solketal with over 99% selectivity.
Rapid modernization and industrialization of our society over the years have resulted in excessive burning of fossil fuels, imparting a detrimental effect on our environment, particularly global warming.1 Therefore, the reduction of fossil carbon consumption and CO2 emission has been a major global concern.2 Biodiesel derived from biomass resources, being eco-friendly, nontoxic, and biodegradable in nature, can be an ideal alternative to fossil fuels.3 However, huge production of crude glycerol as a byproduct set forth high purification costs, limiting the industrial biodiesel production.4 Therefore, in light of economics, searching for new feasible processes to convert glycerol into platform chemicals5 has been a key challenge for researchers. A novel route to exploit the glycerol byproduct is via acetalization with acetone under acidic conditions to produce 2,2-dimethyl-1,3-dioxolane-4-methanol, commonly known as solketal.6 Solketal, a fuel additive, has found versatile application in fields like pharmaceuticals, cosmetics, perfumes, polymers, and food industries.7
Traditionally, the acetalization reaction between glycerol and acetone is conducted in the presence of homogeneous based strong acid catalysts like HCl, H2SO4, p-toluenesulfonic acid, etc. On the contrary, heterogeneous solid acid catalysts are often employed for this reaction as they offer many advantages over homogenous counterparts, which include high recyclability, good thermal stability, high product selectivity, and ease of product separation from the reaction mixture.8 Titanium phosphate materials, owning to their Brønsted and Lewis acid sites, serve as a promising heterogeneous acid catalyst.9 However, the sole acidity of the titanium phosphate framework is not enough to achieve high catalytic efficiency and selectivity. This has motivated us to fabricate a new organic–inorganic hybrid titanium phosphate (H-TiPOx), containing 5-aminosalicylic acid (5-ASA, an anti-inflammatory drug) as a ligand, where chelating ortho –CO2H and –OH groups can coordinate with Ti(IV) and protonated amine moieties of 5-ASA serve as Brønsted acid sites to form a novel microporous framework (Scheme 1). H-TiPOx showed excellent catalytic activity for selective and green synthesis of fuel additive solketal via acetylation of glycerol. Furthermore, in controlled synthesis with salicylate as an additive and in the absence of any organic additive resulted in the same titanium phosphate phase TiPOx, and they showed much lower catalytic activity in this glycerol acetylation reaction, suggesting an unprecedented role of 5-ASA in the formation of a microporous H-TiPOx structure and its acid catalytic property.
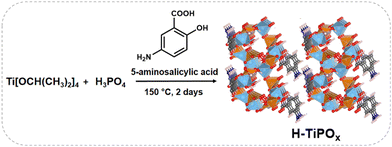 | ||
| Scheme 1 Proposed model structure of H-TiPOx, Ti – light blue, P – orange, O – red, C – grey, N – blue, H – white. | ||
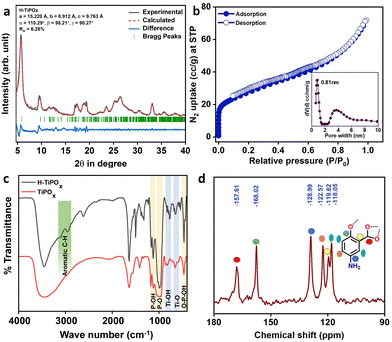
The crystallinity and the unit cell parameters of H-TiPOx were analyzed by using powder XRD patterns in the 2θ range of 2–40 degrees of 2θ and the respective patterns are shown in Fig. 1a. The first peak for this H-TiPOx material appears at 2θ of 5.78 degrees having a d-spacing of 1.506 nm. Other sharp crystalline peaks are detected at the 2θ values of 9.62, 11.56, 12.48, 17.08, 17.42, 18.72, 18.98, 19.44, 22.20, 23.58, 25.32, 26.46, 28.52, 30.44, 33.12, and 34.54 degree, respectively, suggesting significantly high crystalline phase. These above-mentioned peaks were indexed with the help of Expo 2014 software.10 The corresponding unit cell parameters of this titanium phosphate material are as follows, a = 15.220 Å, b = 8.912 Å, c = 9.763 Å, α = 110.29°, β = 98.21°, γ = 90.27°. The unit cell has a triclinic setting with space group Hall: P1; space group Hermann–Mauguin (P1). Rietveld refinements were conducted using the MAUD11 software, adhering to the criteria outlined by Lutterotti,12 with an acceptable Rw value of less than 15%. Our refinement achieved a Rw value of 6.28%, meeting the required criteria and demonstrating a high level of accuracy. Rietveld refinement output plots using MAUD are shown in Fig. 1a. A perspective model of the structure of H-TiPOx (Ti6P7O37C14N2H15) is shown in Scheme 1. Moreover, the first intense peak at around 5.78° of 2θ suggests the existence of micropores within the framework. On the other hand, PXRD patterns of the materials synthesized with salicylate as an additive and in the absence of any organics resulted in the same TiPOx phase (Fig. S1, ESI†). Comparative FTIR data of these three materials (Fig. S2, ESI†) suggested the incorporation of organics (C–H bond) in H-TiPOx, whereas the other two materials are organic-free.
In Fig. 1b the N2 sorption isotherm of H-TiPOx at 77 K is shown, which exhibited a type I BET isotherm at a low P/P0 of N2,13 followed by a steady rise in N2 uptake at higher P/P0. The BET surface area of H-TiPOx was found to be 97 m2 g−1, with a peak pore diameter and pore volume of 0.81 nm and 0.10 cm3 g−1, respectively. The TGA analysis of H-TiPOx suggested a weight loss of about 5% until 128 °C due to surface adsorbed water molecules. Compared to TiPOx, which showed the first weight loss at around 255 °C, H-TiPOx is stable up to 300 °C (Fig. S3, ESI†). The weight loss beyond 350 °C for H-TiPOx, could be due to decomposition of the 5-ASA. The Fourier transform infrared (FTIR) spectroscopic study revealed bands at around 3100–2900 cm−1, suggesting incorporation of the organic moiety within the titanium phosphate framework (Fig. 1c). Other characteristic peaks at 987 cm−1 and 1200 cm−1 were recognized as the symmetric stretching vibration of the P–O and P–OH bonds, respectively.14 Peaks corresponding to 763, 635 and 465 cm−1 could be assigned to non-bonding Ti–OH, Ti–O stretching and O–P–OH bonds, respectively. The 31P MAS NMR spectrum (Fig. S4, ESI†) of H-TiPOx showed four distinct peaks along with a few spinning side bands in the range between −2 to −19 ppm.15 A peak was observed at −18.5 ppm for the (HPO4) species. Three other distinct peaks observed at −2.87, −6.81 and −9.87 ppm, could be assigned to the defect [P(OTi)3OH], [P(OTi)2(OH)2] and P(OTi)(OH)3 species, respectively, present in the material. Sharp peaks at 157.61, 128.99, 122.57, 119.62, and 118.05 ppm were observed in the 13C MAS NMR spectrum (Fig. 1d) of the H-TiPOx material, corresponding to aromatic carbons of the 5-aminosalicylic acid molecule, whereas the peak at 168.02 ppm corresponded to the carboxylic acid group.16 This data clearly suggested the presence of 5-ASA inside the TiPOx framework.
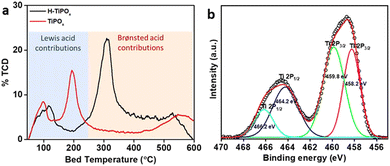
The surface acidity of both H-TiPOx and TiPOx materials was determined from the NH3-TPD (NH3-temperature-programmed desorption) analysis (Fig. 2a). Compared to TiPOx, the H-TiPOx exhibited very intense peaks above 300 °C, confirming the presence of Brønsted acid (strong acid) sites upon incorporation of the 5-ASA molecules into the titanium phosphate framework. The total surface acidities of H-TiPOx and TiPOx were found to be 5.9 and 2.4 mmol g−1, respectively. The oxidation state and chemical environment of the elements in H-TiPOx were determined through X-ray photoelectron spectroscopy (XPS) (Fig. 2b and Fig. S5, ESI†). As seen in Fig. 2b, Ti(IV) exhibited two coordination environments, 458.2 and 464.2 eV peaks for octahedrally coordinated Ti 2p3/2 and 2p1/2, respectively, whereas 459.8 and 466.2 eV peaks corresponded to tetrahedrally coordinated Ti 2p3/2 and 2p1/2 respectively,17 which also supported our proposed model structure (Scheme 1 and cif). The incorporation of the organic moiety was further supported by the presence of the N 1s XPS spectrum (Fig. S6, ESI†). Moreover, it was observed that a greater proportion of the amine moieties were present in protonated form, which contributed significantly to the surface acidity. The P 2p, O 1s and C 1s XPS spectra of H-TiPOx are shown in the ESI,† Fig. S7–S9.
The high-resolution transmission electron microscopic (TEM) images (Fig. S10, ESI†) of H-TiPOx showed crystalline lattice fringes with a d-spacing of 0.47 nm, corresponding to the (−1−12) crystal plane (Fig. S9c, ESI†). The field emission scanning electron microscopic (FE-SEM) images of H-TiPOx revealed a small sheet-like morphology, which agglomerated to form solid spheres (Fig. S11, ESI†). Additionally, the homogeneous distribution of the elements throughout the H-TiPOx material was confirmed from elemental mapping. Also, through CHN analysis, the incorporation of an organic moiety has been confirmed in H-TiPOx (Table S1, ESI†).
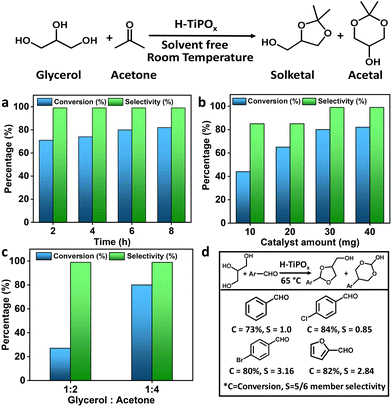
To understand the catalytic activity of the material, initially we took 2 wt% (20 mg) of H-TiPOx and performed the glycerol-acetalization reaction with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of glycerol and acetone at 25 °C. The progress of the reaction was monitored by collecting reaction mixtures at different time intervals and the corresponding products were analyzed through 1H NMR. With increase in the reaction time from 2 to 6 h, the glycerol conversion increased from 17% to 55%. A maximum of 70% conversion with 85% selectivity of a five-membered ring was observed after 8 h of reaction. As we increased the catalyst amount both solketal yield and its selectivity enhanced considerably (Fig. 3a). With 40 mg catalyst, after 8 h we achieved 84% conversion with 99% selectivity of the five-membered solketal ring. Furthermore, upon decreasing the amount of catalyst to 10 mg (1 wt%), the conversion reduced to 43%, with 85% selectivity for the five-membered solketal, after 8 h of reaction (Fig. 3b). This increment in the catalytic activity with increase in catalyst amount is due to the presence of a higher number of Brønsted acid sites, which ultimately increased the glycerol conversion. The high Brønsted acidity originated from the free P–OH and Ar–NH3+ groups, whereas the Ti sites accounted for the Lewis acidity in the H-TiPOx. To elucidate our hypothesis, we performed glycerol-acetalization with TiPOx, which resulted in 67% conversion. Furthermore, with titanium isopropoxide that possesses only Lewis acid sites, the conversion was only ca. 6%, thereby corroborating the role of Brønsted acid sites and porous nanostructure in the catalysis.
4 ratio of glycerol and acetone at 25 °C. The progress of the reaction was monitored by collecting reaction mixtures at different time intervals and the corresponding products were analyzed through 1H NMR. With increase in the reaction time from 2 to 6 h, the glycerol conversion increased from 17% to 55%. A maximum of 70% conversion with 85% selectivity of a five-membered ring was observed after 8 h of reaction. As we increased the catalyst amount both solketal yield and its selectivity enhanced considerably (Fig. 3a). With 40 mg catalyst, after 8 h we achieved 84% conversion with 99% selectivity of the five-membered solketal ring. Furthermore, upon decreasing the amount of catalyst to 10 mg (1 wt%), the conversion reduced to 43%, with 85% selectivity for the five-membered solketal, after 8 h of reaction (Fig. 3b). This increment in the catalytic activity with increase in catalyst amount is due to the presence of a higher number of Brønsted acid sites, which ultimately increased the glycerol conversion. The high Brønsted acidity originated from the free P–OH and Ar–NH3+ groups, whereas the Ti sites accounted for the Lewis acidity in the H-TiPOx. To elucidate our hypothesis, we performed glycerol-acetalization with TiPOx, which resulted in 67% conversion. Furthermore, with titanium isopropoxide that possesses only Lewis acid sites, the conversion was only ca. 6%, thereby corroborating the role of Brønsted acid sites and porous nanostructure in the catalysis.
The progress of the reaction was studied by varying the reaction time from 2 to 8 h, while keeping all other parameters constant. Under the optimum reaction conditions, the yield gradually increased with time and a maximum conversion of 84% was observed at 8 h. However, with further increasing the time from 8 to 10 h, no enhancement in the conversion level was observed, suggesting the saturation point over this catalyst. It is noteworthy that as we continued the reaction for 24 h, only 1% increase in the solketal yield (85%) was observed, indicating 8 h as the optimum reaction time.18 Time dependent 1H NMR analysis for glycerol conversion over the H-TiPOx catalyst has been shown in Fig. S12 (ESI†). The reaction profiles at different reaction times are in good agreement with the previous reports.19,20 For the catalyst amount to 3 wt% and reaction time to 6 h, by varying the molar ratio of acetone to glycerol from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (data plotted in Fig. 3c) increased solketal yield was observed. The result could be attributed to the higher polarity of the reaction mixture; the extra acetone used in this reaction increases the accessibility of glycerol to acetone, which shifts the equilibrium towards the forward direction, improving the conversion of glycerol.
1 (data plotted in Fig. 3c) increased solketal yield was observed. The result could be attributed to the higher polarity of the reaction mixture; the extra acetone used in this reaction increases the accessibility of glycerol to acetone, which shifts the equilibrium towards the forward direction, improving the conversion of glycerol.
In the absence of catalyst, under the optimum reaction conditions, very low glycerol conversion took place, suggesting the crucial role of H-TiPOx towards this acetalization reaction. Primarily the abundance of acidic sites over this microporous hybrid titanium phosphate can be considered responsible for its excellent catalytic performance. Apart from the crystallinity, porosity and textural properties also played a key role in the catalytic efficiency. Additionally, organic free pure titanium phosphate TiPOx, showed only 67% glycerol conversion. High catalytic activity of H-TiPOx over TiPOx could be attributed to the well defined microporous structure and high surface acidity (5.9 mmol g−1) of the former. After successful optimization of the reaction conditions, several substrates were also employed to check the broader scope of the catalyst H-TiPOx. Apart from acetone, other aldehydes such as benzaldehyde and their derivatives were also studied (Table S2, ESI†). For aldehydes, the conversions were quite low at 25 °C, due to less reactivity of the substrates. However, under elevated reaction temperatures for different substrates, we could reach their corresponding maximum conversions (Fig. 3d). Furthermore, the catalytic performance of H-TiPOx has been compared to previously reported state-of-the-art catalysts for acetalization (Table S3, ESI†). Considerably good TON/TOF of H-TiPOx suggested its good future as a heterogeneous acid catalyst.
Recyclability and stability of the heterogeneous catalysts are very demanding for their sustainable operation. The conversion of glycerol decreased little after four consecutive reaction cycles, suggesting good recyclability of H-TiPOx (Fig. S13, ESI†). The leaching test was carried out via a hot filtration method (Fig. S14, ESI†), suggesting almost no leaching of acid sites. Little decrease in the catalytic activity of H-TiPOx after the 4th reaction cycle could be attributed to the decrease in total acidity (5.9 to 5.3 mmol g−1, TPD-NH3 data: Fig. S15, ESI†). Furthermore, the PXRD and FTIR data of the reused catalyst showed no significant change in the framework structure compared to the fresh catalyst (Fig. S16 and S17, ESI†), suggesting high stability of the hybrid framework in H-TiPOx. TGA data of the H-TiPOx catalysts before and after the 4th cycle (Fig. S18, ESI†) also remained the same. Moreover, the selectivity of solketal remained almost unchanged after four reaction cycles. Most polyalcohol-related acetalization reactions generally follow two reversible stages, as shown in Fig. 4.21 Firstly, the lone pair of oxygen atoms of glycerol attacks the positively charged carbonyl carbon to form the tertiary alcohol intermediate, also known as hemiketal. This intermediate interacts with the catalyst's acidic sites through the tertiary alcohol group. In the next step, solketal is formed as a major product by the nucleophilic attack of the glycerol's secondary hydroxyl group on the tertiary carbon of the hemiketal intermediate (Solketal pathway, Fig. 4a). On the other hand, the six-membered compound is produced as a minor product by the nucleophilic attack of the glycerol's primary hydroxyl group (Acetal pathway, Fig. 4b). The selectivity of the five-membered and six-membered ring ketals depends on the acetalization position within the glycerol molecule. Generally the glycerol acetalization with acetone favors the formation of the five-membered ring transition state, leading to the production of solketal. In terms of thermodynamics, the five-membered ring solketal is more stable than the six-membered ring one, as the latter suffers from steric repulsion due to the presence of a methyl group at the axial position.22
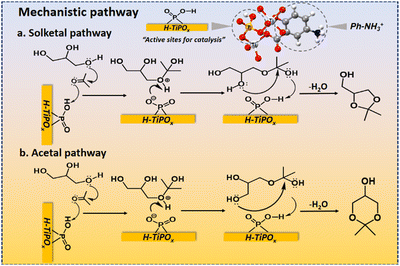 | ||
| Fig. 4 Plausible mechanistic pathways (a and b) for acetalization of glycerol over the H-TiPOx catalyst. Catalytic sites associated with P–OH are shown for clarity. | ||
Our experimental results suggested that a new microporous hybrid titanium phosphate material H-TiPOx can be synthesized by using 5-aminosalicylic acid as an organic ligand. H-TiPOx exhibits good porosity, high surface acidity of 5.9 mmol g−1 and excellent catalytic activity in the acetalization of glycerol into solketal (84% glycerol conversion and 99% solketal selectivity). Synthesis of the new microporous hybrid titanium phosphate framework and its excellent catalytic activity reported herein may contribute significantly in the biodiesel business in future.
B. M. wants to thank UGC, New Delhi for a Junior Research Fellowship. The authors from INOMAR Center (NQMN, TLHD, and TBP) would like to thank Vietnam National University, Ho Chi Minh City (grant no. TX2025-50-01). A. B. would like to thank DST-SERB for a Core Research Grant (project no. CRG/2022/002812).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- D. Tong, Q. Zhang, Y. X. Zheng, K. Caldeira, C. Shearer, C. P. Hong, Y. Qin and S. J. Davis, Nature, 2019, 572, 373–377 CrossRef CAS PubMed.
- M. He, Y. Sun and B. Han, Angew. Chem., Int. Ed., 2022, 61, e202112835 CrossRef CAS PubMed.
- (a) S. K. Das, M. K. Bhunia, A. K. Sinha and A. Bhaumik, ACS Catal., 2011, 1, 493–501 CrossRef CAS; (b) K. Lee, Y. X. Jing, Y. Q. Wang and N. Yan, Nat. Rev. Chem., 2022, 6, 635–652 CrossRef PubMed.
- V. Singh, S. Arumugam, A. P. Tathod and V. Nagabhatla, Chem. Commun., 2022, 58, 4873–4876 RSC.
- (a) Y. Leng, J. Zhao, P. Jianga and D. Lua, Catal. Sci. Technol., 2016, 6, 875–881 RSC; (b) Y. Xu, Q. S. Zhou, T. T. Liu, T. L. Ren, H. J. Yu, K. Deng, Z. Q. Wang, L. Wang and H. J. Wang, Chem. Commun., 2023, 59, 7623–7626 RSC.
- (a) B. Mallesham, P. Sudarsanam and B. M. Reddy, Ind. Eng. Chem. Res., 2014, 53, 18775–18785 CrossRef CAS; (b) B. Saini, A. P. Tathod, S. K. Saxena, S. Arumugam and N. Viswanadham, ACS Sustainable Chem. Eng., 2021, 10, 1172–1181 CrossRef.
- A. L. Olson, M. Tuner and S. Verhelst, Heliyon, 2023, 9, e13041 CrossRef CAS PubMed.
- (a) S. B. Umbarkar, T. V. Kotbagi, A. V. Biradar, R. Pasricha, J. Chanale, M. K. Dongare, A. S. Mamede, C. Lancelot and E. Payen, J. Mol. Catal. A: Chem., 2009, 310, 150–158 CrossRef CAS; (b) S. Ao, L. A. Alghamdi, T. Kress, M. Selvaraj, G. Halder, A. E. H. Wheatley and S. L. Rokhum, Fuel, 2023, 345, 128190 CrossRef CAS.
- (a) A. Dutta, A. K. Patra, S. Dutta, B. Saha and A. Bhaumik, J. Mater. Chem., 2012, 22, 14094–14100 RSC; (b) C. Imparato, E. Finocchio, S. Campisi, M. Bigica, A. Gervasini, A. Bifulco, R. Avolio, N. J. Clayden, M. E. Errico and A. Aronne, Mater. Today Chem., 2024, 38, 102126 CrossRef CAS.
- A. Altomare, C. Cuocci, C. Giacovazzo, A. Moliterni, R. Rizzi, N. Corriero and A. Falcicchio, J. Appl. Cryst., 2013, 46, 1231–1235 CrossRef CAS.
- (a) A. I. Saville, et al. , Integrating Mater. Manuf. Innov., 2021, 10, 461–487 CrossRef PubMed; (b) L. Adhani, A. Fauzi, D. Navanti and T. S. Lestari, Int. J. Adv. Sci. Eng. Inf. Technol., 2023, 13, 141–148 CrossRef.
- L. Luca, MAUD - Materials Analysis Using Diffraction. Department of Industrial Engineering.
- (a) Y. Wan and D. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef CAS PubMed; (b) S. Bhattacharjee, S. Chongdar, A. Modak, P. Bhanja, B. K. Jena and A. Bhaumik, Green Chem., 2022, 24, 8853–8862 RSC.
- (a) M. Kapnisti, A. G. Hatzidimitriou, F. Noli and E. Pavlidou, J. Radioanal. Nucl. Chem., 2014, 302, 679–688 CrossRef CAS; (b) H. Takahashi, T. Oi and M. Hosoe, J. Mater. Chem., 2002, 12, 2513–2518 RSC.
- A. Bhaumik and S. Inagaki, J. Am. Chem. Soc., 2001, 123, 691 CrossRef CAS PubMed.
- C. Volkringer, T. Loiseau, N. Guillou, G. Ferey, M. Haouas, F. Taulelle, E. Elkaim and N. Stock, Inorg. Chem., 2010, 49, 9852–9862 CrossRef CAS PubMed.
- (a) D. Chandra, N. K. Mal, M. Mukherjee and A. Bhaumik, J. Solid State Chem., 2006, 179, 1802–1807 CrossRef CAS; (b) L. Alrais, W. Al Maksoud, B. Werghi, A. Bendjeriou-Sedjerari, E. Abou-Hamad, M. N. Hedhili and J. M. Basset, Chem. Commun., 2023, 59, 12503–12506 RSC.
- M. S. Khayoon and B. H. Hameed, Appl. Catal., A, 2013, 464, 191–199 CrossRef.
- P. S. Reddy, P. Sudarsanam, B. Mallesham, G. Raju and B. M. Reddy, J. Ind. Eng. Chem., 2011, 17, 377–381 CrossRef CAS.
- G. S. Nair, E. Adrijanto, A. Alsalme, I. V. Kozhevnikov, D. J. Cooke, D. R. Brown and N. R. Shiju, Catal. Sci. Technol., 2012, 2, 1173–1179 RSC.
- G. Vicente, J. A. Melero, G. Morales, M. Paniagua and E. Martin, Green Chem., 2010, 12, 899–907 RSC.
- L. P. Ozorio, R. Pianzolli, M. B. S. Mota and C. J. A. Mota, J. Braz. Chem. Soc., 2012, 23, 931–937 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc04799k |
| This journal is © The Royal Society of Chemistry 2025 |