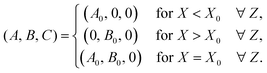Exploring buoyancy-driven effects in chemo-hydrodynamic oscillations sustained by bimolecular reactions
Adam
Bigaj
 *a,
Marcello A.
Budroni
*a,
Marcello A.
Budroni
 *b and
Laurence
Rongy
*b and
Laurence
Rongy
 *a
*a
aNonlinear Physical Chemistry Unit, Service de Chimie Physique et Biologie Théorique, Université libre de Bruxelles (ULB), CP 231 – Campus Plaine, 1050 Brussels, Belgium. E-mail: adam.bigaj@ulb.be; laurence.rongy@ulb.be; Fax: +32 (0)2 650 5767; Tel: +32 (0)2 650 5699
bDepartment of Chemistry and Pharmacy, University of Sassari, Via Vienna 2, 07100 Sassari, Italy. E-mail: mabudroni@uniss.it
First published on 10th December 2024
Abstract
Exotic dynamics, previously associated only with reactions involving complex kinetics, have been observed even with simple bimolecular reactions A + B → C, when coupled with hydrodynamical flows. Numerical studies in two-dimensional reactors have shown that oscillatory dynamics can emerge from an antagonistic coupling between chemically-driven buoyancy and Marangoni convective flows, induced by changes in density and surface tension, respectively, as the reaction occurs. Here, we investigate reactions increasing both surface tension and density, leading to a cooperative coupling between the flows and show how, in this configuration, buoyancy-driven contribution dampens spatio-temporal oscillations of concentration. We finally identify the key parameters controlling the onset and persistence of the oscillatory instability, namely the density and surface tension gradients, and the systems height.
1. Introduction
Chemical oscillations represent captivating examples of a system's spontaneous self-organization and find applications in various fields, including material sciences,1–6 chemical artificial intelligence,7–9 environmental science.10,11 These dynamics are particularly interesting as they also provide understanding of biological functional behaviors12–24 (e.g. morphogenesis, quorum sensing, calcium signaling, circadian rhythms, synchronization, etc.).Throughout years, several reactions in which self-sustained dynamics can be induced have been highlighted such as the Belousov–Zhabotinsky,25 Bray–Liebhafsky,26,27 Briggs–Rauscher,28,29 Bruk Temkin–Gorodsky Novakovic (BT–GN)30,31 and chemiluminescence32–34 reactions. The latter all share a common characteristic essential for inducing oscillatory dynamics: the presence of a nonlinear chemical feedback. Such loops were demonstrated to be crucial in the occurrence of oscillations as in the Brusselator and Oregonator models to name but the most famous.35,36
However, it was recently shown that even in the absence of complex kinetics (e.g. in A + B → C systems) oscillatory dynamics can occur in spatially extended systems37 when coupled with hydrodynamic effects. The pioneering work of Gàlfi and Ràcz showed that when two pools of reactant solutions A and B initially separated in space react upon diffusive mixing, a reaction–diffusion (RD) front emerges.38,39 In systems where both reactants present the same initial characteristics (diffusion rate and concentrations), this RD front is localized at the initial position of contact, but as small discrepancies are introduced in the system, the front starts propagating in time.40,41
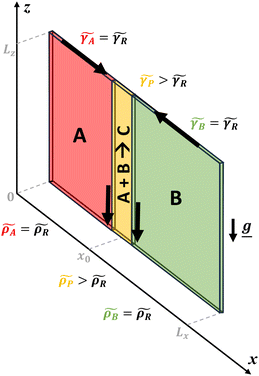
In this context, the occurrence of complex dynamics has been investigated in simple bimolecular reactions in the presence of natural convection.37,42,43 In reactive fluids, local gradients in the physical properties of the system (density and/or surface tension) can be induced as changes in concentration and/or temperature occur in the system, further coupling with the initial RD front and setting the fluid itself in motion. This configuration is modeled in Fig. 1 and will be further discussed in the next section.
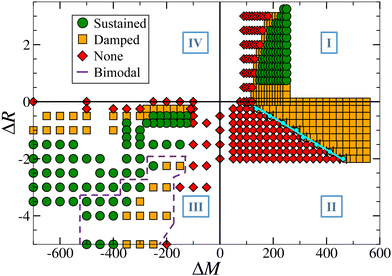
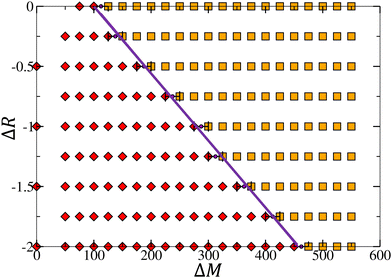
Previous studies have shown that damped oscillations can emerge from a competitive mechanism involving oppositely directed vertical transport processes when the reaction increases surface tension: an upward diffusion relaxation and a downward compressive Marangoni-induced flow. These oscillatory dynamics are further sustained by the addition of so-called antagonistic buoyancy driven flows induced by a local decrease in density and reinforcing the vertical diffusion fluxes [Fig. 2 region I, where ΔM and ΔR are parameters representing the changes in surface tension and density occurring during the reaction, respectively, and will be fully defined in the next section].37,42,43
A dynamical regime is defined as oscillatory if the temporal evolution of a variable (concentration, stream function, vorticity,…) presents at least two successive maxima. Oscillations with rapidly decreasing amplitude (of the variable) over time are defined as damped oscillations, whereas oscillations with quasi-constant amplitude over time are defined as sustained. Since the system is closed and will reach equilibrium at very long times, all the oscillations are eventually damped but in the timeframe considered in this study, it is possible to distinguish damped oscillations from sustained ones. If the system is kept out of equilibrium by introducing an inflow of reactants and an outflow of the products, sustained oscillations will persist.37
In the opposite antagonistic case where a decrease in surface tension is coupled to an increase in density [Fig. 2 region III], the competition between both convective flows is essential for the occurrence of oscillatory dynamics.44 In both antagonistic cases, sustained oscillations were observed in an optimal range of ΔM and ΔR, eventually dampening and going extinct as one of the convective effects becomes predominant.
Similar effects have been seen, both numerically and experimentally, in non-reactive systems where the introduction of a droplet inducing changes in surface tension led to nonlinear oscillatory dynamics of the surface tension. These oscillations were further affected when buoyancy effects due to a difference in density between the droplet and the solvent were at play, modifying for example the period/amplitude of oscillations or the onset of oscillatory dynamics.45–47
In this paper, we review the dynamics observed when a local production of C increases both surface tension and density [Fig. 2 region II], completing the classification of oscillatory behavior observed in chemo-Marangoni-buoyancy regime for equal diffusion coefficients and initial properties of all species. Here, both convective flows are acting cooperatively, i.e. we expect the convection rolls to reinforce themselves. In autocatalytic fronts, such a coupling between chemically-induced flows has highlighted particular dynamics (steady asymptotic regimes, also observed in the pure solutal Marangoni effect), different to the ones observed in an antagonistic configuration (spatio-temporal oscillations).48,49 We note that the opposite cooperative case (i.e. when a local production of C decreases both surface tension and density [Fig. 2 region IV]), has also been investigated but showed no oscillations and will therefore not be discussed.
2. Model
The model system, represented in Fig. 1 is a two-dimensional solution layer of length LX and height LZ in the (X, Z) reference frame, where Z is oriented against the gravitational acceleration g = (0, −g). We suppose the reactor isothermal, closed at the bottom and lateral borders and open at the top border with an air–liquid interface supposed non-deformable. Any evaporation is neglected. The system consists of two miscible reactant solutions (A and B) initially separated horizontally asAs the reactant solutions, assumed to have the same properties (density, surface tension and equal initial concentrations), react upon diffusive mixing across the contact line, the product C is formed following the bimolecular A + B → C reaction. The coupling of the latter with molecular diffusion allows for the propagation of a planar reaction–diffusion front, inducing local changes in surface tension and density. The resulting gradients in surface tension and density further trigger convective transport in the system.
The nonlinear dynamics is governed by a set of partial differential reaction–diffusion–convection (RDC) equations for the chemical species A, B, C (eqn (1)–(3)), coupled to the incompressible Navier–Stokes equations for the fluid velocity (eqn (4) and (5))
| ∂TA + (V·∇)A = D∇2A − kAB, | (1) |
| ∂TB + (V·∇)B = D∇2B − kAB, | (2) |
| ∂TC + (V·∇)C = D∇2C + kAB, | (3) |
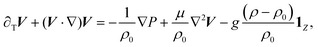 | (4) |
| ∇·V = 0, | (5) |
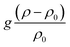 . The chemo-hydrodynamical coupling is made via the state equation for the density
. The chemo-hydrodynamical coupling is made via the state equation for the density 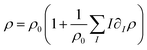 , and the surface tension,
, and the surface tension, 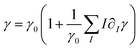 , where I = A, B, C are the dimensional concentrations of the chemical species. In diluted solutions, these equations are assumed to be linear combinations of the chemical concentrations, with
, where I = A, B, C are the dimensional concentrations of the chemical species. In diluted solutions, these equations are assumed to be linear combinations of the chemical concentrations, with 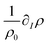 and
and 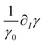 representing respectively the density and surface tension solutal coefficient of the Ith species.51ρ0 and γ0 represent the solvent density and surface tension respectively.
representing respectively the density and surface tension solutal coefficient of the Ith species.51ρ0 and γ0 represent the solvent density and surface tension respectively.
A Marangoni boundary condition (eqn (6)) is imposed at the free surface to describe the chemically-induced shear stress
| μ∂ZU = ∂Xγ, V = 0 at Z = LZ. | (6) |
The system's dimensionless form is expressed by using the reaction–diffusion scales for concentration, A0, time, t0 = 1/kA0, length, 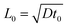 , and the derived scales for velocity,
, and the derived scales for velocity, 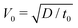 , pressure, P0 = μ/t0, density,
, pressure, P0 = μ/t0, density, 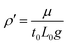 , and surface tension,
, and surface tension, 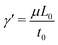 . The dimensionless solutal Rayleigh, Ri, and Marangoni, Mi, numbers of the Ith species represent the contribution of each species to the density, (∂Iρ), and surface tension, (∂Iγ), respectively. They are defined as
. The dimensionless solutal Rayleigh, Ri, and Marangoni, Mi, numbers of the Ith species represent the contribution of each species to the density, (∂Iρ), and surface tension, (∂Iγ), respectively. They are defined as
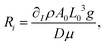 | (7) |
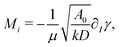 | (8) |
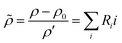 , and the dimensionless surface tension,
, and the dimensionless surface tension, 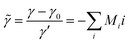 (with i representing the dimensionless concentration of A, B and C).37,42,43
(with i representing the dimensionless concentration of A, B and C).37,42,43
When the reactants have same initial concentrations and identical diffusion coefficients, the conservation of mass37 implies that a + b + 2c = 1 ∀ ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x, z, t. This allows to reconstruct the dimensionless concentration field of the product c from the reactants concentrations a and b by introducing two key parameters ΔR and ΔM, defined as, ΔR = R − Rc/2 and ΔM = M − Mc/2 respectively, where R = RA = RB, M = MA = MB. ΔR (ΔM) tunes the relative importance of buoyancy (surface tension) to the convective flows.
x, z, t. This allows to reconstruct the dimensionless concentration field of the product c from the reactants concentrations a and b by introducing two key parameters ΔR and ΔM, defined as, ΔR = R − Rc/2 and ΔM = M − Mc/2 respectively, where R = RA = RB, M = MA = MB. ΔR (ΔM) tunes the relative importance of buoyancy (surface tension) to the convective flows.
The final dimensionless RDC equations, in which the lowercase characters represent dimensionless variables, therefore read
| ∂ta + (v·∇)a = ∇2a − ab, | (9) |
| ∂tb + (v·∇)b = ∇2b − ab, | (10) |
| ∂tv + (v·∇)v = Sc(−∇p + ∇2v − ΔR(∂xa + ∂xb)1z), | (11) |
| ∇·v = 0, | (12) |
| ∂zu = ΔM(∂xas + ∂xbs) at z = Lz. | (13) |
Eqn (9)–(12) are solved numerically by using the alternating direction implicit (ADI) method52,53 with the defined initial and boundary conditions. Typical simulations are run over a spatial domain of dimensionless length Lx = 256 and variable dimensionless height Lz, discretized over a grid with the integration space steps hx = hz = 0.25 and the integration time step ht = 10−5.
3. Results
3.1. Explaining the role of buoyancy in the cooperative region II
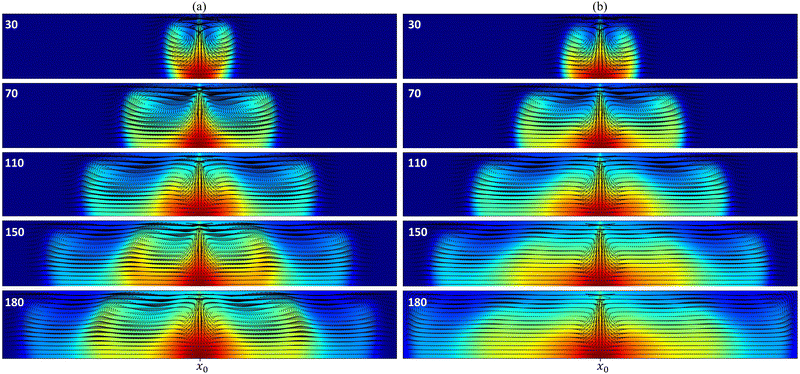
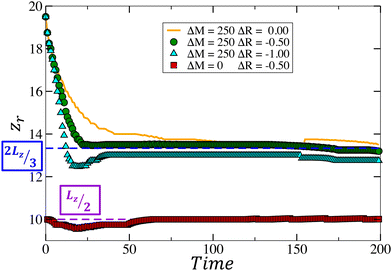
In the pure chemo-Marangoni regime, when the reaction increases surface tension, the mechanism at the core of the oscillatory dynamics is the competition between the Marangoni-induced flow and the vertical diffusion relaxation. The addition of an upward buoyancy-driven flow (ΔR > 0) reinforces the mechanism leading to sustained oscillations for a range of optimal values of ΔR. In contrast with region I, the addition of a downward buoyancy-driven flow (ΔR < 0) not only fails in providing sustained oscillations in the cooperative case (region II) but also can quench the dynamics. The underlying role of ΔR in this cooperative coupling is described below.As the reaction occurs, density and surface tension locally increase in the reactive zone, giving rise to a velocity field initially characterized by two convection rolls, one on either side of the reactive zone. The position of the center of a convective roll along the z-axis (zr) is computed as the position in the system where both the horizontal and vertical velocities are null. In the absence of gravitational forces [Fig. 3a], the convective rolls are centered around 2Lz/3 [Fig. 4 ΔM = 250, ΔR = 0.00] and oscillations develop according to the mechanism summarized above. By contrast, in the other limit case with no Marangoni contribution (i.e. in systems closed at the top surface), the convective rolls resulting from the density gradient are centered at Lz/2 [Fig. 4 ΔM = 0, ΔR = −0.50] but no oscillatory dynamics have been observed.
When both effects are coupled, as |ΔR| increases the convective cells initially centered at 2Lz/3 are gradually pushed toward the bottom of the system [Fig. 4 ΔM = 250, ΔR = −0.50 and ΔM = 250, ΔR = −1.00]. The amount of product c reaching the surface of the system thus decreases [as can be seen in Fig. 3 at t = 110–150–180 by comparing the convection roll's height in both cases] and, past a certain intensity of the buoyancy-driven flow, becomes insufficient to restore surface tension gradients, further disabling the oscillations. Thus, the coupling with an downward buoyancy flow does not sustain the oscillatory dynamics. Indeed, it dampens them by modifying the velocity field and preventing the renewal of the necessary conditions (i.e. sufficient surface tension gradients) for the emergence of new convective rolls.
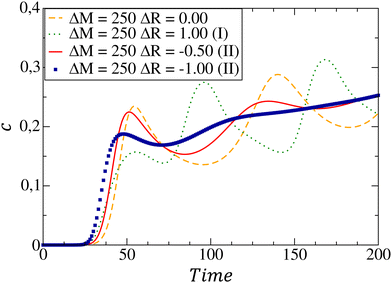
The temporal evolution of the local concentration of C is illustrated in Fig. 5 for different combinations of Marangoni and buoyancy contributions. In the pure chemo-Marangoni-driven dynamics (ΔM = 250, ΔR = 0.00), damped oscillations are observed as long as a sufficient gradient in surface tension (ΔM >ΔMcrit) is created by the reaction.37,42 The addition of antagonistically oriented buoyant flows (ΔM = 250, ΔR = 1.00), allows for sustained oscillations37,42,43 to be reached in particular ranges of ΔM and ΔR. In the presently studied case, even if both flows reinforce each other, damped oscillatory dynamics are observed for specific combinations of the hydrodynamic parameters. However, the oscillation's amplitude decreases as buoyancy-induced flows are added (ΔM = 250, ΔR = −0.50), further quenching the oscillatory dynamics (ΔM = 250, ΔR = −1.00) due to the pushing of the convective cells to the bottom of the system.
3.2. Conditions to induce oscillations
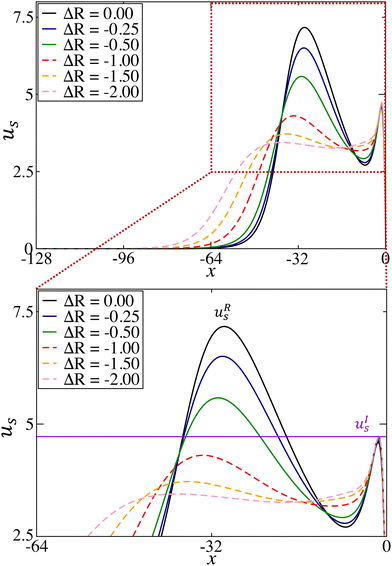
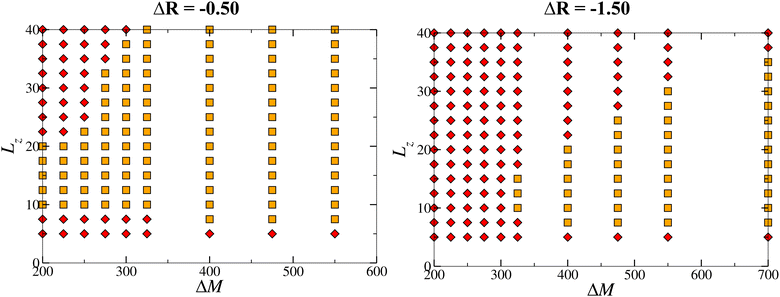
The complete picture of the dynamical regimes obtained in region II by varying the convective flows intensity is represented in Fig. 6 and highlights major differences with regions I and III. First, the addition of buoyancy flows allows for the emergence of sustained oscillations in previously studied regions I and III, but can only lead to the dampening of the dynamics in this cooperative case. This shows that the coupling can either have a positive/negative impact on the dynamics by sustaining/quenching them, depending on the changes in density occurring during the reaction. More importantly, it emphasizes that no sustained oscillations are observed in the cooperative case. Secondly, there is a threshold separating non-oscillatory and oscillatory scenarios which scales linearly with the two hydrodynamic parameters ΔM and ΔR.To observe oscillatory dynamics in the case of a cooperative coupling between flows, several conditions have to be fulfilled. Firstly, the surface tension gradient induced by the reaction must be sufficient to initiate an oscillatory motion, which means that the convection rolls formed must have sufficient horizontal surface velocity to carry a sufficiently large quantity of product away from the center of the reaction front (by the return flow). In particular, we found that at the surface, in both the pure chemo-Marangoni and in the chemo-Marangoni-buoyancy driven systems, the horizontal velocity associated with the first convective roll uRs must be greater than the initial horizontal velocity uIs (close to the center of the system, in the reactive zone) [Fig. 7]. Since the role of buoyancy is to displace the center of the convective rolls to the lower part of the system, it effectively reduces the horizontal velocity, thereby decreasing the amount of product c brought to the surface and quenching the oscillations.
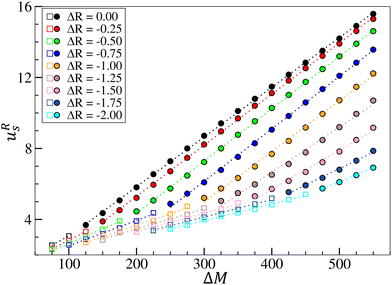
Based on the Marangoni boundary condition (eqn (13)), it is possible to identify a scaling between ΔM and the horizontal surface velocity. In fact, the typical form of the horizontal component of the Marangoni return flow scales as 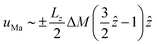 and fits well with the velocity profiles developed in pure chemo-Marangoni systems.42Fig. 8 shows that the maximum horizontal surface velocity linearly increases with ΔM in the different regimes, as predicted by the analytical solution for the Marangoni return flow uMa. A higher slope is observed in the presence of oscillatory dynamics and the velocity field is more intense than in their absence, revealing two distinct linear relations.
and fits well with the velocity profiles developed in pure chemo-Marangoni systems.42Fig. 8 shows that the maximum horizontal surface velocity linearly increases with ΔM in the different regimes, as predicted by the analytical solution for the Marangoni return flow uMa. A higher slope is observed in the presence of oscillatory dynamics and the velocity field is more intense than in their absence, revealing two distinct linear relations.
In this regime II, oscillations are associated with high horizontal velocities as long as there is a dominating effect of the Marangoni contribution (ΔM). High flows do not trivially imply oscillations, as can be seen in Fig. 8 where non-oscillating systems (e.g. ΔM = 450 ΔR = −2.00) present higher maximum horizontal velocities than oscillating systems (e.g. ΔM = 175 ΔR = −0.25). A similar observation applies to previously studied regimes as well.37,42–44
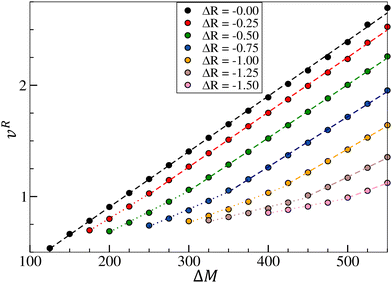
A second condition arising from the first one is that the product must arrive at the surface in sufficient quantity to recreate gradients and thus re-initiate oscillations. Fig. 9 shows that the maximum vertical velocity vR in the system decreases as density effects are increased, whatever the values of ΔM considered. The denser the species produced, the lower the maximum vertical velocity, due to the intensification of downward buoyancy-driven flow. As the intensity of the return flow decreases, less product is brought back to the surface of the system, inhibiting the reconstruction of a gradient at the surface and driving the dampening of the oscillatory dynamics.
Furthermore, Fig. 9 highlights two distinct linear relationships between the flow intensity and the control hydrodynamic parameter ΔM, with the maximum vertical velocity increasing with the surface tension variation. In the pure chemo-Marangoni case (ΔR = 0), a linear relationship is observed between vR and ΔM. As buoyancy-driven effects are added, a deviation from linearity appears for oscillating systems close to the threshold separating the dynamics (defined by the purple line in Fig. 6) and two linear regimes are thus distinguished. The first one (dotted curves) is close to the boundary where oscillations start to appear and hydrodynamic parameters counterbalance each other. In the second regime (dashed curves), systems are assimilated to pure chemo-Marangoni cases, since density effects are significantly weaker than surface tension effects and are therefore annihilated.
3.3. Influence of Lz
As the amount of product c reaching the surface appears to be a key ingredient for the onset and the control of the oscillatory dynamics, and as it is directly related to the system's height (Lz), we expect Lz to be an important control parameter. We therefore explore the influence of the latter.Previous studies in the pure chemo-Marangoni case have shown that, in thin layers, the initial convective rolls responsible for the oscillatory dynamics cannot develop and a minimum cell's height (Lminz) below which wave formation is hindered has been highlighted. Furthermore, Lminz varies with the initial change in surface tension necessary to induce oscillatory dynamics and scales as Lminz ∼ ΔM−1. An additional scaling between the period τ at which oscillations are emitted from the center and Lz has been identified42 as τ ∼ Lz2.
In region I, buoyancy contributions to the flow are at play and the velocity field consists not only of two counter-rotating rolls due to the converging Marangoni flow, but also of two counter-rotating rolls at the bottom of the reactor, pushing the fluid upwardly at x0.37,42 Increasing the thickness of the cell, increases the size and intensity of buoyancy-driven forces, which can enhance the oscillatory mechanism (reinforcing the relaxation phase of the cycle) when moderate but, eventually, suppress oscillations as soon as they become dominant and prevail over Marangoni forces. Correspondingly, the characteristic period of the oscillation, τ, first increases as the cell enlarges due to a larger displacement of the concentration field during each spatio-temporal oscillation. However, above a threshold height LMaxz, linked to a maximum value of τ, the period further decreases linearly as the system gets thicker, marking a switch from Marangoni- to buoyancy-controlled dynamics.
In the case studied here (region II), a range of Lz in which oscillatory dynamics are observed has been highlighted for different values of ΔM and ΔR (Fig. 10). This range increases (decreases) as ΔM (ΔR) increases at constant ΔR (ΔM), between LMinz and LMaxz. In agreement with previous observations, if the system is too thin, the initial Marangoni convective rolls cannot develop, whereas if the system is too thick, the latter rolls are shrunk due to the extend of buoyancy convective rolls and the oscillatory dynamics are inhibited.
However, Fig. 11 shows that the relation τ(Lz) takes the form τ ∼ Lz2 for all values of ΔM and ΔR tested, and an increase in ΔM (|ΔR|) translates in an increase (decrease) in the oscillatory period. In contrast to the antagonistic coupling in region I, the period evolves here similarly to the pure Marangoni case. This observation is in line with results obtained in the previous section, showing that the cooperatively coupled cases actually behave like pure Maragoni-driven systems, in which the addition of buoyancy-driven flows has no other effect than to inhibit the complex dynamics.
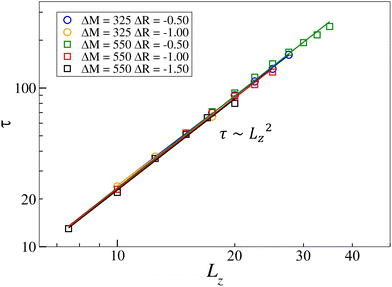 | ||
| Fig. 11 Characterization of the oscillation period, τ, as a function of the system height, Lz, for different values of ΔM and ΔR. The solid lines represent linear regressions. | ||
4. Conclusions
In this paper, we have expanded the realm of complex dynamics in bimolecular reactions where Marangoni- and buoyancy-driven flows are triggered by the chemical process. In particular, we have shown that chemo-hydrodynamic oscillations, known to develop in the presence of an antagonistic coupling between Marangoni- and buoyancy-driven contributions, can persist even in a cooperative configuration though the buoyancy effect plays here a quenching role.The dynamics observed in regions I (antagonistic) and II (cooperative) present morphological similarities, as the core mechanism responsible for the onset of oscillatory dynamics is the same (i.e. introduced in the pure Marangoni case). However, when a bimolecular reaction increases surface tension, the precise nature of the dynamics is determined by the way Marangoni-driven flows are coupled to density effects. When antagonistically coupled (ΔR > 0), the initial oscillatory dynamics can be sustained. When a cooperative coupling is at play (ΔR < 0), the oscillations will always be dampened as the reinforcement of the initial flow pushes the convective cells to the bottom of the system.
The key role of the surface tension gradient in inducing oscillations has already been demonstrated from previous studies.37,42,44 Here, we deepen the physical understanding of the gradients' key role by showing the importance of the horizontal velocity at the surface associated to the convective roll (uRs). In fact, in order for the system to present oscillations, the latter must be greater than the initial horizontal velocity (uIs) close to the system's center. Furthermore, distinct linear relations between uRs and ΔM have been highlighted in both oscillatory and non-oscillatory regimes. These relations match the analytical scaling predicted for the Marangoni return flow.
In addition, the maximum vertical velocity in the system decreases as the density effects intensify. In turn, less product is brought back to the surface, thus preventing the renewal of a gradient at the surface, which is essential for further oscillations. Moreover, two linear relationships between the maximum vertical velocity and ΔM have been demonstrated, separating two distinct regimes. In the first regime hydrodynamic parameters counterbalance each other, while in the second one coupled systems can be assimilated to pure chemo-Marangoni systems when surface tension effects are significantly stronger than density effects.
Finally, the variation of the system's height Lz shows that oscillatory dynamics are observed only in a range of Lz, which depends on both hydrodynamic parameters ΔM and ΔR. A scaling between the period of oscillation τ and Lz has been identified and takes the form τ ∼ Lz2, characteristic of pure Marangoni cases. The latter observation reinforces the idea that the cooperatively coupled cases behaves like pure Marangoni systems.
This work completes the general classification of oscillatory dynamics in a (ΔM, ΔR) plane, paving the way for further theoretical studies in asymmetric systems (different physical properties/diffusion coefficients/concentrations for all the chemical species). This complete picture also extends the range of chemical reactions that can be explored to demonstrate chemo-hydrodynamic oscillations experimentally.
Data availability
The data supporting the findings of this study have been included as part of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Fundings by the PDR-FNRS DYNAMO project and Fondation ULB are gratefully acknowledged. M. A. B. gratefully acknowledges funding from the University of Sassari (Fondo di Ateneo per la Ricerca 2020).Notes and references
- G. P. Misra and R. A. Siegel, J. Controlled Release, 2002, 79, 293–297 CrossRef CAS PubMed.
- R. Yoshida, Adv. Mater., 2010, 22, 3463–3483 CrossRef CAS PubMed.
- V. V. Yashin, O. Kuksenok and A. C. Balazs, Prog. Polym. Sci., 2010, 35, 155–173 CrossRef CAS.
- A. Isakova and K. Novakovic, J. Mater. Chem. B, 2018, 6, 5003–5010 RSC.
- T. Geher-Herczegh, Z. Wang, T. Masuda, R. Yoshida, N. Vasudevan and Y. Hayashi, Macromolecules, 2021, 54, 6430–6439 CrossRef CAS.
- S. Li, M. M. Lerch, J. T. Waters, B. Deng, R. S. Martens, Y. Yao, D. Y. Kim, K. Bertoldi, A. Grinthal, A. C. Balazs and J. Aizenberg, Nature, 2022, 605, 76–83 CrossRef CAS PubMed.
- D. Przyczyna, P. Zawal, T. Mazur, P. L. Gentili and K. Szacilowski, Jpn. J. Appl. Phys., 2020, 59, 050504 CrossRef CAS.
- P. L. Gentili, M. S. Giubila and B. M. Heron, ChemPhysChem, 2017, 18, 1831–1841 CrossRef CAS.
- P. L. Gentili, M. S. Giubila, R. Germani and B. M. Heron, Dyes Pigm., 2018, 156, 149–159 CrossRef CAS.
- Z. Neufeld, P. H. Haynes, V. Garçon and J. Sudre, Geophys. Res. Lett., 2002, 29, 1534–1538 CrossRef.
- E. Hernández-Garcia and C. López, Ecol. Complex., 2004, 1, 253–259 CrossRef.
- A. M. Turing, Phil. Trans. R. Soc. Lond. B, 1952, 237, 37–72 Search PubMed.
- P. Richard, B. M. Bakker, B. Teusink, K. Van Dam and H. V. Westerhoff, Eur. J. Biochem., 1996, 235, 238–241 CrossRef CAS PubMed.
- A. Goldbeter and M. J. Berridge, Biochemical Oscillations and Cellular Rhythms: The Molecular Bases of Periodic and Chaotic Behaviour, Cambridge University Press, 1996 Search PubMed.
- J. D. Murray, Mathematical Biology: I. An Introduction, Springer, New York, 2002, vol. 17 Search PubMed.
- D. Gonze, J. Halloy and A. Goldbeter, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 673–678 CrossRef CAS PubMed.
- S. Jelić, Ž. Cupić and L. Kolar-Anić, Math. Biosci., 2005, 197, 173–187 CrossRef.
- A. F. Taylor, M. R. Tinsley, F. Wang, Z. Huang and K. Showalter, Science, 2009, 323, 614–617 CrossRef CAS.
- M. Toiya, H. O. González-Ochoa, V. K. Vanag, S. Fraden and I. R. Epstein, J. Phys. Chem. Lett., 2010, 1, 1241–1246 CrossRef CAS.
- A. Goldbeter, C. Gérard, D. Gonze, J.-C. Leloup and G. Dupont, FEBS Lett., 2012, 586, 2955–2965 CrossRef CAS PubMed.
- M. Wickramasinghe and I. Z. Kiss, PLoS One, 2013, 8, e80586 CrossRef PubMed.
- G. Dupont, M. Falcke, V. Kirk and J. Sneyd, Models of Calcium Signalling, Springer International Publishing, Cham, 2016, vol. 43 Search PubMed.
- M. A. Budroni, K. Torbensen, S. Ristori, A. Abou-Hassan and F. Rossi, J. Phys. Chem. Lett., 2020, 11, 2014–2020 CrossRef CAS PubMed.
- M. A. Budroni, G. Pagano, D. Conte, B. Paternoster, R. D’ambrosio, S. Ristori, A. Abou-Hassan and F. Rossi, Phys. Chem. Chem. Phys., 2021, 23, 17606–17615 RSC.
- B. Belousov, Sbornik Referatov po Radiatsionni Meditsine, 1958, pp. 145–147 Search PubMed.
- W. Bray and H. Liebhafsky, J. Am. Chem. Soc., 1931, 53, 38–44 CrossRef CAS.
- L. Negrojević, A. Lončar, J. Maksimović, S. Anić, Ž. Čupić, L. Kolar-Anić and N. Pejić, React. Kinet. Mech. Catal., 2022, 135, 1147–1162 CrossRef.
- Z. Nagy-Ungvarai, S. Müller and B. Hess, Chem. Phys. Lett., 1989, 156, 433–437 CrossRef CAS.
- Z. Li, L. Yuan, M. Liu, Z. Cheng, J. Zheng, I. R. Epstein and Q. Gao, J. Chem. Educ., 2021, 98, 665–668 CrossRef CAS.
- K. Novakovic, L. Bruk and O. Temkin, RSC Adv., 2021, 11, 24336–24344 RSC.
- Ž. Cupić, S. Maćešić, S. Anić, L. Kolar-Anić, A. Ivanović-Šašić and K. Novakovic, React. Kinet. Mech. Catal., 2022, 135, 3–14 CrossRef.
- K. Pȩkala, R. Jurczakowski, A. Lewera and M. Orlik, J. Phys. Chem. A, 2007, 111, 3439–3442 CrossRef PubMed.
- A. Wiśniewski, M. T. Gorzkowski, K. Pekala and M. Orlik, J. Phys. Chem. A, 2013, 117, 11155–11166 CrossRef.
- E. Toki, S. Osaki, M. Nakamura, K. Tsuoka, N. Samejima, T. Nakashima, R. Abe, T. Oyama and K. Tsukiyama, Int. J. Chem. Kinet., 2020, 52, 907–917 CrossRef CAS.
- I. Prigogine and R. Lefever, J. Chem. Phys., 1968, 48, 1695–1700 CrossRef.
- R. J. Field and R. M. Noyes, J. Chem. Phys., 1974, 60, 1877–1884 CrossRef CAS.
- M. A. Budroni, V. Upadhyay and L. Rongy, Phys. Rev. Lett., 2019, 122, 244502 CrossRef CAS.
- L. Gálfi and Z. Rácz, Phys. Rev. A, 1988, 38, 3151–3154 CrossRef.
- Z. Jiang and C. Ebner, Phys. Rev. A, 1990, 42, 7483–7486 CrossRef CAS.
- Y. E. L. Koo and R. Kopelman, J. Stat. Phys., 1991, 65, 893–918 CrossRef.
- H. Taitelbaum, Y.-E. L. Koo, S. Havlin, R. Kopelman and G. H. Weiss, Phys. Rev. A, 1992, 46, 2151–2154 CrossRef CAS PubMed.
- M. A. Budroni, A. Polo, V. Upadhyay, A. Bigaj and L. Rongy, J. Chem. Phys., 2021, 154, 114501 CrossRef CAS.
- M. A. Budroni, F. Rossi and L. Rongy, ChemSystemsChem, 2021, 4, e202100023 CrossRef.
- A. Bigaj, M. A. Budroni, D. M. Escala and L. Rongy, Phys. Chem. Chem. Phys., 2023, 25, 11707–11716 RSC.
- N. M. Kovalchuk and D. Vollhardt, J. Phys. Chem. B, 2003, 107, 8439–8447 CrossRef CAS.
- N. M. Kovalchuk and D. Vollhardt, Phys. Rev. E, 2004, 69, 016307 CrossRef CAS PubMed.
- N. M. Kovalchuk and D. Vollhardt, J. Phys. Chem. B, 2005, 109, 15037–15047 CrossRef CAS PubMed.
- L. Rongy and A. De Wit, J. Chem. Phys., 2006, 124, 164705 CrossRef CAS PubMed.
- M. A. Budroni, L. Rongy and A. De Wit, Phys. Chem. Chem. Phys., 2012, 14, 14619–14629 RSC.
- D. Tritton, Physical Fluid Dynamics, Clarendon Press, 1988 Search PubMed.
- P. Cheng, M. Bestehorn and A. Firoozabadi, Water Resour. Res., 2012, 48, 10902 CrossRef.
- D. W. Peaceman and H. H. Rachford, J. Soc. Indus. App. Math., 1955, 3, 28–41 CrossRef.
- C. Fletcher and K. Srinivas, Computational Techniques for Fluid Dynamics 1, Springer, Berlin Heidelberg, 1991 Search PubMed.
| This journal is © the Owner Societies 2025 |

![[small gamma, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0dd.gif)
![[small rho, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e4.gif)