Strongly coupled and highly-compacted zirconium aminobenzenedicarboxylate crystal membranes for accelerating carbon dioxide capture†
Qi
Li
 *,
Liangmei
Luo
*,
Liangmei
Luo
 ,
Zhiwei
Wu
,
Yufei
Cao
,
Qiyang
Guo
* and
Yanqing
Wang
,
Zhiwei
Wu
,
Yufei
Cao
,
Qiyang
Guo
* and
Yanqing
Wang
 *
*
School of Chemistry and Chemical Engineering, Nantong University, Nantong, 226019, China. E-mail: zhuiqiuzhizhuo@163.com; qyguo@ntu.edu.cn; ccewyq@ntu.edu.cn
First published on 3rd December 2024
Abstract
This study presents the fabrication of a UiO-66-NH2 composite membrane on a porous carbon cloth–polypyrrole substrate (CC–PPy@UiO-66-NH2) that demonstrates strongly coupled and highly ordered features to enhance CO2 capture efficiency. This free-standing configuration exhibits a higher adsorption kinetics with a slope of 0.48 mg g−1 min−1 for CO2 and superior adsorption selectivity, demonstrating that the adsorption kinetics rate of the CC–PPy@UiO-66-NH2 composite membrane for CO2 is approximately twice that of bulk UiO-66-NH2 (0.27 mg g−1 min−1) crystals at a bar.
Porous membrane separation technologies offer high throughput, selectivity, and separation efficiency, as well as low energy consumption, positioning them as green and efficient solutions for molecular separation. However, traditional organic/inorganic porous membranes face limitations owing to their fixed pore sizes, complex medium environments, material stability, etc., often failing to achieve effective separation in many applications. Metal–organic frameworks (MOFs) have excellent thermal stability, customizable pore structures, and permanent internal voids, making them uniquely suitable for molecular separation.1–3 However, MOFs, particularly in nanocrystal form, are typically produced powders with high surface energy, which considerably limits their development and practical application in the field of separation.
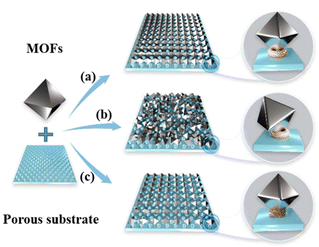
The development of MOF hybrid membranes has progressed beyond the design and exploration of powder and single-crystal materials. These membranes integrate the excellent selectivity and permeability of MOF crystals, addressing the challenge of low powder utilization.4 The primary challenge now lies in preparing robust highly ordered hybrid membranes that leverage the strong coupling and directional characteristics of individual MOF crystals. Advances in this regard have led to the production of membranes with closely packed structures and stable crystal orientations.5–7 By controlling these orientations, the MOF channels within the crystals can be aligned in specific directions, endowing MOF membranes with anisotropic properties.8–10 These properties include enhanced diffusion along preferred directions, preferential orientation of guest molecules, and protection of functional guest molecules. As depicted in Scheme 1, the coupling configuration, interactions, and arrangement density of MOF crystals on the surface of porous substrates considerably influence their separation performance. The configurations of MOF crystals can be categorized into three types: (a) strongly coupled and highly ordered, resulting in high selectivity and permeability; (b) uneven size and random arrangement, leading to low selectivity and permeability; and (c) weakly coupled and loosely arranged, also resulting in low selectivity and permeability.
UiO-66, a distinguished member of the Zr-MOF family with a three-dimensional topology, has emerged as a promising candidate for membrane-based CO2 separation and utilization owing to its abundant and interconnected pore channels, high chemical affinity for CO2, and exceptional thermal stability.11–15 It has been demonstrated that reducing the thickness of UiO-66 membranes considerably enhances the CO2/N2 separation performance, particularly in terms of dynamic kinetics.16,17 Furthermore, downsizing UiO-66 crystals further improves the CO2/N2 separation performances.18,19 However, the fabrication of high-performance UiO-66 hybrid membranes, which maintain small size and octahedral shape, face many challenges, such as weak interactions between crystals and substrates, irregular arrangement, and a loose state. These disadvantages impede further enhancement of CO2 selectivity and kinetics. To overcome these challenges, designing a new synthesis route for achieving strongly coupled and highly ordered UiO-66 hybrid membranes has become indispensable. Despite numerous efforts devoted to the configuration and arrangement of UiO-66 crystals supported on porous substrates, advancement with respect to the development of hybrid membranes exhibiting high efficiency remains stagnant.20–22
Over the past few decades, in situ growth based on chemical grafting has proven considerably effective for fabricating highly coupled and ordered MOF hybrid membranes through strong covalent bonding and surface confinement effects.23–25 For example, Tan et al. synthesized carboxyl-functionalized cotton fabric-supported Cu–BTC crystals via a layer-by-layer synthesis route through covalent bonding between Cu–BTC and modified cotton fiber.26 Furthermore, our group has demonstrated that ligands (1,4-benzenedicarboxylic acid, BDC) can be grafted onto the N sites of polypyrrole (PPy) via nucleophilic substitution reactions, resulting in the formation of covalently bonded UiO-66 anchored on PPy.17 Inspired by these achievements, we expected that strongly coupled and ordered UiO-66 composite membranes may be obtained through a two-step nucleophilic substitution reaction based on bridged molecules. Benefiting from the small-size effect of UiO-66-NH2 nanocrystals, the as-prepared single-layer UiO-66-NH2 composite membrane with a superior adsorption kinetics rate was demonstrated in the CO2 capture test. To the best of our knowledge, taking advantage of the covalent grafting strategy to fabricate strongly coupled and highly ordered UiO-66-NH2 composite membranes has not yet been reported.
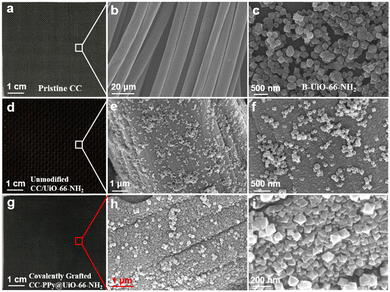
In this study, we fabricated a strongly coupled and highly ordered carbon cloth-supported UiO-66-NH2 composite membrane with an octahedral shape and small size (Fig. S1–S3, ESI†). The details for the synthesis route of the CC–PPy@UiO-66-NH2 membrane are provided in the Experimental section. To characterize the microstructure of the in situ grown UiO-66-NH2 crystals, the pristine CC and bulk UiO-66-NH2 (denoted as B-UiO-66-NH2), synthesized using the solvothermal method, were characterized via SEM. As shown in Fig. 1a and b, the CC comprises numerous fibers with an average size of 10 μm. B-UiO-66-NH2 crystals display a typical octahedral shape with an average size exceeding 300 nm (Fig. 1c). To assess the effect of chemical grafting on UiO-66-NH2 crystals, we fabricated CC/UiO-66-NH2 membranes using unmodified CC under the same conditions. These membranes exhibited a black appearance (Fig. 1d). The magnified SEM images presented in Fig. 1e and f reveal an irregular and loose arrangement of unmodified CC-supported UiO-66-NH2 crystals having an average size of 200 nm. Fig. 1g shows the CC–PPy@UiO-66-NH2 hybrid membrane, wherein 1,4-dibromobutane ligand molecules were covalently grafted onto the CC–PPy composite substrate. The SEM images presented in Fig. 1h and i display octahedral-shaped UiO-66-NH2 crystals (100 nm) orderly arranged on the surface of the CC composite substrate, demonstrating that covalent grafting positively effects the growth and configuration of UiO-66-NH2 crystals.
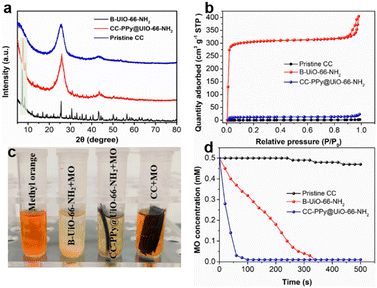
The crystal phase and high crystallinity of the as-prepared hybrid membrane were verified via X-ray diffraction (XRD) patterns (Fig. 2a), wherein two characteristic peaks observed at 6° and 8° could be indexed to the (111) and (002) planes, respectively, indicating that chemical grafting and subsequent in situ growth exerted no adverse effects on the UiO-66-NH2 crystals. Specific surface area is a critical parameter for evaluating the physical properties of hybrid membranes or textiles. Fig. 2b illustrates the N2 sorption isotherms measured at 77 K. B-UiO-66-NH2 crystals exhibited a type I isotherm with a hysteresis loop at P/P0 = 0.85–1.0, indicative of a micro–mesoporous structure with a pore size distribution ranging from 1.5 to 5 nm (Fig. S4, ESI†). The BET surface area of B-UiO-66-NH2 was determined to be 1258 m2 g−1, underscoring its potential for gas separation applications. CC–PPy@UiO-66-NH2 demonstrated a BET surface area of 48.5 m2 g−1, significantly higher than that of pristine CC (2.4 m2 g−1), which highlights the substantial contribution of UiO-66-NH2 nanocrystals to the overall BET surface area of the membrane. With an actual loading of 7 mg of UiO-66-NH2 in the 215 mg membrane, the adjusted BET surface area of the UiO-66-NH2 nanocrystals was calculated to be 1280 m2 g−1, with an average pore size of 2 nm.
The thermal stability of the CC–PPy@UiO-66-NH2 was assessed using thermogravimetric analysis (TGA) under N2 flow. Both the CC–PPy@UiO-66-NH2 hybrid membrane and B-UiO-66-NH2 exhibited minimal weight loss from room temperature (25 °C) up to 300 °C (Fig. S5, ESI†), confirming their exceptional thermal stability. The adsorption performance of the CC–PPy@UiO-66-NH2 membrane was evaluated using a 0.5 mM methyl orange (MO) solution, showing that both CC–PPy@UiO-66-NH2 and B-UiO-66-NH2 have excellent adsorption capabilities for MO (Fig. 2c). Notably, the adsorption kinetics of the CC–PPy@UiO-66-NH2 membrane reached 5 μM s−1, approximately four times faster than that of B-UiO-66-NH2, which was 1.3 μM s−1 (Fig. 2d). Due to the robust structure of the CC, the CC–PPy@UiO-66-NH2 hybrid membrane demonstrated remarkable flexibility, capable of enduring 360° bending and multiple twisting actions without damage (Fig. S6, ESI†).
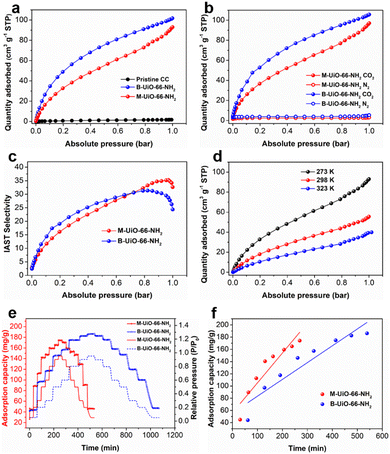
The CO2 capture and separation performance of the CC–PPy@UiO-66-NH2 hybrid membrane was assessed using adsorption kinetics curves and a vacuum vapor/gas sorption method at various temperatures. To elucidate the contributions of UiO-66-NH2 nanocrystals grown in situ on carbon fibers, this hybrid membrane is referred to as M-UiO-66-NH2, and the corresponding data were normalized based on the weight percentage of UiO-66-NH2. Fig. 3a presents the CO2 adsorption isotherms for three samples measured at 273 K. It was found that the pristine CC exhibited the lowest CO2 adsorption capacity (0.2 cm3 g−1), which can be attributed to its low porosity and insufficient adsorption sites. In contrast, M-UiO-66-NH2 demonstrated a high CO2 uptake capacity of 98 cm3 g−1, comparable to that of B-UiO-66-NH2 (105 cm3 g−1). This indicates that the covalently grafted UiO-66-NH2 nanoparticles retain the complete topological structure and abundant –NH2 sites, contributing almost entirely to the total CO2 adsorption capacity of the hybrid membrane. As shown in Fig. 3b, both the M-UiO-66-NH2 and B-UiO-66-NH2 displayed relatively low N2 adsorption capacities at 273 K, which is capable of improving the CO2/N2 separation potential. Notably, M-UiO-66-NH2 showed higher ideal adsorbed solution theory (IAST) selectivity compared to B-UiO-66-NH2, particularly at higher pressures with an IAST of 35.2, which is advantageous for accelerating CO2/N2 separation (Fig. 3c). The superior IAST selectivity of M-UiO-66-NH2 at high pressures can be attributed to the ordered arrangement of UiO-66-NH2 nanocrystals, which not only restricts N2 diffusion into the pores, but also enhances stronger quadrupolar interactions with CO2.18 This structural arrangement has been shown to confer a preferential binding affinity toward CO2, resulting in higher IAST selectivity under elevated pressures.
The CO2 adsorption capacities of M-UiO-66-NH2 at various temperatures were measured to evaluate its practicality. As shown in Fig. 3d, M-UiO-66-NH2 exhibited a high CO2 uptake capacity of 52 cm3 g−1 at 298 K (room temperature). Even at 323 K, M-UiO-66-NH2 also maintained a relatively high CO2 adsorption capacity of 41 cm3 g−1, surpassing that of B-UiO-66-NH2, which was 40 cm3 g−1, as shown in Fig. S7 (ESI†). It is recognized that the size of MOF nanoparticles significantly influences their gas separation performance, particularly the gas adsorption kinetics.16 In this study, the vacuum vapor/gas sorption method was employed to analyze the adsorption kinetics of the hybrid membrane. It was observed that both M-UiO-66-NH2 and B-UiO-66-NH2 demonstrated increased CO2 adsorption capacities with the increasing of relative pressure (Fig. S8, ESI†), confirming their excellent CO2 capture performance. Fig. 3e displays the CO2 adsorption capacity–time curves across eight pressure values. Compared to B-UiO-66-NH2, M-UiO-66-NH2 reached system weight equilibrium more rapidly at each relative pressure level, attributed to the faster diffusion of CO2 into the pores of the smaller UiO-66-NH2 framework. This rapid diffusion is likely due to reduced physical or chemical resistance encountered by CO2 molecules entering the pores, facilitating quicker adsorption equilibrium in the smaller M-UiO-66-NH2 crystals. Further analysis is shown in Fig. 3f, where the average adsorption capacity–time curves are plotted. The slope of these curves represents the adsorption kinetics, with M-UiO-66-NH2 exhibiting a slope of 0.48 mg g−1min−1, higher than the 0.27 mg g−1min−1 observed for B-UiO-66-NH2. These results indicate that the CC–PPy@UiO-66-NH2 hybrid membrane with small-sized nanocrystals not only retains the intrinsic adsorption capacity but also achieves faster adsorption equilibrium compared to bulk UiO-66-NH2 crystals.
To evaluate the stability of this hybrid membrane under open flame conditions, infrared thermal imaging was used to monitor temperature changes on the side far from the fire source (denoted as the nightside), using a lighted candle as the fire source (see details in Fig. S9, S10 and Videos S1–S4, ESI†). These results indicate that densely packed UiO-66-NH2 nanocrystals effectively reduced the heat transfer rate through the carbon fibers in the CC. Furthermore, we anticipate that the CC–PPy@UiO-66-NH2 hybrid membrane will be effective for CO2 separation in flue gases and for preventing the ingestion of thick smoke at fire scenes.
It has been well accepted that the spatial orientation of MOF crystals plays a substantial role in regulating the separation efficiency of MOF hybrid membranes. To investigate the effect of bridged molecules on the spatial orientation of UIO-66-NH2 crystals, molecular dynamics simulation, based on the molecular plane model of PPy, was adopted to elucidate the relationships between molecular structure and spatial orientation (Fig. S11, ESI†). These results indicate that brominated alkanes with short chains and asymmetry can regulate the spatial orientation of the BDC-NH2 molecule. The above theoretical calculations can be used to guide the synthesis of MOF crystal membranes in future work.
In summary, we have demonstrated that the strongly coupled and highly ordered CC–PPy@UiO-66-NH2 hybrid membrane was fabricated using a universal covalent grafting method. The as-prepared hybrid membrane exhibited excellent CO2 capture performances (high IAST selectivity and fast adsorption kinetics) while maintaining intrinsic adsorption capacity. Benefiting from its structural superiority, this hybrid membrane exhibited good separation performance for thick smoke, highlighting its considerable potential application in fire accidents.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Jiangsu Specially-Appointed Professor Program and the National Natural Science Foundation of China (22402090) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX24_1991) and Large Instruments Open Foundation of Nantong University (KFJN2458) for financial support.Notes and references
- A. Betard and R. A. Fischer, Chem. Rev., 2012, 112, 1055–1183 CrossRef CAS.
- T. Wen, G. Quan, B. Niu, Y. Zhou, Y. Zhao, C. Lu, X. Pan and C. Wu, Small, 2021, 17, 2005064 CrossRef CAS PubMed.
- Q. Li, F. Yan and J. Texter, Chem. Rev., 2024, 124, 3813–3931 CrossRef CAS PubMed.
- J. Yan, Y. Sun, T. Ji, C. Zhang, L. Liu and Y. Liu, J. Membrane Sci., 2022, 653, 120496 CrossRef CAS.
- D. L. Gin and R. D. Noble, Science, 2011, 332, 674–676 CrossRef CAS.
- S. Hermes, F. Schröder, R. Chelmowski, C. Wöll and R. A. Fischer, J. Am. Chem. Soc., 2005, 127, 13744 CrossRef CAS PubMed.
- H. Bux, F. Liang, Y. Li, J. Cravillon, M. Wiebcke and J. Caro, J. Am. Chem. Soc., 2009, 131, 16000–16001 CrossRef CAS PubMed.
- S. Panda, S. Kundu, P. Malika and R. Haldar, Chem. Sci., 2024, 15, 2586–2592 RSC.
- H. Sun, F. Wang, X. Li, J. Caro, H. Meng, N. Wang and Q.-F. An, Angew. Chem., Int. Ed., 2023, 62, e202300262 CrossRef CAS PubMed.
- W.-J. Li, J. Liu, Z.-H. Sun, T.-F. Liu, J. Lü, S.-Y. Gao, C. He, R. Cao and J.-H. Luo, Nat. Commun., 2016, 7, 11830 CrossRef CAS PubMed.
- S. Friebe, B. Geppert, F. Steinbach and J. Caro, ACS Appl. Mater. Interface, 2017, 9, 12878–12885 CrossRef CAS.
- F. Liang, S. Wei, L. Xue and S. Yan, Green Carbon, 2024, 2, 252–261 CrossRef.
- S. Fakhraie, H. Rajabi and A. Rashidi, Chem. Eng. J., 2023, 452, 139001 CrossRef CAS.
- J. Zhu, Y. Zhang, Z. Chen, Z. Zhang, X. Tian, M. Huang, X. Bai, X. Wang, Y. Zhu and H. Jiang, Nat. Commun., 2024, 15, 1565 CrossRef CAS PubMed.
- M. Chen, K. Chang, Y. Zhang, Z. Zhang, Y. Dong, X. Qiu, H. Jiang, Y. Zhu and J. Zhu, Angew. Chem., Int. Ed., 2023, 62, e202305530 CrossRef CAS.
- L. Luo, C. Xu, W. Shi, Q. Liu, Y. Ou-Yang, J. Qian, Y. Wang and Q. Li, J. Phys. Chem. Lett., 2023, 14, 8437–8443 CrossRef CAS.
- Q. Li, H. Zhu, Y. Tang, P. Zhu, H. Ma, C. Ge and F. Yan, Chem. Commun., 2019, 55, 12108–12111 RSC.
- Y. Sun, J. Yan, Y. Gao, T. Ji, S. Chen, C. Wang, P. Lu, Y. Li and Y. Liu, Angew. Chem., Int. Ed., 2024, 62, e202216697 CrossRef.
- G. Liu, Y. Guo, C. Chen, Y. Lu, G. Chen, G. Liu, Y. Han, W. Jin and N. Xu, Nat. Mater., 2023, 22, 769–776 CrossRef CAS PubMed.
- S. Rasoul, S. Mahmoud and P. Vahid, Polym. Sci., Ser. A, 2022, 64, 781–793 CrossRef.
- X. Li, G. Jiang, M. Jian, C. Zhao, J. Hou, A. W. Thornton, X. Zhang, J. Z. Liu, B. D. Freeman, H. Wang, L. Jiang and H. Zhang, Nat. Commun., 2023, 14, 286 CrossRef CAS.
- L.-C. Jheng, J. Park, H. W. Yoon and F.-C. Chang, Sep. Purif. Technol., 2023, 310, 123–126 CrossRef.
- K. Barcus, P.-A. Lin, Y. Zhou, G. Arya and S. M. Cohen, ACS Nano, 2022, 16, 18168–18177 CrossRef CAS PubMed.
- M. Taddei, G. M. Schukraft, M. E. A. Warwick, D. Tiana, M. J. McPherson, D. R. Jones and C. Petit, J. Mater. Chem. A, 2019, 7, 23781–23786 RSC.
- X. Pei and W. Song, Langmuir, 2023, 39, 15046–15054 CrossRef CAS.
- W. Li, Y. Zhang, Z. Yu, T. Zhu, J. Kang, K. Liu, Z. Li and S. C. Tan, ACS Nano, 2022, 16, 14779–14791 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp03259d |
| This journal is © the Owner Societies 2025 |