Inversion of circularly polarized luminescence by electric current flow during transition†
Ayumi
Imayoshi
 *a,
Shinya
Fujio
a,
Yuuki
Nagaya
a,
Misato
Sakai
a,
Atsushi
Terazawa
a,
Misa
Sakura
a,
Keita
Okada
b,
Takahiro
Kimoto
b,
Tadashi
Mori
*a,
Shinya
Fujio
a,
Yuuki
Nagaya
a,
Misato
Sakai
a,
Atsushi
Terazawa
a,
Misa
Sakura
a,
Keita
Okada
b,
Takahiro
Kimoto
b,
Tadashi
Mori
 c,
Yoshitane
Imai
c,
Yoshitane
Imai
 b,
Masahiko
Hada
b,
Masahiko
Hada
 a and
Kazunori
Tsubaki
a and
Kazunori
Tsubaki
 *a
*a
aGraduate School of Life and Environmental Sciences, Kyoto Prefectural University, 1-5 Hangi-cho, Shimogamo, Sakyo-ku, Kyoto 606-8522, Japan. E-mail: imayoshi@kpu.ac.jp; tsubaki@kpu.ac.jp
bDepartment of Applied Chemistry, Faculty of Science and Engineering, Kindai University, 3-4-1 Kowakae, Higashi-Osaka, Osaka 577-8502, Japan
cDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita, Osaka 565-0871, Japan
First published on 21st November 2024
Abstract
The development of chiral compounds exhibiting circularly polarized luminescence (CPL) has advanced remarkably in recent years. Designing CPL-active compounds requires an understanding of the electric transition dipole moment (μ) and the magnetic transition dipole moment (m) in the excited state. However, while the direction and magnitude of μ can, to some extent, be visually inferred from chemical structures, m remains elusive, posing challenges for direct predictions based on structural information. This study utilized binaphthol, a prominent chiral scaffold, and achieved CPL-sign inversion by strategically varying the substitution positions of phenylethynyl (PE) groups on the binaphthyl backbone, while maintaining consistent axial chirality. Theoretical investigation revealed that the substitution position of PE groups significantly affects the orientation of m in the excited state, leading to CPL-sign inversion. Furthermore, we propose that this CPL-sign inversion results from a reversal in the rotation of instantaneous current flow during the S1 → S0 transition, which in turn alters the orientation of m. The current flow can be predicted from the chemical structure, allowing anticipation of the properties of m and, consequently, the characteristics of CPL. This insight provides a new perspective in designing CPL-active compounds, particularly for C2-symmetric molecules where the S1 → S0 transition predominantly involves LUMO → HOMO transitions. If μ represents the directionality of electron movement during transitions, i.e., the “difference” in electron locations before and after transitions, then m could be represented as the “path” of electron movement based on the current flow during the transition.
Introduction
Circularly polarized luminescence (CPL) has attracted significant interest in recent years owing to, alongside its potential applications,1 its ability to provide insights into the structure–property relationship of molecules in their excited states. The binaphthyl motif has emerged as a prominent scaffold for integrating chiral elements, and numerous chiral binaphthyl derivatives exhibiting robust CPL have been documented,2–6 including its uses as additives,7,8 ligands9 and polymers.10Theoretically, the sign of CPL is expected to reverse upon the introduction of a chiral element with an opposite configuration. However, binaphthyls with identical axial handedness can also invert their chiroptical properties, depending on factors such as the dihedral angle (ϕ) between the binaphthyl units or the structure of the linker in the binaphthol's hydroxy groups.11 Takaishi and Ema et al. demonstrated through computational investigations that the CPL sign of (S)-1,1′-binaphthyl reverses at a dihedral angle of around 90°.12
The sign of CPL can be inverted not only through structural modifications5,6,13 but also by varying the environmental conditions of the molecule,14 such as solvent3,15 and temperature.4,8 This inversion occurs while maintaining the same handedness in the binaphthyl core. However, a deeper understanding of the relationship between these chemical structures and the CPL sign remains elusive, particularly regarding the electronic (μ) and magnetic (m) transition dipole moments crucial for enhancing the dissymmetry (glum) value. This value is defined as 2 (IL – IR)/(IL + IR), where IL and IR represent the intensity of left and right-handed CPL, respectively. Especially challenging is the prediction of the properties of the magnetic (m) moment from chemical structures, a methodology that is critically needed.16
Recently, we reported a complete series of binaphthyl derivatives with a methylene tether, incorporating phenylethynyl (PE) groups at the 3,3′- to 8,8′-positions of a 1,1′-bi-2-naphthol backbone (3-PE1 to 8-PE1). Among these, only 7-PE1 exhibited a reversal in the CPL sign (Fig. 1).17 In this study, we specifically focused on 7-PE1 and 6-PE1, which exhibit positive and negative CPL, respectively, to elucidate the details behind these observations.
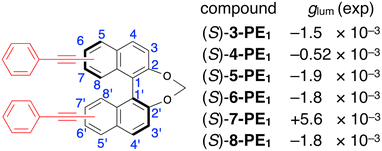 | ||
| Fig. 1 Summary of our recent study17 on methylene-linked binaphthol derivatives (S)-3-PE1 to (S)-8-PE1 with phenylethynyl (PE) groups at from 3,3′ to 8,8′ positions on the binaphthyl backbone and their dissymmetry (glum) values for CPL. | ||
Results and discussion
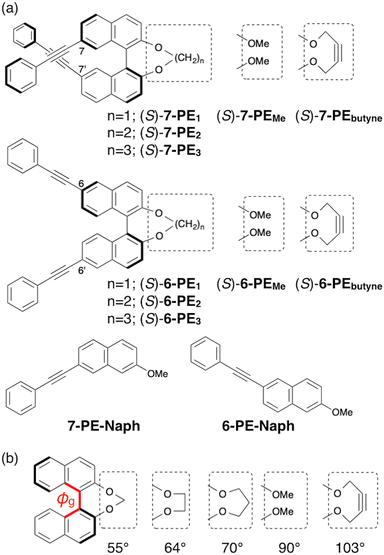
We performed further CPL studies on binaphthyl derivatives with varied tether groups and PE-substitution locations. The binaphthol derivatives 7-PEn and 6-PEn feature free methoxy groups (n = Me) or are connected by methylene, ethylene, and propylene chains (n = 1, 2, or 3, represented as –(CH2)n–) along with a –CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2– linker (n = butyne) (Fig. 2a). This systematic alteration affects the dihedral angle between the naphthalenes (7-PE-Naph and 6-PE-Naph) in their ground (ϕg) and excited (ϕex) states. The former was promptly confirmed by density functional theory (DFT) calculations at the B3LYP/6-31G(d,p) level (Fig. 2b).
CCH2– linker (n = butyne) (Fig. 2a). This systematic alteration affects the dihedral angle between the naphthalenes (7-PE-Naph and 6-PE-Naph) in their ground (ϕg) and excited (ϕex) states. The former was promptly confirmed by density functional theory (DFT) calculations at the B3LYP/6-31G(d,p) level (Fig. 2b).
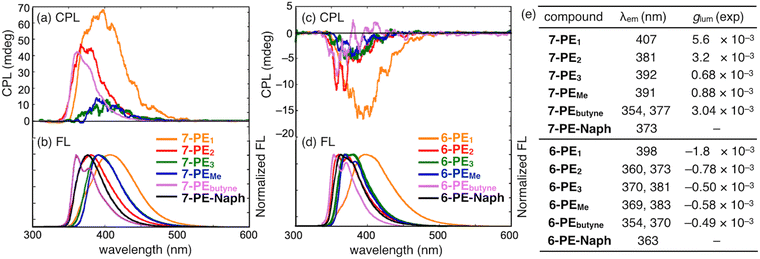
Fig. 3a–d shows the fluorescence (FL) and CPL spectra of 7-PEn and 6-PEn in chloroform. To ensure clarity, axial chirality throughout this study consistently refers to the (S)-configuration for both 7-PEn and 6-PEn. Among the derivatives of 7-PEn and 6-PEn (n = 1, 2, 3, Me, and butyne), the methylene-tethered binaphthyls 7-PE1 and 6-PE1, featuring the smallest dihedral angles, exhibit distinct fluorescence behavior characterized by low-energy and broad emissions at λmax = 407 and 398 nm, respectively. Both the CPL signals of 7-PE1 and 6-PE1 have higher intensities compared with derivatives having other linker groups,18 with substantial glum values of +5.6 × 10−3 and −1.8 × 10−3, respectively. The 7-PEn series tends to consistently exhibit higher glum values compared with the 6-PEn series with identical linkers,19 as depicted in Fig. 3e. Interestingly, the glum values for 7-PEn are more affected by the linker groups, while 6-PE1 shows a significantly higher glum value in the 6-PEn series.
The main distinction between 7-PEn and 6-PEn derivatives lies in the inherent difference in the CPL sign, despite having the same axial chirality (compare Fig. 3a and c). Thus, all (S)-7-PEn compounds exhibited CPL with positive (+) signs, while all (S)-6-PEn compounds exhibited CPL with negative (−) signs, regardless of their respective linker groups. In essence, the inversion of CPL sign was achieved solely by altering the PE-substitution positions on the binaphthyl backbone.
To better understand the origin of this sign inversion, theoretical investigations were conducted as follows:20 The chiroptical and structural computations for 7-PE1 and 6-PE1 in their excited states were initially performed using the TD-DFT approach, which successfully reproduced the observed trends, including the CPL-sign inversion (Table S2, ESI†). To enhance the accuracy of our calculations, we subsequently employed time-dependent approximate coupled cluster calculations at the RI-CC2/def2-TZVP level21 for 7-PE1 and 6-PE1.
Table 1 shows a comparison between the calculated and experimental glum values as derived from the optimized excited state structures. While slightly larger discrepancies were observed for 7-PE1, the calculated values successfully reproduce the trends in both intensity and sign of the glum value.
| Compound | ϕ g (°) | ϕ ex (°) | μ (10−18 esu cm) | m (10−20 esu cm) | θ μm (°) | g lum (calc) | g lum (exp) |
|---|---|---|---|---|---|---|---|
| ϕ g: Dihedral angle of the binaphthyl in the ground state. Calculated at the CC2/def2-TZVP level. ϕex: Dihedral angle of the binaphthyl in the excited state. μ: Electric transition dipole moment in the excited state. m: Magnetic transition dipole moment in the excited state. θμm: Angle of vectors between μ and m. glum (calc): Theoretically calculated glum value. glum (exp): Experimentally observed glum value. | |||||||
| 7-PE1 | 51.9 | 38.7 | 2.23 | 6.09 | 80.5 | 9.0 × 10−3 | 5.6 × 10−3 |
| 6-PE1 | 58.6 | 33.2 | 4.37 | 2.67 | 97.8 | –1.7 × 10−3 | −1.8 × 10−3 |
Crucial structural features relevant to the electronic transitions are also summarized in Table 1. The dihedral angles between the binaphthyl units are lower in the excited state (ϕex) compared with the ground state (ϕg). This structural adjustment renders the binaphthyl moieties more planar in the excited state, facilitating enhanced interaction between the naphthalene groups compared with that in the ground state.
The theoretical calculations also assessed the electric (μ) and magnetic (m) transition dipole moments in the excited state, relevant for the glum values, approximately derived for isotropic solutions as 4 (|μ| |m| cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θμm)/(|μ|2 + |m|2), where θμm represents the angle between μ and m. The angles θμm for 7-PE1 and 6-PE1 deviated by 9.5° less and 7.8° more than 90°, respectively. Thus, the deviation from a right angle was primarily responsible for the reversal in CPL sign between 7-PEn and 6-PEn.
θμm)/(|μ|2 + |m|2), where θμm represents the angle between μ and m. The angles θμm for 7-PE1 and 6-PE1 deviated by 9.5° less and 7.8° more than 90°, respectively. Thus, the deviation from a right angle was primarily responsible for the reversal in CPL sign between 7-PEn and 6-PEn.
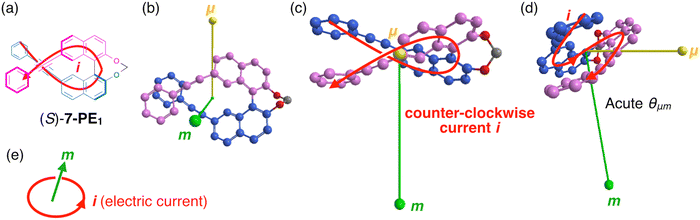
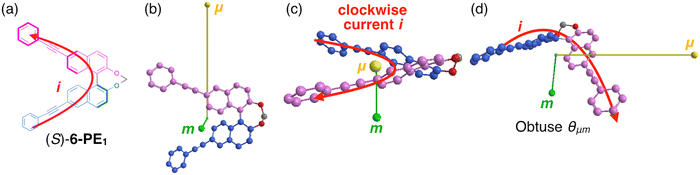
To further understand why the orientation of θμm varies dramatically spanning a right angle between 7-PE1 and 6-PE1, we examine in detail the relationship between molecular structures and the orientations of μ and m (see Fig. 4 and 5). During the S1 → S0 transition, when electrons move from the upper to the lower PE-Naph unit, μ is directed upwards, indicating the opposite direction to the electron movement (Fig. 4b and 5b). In a classical explanation, the generated current flows in the opposite direction to the electron movement. Thus, it is expected that the instantaneous current (i)16,22 generated by μ during an electron transition in these molecular systems will flow along μ (from the lower to upper PE-Naph units), as indicated by the red arrows in Fig. 4a and 5a. Importantly, in 7-PE1, the current flows counterclockwise relative to the origin-μ axis (Fig. 4c), and clockwise in 6-PE1 (Fig. 5c). Despite similar directions of electron movement from the upper to lower PE-Naph units in both 7-PE1 and 6-PE1, the direction of current rotation is apparently reversed. According to the classic loop model (Fig. 4e), the reversal in current-flow rotation inversely affects the direction of m. Consequently, this reversal in current rotation and thus in orientation of m between 7-PE1 and 6-PE1 accounts for the angle θμm being acute in 7-PE1 and obtuse in 6-PE1 (Fig. 4d and 5d). Thus, 7-PE1 exhibited left-handed CPL, while 6-PE1 showed right-handed CPL. Additionally, the more pronounced coil-like flow of current in 7-PE1 results in a larger m and thus a higher glum value compared to that in 6-PE1 (compare Fig. 4d and 5d).
Our rationale may aid in understanding the structure–property relationship of m, especially for C2-symmetric molecules like 7-PE1 and 6-PE1, where the S1 → S0 transition mainly involves LUMO → HOMO transitions. Since the value of m depends on the position of the origin, it is recommended to place the origin, in this case, in the middle of the electric current flow (or thereabout) for better analysis of the correlation between the electric current flow and m. Similarly, this reasoning would explain why compounds such as 3-PE1, 4-PE1, 5-PE1, and 8-PE1 also exhibit negative CPL like 6-PEn (see Fig. S3–S6, ESI†).
As mentioned above, among the 6-PEn series, the glum value of 6-PE1 exhibited a significantly higher value, while the glum values of 7-PEn were considerably influenced by the linker groups (Fig. 3e). Interestingly, 6-PE1 has a helicene-like twisted structure in the excited state (Fig. 6c), while in the ground state, it bears the typical binaphthyl conformation. Indeed, the trend in the degree of torsional angles considerably differs in these systems (ϕ and ϕ′/ϕ′′ in Fig. 6a). Both 7-PE1 and 6-PEMe having typical binaphthyl conformations in the excited state show angles of 39° and 9/9° or 65° and 3/3°, respectively (Fig. 6b and d). In contrast, these angles were found to be 33° and 31/20° in 6-PE1, resulting in a greatly twisted conformation similar to that of a typical helicene structure.23 This unexpected structural change in the excited state of 6-PE1 is most likely responsible for its red-shifted emission and better glum value compared with the other 6-PEn derivatives.
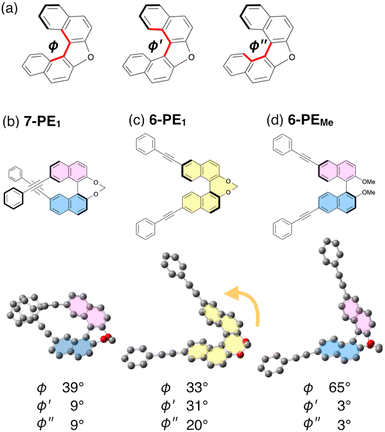 | ||
| Fig. 6 (a) Definition of torsional angles (φ, φ′ and φ′′) of binaphthyls. Optimized structures in the excited state and the corresponding angles for (b) 7-PE1, (c) 6-PE1 and (d) 6-PEMe. | ||
Conclusions
The introduction of PE groups at the 6,6′- or 7,7′-positions of the (S)-binaphthyl backbone results in oppositely signed CPL responses. While the methylene-tethered 7-PE1 and 6-PE1 derivatives display superior glum values, a uniform sign inversion is observed across all related derivatives. Theoretical calculations provided a rationale for the sign inversion based on the orientations of μ and m, as well as other differences in chiroptical responses.Further analysis revealed that the direction of instantaneous current-flow rotation during transitions can reverse the orientation of m, thereby reversing the CPL sign. Previously, the properties of m were elusive, but for C2-symmetric molecules like ours, where the major S1 → S0 transition involves LUMO → HOMO transitions, the orientation of m can be predicted directly from the chemical structure. If μ represents the directionality of electron movement during transitions, i.e., the “difference” in electron presence before and after transitions, then m could perhaps be represented as the “path” of electron movement based on current flow during the transition.
While this approach may not be universally applicable, we anticipate that our observations and the insights derived from our detailed structural analyses of binaphthyls in the excited state will contribute to the understanding and design of other novel CPL phenomena.
Author contributions
A. Imayoshi contributed to the conceptualization and methodology, and wrote the original draft of the manuscript. K. Tsubaki managed the overall project administration, provided supervision, and was responsible for the review and editing of the manuscript. S. Fujio, Y. Nagaya, and M. Sakai played major roles in the investigation. A. Terazawa, M. Sakura, K. Okada, and T. Kimoto supported the investigation. T. Mori contributed to software and investigation, and also reviewed and edited the manuscript. Y. Imai provided resources and conducted investigations. M. Hada contributed to conceptualization, methodology and software, and reviewed and edited the manuscript.Data availability
All synthetic procedures and/computational/analytical data related to this article are provided in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Prof. Keiji Hirose and Dr Rika Miyake (Graduate School of Engineering Science, Osaka University), Prof. Yasunao Hattori (Center for Instrumental Analysis, Kyoto Pharmaceutical University) and Prof. Takumi Furuta (Laboratory of Pharmaceutical Chemistry, Kyoto Pharmaceutical University), and Prof. Makoto Oba and Dr Tomohiro Umeno (Graduate School of Medical Science, Kyoto Prefectural University of Medicine) for the HRMS measurements. This study was carried out using the FT-ICR mass spectrometer and the NMR spectrometer in the Joint Usage/Research Center at the Institute for Chemical Research, Kyoto University. The computation was performed using Research Center for Computational Science, Okazaki, Japan (Project: 23-IMS-C043). This study was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI (No. 24K18254, 22K15256, 19K23803, 22H02746 and 19H03355), Japan Science and Technology Agency (JST) CREST (JPMJCR2001), and Grant-in-Aid from the Naito Foundation. We also thank Dr Jay Freeman at Edanz for editing a draft of this manuscript.Notes and references
- (a) J. R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem., 2017, 1, 1 CrossRef; (b) Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2020, 32, e1900110 CrossRef PubMed; (c) Y. Deng, M. Wang, Y. Zhuang, S. Liu, W. Huang and Q. Zhao, Light: Sci. Appl., 2021, 10, 1 CrossRef; (d) J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef; (e) X. Wang, S. Ma, B. Zhao and J. Deng, Adv. Funct. Mater., 2023, 33, 2214364 CrossRef CAS; (f) Y. Yang, N. Li, J. Miao, X. Cao, A. Ying, K. Pan, X. Lv, F. Ni, Z. Huang, S. Gong and C. Yang, Angew. Chem., Int. Ed., 2022, 61, e202202227 CrossRef CAS; (g) D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331 RSC; (h) I. Song, J. Ahn, H. Ahn, S. H. Lee, J. Mei, N. A. Kotov and J. H. Oh, Nature, 2023, 617, 92 CrossRef CAS PubMed; (i) L. E. MacKenzie and R. Pal, Nat. Rev. Chem., 2020, 5, 109 CrossRef PubMed; (j) Y. Shi, J. Han, X. Jin, W. Miao, Y. Zhang and P. Duan, Adv. Sci., 2022, 9, e2201565 CrossRef.
- (a) Y. Nojima, M. Hasegawa, N. Hara, Y. Imai and Y. Mazaki, Chem. – Eur. J., 2021, 27, 5923 CrossRef CAS; (b) K. Miki, T. Noda, M. Gon, K. Tanaka, Y. Chujo, Y. Mizuhata, N. Tokitoh and K. Ohe, Chem. – Eur. J., 2019, 25, 9211 CrossRef CAS PubMed; (c) K. Takaishi, S. Murakami, F. Yoshinami and T. Ema, Angew. Chem., Int. Ed., 2022, 61, e202204609 CrossRef CAS PubMed; (d) W. Duan, K. Li, H. Ji, Y. Huo, Q. Yao, H. Liu and S. Gong, Dyes Pigm., 2021, 193, 109538 CrossRef CAS; (e) Z. Jiang, X. Wang, J. Ma and Z. Liu, Sci. China: Chem., 2019, 62, 355 CrossRef CAS; (f) J. Han, Y. Shi, X. Jin, X. Yang and P. Duan, Chem. Sci., 2022, 13, 6074 RSC; (g) H. Feng, J. Pu, S. Wang, S. Jiang, W. Yang, D. Cao and Y.-S. Feng, Dyes Pigm., 2023, 217, 111422 CrossRef CAS; (h) F. Song, Z. Xu, Q. Zhang, Z. Zhao, H. Zhang, W. Zhao, Z. Qiu, C. Qi, H. Zhang, H. H. Y. Sung, I. D. Williams, J. W. Y. Lam, Z. Zhao, A. Qin, D. Ma and B. Z. Tang, Adv. Funct. Mater., 2018, 28, 1800051 CrossRef; (i) Y. Wang, X. Li, F. Li, W.-Y. Sun, C. Zhu and Y. Cheng, Chem. Commun., 2017, 53, 7505 RSC; (j) Q. Ye, D. Zhu, L. Xu, X. Lu and Q. Lu, J. Mater. Chem., 2016, 4, 1497 CAS.
- Z.-B. Sun, J.-K. Liu, D.-F. Yuan, Z.-H. Zhao, X.-Z. Zhu, D.-H. Liu, Q. Peng and C.-H. Zhao, Angew. Chem., Int. Ed., 2019, 58, 4840 CrossRef CAS.
- K. Takaishi, F. Yoshinami, Y. Sato and T. Ema, Chem. – Eur. J., 2024, e202400866 CrossRef CAS.
- K. Takaishi, K. Iwachido, R. Takehana, M. Uchiyama and T. Ema, J. Am. Chem. Soc., 2019, 141, 6185 CrossRef CAS PubMed.
- J. Li, C. Hou, C. Huang, S. Xu, X. Peng, Q. Qi, W.-Y. Lai and W. Huang, Research, 2020, 2020, 3839160 CAS.
- (a) Y. He, S. Lin, J. Guo and Q. Li, Aggregate, 2021, 2, e141 CrossRef CAS; (b) S. Yang, F. Li, Q. Li, J. Han and Y. Cheng, ACS Appl. Opt. Mater., 2023, 1, 1492 CrossRef CAS.
- Y. Zhang, H. Li, Z. Geng, W.-H. Zheng, Y. Quan and Y. Cheng, ACS Nano, 2022, 16, 3173 CrossRef CAS PubMed.
- (a) N. F. M. Mukthar, N. D. Schley and G. Ung, J. Am. Chem. Soc., 2022, 144, 6148 CrossRef CAS PubMed; (b) Y. Zhou, H. Li, T. Zhu, T. Gao and P. Yan, J. Am. Chem. Soc., 2019, 141, 19634 CrossRef CAS.
- (a) Z. Geng, Y. Zhang, Y. Zhang, Y. Li, Y. Quan and Y. Cheng, J. Mater. Chem., 2021, 9, 12141 CAS; (b) Z. Geng, Y. Zhang, Y. Zhang, Y. Quan and Y. Cheng, Angew. Chem., Int. Ed., 2022, 61, e202202718 CrossRef CAS PubMed.
- (a) Y. Nojima, M. Hasegawa, N. Hara, Y. Imai and Y. Mazaki, Chem. Commun., 2019, 55, 2749 RSC; (b) T. Sato, N. Tajima, H. Ueno, T. Harada, M. Fujiki and Y. Imai, Tetrahedron, 2016, 72, 7032 CrossRef CAS; (c) T. Kinuta, N. Tajima, M. Fujiki, M. Miyazawa and Y. Imai, Tetrahedron, 2012, 68, 4791 CrossRef CAS; (d) T. Kimoto, N. Tajima, M. Fujiki and Y. Imai, Chem. Asian J., 2012, 7, 2836 CrossRef CAS.
- K. Takaishi, T. Matsumoto, M. Kawataka and T. Ema, Angew. Chem., Int. Ed., 2021, 60, 9968 CrossRef CAS.
- (a) K. Takaishi, S. Murakami, K. Iwachido and T. Ema, Chem. Sci., 2021, 12, 14570 RSC; (b) D. Kaji, S. Ikeda, K. Takamura, N. Tajima, M. Shizuma, T. Mori, M. Miyasaka and Y. Imai, Chem. Lett., 2019, 48, 874 CrossRef CAS; (c) T. Amako, T. Kimoto, N. Tajima, M. Fujiki and Y. Imai, RSC Adv., 2013, 3, 6939 RSC; (d) N. Hara, D. Kaji, K. Okuda, M. Shizuma, N. Tajima and Y. Imai, Chem. Lett., 2018, 47, 894 CrossRef CAS; (e) K. Nakabayashi, S. Kitamura, N. Suzuki, S. Guo, M. Fujiki and Y. Imai, Eur. J. Org. Chem., 2016, 64 CrossRef CAS.
- (a) Y. Imai, Chem. Lett., 2021, 50, 1131 CrossRef CAS; (b) Y. Sheng, D. Shen, W. Zhang, H. Zhang, C. Zhu and Y. Cheng, Chem. – Eur. J., 2015, 21, 13196 CrossRef CAS; (c) T. Kimoto, T. Amako, N. Tajima, R. Kuroda, M. Fujiki and Y. Imai, Asian J. Org. Chem., 2013, 2, 404 CrossRef CAS; (d) K. Nakabayashi, T. Amako, N. Tajima, M. Fujiki and Y. Imai, Chem. Commun., 2014, 50, 13228 RSC; (e) Y. Li, C. Xue, M. Wang, A. Urbas and Q. Li, Angew. Chem., Int. Ed., 2013, 52, 13703 CrossRef CAS PubMed; (f) H. Okada, N. Hara, D. Kaji, M. Shizuma, M. Fujuiki and Y. Imai, Phys. Chem. Chem. Phys., 2020, 22, 13862 RSC.
- (a) K. Takaishi, K. Iwachido and T. Ema, J. Am. Chem. Soc., 2020, 142, 1774 CrossRef CAS PubMed; (b) S. Nakanishi, N. Hara, N. Kuroda, N. Tajima, M. Fujiki and Y. Imai, Org. Biomol. Chem., 2018, 16, 1093 RSC; (c) M. Okazaki, T. Mizusawa, K. Nakabayashi, M. Yamashita, N. Tajima, T. Harada, M. Fujiki and Y. Imai, J. Photochem. Photobiol., A, 2016, 331, 115 CrossRef CAS.
- R. G. Uceda, C. M. Cruz, S. Miguez-Lago, L. A. de Cienfuegos, G. Longhi, D. A. Pelta, P. Novoa, A. J. Mota, J. M. Cuerva and D. Miguel, Angew. Chem., Int. Ed., 2024, 63, e202316696 CrossRef CAS PubMed.
- M. Sakai, S. Fujio, A. Imayoshi, T. Sasamori, K. Okada, Y. Imai, M. Hasegawa and K. Tsubaki, Chem. – Asian J., 2024, e202400159 CrossRef CAS PubMed.
- (a) K. Ma, W. Chen, T. Jiao, X. Jin, Y. Sang, D. Yang, J. Zhou, M. Liu and P. Duan, Chem. Sci., 2019, 10, 6821 RSC; (b) K. Takaishi, S. Hinoide, T. Matsumoto and T. Ema, J. Am. Chem. Soc., 2019, 141, 11852 CrossRef CAS PubMed; (c) Y. Wang, Q. Liao, Y. Feng and Q. Meng, J. Mol. Struct., 2024, 1304, 137695 CrossRef CAS; (d) Y. Wang, Y. Li, S. Liu, F. Li, C. Zhu, S. Li and Y. Cheng, Macromolecules, 2016, 49, 5444 CrossRef CAS; (e) T. Ikai, N. Mishima, T. Matsumoto, S. Miyoshi, K. Oki and E. Yashima, Angew. Chem., Int. Ed., 2024, 63, e202318712 CrossRef CAS PubMed; (f) W. Huang, Y. Zhu, K. Zhou, L. Chen, Z. Zhao, E. Zhao and Z. He, Chem. – Eur. J., 2023, e202303667 Search PubMed; (g) S. Jena, A. Thayyil Muhammed Munthasir, S. Pradhan, M. Kitahara, S. Seika, Y. Imai and P. Thilagar, Chem. – Eur. J., 2023, 29, e202301924 CrossRef CAS PubMed.
- K. Zhang, J. Zhao, N. Zhang, J.-F. Chen, N. Wang, X. Yin, X. Zheng and P. Chen, J. Mater. Chem., 2022, 10, 1816 CAS.
- TURBOMOLE V7.8 2023, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, TURBOMOLE GmbH, since 2007; available from https://www.turbomole.org.
- (a) C. Hättig and A. Köhn, J. Chem. Phys., 2002, 117, 6939 CrossRef; (b) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC; (c) F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057 RSC.
- (a) M. Fortino, G. Schifino and A. Pietropaolo, Chirality, 2023, 35, 673 CrossRef CAS PubMed; (b) F. Furche, R. Ahlrichs, C. Wachsmann, E. Weber, A. Sobanski, F. Vögtle and S. Grimme, J. Am. Chem. Soc., 2000, 122, 1717 CrossRef CAS.
- P. Ravat, R. Hinkelmann, D. Steinebrunner, A. Prescimone, I. Bodoky and M. Juríček, Org. Lett., 2017, 19, 3707 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp02968b |
| This journal is © the Owner Societies 2025 |