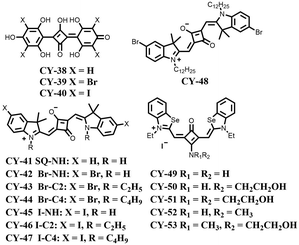
Design strategies and applications of cyanine dyes in phototherapy
Jie
Yuan
a,
Hanxue
Yang
a,
Wenhui
Huang
 a,
Shilong
Liu
a,
Shilong
Liu
 a,
Hua
Zhang
a,
Hua
Zhang
 *a,
Xiaobing
Zhang
*a,
Xiaobing
Zhang
 *b and
Xiaojun
Peng
*b and
Xiaojun
Peng
 *cd
*cd
aKey Laboratory of Green Chemical Media and Reactions, Ministry of Education, Henan Key Laboratory of Organic Functional Molecule and Drug Innovation, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453007, P. R. China. E-mail: zhh1106@htu.edu.cn
bState Key Laboratory for Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China. E-mail: xbzhang@hnu.edu.cn
cState Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian, China. E-mail: pengxj@dlut.edu.cn
dCollege of Materials Science and Engineering, Shenzhen University, Shenzhen University, Shenzhen 518035, China
First published on 22nd November 2024
Abstract
Cyanine dyes have been widely used in phototherapy in recent years due to their excellent optical properties and diverse modifiable structures. This review provides detailed descriptions of the basic structures of various cyanines and their derivatives as well as their optical properties. It summarizes the strategies for constructing cyanine dyes for phototherapy and discusses their structure–effect relationship. Furthermore, a comprehensive classification and summary of the applications of cyanine dyes in phototherapy are presented. Importantly, this review also addresses both the advances made in this field as well as the challenges that need to be overcome. We hope that these profound insights into phototherapy using cyanine dyes will facilitate the design of future systems for clinical applications based on these compounds.
1. Introduction
Compared with traditional treatments such as chemotherapy, radiotherapy and surgery, phototherapy (including photodynamic therapy (PDT) and photothermal therapy (PTT)) has emerged as a minimally invasive cancer treatment that effectively eliminates cancer cells and improves clinical outcomes.1–3 This is due to their advantages, such as non-drug resistance, non-invasiveness, safety and selectivity. In PDT, a photosensitizer in the excited triplet state interacts with oxygen or other nearby molecules under light irradiation to generate reactive oxygen species, which kill cancer cells through oxidative damage.4,5 In PTT, a photothermic agent is excited by a specific wavelength of light (usually in the near-infrared (NIR) spectrum range) and then returns to the ground state from its excited state accompanied by heat release to kill cancer cells.6,7 Although the action mechanisms of PDT and PTT are not exactly the same, phototherapy reagents are one of the indispensable elements in both therapies and play a key role in determining their therapeutic effects.8Ideal phototherapy agents possess the following properties: (1) strong absorption in the near-infrared region, (2) low or negligible dark toxicity, (3) good photostability, (4) effective tumour targeting ability, and (5) high capacity for generating reactive oxygen species or efficient conversion of light into heat energy.9 The ability to generate reactive oxygen species or heat is a prerequisite for being considered as a phototherapy agent. Recently, various types of phototherapy agents have been developed for PDT or PTT, including organic small molecules, such as cyanine dyes,10 porphyrins,11 BODIPY,12etc., as well as inorganic materials like transition metal compatibilizers,13 gold nanoscale,14 transition metal nanomaterials,15etc.
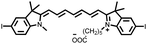
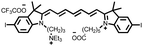
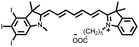
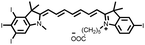
Among them, cyanine dyes (Fig. 1A), first reported by Charles Hanson Greville Williams in 1856, are most widely used in the phototherapy field. Cyanine dyes are characterized by two nitrogen heterocyclic rings connected via a π-conjugated polymethine chain.16–18 The nitrogen heterocyclic rings in the structure can be pyrrole, imidazole, thiazole, benzothiazole, pyridine, quinoline, etc.,18,19 and the hydrogen in the single carbon atom on the conjugated chain can also be replaced by other groups to produce different substituted dyes.20 The changes of nitrogen substituents in polymethine chains and heterocycles lead to the structural diversity of cyanine derivatives. Based on different charges on the cyanine conjugated system, cyanine dyes are divided into the following: (1) cationic polymethine cyanines and semicarbocyanine dyes, (2) anionic polymethine cyanines, (3) neutral polymethine cyanines, and (4) amphoteric ionic squaraine dyes.21,22 Because of the special molecular structure, most cyanine dyes exhibit unique characteristics such as high molar absorption coefficients and narrow absorption/emission bands in the NIR region, inherent photosensitizing or photothermal properties. Additionally, they demonstrate good biocompatibility, making them closer to meeting requirements for ideal phototherapy reagents.23 Thus, they have been widely used in biomedical fields.24–27 More importantly, indocyanine green (ICG) is a classical structure of cyanine dye compounds, is still the only NIR fluorophore approved by the U.S. Food and Drug Administration (FDA) for in vivo clinical usage and has been extensively utilized for guiding sentinel lymph node biopsies, lymphography, intraoperative imaging of certain cancers, and other clinical applications.28–31 Therefore, cyanine dye-based phototherapy agents have been extensively reported and have become preferred candidates for constructing phototherapy platforms. Especially, in recent years, researchers have successfully combined cyanine dyes with nanotechnology to enhance the effectiveness of phototherapy and have established numerous multiple phototherapy systems.
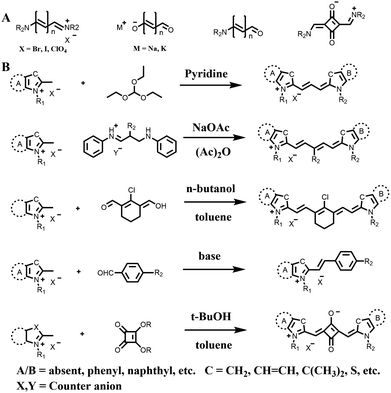 | ||
| Fig. 1 (A) The general structure formula of cyanine dyes. (B) Classical synthetic routes of polymethine cyanines, hemicyanines and squarylium cyanines. | ||
According to the application requirements of phototherapy, the excitation wavelength of cyanine dyes should fall within the optical window of biological tissue (650–1350 nm), which refers to the range of wavelengths where light can penetrate biological tissue most effectively. Therefore, this review will focus on polymethine cyanines, hemicyanines and squarylium cyanines, which are widely used cyanine dyes with excitation wavelengths in the 650–1350 nm range (Fig. 1B). We will describe their design strategies and explore their applications in PDT, PTT and multimode phototherapy. Additionally, we will provide personal perspectives on opportunities for advancing phototherapy agents and improving their practical translation into the clinic regarding PDT, PTT and multimode phototherapy. Finally, we will outline key challenges that need to be overcome for the continued and improved development of phototherapy agents.
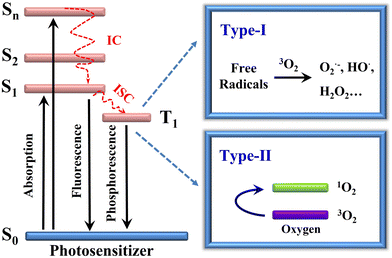
2. Cyanine dyes for PDT
To enhance the photosensitizing performance of photosensitizers, the PDT mechanism of photosensitization must be summarized and analyzed in detail. In a typical photosensitization PDT process (Fig. 2), the photosensitizer in the ground state absorbs photon energy and transitions from the ground state (S0) to the excited state (S1). Subsequently, it undergoes intersystem crossing (ISC), transferring from the excited singlet state (S1) to the excited triplet state (T1). The photosensitizer in the T1 state generates a large amount of reactive oxygen species by electron transfer (type I photochemical reaction) or energy transfer (type II photochemical reaction). The key factors for achieving effective PDT rely on two main aspects: (1) the occurrence of effective ISC and (2) the efficient transfer of electrons or energy in the T1 state. Furthermore, efficient electron or energy transfer in the T1 state depends on high ISC efficiency.32,33 Therefore, enhancing ISC becomes an effective approach for improving the photosensitization performance of photosensitizers. The occurrence of this process is closely related to both rates from the excited singlet state to the excited triplet state and differences in energies between them. Current strategies used to enhance ISC in cyanine dyes mainly include: (1) heavy-atom effects, (2) spin–orbit charge-transfer intersystem crossing (SOCT-ISC), (3) radical-enhanced intersystem crossing (RE-ISC), (4) reduction of the energy gap between the singlet and triplet states (ΔES–T), and (5) photoinduced molecular vibrational torsion-enhanced spin–orbit coupling-induced intersystem crossing (PVT-SOC-ISC). Next, we will summarize in detail the design strategies of cyanine dyes and their applications in PDT through typical cases focusing on these strategies.2.1 Heptamethine cyanines
Classical polymethine cyanines mainly contain cyanine 2 (Cy2), cyanine 3 (Cy3), cyanine 3.5 (Cy3.5), cyanine 5 (Cy5), cyanine 5.5 (Cy5.5), cyanine 7 (Cy7) and cyanine 7.5 (Cy7.5), and their basic excitation and emission wavelengths gradually increase with the increase of the conjugated methylidyne chain (Fig. 3A and B).34 The absorption wavelengths of parts of Cy5, Cy5.5 and most Cy7, Cy7.5 are in the optical window of biological tissues (650–1350 nm). They also cause less photodamage to organisms, minimize fluorescence background, and exhibit reduced light scattering. Therefore, they are often used as the parent structure for designing of photosensitizers in biomedical applications due to their specific photosensitizing properties.27,35–44 The promotion of the ISC process has become an effective strategy to improve the photosensitizing properties of polymethine cyanines. For polymethine cyanines, with the increase of the methine chain, the ISC process showed an enhanced trend. Therefore, among polymethine cyanines, Cy7 has a better ability of ISC, and it is easier to improve their photosensitizing performance by regulating its ISC process. Therefore, most phototherapeutic reagents are designed and synthesized based on Cy7 and its derivatives, which are the most widely used, so the main focus of this paper is summarized and elaborated around heptamethine cyanines.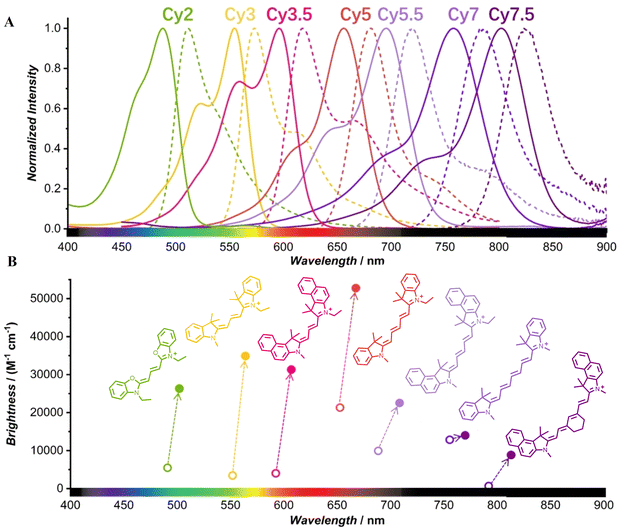 | ||
| Fig. 3 (A) Photophysical properties of supra-cyanines. Normalized UV/vis absorption spectra (solid lines) and fluorescence emission spectra (dash lines) of supra-cyanines in solution. (B) Comparison of the brightness between supra-cyanines (closed circles) and free cyanines (open circles). Adapted from ref. 34 with permission from the John Wiley and Sons, copyright 2023. | ||
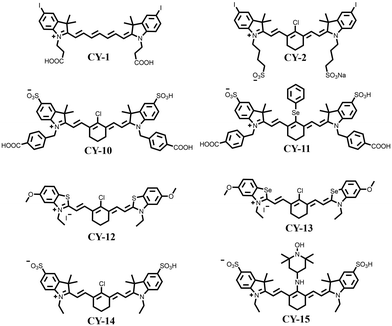
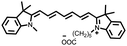
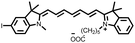
To enhance the photosensitizing properties of heptamethine cyanines with excitation wavelengths greater than 650 nm, researchers have modified their structure using a heavy atom strategy that enhances orbital spin–orbit coupling and facilitates the ISC process of the molecules.45–48 The introduction of heavy atoms like Br, I, and Se increases the rate of ISC, resulting in improved production of reactive oxygen species and enhanced performance as photosensitizers. For example, Cao et al. introduced iodine into a Cy7 dye using a heavy-atom strategy to obtain a novel near-infrared dye, CY-1 (Fig. 4).49 This modification significantly increased reactive oxygen species production with a 75% increase in a singlet oxygen (1O2) yield compared to an unmodified dye. Atchison et al. substituted hydrogen with iodine in IR783 to obtain CY-2 (Fig. 4),50 which exhibited significantly enhanced 1O2 generation under NIR excitation compared to ICG.
Differences in the number of iodine substitutions may affect the photophysical properties of the resulting compounds. Semenova et al. designed and thoroughly examined a series of novel NIR iodinated heptamethylo cyanine dyes for their photosensitizing properties (CY-3 to CY-9, Table 1).51 As expected, the 1O2 yields (ΦΔ) increased with an increasing number of iodine atoms (Table 1). It is noteworthy that the dyes CY-5 and CY-6 containing two iodines exhibit approximately the same ΦΔ, indicating that the photosensitizing properties are mainly affected by the heavy atoms in similar molecular structures. This is due to an increase in spin–orbit coupling, improvement in ISC efficiency, and consequently an increase in their 1O2 yields.
Motivated by the above, different heavy atoms such as Br, Se, Pt, etc. have also been investigated and introduced into the molecular structure of heptamethine cyanines to enhance their photosensitizing properties.52–56 Obviously, the introduction of these heavy atoms has significantly enhanced spin–orbit coupling to different extents, thereby facilitating the ISC process and ultimately leading to an increase in the 1O2 yield of the target structures. For example, Sun et al. introduced selenium into CY-10 to obtain CY-11.54 The incorporation of heavy atoms increased the ISC rate by enhancing spin–orbit coupling and consequently prolonged its excited triplet state lifetime (τ = 4.3 μs). A comparison between CY-10 and CY-11 revealed a remarkable 18-fold increase in the yield of 1O2 along with excellent PDT effects at the live cell level. Recently, Liu et al. also introduced S and Se into polymethine cyanines to obtain CY-12 and CY-13, respectively.57 Compared with ICG, the 1O2 yields of CY-12 and CY-13 increased 4.3 times and 24.5 times, respectively. All the above studies have proved that the introduction of heavy atoms is an effective means to facilitate the ISC process of the molecules and improve the photosensitivity of molecules.
Although the introduction of I atoms promotes the ISC process, it also leads to poor water solubility, causing molecule aggregation and reducing their reactive oxygen species yield.50–52 To overcome this problem and enhance its applicability in vivo tumour therapy, Dong et al. combined CY-1 with liposomes to construct a novel liposome (LCT) co-loaded with CY-1 and the chemotherapeutic drug (TPZ).58 This facilitates the accumulation of CY-1 at the tumour site and effectively eliminates the tumour by efficiently generating reactive oxygen species and heat.
The introduction of heavy atoms into the skeleton structure of heptamethine cyanines can effectively increase the 1O2 yield, but at the same time it may bring additional dark toxicity, which is not favourable for further biological applications. To address this issue, Jiao et al. incorporated a stable radical substituent, namely 4-amino-TEMPO, into CY-14 to obtain CY-15 (Fig. 4),59 resulting in significant enhancement of the ISC process by the RE-ISC mechanism. Compared with CY-14, CY-15 not only exhibits a greater increase in the 1O2 yield (about a 33-fold increase) but also has greater photostability and lower dark toxicity, thus displaying greater potential for clinical applications.
Hao et al. developed an orthorhombic molecular cationization system,60 in which the cationic pyridine was introduced at the middle position of the asymmetric CY-16 to construct an atypical electron-transferring triad, CY-17 (Fig. 5). This strategy reduces ΔES1–S0, avoids rapid charge recombination and simultaneously enhances ISC based on the SOCT-ISC mechanism. Time-varying density-functional theory calculations for both compounds show that the ΔES–T value of CY-17 is 0.528 eV with a high spin–orbit coupling constant (SOC (S1–T1) = 1.085 cm−1), while the ΔES–T value of CY-16 is determined to be 0.713 eV with a relatively smaller SOC (S1–T1) at 0.028 cm−1. Smaller ΔES–T values and higher SOC constants favored the ISC process. Researchers used 1,4-diphenyl-2,3-benzofuran (DPBF) as a probe and ICG as a control to determine the reactive oxygen species-generating ability of both compounds. The results showed that CY-16 had almost the same reactive oxygen species generation capacity as ICG (ΦΔ = 0.2%), while that of CY-17 exhibited an elevated capacity by nearly five-fold (ΦΔ = 1.1%). All these factors fully demonstrate the effectiveness of the SOCT-ISC mechanism.
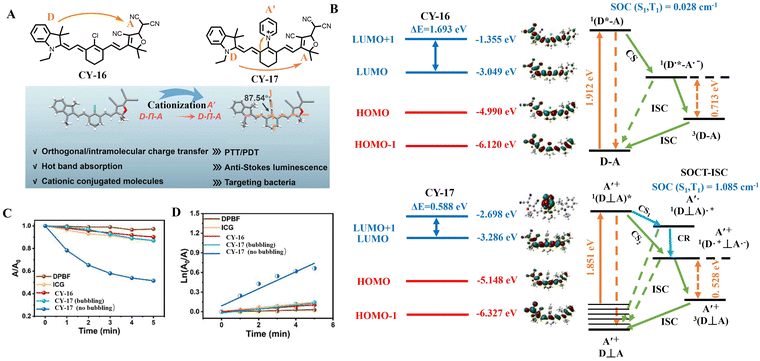 | ||
| Fig. 5 (A) Molecular design and the crystal structure of triad CY-16 based on ICT and SOCT-ISC mechanisms. (B) The frontier molecular orbital energy level diagrams of CY-16 and CY-17. (C) Time-dependent absorbance (A/A0) of DPBF at 420 nm in ICG, CY-16, CY-17 (bubbling) or CY-17 (no bubbling) solution under laser irradiation (808 nm, 0.3 W cm−2) for 5 min. (D) The linear-fit results are based on the absorbance of DPBF at 420 nm at different periods of light irradiation. Adapted from ref. 60 with permission from the Elsevier, copyright 2024. | ||
The PVT-SOC mechanism as another typical regulatory strategy could avoid the additional cytotoxicity40,61–63 and the interference from the solvent environment to some extent.64 Zhou et al. revealed for the first time the PVT-SOC mechanism in CY-18 and its contribution to enhancing ISC capability, and successfully applied it to PDT (Fig. 6).65 A series of NIR photosensitizers with a high 1O2 yield (33.8%) and a reasonable fluorescence quantum yield (31%) were successfully obtained by ester-based modification of CY-18 using intramolecular vibrational torsion motion. The enhancement of the ISC efficiency was logically interpreted by the PVT-SOC mechanism: the molecule undergoes light-induced vibrational torsional motion in the S1 state, leading it to reach another energetically close Franck–Condon minimum (S1V) on the potential energy surface. In this case, the 1ππ*–3nπ* transition between S1V and T2 dramatically increases SOC and thus enhances kISC (∼108 s−1). Compared with a commercial photosensitizer (Ce6), CY-19 exhibits lower cytotoxicity and broad spectrum cell membrane disruption, thereby demonstrating superior potential for PDT with 11-fold enhancement at low light doses. The inherent structural targeting of tumours confers CY-19 with a strong accumulation capacity at the tumour site and enables effective photoablation of tumours under NIR irradiation (95%). These findings not only break the limitations of conventional quinoid cyanine (QCy) dyes for cellular and molecular recognition, but also provide an effective strategy for constructing highly efficient photosensitizers.
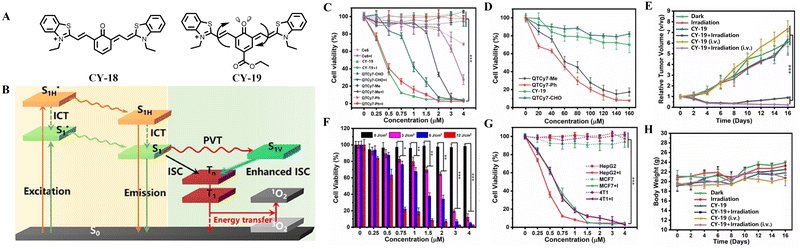 | ||
| Fig. 6 (A) Molecular structures of CY-18 to CY-19. (B) The light-induced molecular vibrational torsion (PVT)-enhanced spin–orbit coupling in the CY-19 (PVT-SOC)-induced ISC mechanism and its contribution to the enhancement of ISC capability. (C) Quantitative detection of cell viability (MTT assay) for QTCy7-R and Ce6 with/without 660 nm irradiation (12 J cm−2). (D) Cell viability of HepG2 cells treated with QTCy7-R at different concentrations under dark conditions. (E) Relative tumour volumes with different treatments at different days. (F) Cell viability of HepG2 cells treated with CY-19 at different light doses. Data are expressed as mean ± SD. **, P < 0.01; ***, p < 0.001; ****, P < 0.0001 as determined by Student's t test. (G) Cell viability of different cancer cells (HepG2, MCF7, and 4T1) treated with CY-17 under 660 nm irradiation (12 J cm−2). (H) Body weights of the mice in various groups at different days. Adapted from ref. 65 with permission from the American Chemical Society, copyright 2023. | ||
All the strategies can effectively enhance the reactive oxygen species generation capacity of polyimide dyes in different degrees. Unfortunately, compared with strategy (1), there are fewer reports on strategies (2)–(4). Consequently, further research efforts are required in this field to design and develop heptamethine cyanine-based photosensitizers with excellent performance.
2.2 Hemicyanines
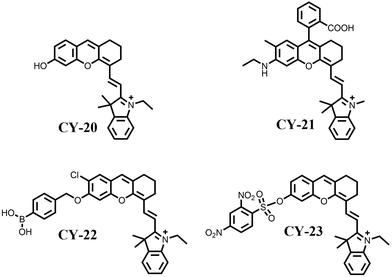
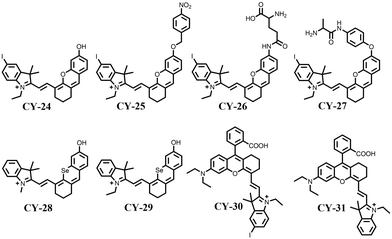
As early as the 1990s, hemicyanines were used for studying leech neuronal cells due to their modulation of excitation and emission spectra in response to external stimuli such as electric fields and solvents.66–68 However, the short excitation and emission wavelengths (λmax < 600 nm) and the lack of modifiable sites of traditional semicarbocyanine dyes have seriously limited their applications in the biological field. For this reason, after a long-term study, researchers have constantly adjusted the structure of semi-cyanine dyes to improve their spectral properties. Yuan et al. reported a series of semicarbocyanine systems by hybridizing traditional hemicyanine with fluorescein and rhodamine, respectively, in 2012, namely ‘Huda’ (CY-20)69 and ‘Changsha’ (CY-21).70 The excitation and emission wavelengths of these dyes have red-shifted to the near-infrared region (λmax ≥ 680 nm) exhibiting modifiable photochemical sites distinguishing them from traditional ones. This advancement promotes the expansion of hemicyanines into the life sciences field. For example, introducing analyte recognition sites to construct semicarbocyanine diagnostic reagents (e.g., CY-22 and CY-23 in Fig. 7) with excellent performance can target and recognise tumour overexpression substances (e.g., hydrogen peroxide, biothiols, etc.) very well, which lays a good foundation for its application in the PDT of tumours.69To improve the photosensitive properties of hemicyanines, the heavy atom iodine was introduced into the indole ring of CY-20 to obtain CY-24 (Fig. 8).71 The introduction of heavy atoms increased the spin–orbit coupling, facilitating the ISC process of CY-24 and effectively increasing the yield of 1O2 (1.8%). To enhance the tumour targeting properties of CY-24, a nitroreductase recognition group was incorporated into its structure to create an activated photosensitizer CY-25. This activated form could be present at the tumour site to release the reduction product CY-24, which specifically localizes in the mitochondria and induces apoptosis by generating a large amount of 1O2 under irradiation with light at 660 nm (IC50 = 0.63 μM). Subsequently, the team designed a series of activated hemicyanine-based photosensitizers (CY-26 and CY-27) for precise tumour therapy based on the heavy atom strategy.72,73 Recently, Cheng et al. also developed an enzyme-activatable NIR hemicyanine-based photosensitizer for tumour-targeted PDT and achieved excellent therapeutic effects.74 This approach greatly facilitates the development of hemicyanines for applications in PDT.
To perform in-depth PDT and achieve better therapeutic outcomes, Wang et al. substituted heavy atom selenium for oxygen in CY-20 to obtain CY-29, which not only extended its excitation wavelength, but also effectively increased its 1O2 generation.75 Tian et al. obtained the first single-photon excited molecular photosensitizer CY-30 based on the frequency upconversion luminescence mechanism by utilizing CY-3176 as the parent structure for the reported frequency upconversion luminescence molecular dye and incorporating iodine atoms into its structure (Fig. 8).77 The introduction of iodine atoms significantly enhanced ISC based on upconversion excitation (808 nm) and facilitated the generation of 1O2, enabling effective implementation of deep PDT.
It was found that the type of heavy atom and the introduction position have different effects on the photosensitive properties of dye molecules.78–80 To investigate the effect of introducing and adjusting positions of halogen elements (Br and I) on improving hemicyanine's photosensitive properties, Zhang et al. designed and optimized a set of hemicyanine derivatives, CY-20, CY-32, CY-33 and CY-34 (Fig. 9).81 According to density-functional theory (DFT), time-varying density-functional theory (TD-DFT) and SOC constants, CY-20, CY-32, CY-33 and CY-34 have similar values of ΔES–T. However, the SOC constants of CY-20, CY-32, CY-33 and CY-34 increase, respectively, to 0.06, 0.08, 0.24 and 0.42 cm−1. The larger SOC constants of CY-33 and CY-34 compared to CY-20 and CY-32 result in a more effective ISC process that promotes excited state formation and is more favourable for reactive oxygen species generation. Gibbs free energy (ΔG) calculations revealed that the ΔG values of CY-20, CY-32, CY-33 and CY-34 were −94.08, −98.88, −104.64 and −113.28 kJ mol−1, respectively. The smaller ΔG values observed for CY-33 and CY-34 compared to those of CY-20 and CY-32 suggest a higher likelihood of the occurrence of the electron transfer process. Therefore, halogen-substituted hemicyanines CY-33 and CY-34 exhibit enhanced efficiency in both ISC processes and electron transfer reactions leading to type-I reactive oxygen species generation. Experimental results further confirmed that out of the four tested probes, CY-34 demonstrates the most efficient superoxide anion (O2˙−) generation ability. The introduction of halogens into the skeleton structure of hemicyanines increases the SOC constant, which promotes more efficient ISC processes resulting in an unfavourable formation of triplet excitons for sensitizing O2 to 1O2 production, and this facilitates O2˙− generation via a type-I electron transfer pathway accompanied by a decrease in fluorescence quantum yields.
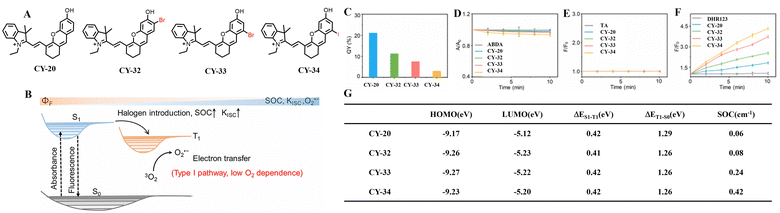 | ||
| Fig. 9 (A) Molecular structures of CY-20, CY-32, CY-33, and CY-34 for antihypoxic PDT. (B) O2˙− generation through the electron transfer process. (C) Quantum yields of CY-20, CY-32, CY-33, and CY-34. (D) Relative absorbance intensity (A/A0) of ABDA at 378 nm as a function of irradiation time in the presence of CY-20, CY-32, CY-33, and CY-34 in PBS. (E) Relative fluorescence intensity (F/F0) of TA at 425 nm as a function of irradiation time in the presence CY-20, CY-32, CY-33, and CY-34 in PBS. (F) Relative fluorescence intensity (F/F0) of DHR 123 at 525 nm as a function of the irradiation time in the presence of CY-20, CY-32, CY-33, and CY-34 in PBS. (G) Calculated frontier orbital distributions, ΔES–T, and SOC values of CY-20, CY-32, CY-33, and CY-34. Light irradiation: 660 nm, 30 mW cm−2. The error bars represent the standard deviation from three separate measurements (n = 3). Adapted from ref. 81 with permission from the American Chemical Society, copyright 2023. | ||
Reducing the energy difference between the excited singlet state and the excited triplet state is also an effective strategy for promoting the ISC process and increasing the production of reactive oxygen species. Zhang et al. selected indole hemicyanine-based CY-35 as the molecular backbone and replaced indole with a benz[c,d]indolium fragment to inhibit fluorescence emission and extend the wavelength of the molecule.82 Subsequently, they introduced a phenyl group at the middle position of CY-36 based on the SOCT-ISC mechanism between compact electron donor–acceptor (D–A) diatoms to obtain CY-37. Experimental tests demonstrated a 12-fold higher 1O2 quantum yield of CY-37 compared to ICG. In contrast, no significant production of 1O2 was observed for CY-36. ΔES–T calculations revealed that CY-37 has a lower ΔES–T value (0.057 eV) than CY-35 (0.081 eV) and CY-36 (0.087 eV), attributed to the involvement of electron-rich phenyls which facilitates the ISC process and enhances the efficiency in producing 1O2, further confirming that lowering ΔES–T is effective in promoting the ISC process (Fig. 10). To our knowledge, fewer strategies have been reported for improving the photosensitizing properties of hemicyanines. We believe that as research continues, more strategic mechanisms will be developed and used to enhance the development of hemicyanine-based photosensitizers.
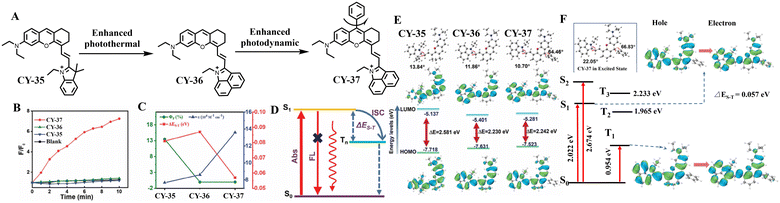 | ||
| Fig. 10 (A) Molecular structures of CY-35, CY-36 and CY-37. (B) 1O2 generation by CY-35, CY-36 and CY-37 with SOSG as indicators under 808 nm laser irradiation. (C) Fluorescence quantum yields (ΦF), singlet–triplet energy gaps (ΔES–T), and molar extinction coefficients of CY-35, CY-36 and CY-37 in DMSO. (D) Schematic illustration of the strategy for suppressing fluorescence emission to enhance PTT/PDT with Rh-BI. (E) Frontier molecular orbitals and energies (eV) of CY-35, CY-36 and CY-37 in their ground state (S0). (F) The spin–orbit coupling (SOC) and natural transition orbitals (NTO) of CY-37. Adapted from ref. 82 with permission from the John Wiley and Sons, copyright 2023. | ||
2.3 Squarylium cyanines
Squaraine and its derivatives usually exhibit distinct and intense absorption and emission bands associated with donor–acceptor–donor (D–A–D) electron transfer structures (Fig. 11).21 To date, squaraines have been extensively utilized in various photonic materials for nonlinear optics, photovoltaics, biomarkers, and PDT.83–87The heavy atom substitution strategy is an important method used to enhance the photosensitive properties of squarylium cyanine dyes. In 1997, Ramaiah et al. introduced bromine and iodine into the structure of the squaraine dye CY-38 to obtain squarate amine derivatives CY-39 and CY-40, respectively.86 They then investigated the ability of CY-39 and CY-40 to generate 1O2 through laser flash photolysis studies, revealing that substituting heavy atoms (bromine or iodine) for hydrogen in the parent dye CY-38 significantly enhances ISC efficiency from the first excited singlet state (S1) to the first excited triplet state (T1) in both the bromine derivative and the iodine derivative CY-40. Higher yields of excited triplet and singlet oxygen were observed in the iodine derivative CY-40 compared to the bromine derivative CY-39, confirming that spin–orbit coupling increases with an increasing heavy-atom size, thereby increasing intersystem crossover and triplet yields. This work demonstrated that using a heavy atom strategy can effectively increase the reactive oxygen species production capacity of square acid dyes. In addition, Serpe et al., in their study conducted in 2016, synthesized a series of symmetric indolenine-based squaraine dyes (CY-41 to CY-47, Fig. 12) by introducing different halogen atoms into SQ-NH and evaluated their ability to produce 1O2.88 The results revealed that the heavy atom effect promotes ISC by enhancing spin–orbit coupling. This enhancement effectively promotes 1O2 generation and makes these dyes excellent photosensitizers for PDT. Bordignon et al. also designed and synthesized brominated squaraine CY-48,89 which exhibited strong absorption in the red/near-infrared region using a heavy-atom strategy. These compounds were further integrated into Quatsome nanovesicles under different loadings to further expand their applications in organisms. The poor water solubility of brominated squaraine was not only overcome, but its ability to generate reactive oxygen species was also maintained. Moreover, by loading CY-48 highly localized within the Quatsome, the effectiveness of PDT was maximized, resulting in higher photostability, increased cellular uptake, and significantly enhanced local concentration. As a result, better therapeutic efficacy could be achieved at lower concentrations (up to 10–1000 times lower).
Moreover, introducing heavy atoms such as S or Se can also promote the ISC in square squarylium cyanine molecules further enhancing their capacity for reactive oxygen species production.90 Magalhães et al. modified several symmetrical squarylium cyanines derived from benzothiazole to improve their 1O2 generation by replacing sulphur with selenium atoms.91 This led to the synthesis of squarylium cyanines (CY-49 to CY-53, Fig. 12) that exhibit strong absorption in the range of 665–685 nm and demonstrate photodynamic activity against human tumour cell lines (MCF-7, NCI-H460, HeLa, and HepG2). These dyes inhibited cell growth more effectively in the presence of light than in its absence, with GI50 values being below 5.0 μM.
In addition to modifying the end-group structure, which can enhance the photosensitivity of square acid dyes, structural modification of their square moiety has been found to further enhance the capacity for generating reactive oxygen species. Santos et al. conducted a study where they designed and synthesized a series of square acid dyes (CY-54 to CY-57, Fig. 13A).92 Among them, all the dyes containing N-methylamine groups on the four-membered ring exhibited higher quantum yields of 1O2 than the corresponding N,N-diethylamine-substituted dyes. This could be attributed to the stronger intramolecular hydrogen bonding in the molecules containing sec-amine. Thus, a higher degree of rigidity in the dye structure can lead to a decrease in the nonradiative decay efficiency of photoisomerisation and an inherent increase in the ISC efficiency. Additionally, different n-alkyl chains also affect 1O2 yields to some extent, with dyes having longer n-alkyl groups showing larger ΦΔ values. For example, the 1O2 yields of benzoselenazole derivatives increased from 0.45 for CY-54c to 0.68 for CY-55c. The presence of stronger intramolecular hydrogen bonding in the former inhibits photoisomerization of olefinic bonding and leads to a greater structural rigidity in benzoselenazole derivatives' yield increase for 1O2. The resulting reduction in non-radiative decay processes leads to an intrinsic increase in the crossover efficiency between these systems. Webster et al. facilitated the ISC process by reducing ΔES–T through another modification strategy: substitution of O with S effectively overlaps strong π–π* transitions and converts the lowest π–π* transitions into n–π* transitions;93 this modification lowers ΔES–T by reducing the energy level of the excited singlet state, thereby enhancing ISC efficiency (Fig. 13B).
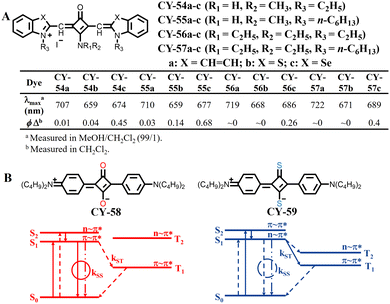 | ||
| Fig. 13 (A) Molecular structures and UV spectral data and φ△ of CY-54 to CY-57. (B) Schematic of energy level structures and the nature of transitions for CY-58 and CY-59. Adapted from ref. 93 with permission from the American Chemical Society, copyright 2010. | ||
3. Cyanine dyes for PTT
PTT is a type of heat therapy in which a photothermic agent is excited by a specific wavelength of light (usually in the near infrared spectrum). The excited state of the photothermic agent then returns to the ground state and generates heat to kill the cancer cells. The mechanism of action is shown in Fig. 14.94 The mechanism diagram reveals that enhancing the non-radiative decay of molecules is crucial for improving the photothermal conversion efficiency (PCE). Current strategies employed to promote the non-radiative decay of cyanine dyes are as follows: (1) wavelength extension, (2) enhanced conjugation, (3) aggregation strategies, (4) self-assembly strategies, and (5) modulation of intramolecular motions.95–99 Subsequently, we will provide detailed summaries on design strategies used with cyanine dyes and their applications in PTT with particular emphasis on exemplary cases.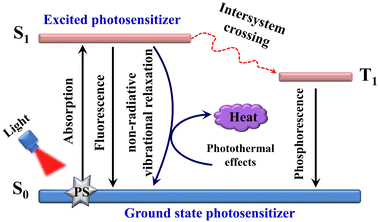 | ||
| Fig. 14 The working principle underlying organic PTT agents is illustrated using a modified Jablonski diagram. Adapted from ref. 94 with permission from the Royal Society of Chemistry, copyright 2018. | ||
3.1 Heptamethine cyanines
The parts of Cy5, Cy5.5 and most Cy7, Cy7.5 that have absorption wavelengths in the optical window of biological tissues (650–1350 nm) have become ideal candidates for the development of photothermal reagents with excellent performance. To this end, researchers have used various strategies to enhance their photothermal conversion ability and expand their applications in PTT.100–103One effective strategy to improve the PCE of heptamethine cyanines is to extend the absorption wavelength by reducing electronic excitation energy and promoting non-radiative decay. The absorption wavelength of a molecule is determined by the electrons jumping from the lowest unoccupied molecular orbital (LUMO) to the lowest unoccupied molecular orbital (HOMO), and extending the wavelength can be achieved by decreasing the molecular energy gap. Additionally, the non-radiative attenuation rate usually increases exponentially as the molecular energy gap decreases,104,105 indicating that extending the molecular wavelength can effectively improve its PCE. Zhao et al. obtained a series of heptamethine cyanines by increasing both conjugated structures of bipartite indole salts and number of rigid polymethine units CY-60 to CY-65 (Fig. 15).106 Comparing the UV absorption spectra of CY-60>, CY-61 and CY-62 showed gradual red-shifts in absorption wavelengths with the increase of the conjugated structure of indole salts. Compared with CY-63, CY-65 exhibited a significant red-shift (164 nm) in its absorption wavelength, suggesting that incorporating conjugates in polymethine can effectively reduce the molecular energy gap leading to red-shifts in absorption wavelengths. The density functional theory calculations similarly confirmed these results, specifically showing that the increasing conjugate structure reduces molecular energy gaps for CY-60 to CY-65. The data are 1.946 eV, 1.875 eV, 1.579 eV, 1.559 eV, 1.515 eV, and 1.241 eV, respectively. This confirms that an increase in the conjugate structure will reduce the molecular energy gap, which is more favourable for improving of PCE. Subsequently, researchers tested the photothermal performance of CY-65 with a large wavelength and found that its PCE greatly improved to 83.2% compared with ICG (20%). These statements indicate that reducing the molecular excitation energy gap by extending the absorption wavelength is an effective strategy to improve the PCE.
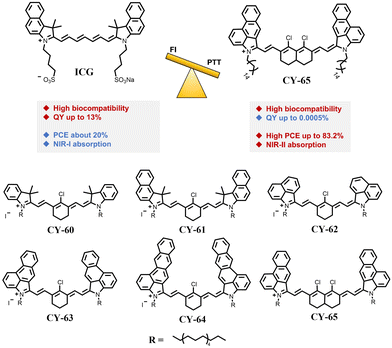 | ||
| Fig. 15 Molecular structures of CY-60 to CY-65. Adapted from ref. 106 with permission from the John Wiley and Sons, copyright 2024. | ||
In addition to prolonging the absorption wavelength, the formation of aggregates (including J-aggregates and H-aggregates) is also an effective strategy for improving the PCE.107–109 When multiple molecules are arranged head-to-tail by non-covalent interactions, J-aggregates may form, leading to a red-shift in absorption and emission spectra, narrow absorption and emission bands, enhanced absorption coefficients (ε), and shortened fluorescence lifetimes. These characteristics are more favorable for enhancing the PCE. In 2014, Song et al. used the J-aggregation strategy to improve the PCE of the heptamethine cyanine (IR825) for the first time.110 In this work, IR825 formed J-aggregates with a red-shift and significantly enhanced absorbance in the presence of the cationic polymer polyallylamine hydrochloride (PAH). It was further functionalized and complexed with ultra-small iron oxide nanoparticles (IONPs) and polyethylene glycol (PEG) to obtain composite nanoparticles IR825@PAH-IONP-PEG for PTT of tumours. Subsequently, Liu et al. also successfully prepared an aggregate of ICG by a simple method that resulted in a red-shifted absorption peak, better photostability, and enhanced PCE compared to ICG alone.111 Millard et al. also prepared nanoscale (ICG-J/CX) by co-assembling anionic ICG J-aggregates with cationic tetraguanidine cupro[4]aryl hydrocarbons to improve the PCE and photostability of ICG.112
Unlike J-aggregates, H-aggregates exhibit a parallel face-to-face stacking arrangement, which facilitates close molecular packing and the specific alignment of jump dipole moments. Consequently, this unique configuration enables efficient non-radiative energy transfer among molecules while reducing luminescence. Compared to J-aggregates, the lowest energy level of H-aggregates is forbidden and therefore more likely to emit energy in a non-radiative form. Therefore, researchers are more likely to adopt H-aggregation to improve the PCE of molecules.113 For example, Wu et al. coupled planar pyrene with the Cy7 dye, inducing the formation of H-aggregates assembled into CY-66 through strong π–π stacking (Fig. 16A).114 Compared with free phloroglucinic dyes, CY-66 exhibited H-aggregation leading to complete quenching and ultimately enabling efficient photothermal conversion with a remarkable PCE value of up to 22.3%.
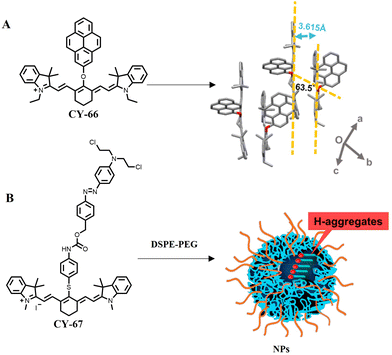 | ||
| Fig. 16 The H-aggregation of CY-66 (A) (adapted from ref. 114 with permission from the American Chemical Society, copyright 2020) and CY-67 (B) (adapted from ref. 115 with permission from the American Chemical Society, copyright 2022). | ||
CY-67 could achieve the PCE at 39.3% by introducing nitrogen mustard into the near-infrared fluorescent moiety heptamethine cyanine through an azo bond and enhancing its H-aggregation ability by π–π stacking.115 This improvement was significant compared to the conventional cyanine dye (Cy-NH2), which had a PCE of only 22.5%. The presence of nitrogen mustard in CY-67 resulted in stronger H-aggregation than Cy-NH2 in aqueous solution, promoting non-radiative dissipation of molecular energy and generating more heat under NIR light irradiation (Fig. 16B). This was confirmed by conducting geometry optimization and filling models using the DFT method for both CY-67 and Cy-NH2. To further enhance the accumulation of photosensitive prodrugs at the tumour site and achieve better therapeutic effects, researchers encapsulated CY-67 in polymer nanoparticles to stabilize its H-aggregates and increase its accumulation within the tumour by enhancing the permeability and retention effect (EPR). Ultimately, good anticancer therapeutic effects were achieved under 808 nm light irradiation.
In addition to the above strategies, Mu et al. designed the asymmetric CY-68 as a photothermic agent (Fig. 17) by introducing 2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran as an acceptor and an indole as a donor.116 The moiety of 2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran in CY-68 exhibits a high degree of electron delocalization, which not only functions as a high electron acceptor group for the classical “D–π–A” molecular configuration but also serves as a multivalent hydrogen-bonding acceptor driving the assembly of CY-68. In an aqueous solution, stable nanostructures can spontaneously form from CY-68 through self-assembly. This self-assembly not only leads to reduced intrinsic fluorescence but also generates low monoclinic oxygen, thereby effectively contributing to the improvement of PCE.
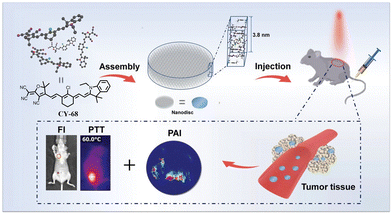 | ||
| Fig. 17 Schematic illustration of CY-68 self-assembly into a nanodisc to exhibit the phototheranostic functions and accumulation at the tumour site. Adapted from ref. 116 with permission from the John Wiley and Sons, copyright 2019. | ||
3.2 Hemicyanines
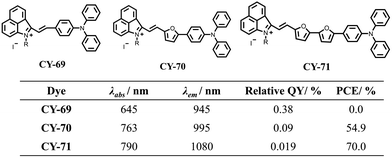
The production of fluorescence involves the movement of electrons in a molecule from the single excited state to the ground state, while the production of photothermal involves the nonradiative relaxation of electrons in a molecule.117,118 However, balancing the two competing photophysical processes, radiative and non-radiative relaxation, remains challenging. Twisted intramolecular charge transfer (TICT) is a torsionally excited electron transfer process that consists of a molecular donor and an acceptor connected by a single bond. Molecules with TICT properties may exhibit greater Stokes shifts and enhanced photothermal properties compared to other organic molecules.119–125 Hemicyanines have been widely used in biomedical imaging due to their unique D–A structure, large Stokes shift and biocompatibility.126–135 They have tunable optical properties in the near-infrared region and great potential for development as photothermal reagents based on the intramolecular charge transfer (ICT) mechanism. Based on this concept, Zhao et al. designed three NIR-II semicarbocyanines CY-69 to CY-71 by incorporating positively charged pyridinium salts as electron acceptors and triphenylamine as the electron donor, with oxacyclopentadiene serving as a conjugated π-bridge (Fig. 18).136 Increasing the number of oxole introductions leads to a gradual red-shift in absorption wavelengths CY-69 to CY-71, favouring non-radiative relaxation dissipation of molecular energy. DFT calculations show that the energy difference between the HOMO and the LUMO decreases in the order from 2.13 eV for CY-69 to 1.81 eV for CY-70 and further down to 1.67 eV for CY-71 respectively, indicating that increased conjugation extends the absorption wavelength while reducing the energy gap which promotes generation of non-radiative heat. The subsequent test results showed that CY-70 had a quantum yield of 0.09% and a PCE of 54.90%, making it suitable for PTT and high-resolution imaging. Gu et al. also constructed a series of hemicarbocyanine derivatives CY-72 to CY-74 and synthesized them by introducing a triphenylamine rotor and a thiophene conjugated structure into the parent structure of hemicarbocyanine (Fig. 19).137 The incorporation of thiophene enhanced the push–pull effect of the molecules and extended the conjugated structure, resulting in a shift of the emission spectra of CY-74 further towards the NIR-II region. CY-74 has smaller DES-T values and longer wavelengths, indicating a higher capacity for 1O2 generation and higher PCE.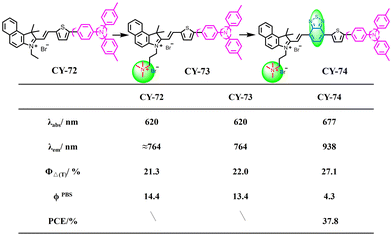 | ||
| Fig. 19 Molecular structures, singlet oxygen yields and the absolute fluorescence quantum yields of CY-72 to CY-74. | ||
Zhou et al. synthesized a NIR-absorbing hemicyanine (CY-75) by hybridizing rhodamine and hemicarbocyanine derivatives to extend their conjugated structure (Fig. 20).138 Compared to the rhodamine and hemicarbocyanine parent structures, CY-75 showed significant prolongation in the absorption wavelength with a strong broad absorption band with peaking at 868 nm and an additional shoulder peak at 950 nm. However, when RC was incorporated into bovine serum albumin nanoparticles (CY-75-BSA NPs), its absorption wavelength significantly increased to 868 nm with an observed shoulder peak at 950 nm while maintaining high PCE (28.7%). This indicates that enhancing conjugation can effectively prolong the absorption wavelength, thereby improving its PCE. Cao et al. designed and synthesized CY-76 with a more larger planar π-conjugated structure (Fig. 21),139 which not only enables it to have longer near-infrared absorption/emission at 770/915–1200 nm, but also lowers the energy levels of excited singlet/triplet states, facilitating intersystemic crossover processes and enhancing its ability to generate 1O2 and photothermal effects. Subsequently, researchers assembled CY-76 with DSPE–PEG2000 to form homogeneous nanoparticles, which effectively improved the tumour-targeting and photothermal properties of CY-76, resulting in a PCE of 55.1%. Under 808 nm laser irradiation, homogeneous nanoparticles showed excellent anti-tumour effects in vitro and in vivo.
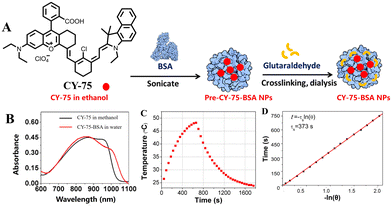 | ||
| Fig. 20 (A) A schematic diagram of the fabrication of CY-75-BSA NPs. (B) Absorption spectra of RC in methanol and CY-75-BSA NPs in water. (C) The photothermal effect of CY-75-BSA NPs in an aqueous suspension (CY-75 content: 0.1 mM). After 10 min exposure, the laser was switched off. (D) The time constant for heat transfer of the system (τs). Adapted from ref. 138 with permission from the American Chemical Society, copyright 2016. | ||
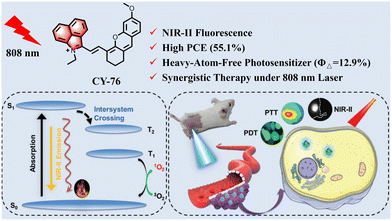 | ||
| Fig. 21 CY-76 with NIR-II fluorescence for synergistic PDT/PTT cancer therapy. Adapted from ref. 139 with permission from John Wiley and Sons, copyright 202. | ||
3.3 Squarylium cyanines
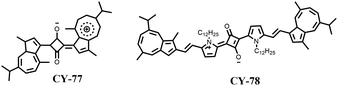
Square quinone dyes contain π-scaffolds of donor–acceptor–donor (D–A–D) sequences with a unique planar structure of amphiphiles. The stable quinone structure has strong planarity and rigidity, giving the square arrays near-infrared absorbance and high ε values.140,141 The excellent photophysical properties and photostability make them attractive candidates for many optoelectronic and biomedical applications.142–144 However, squarylium cyanines have some shortcomings when used as a photothermal agent for PTT, such as poor PCE. To expand the application of squarylium cyanines in PTT, researchers have developed a series of PTT platforms based on squarylium cyanines through structural modification and modulation of the molecular orbital energy level to improve their PCE. It is well known that squarylium cyanines tend to exhibit strong fluorescence emission, which is unfavourable for photothermal conversion according to the Jablonski diagram.97 For this reason, Yao et al. constructed squarylium cyanine-based photothermic agents CY-77 and CY-78 by introducing a dipole molecular unit (guaiazulene) consisting of an electron-rich five-membered ring and an electron-deficient seven-membered ring into square dyes in order to reduce the molecule's radiative decay (Fig. 22).145 To enhance biocompatibility and enable in vivo tumour therapy, both compounds were prepared as nanoparticles, CY-77 NPs and CY-78 NPs, respectively. The results showed that CY-77 and CY-78 and their nanoparticles exhibited strong near-infrared absorption while having negligible fluorescence emission in organic solvents and water; these characteristics are more favourable for efficient photothermal energy generation with high PCE values (η) reaching up to 47.3% and 53.2%, respectively.To extend the wavelength of square squarylium cyanine and enhance its PCE (Fig. 23), Yao et al. introduced a strong electron-absorbing group, malononitrile, into the structure of CY-79 to obtain a square amine dye CY-80.146 Compared with CY-79 (λex = 870 nm, λem = 915 nm), both the absorption and emission of CY-80 exhibited a significant redshift (λex = 930 nm, λem = 970 nm). The density functional theory (DFT) results indicated that the HOMOs in CY-80 were well delocalized along its conjugated structure, while the LUMOs were concentrated on the acceptor unit. This suggests that effective intramolecular charge transfer along the conjugated structure resulted in long-wavelength absorption. The high D–A intensity and D–A–D modes contribute to a narrow band gap and long-wavelength absorption within the NIR-I window, which is beneficial for enhancing the PCE. Photothermal performance tests confirm that CY-80 exhibits superior heat production capability compared to CY-79.
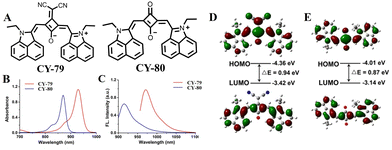 | ||
| Fig. 23 (A) Molecular structures of CY-79 and CY-80. (B) The absorbance and (C) emission of spectra CY-79 and CY-80 in tetrahydrofuran (THF). The excitation wavelengths are 860 and 915 nm for CY-79 and CY-80, respectively. (D) and (E) In the density functional theory (DFT) calculation, the HOMO and the LUMO of (D) CY-80 and (E) CY-79 (B3LYP/6-31G(d,p)). Adapted from ref. 146 with permission from the American Chemical Society, copyright 2020. | ||
Co-planar stacked H-aggregation has been reported to induce rapid internal transitions from high-energy to low-energy excited states, resulting in burst fluorescence, which may promote heat generation.147–149 Based on this, Wang et al. synthesized a NIR-absorbing amphiphilic CY-81 by attaching PEG chains as hydrophilic anchors to the central nucleus of CY-80 (Fig. 24),150 which is used as a highly efficient organic photothermic agent for cancer therapy. In an aqueous solution, CY-81 was able to spontaneously self-assemble into homogeneous nanospheres consisting of H-dimeric substructures. Recombination energy calculations show that the low-frequency out-of-plane vibrational modes of the H-dimer accelerate non-radiative decay, leading to rapid dissipation of the excited state energy into heat. As a result, the resulting H-dimer PSQ-NSs exhibited an ultra-high PCE (81.2%) under aqueous laser irradiation at a power density of 0.3 W cm−2 and a wavelength of 808 nm.
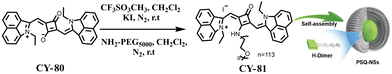 | ||
| Fig. 24 Synthetic route from CY-80 to the target product CY-81, and schematic illustration for CY-81 self-assembly in aqueous solution. Adapted from ref. 150 with permission from John Wiley and Sons, copyright 2022. | ||
In addition, Cai et al. developed a new strategy to enhance the PCE by harnessing intermolecular hydrogen bonding,151 which involves the interaction between individual dye molecules and water. This strategy effectively promotes the conversion of absorbed energy into heat. Compared to pentamethylene dyes CY-82 and CY-85, the presence of “–O–” in the middle of the square ring allows CY-83 to form intermolecular hydrogen bonds with water, thereby extending its absorption wavelength and promoting the formation of energy dissipation pathways. Moreover, the incorporation of a long-chain benzenesulfonic acid group not only improves the water solubility but also further enhances the PCE through enhanced fluorescence. Compared with CY-84 (50.5%), CY-85 (49.5%) and CY-82 (44.5%), CY-83 exhibits an exceptionally high PCE (74.0%) in aqueous environments and demonstrates promising therapeutic effects when subjected to PTT in ruffed tumour-bearing mice under 915 nm laser irradiation treatment, as shown in Fig. 25.
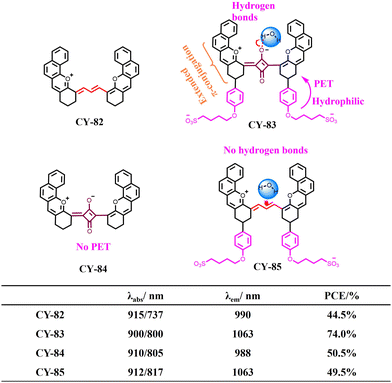 | ||
| Fig. 25 Comparison of the properties of four probe molecules CY-82 to CY-85. Adapted from ref. 151 with permission from Elsevier, copyright 2024. | ||
4. Cyanine dyes for multimodal combination therapy
The incidence and mortality rates of tumours are increasing globally every year. A study shows that by 2030, cancer will cause an additional 17 million deaths and worsen life expectancy.152 The treatment of tumours has been a pressing issue in various countries. In addition to the continuous improvement and innovation of traditional treatments such as surgical resection, radiotherapy and chemotherapy,153,154 many new therapeutic methods have been gradually researched, developed and applied to cancer treatment.155–167 However, due to the heterogeneity of tumours and the diversity of pathways involved in tumour pathogenesis, it is widely believed that single therapies cannot achieve satisfactory therapeutic effects. To overcome the inherent shortcomings of each monotherapy, more efforts have been directed towards research at developing multimodal therapies. Combination therapies have been considered a promising strategy to take advantage of the strengths of each therapy while minimizing adverse effects and improving therapeutic efficiency.168–181 In recent years, cyanine dyes have been used to develop multi-modal therapy platforms based on their excellent therapeutic properties. Next, we will detail the design strategy and application of building a combined therapeutic platform based on cyanine dyes by providing examples.4.1 PDT and PTT
PDT consists of three main elements: light, photosensitizers and oxygen. Its mechanism of action is to deplete intracellular oxygen and produce toxic mono-linear oxygen to kill cells, a process that inevitably exacerbates cellular hypoxia. Therefore, the implementation of PDT in the hypoxic microenvironment of tumours often leads to increased hypoxia and inefficient reactive oxygen species generation, which hinders the PDT process and results in poor therapeutic outcomes.182–184 This problem can be overcome by enhancing tumour therapy with combined PTT. PTT induces an increase in local temperature, promoting blood flow and facilitating the entry of photosensitizers into tumour cells while alleviating tumour tissues hypoxia and improving the therapeutic efficacy. In addition, PTT is independent of oxygen, complementing PDT's reliance on oxygen for reactive oxygen species generation and further augmenting therapeutic efficacy through synergistic mechanisms.185The main approach mentioned above for developing drugs for combinatorial phototherapy is to integrate separate PTT and PDT drugs into a single nanocarrier. However, this strategy often faces two challenges: (1) complex assembly and preparation processes, and (2) difficulties in matching the absorption of the drugs used for PTT and PDT. Therefore, the development of a single NIR phototherapy system capable of achieving both photodynamic and photothermal synergism has become a current hot topic of research. It is well known that ICG is an FDA-approved NIR dye for clinical use. It can be used not only for NIR fluorescence and photoacoustic imaging but also for converting absorbed light energy into reactive oxygen species and localized heat for PDT and PTT, respectively. Therefore, ICG and its derivatives are frequently employed as excellent candidates as single phototherapy reagents in constructing combined PDT/PTT systems (Fig. 26A).186
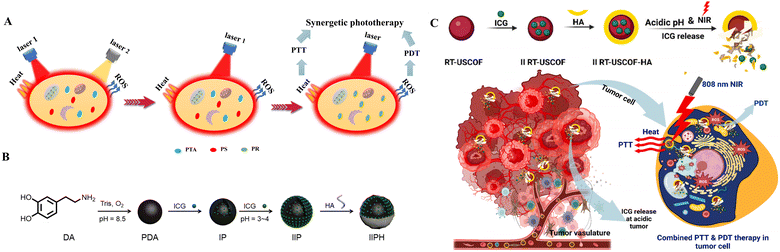 | ||
| Fig. 26 (A) A schematic diagram of the development of PDT/PTT dual-modality therapy. (B) Preparation route of IIPH. Adapted from ref. 187 with permission from the Royal Society of Chemistry, copyright 2020. (C) pH dependent tumour targeting II RT-USCOF-HA for PTT/PDT combination therapy. Adapted from ref. 188 with permission from the Elsevier, copyright 2024. | ||
Early design strategies for combining PDT and PTT typically involved integrating separate photosensitisers and photothermal reagents into a single nanocarrier.189–192 Photodynamic and photothermal effects occur under the irradiation of two different wavelengths or a single wavelength of laser light, respectively, resulting in the production of reactive oxygen species and heat. However, these strategies often face challenges such as complex assembly and preparation processes, as well as difficulties in matching the absorption wavelengths used for PTT and PDT drugs. Therefore, currently, there is significant research interest in using a single light source to activate a single phototherapy reagent (PR) capable of simultaneous PDT and PTT. For example, Chien et al. used ICG as the sole phototherapy reagent to obtain ICGMM3,193 which can convert light energy into heat and generate reactive oxygen species under NIR irradiation. This enables combined PTT and PDT therapy under NIR laser irradiation. Liu et al. successfully prepared a novel ICG-loaded polydopamine nanoparticle (IIPH) with an outer layer of hyaluronic acid using ICG as the only phototherapy reagent (Fig. 26B).187 Both in vitro and in vivo studies demonstrated that IIPH could significantly inhibit tumour growth by PTT-PDT combination therapy. Thankachan et al. also developed a combined PDT/PTT platform (II RT-USCOF-HA) using ICG as a single phototherapy reagent (Fig. 26C).188 The system performed both 1O2 generation and thermal ablation of tumour cells, further demonstrating its effectiveness as a combined PDT/PTT therapeutic platform. Cell uptake studies and flow cytometry analysis of apoptosis and necrosis rates further demonstrated the carrier system's ability in combined tumour therapy.
To enhance the therapeutic efficiency of combined PDT/PTT, Prince et al. encapsulated ICG and near-infrared light-absorbing GNR into niosomes to create a novel phototherapy agent named ICG-GNR@Nio.194 This system combines the photothermal properties of gold nanorods with the photothermal and photodynamic properties of indocyanine green for improved therapeutic efficacy. ICG-GNR@Nio demonstrated excellent storage stability, photostability, high temperature rise and reactive oxygen species generation under 1064 nm laser irradiation. Ye et al. developed a multifunctional system capable of real-time imaging of cancer cells using upconversion fluorescence triggered by NIR laser irradiation followed by effective PDT-assisted PTT using the same laser.195 First, they synthesized upconversion nanoparticles through thermal decomposition and introduced polydopamine (PDA) to improve their biocompatibility and water dispersibility via a ligand exchange technique. Then ICG molecules were electrostatically attached to the surface of UCNP-polydopamine (UCNP@PDAs) complexes to enhance PDT and PTT. Subsequently, PEG-FA was added to the surface of UCNP@PDA-ICG to obtain the final UPIPF. The prepared UPIPF possessed excellent water dispersibility, cancer targeting ability, remarkable UCL imaging capabilities, and efficient photothermal properties that effectively inhibited HepG2 cancer cells through NIR-triggered PDT/PTT. Zhu et al. prepared the injectable agarose in situ forming NIR-responsive hydrogel (CIH) containing Cu-Hemin and ICG for the first time.196 When the CIH system was localized to the tumour tissue by local injection, the ICG in the hydrogel effectively converted the light energy emitted by the 808 nm laser into thermal energy, heating and softening the hydrogel matrix and releasing Cu-Hemin. The overexpressed glutathione (GSH) in the tumour could be down-regulated by the Cu-Hemin, which attenuates reactive oxygen species neutralisation and amplifies ICG-mediated PDT, further enhancing its therapeutic effect. In vivo experiments confirmed that ICG-based PDT/PTT combined with Cu-Hemin-mediated glutathione depletion safely and effectively eliminated tumour tissues. Pang et al. designed and prepared calcium-rich carbon nanoparticles (Ca-CNPs) that can effectively deplete GSH and release calcium ions from tumours, thereby enhancing PDT efficacy while inducing mitochondrial dysfunction due to calcium overload effects.197 Subsequently, Ca-CNPs@ICG was obtained by loading ICG through electrostatic interactions, hydrogen bonding, p–p stacking interactions, and microporous structures. The resulting Ca-CNPs@ICG significantly improved the photostability of ICG while maintaining its ability to generate 1O2 and perform photothermal conversion compared to pure ICG (45.1% vs. 39.5%). Additionally, Ca-CNPs@ ICG consumed GSH, leading to increased 1O2 utilization and enhanced PDT efficacy. In vitro and in vivo experiments demonstrated that Ca-CNPs@ICG could be used for synergistic calcium overload-induced PTT combined with GSH consumption-enhanced PDT guided by near-infrared fluorescence imaging.
If a phototherapy reagent is kept “on”, even in healthy cells, once exposed to light, it can generate reactive oxygen species or heat, causing damage to healthy tissues and resulting in unwanted side effects. The development of activated phototherapy reagents can effectively address this problem by selectively releasing photothermic agents that target the generation of reactive oxygen species and heat to minimize phototoxic side effects.198 For example, recently, Wei et al. designed a near-infrared fluorescent double-locked activatable photothermic agent (CY-88) based on CY-86 for in vivo imaging and automated modulation of PDT–PTT cancer therapy. CY-88 comprises a double-locked hemicarbocyanine (DHCy) molecule consisting of two enzymatically reactive portions and a poly(ethylene glycol) (PEG) chain for enhanced biocompatibility.199 Structural changes can occur in tumours undergoing two enzymatic cleavage pathways. Around the tumour, the γ-glutamyltransferase (GGT)-responsive portion of CY-88 can be cleaved by abundant GGT at the amide moiety to produce the non-fluorescent PDT-active molecule CY-87. Subsequently, in the core of a highly hypoxic tumour where nitroreductase (NTR) is abundantly expressed, the NTR-responsive portion of CY-87 can undergo reduction of nitroxyl groups to amines by 1,6-self-elimination to produce the non-fluorescent PTT-active CY-86, but not the PDT-active molecule. This auto-modulated PDT–PTT photothermic agent presents complete tumour ablation by generating cytotoxic 1O2 around the tumour and thermotherapy in the tumour core under light irradiation from a single laser source (Fig. 27).
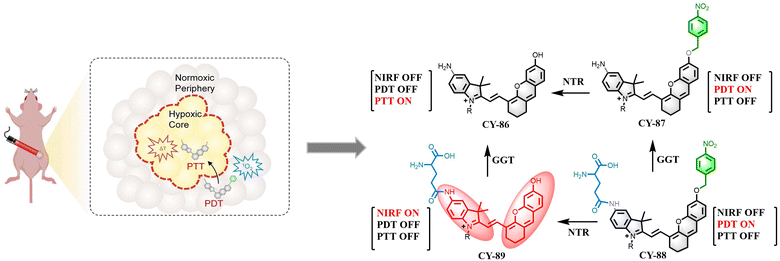 | ||
| Fig. 27 The molecular mechanism of CY-88 for real-time imaging of tumour and auto-regulated PDT-PTT in the presence of GGT and NTR. Adapted from ref. 199 with permission from John Wiley and Sons, copyright 2022. | ||
4.2 PDT and chemotherapy
Chemotherapy has long been the cornerstone of cancer treatment in clinical practice. Repeated administration of high-dose chemotherapy is often used to suppress aggressive tumours, but this approach leads to severe side effects and even triggers multi-drug resistance.200 On the other hand, the diversity, complexity, heterogeneity, and drug resistance of tumours present challenges for a single treatment modality to effectively address the clinical needs. Combining chemotherapy with other treatment modalities is considered a promising strategy to reduce toxicities and achieve the desired anticancer effect.201–203 One way to overcome the limitations of chemotherapy agents is by combining conventional chemotherapy with PDT. The introduction of PDT in chemotherapy can accomplish the following: (1) increase chemosensitivity of cancer cells, (2) significantly reduce the drug dose, thus minimizing toxic side effects, and (3) enhance apoptosis and necrosis of tumour cells through multiple anticancer mechanisms when combined with PDT, which helps overcome multidrug resistance of tumours. Over the past few decades, cyanine dyes have emerged as one of the most promising photosensitisers for PDT and have been utilized in constructing platforms for combination treatments involving chemotherapy/PDT. For example, Zhang et al. synthesized erlotinib (Er)-modified chitosan (ECs) by click-coupling and developed ECs/ICG-self-assembled nanoparticles for targeted chemo-photodynamic combination therapy against non-small cell lung cancer.204In vitro release experiments demonstrated the slow release of Er and ICG from ECs/ICG-self-assembled nanoparticles under acidic conditions. Under near-infrared laser irradiation, ECs/ICG-self-assembled nanoparticles were able to generate more reactive oxygen species for PDT purposes. Cytotoxicity and apoptosis studies confirmed the synergistic molecularly-targeted therapeutic effects as well as photodynamic therapeutic effects of GECs in inhibiting cell growth and inducing apoptosis. The GECs were used for the treatment of non-small-cell lung cancer and in combination with targeted chemo-PDT for non-small-cell lung cancer treatment. Hou et al. successfully developed a BSA-assisted multifunctional CDDP/ICG/HHA-ligand nanopremedicine for combined PDT/chemotherapeutic treatment of hepatocellular carcinoma.205 The nanopremedicine exhibited an ultra-high drug-loading efficiency and a dual-responsive drug release behavior to glutathione/near-infrared light. In vitro experiments demonstrated that under 808 nm laser irradiation, the nanopremedicine effectively internalized into HepG2 cells, generated excessive intracellular reactive oxygen species, and induced mitochondria-mediated apoptosis to effectively kill cancer cells. Results from in vivo experiments confirmed the effective tumour accumulation and biosafety of HBCI. Moreover, the tumour inhibition efficiency of the nanopremedicine was significantly higher than that of CDDP alone and PDT alone, indicating a strong synergistic effect when combining PDT/chemotherapy to inhibit tumour growth.Chaudhuri et al. also developed photoresponsive fluorescent organic nanoconjugates CY-90 for the effective treatment of cancer through a synergistic combination of PDT and chemotherapy (Fig. 28).206 The one-component system was coupled with squaric acid and a coumarin–chlorobenzoic acid coupler. Under visible light, the prepared CY-90 nanoconjugates produced 1O2 while releasing chlorobenzene. Interestingly, the photoproducts formed during photolysis similarly produced 1O2. Cytotoxicity experiments showed that the CY-90 nanoconjugates possessed enhanced anticancer activity due to the synergistic effect of the released anticancer drugs, chlorobenzene and PDT, as compared to the CY-91 nanoconjugates.
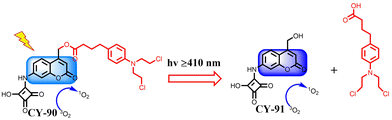 | ||
| Fig. 28 The multifunctional nanoconjugates CY-90 for combination therapy. Adapted from ref. 206 with permission from the American Chemical Society, copyright 2018. | ||
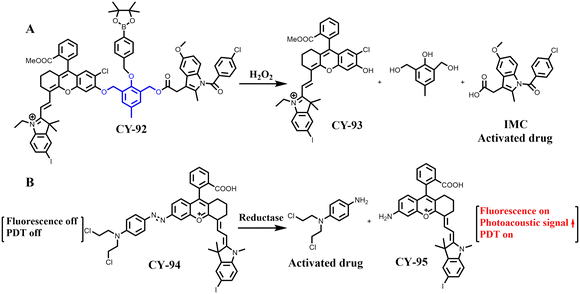
The development of activated prodrugs is an effective approach for enhancing tumour targeting and improving therapeutic precision. Based on this concept, Xiong et al. developed a reactive oxygen species-triggered therapeutic prodrug CY-92 based on CY-93 for chemo-PDT of tumours (Fig. 29A).207 Interestingly, CY-92 selectively targets tumours by releasing both CY-93 and indomethacin when exposed to reactive oxygen species specifically found in tumours. This leads to the production of high levels of phototoxic 1O2, which kills most non-CSCs (cancer stem cells), effectively responding to PDT treatment while also inhibiting CSC growth through IMC administration. Consequently, this multiplies the effectiveness or “therapeutic index” achieved by this approach. In vivo experiments demonstrate that combining CY-92 with light achieves optimal tumour therapy by simultaneously employing chemotherapy and PDT against CSCs within tumours.
Our team also developed a strategy to enhance the efficiency of releasing molecular chemotherapeutic prodrugs via PDT, based on hemicarbocyanine derivatives. We synthesized a molecular prodrug CY-94 by linking a chemotherapeutic drug with a near-infrared photosensitiser CY-95 through a low-oxygen azo bond (Fig. 29B).208 Experimental results demonstrated its selective activation in hypoxic tumours for the simultaneous release of both the chemotherapeutic drugs and CY-95. The release process was successfully monitored in real-time using fluorescence and photoacoustic dual-mode imaging. Furthermore, the activation of CY-94 could be further enhanced under light conditions, leading to the increased release of both chemotherapeutic drugs and photosensitisers. In addition, CY-94 could target mitochondria and induce cell death by destroying them. In vivo experiments showed that the PDT-assisted drug release strategy had a more effective inhibitory effect on tumour growth.
4.3 PTT and chemotherapy
To date, most of the commonly used chemotherapeutic drugs, such as adriamycin, anthracyclines, paclitaxel, etc.,209–212 do not specifically target cancer cells and their non-specific distribution during tumour treatment can cause irreversible damage to normal tissues, resulting in adverse side effects. Additionally, the long-term use of chemotherapeutic agents may lead to multidrug resistance.213–215 Numerous studies have reported that PTT can increase the cellular uptake of drugs, overcome drug resistance, reduce side effects and improve the anticancer efficacy of chemotherapeutic drugs.216–220 Therefore, combining PTT with chemotherapy for the treatment of tumours has the following advantages: (1) PTT-induced thermotherapy can enhance the permeability of cell membranes, which promotes the uptake of chemotherapeutic drugs by tumours and increases the sensitivity of tumours to chemotherapy; (2) it can effectively reduce the dosage of chemotherapeutic drugs; (3) it can complement single treatment modalities' limitations; and (4) chemotherapy can compensate for intrinsic defects of PTT, such as limited tissue penetration depth by lasers preventing deep tumour entry or poor therapeutic effects on metastatic tumours leading to easy recurrence. For example, Liu et al. designed a novel biocompatible nanoparticle therapeutic agent (FA-PEG-PLGA-Ptx@ICG-Pfh NPs) loaded with paclitaxel (Ptx) and ICG. Under laser irradiation,221 FA-PEG-PLGA-Ptx@ICG-Pfh NPs could achieve efficient PTT while also achieving a dramatic release of Ptx in regions with high folate receptor expression ensuring a higher concentration in the tumour region thus achieving synergistic anti-tumour effects through chemotherapy/PTT combination therapy. Researchers evaluated their anticancer therapeutic effects in vitro and in vivo using the CCK8 assay and the MDA-MB231 hormonal mouse model. The results of both in vitro and in vivo experiments showed that FA-PEG-PLGA-Ptx@ICG-Pfh NPs exhibited superior anticancer effects. Ma et al. used bovine serum albumin (BSA) encapsulated with ICG and loaded with the chemotherapeutic drug doxorubicin (DOX) to construct an ICG-based therapeutic nanoplatform (BSA@ICG-DOX NPs).222 The therapeutic efficacy of BSA@ICG-DOX NPs was evaluated by establishing a subcutaneous transplantation model in nude mice, demonstrating their ability to achieve effective chemotherapy/PTT synergistic treatment for cervical tumours under 808 nm laser irradiation, resulting in significant anticancer effects.To promote drug targeting and enrichment at the tumour site and enhance therapeutic efficacy, Chen et al. designed nanoparticles GNP-DOX/ICG with dual photothermal/matrix metalloproteinase-2 (MMP-2)-responsive, tumour-targeting, and size-variable capabilities by encapsulating ICG and DOX using a size-variable gelatin nanoparticle (GNP).223 This design aims to enhance anti-tumour efficacy and achieve the “on-demand” release of the drugs. GNP-DOX/ICG exhibits microenvironmentally responsive tumour-targeting and size-variable capabilities to enhance anti-tumour efficacy and enable the “on-demand” release of the drug. Under 808 nm laser irradiation, GNP-DOX/ICG is photothermally responsive, expanding in size from 71.58 ± 4.28 nm to 160.80 ± 9.51 nm, which facilitates particle retention at the tumour site and the release of loaded therapeutic drugs. In addition, GNP-DOX/ICG showed a particle size reduction to 33.24 ± 4.11 nm along with the improved drug release due to degradation of overexpressed MMP-2 in the tumour. GNP-DOX/ICG exhibited a good photothermal effect under NIR laser irradiation, which significantly enhanced the uptake of DOX and ICG by 4T1 cells. In subsequent therapeutic experiments on tumour models, it was confirmed that GNP-DOX/ICG could provide enhanced and synergistic therapeutic effects through the combination of PTT and chemotherapy, effectively inhibiting the growth of 4T1 tumours.
Yu et al. also promoted drug accumulation and release at the tumour site by constructing a tumour-targeting, dual-stimulation response platform (PSC/ICG@+DOX).224 Due to the presence of calcium carbonate and disulfide-bonded copolymers, the PSC/ICG@+DOX nanoparticles were pH and reduction-sensitive, degrading in slightly acidic solutions and reducing environments to increase the drug release. Under NIR laser irradiation, PSC/ICG@+DOX exhibited effective photothermal effects in vitro and enhanced cellular uptake and cytotoxicity in 4T1 cells. Gao et al. designed and prepared a drug delivery system with synergistic chemotherapy, PTT and magnetic targeting (ICGPTL-Lips@MNPs).212 This system consisted of Parthenolide (PTL), ICG, liposomes (Lips) and Fe3O4 nanoparticles (MNPs) for magnetic targeting. Based on the magnetic targeting effect of MNPs, the drug can rapidly accumulate at the tumour site under the influence of the magnetic field, enhancing chemotherapy–PTT. Simultaneously, ICG converted near-infrared light into heat to kill tumour cells and promote PTL release. Through the synergistic effect of chemotherapy, photothermal therapy and targeted therapy, it effectively killed tumour cells more effectively while improving anti-tumour efficacy.
In addition to the FDA-approved ICGs, an increasing number of cyanine dye derivatives have been developed and used for constructing multimodal therapeutic platforms. Zou et al. designed a GSH-activated prodrug CY-96 based on CY-98 (Fig. 30A).225 The strategy involves covalently linking an alkylating reagent (Cbl) to CY-98 for synergistically enhancing photothermal chemotherapy. In cancer cells with overexpressed GSH, CY-96 is converted to CY-98, which achieves enhanced photothermal effects through the photo-induced electron transfer (PET) effect and releases the alkylating reagent. Additionally, the photothermal effect of CY-98 can further enhance DNA alkylation by chemotherapeutic drugs. Surprisingly, the team discovered that chemotherapy could enhance PTT efficacy by down-regulating HSP70 expression, which was experimentally confirmed. The two treatments had a synergistic promoting effect, resulting in higher efficiency and selectivity in killing cancer cells. In vitro and in vivo evaluations demonstrated that under 808 nm light irradiation, CY-96 effectively and selectively killed cancer cells while inhibiting tumour growth.
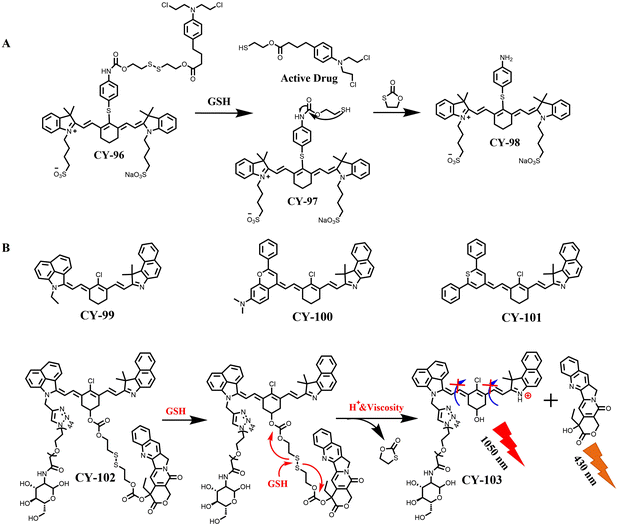 | ||
| Fig. 30 (A) The activation mechanism of CY-96. (B) Molecular structures of CY-99 to CY-101 and the turn-on mechanism of CY-102. | ||
Lu et al. prepared Cy5.5-MSA-G250 NPs by mixing mouse serum albumin (MSA) with koammas brilliant blue (G250), and then coupling it with cyanine 5.5 (Cy5.5).226 In addition to exhibiting good in vivo and in vitro photothermal properties, Cy5.5-MSA-G250 NPs demonstrate cancer-specific chemotherapeutic effects. Compared with clinically used anticancer drugs, the NPs exhibit a stronger cytotoxic effect on cancer cells while showing negligible toxicity on normal cells. Furthermore, in vivo experiments have also demonstrated that the effective removal of residual tumour cells and prevention of tumour metastasis by Cy5.5-MSA-G250 NPs. The platform of H+/viscosity-responsive asymmetric cyanine (NSCyanine) dyes CY-99 to CY-101 (Fig. 30B), which were designed and synthesised by Tian et al.,227 is highly sensitive to pH/viscosity and can be activated by a “double key and lock” strategy. In contrast to the conventional “always-on” heptamethylocyanine, these NSCyanine dyes can be sensitively activated by a “dual-key-and-lock” strategy, exhibiting “off–on” NIR-II fluorescence, larger Stokes shifts, higher chemical and photostability, and better photothermal properties. Liver and intestinal shifts were observed after treatment with CY-99 based on its H+/viscosity dual-stimulation response properties. Notably, by incorporating water-soluble peg-amino glucose and degradable CPT drugs into CY-99, the therapy dye CY-102 was able to successfully target and illuminate subcutaneous 4T1 breast cancer tumours with tumour-to-liver contrast of 19.5/1. High-contrast phototherapy for extrahepatic disease was achieved by targeting combined with PTT and chemotherapy. This asymmetric strategy will further broaden the design and application of NIR-II phalloidin dyes.
4.4 Others
In addition to the more common combination therapy modalities mentioned above, an increasing number of multi-modality combination therapy platforms are being developed. These include a combination of phototherapy and immunotherapy, as well as combinations of phototherapy and radiotherapy. For instance, Zhang et al. addressed the challenge of effectively inhibiting tumour metastasis and recurrence with conventional single PTT by developing multimodal imaging and therapeutic integrated nanoparticles called I-HSA-ICG.228 They achieved this by non-covalently combining I-HSA with ICG for diagnostic and therapeutic purposes. The use of I-HSA-ICG nanoparticles resulted in prolonged retention within tumour cells, enabling sustained induction of cellular damage through radiotherapy during and after PTT, thereby further enhancing the tumour ablation rate. Effective treatment for iodine-refractory thyroid cancer was achieved by combining radionuclide therapy with PTT.Excessive medication dosage can also lead to toxic side effects and impact treatment effectiveness. To reduce drug dosage and achieve better therapeutic outcomes, multimodal combination therapy strategies that incorporate multiple therapeutic modalities have been developed.229 Zhou et al. constructed a tumour microenvironment-responsive supramolecular nanoplatform (CyCA@POPD) using host–guest interactions between the pillar arene-based amphiphilic polymer POPD, the phototherapy agent (Cy7-CN), respiratory medication atovaquone (ATO) and chemotherapeutic drug pyridinyl camptothecin (CPT-Py).230 This platform enables combined respiratory inhibition-enhanced PDT, PTT and chemotherapy. Inhibition of π–π stacking due to host–guest interactions significantly improved the photochemical and photophysical properties of alanine. Triggered by the acidic microenvironment at the tumour site, the supramolecular nanoplatform dissociates and releases CPT-Py and ATO. This inhibits mitochondria-associated oxidative phosphorylation (OXPHOS) while facilitating enhanced PDT through increased oxygen utilization. In vitro and in vivo studies confirm the enhanced efficacy of PDT and PTT with a rational combination of ATO, overcoming limitations associated with single phototherapy. This synergistic approach not only sensitizes chemotherapy drugs but also achieves a higher level of tumour suppression. Li et al. established a chitosan-based nanocomplex called CE7Q/CQ/S, which delivers the molecular-targeted drug erlotinib (Er), survivin shRNA-expressing plasmid (SV), and photothermal agent heptamethine cyanine dye (Cy7) in a single platform for targeted therapy against tumours expressing survivin protein levels above the normal range.231 By integrating chemo/gene/photothermal therapies into one therapeutic nanoplatform, CE7Q/CQ/S could significantly inhibit non-small cell lung cancer (NSCLC) bearing epidermal growth factor receptor (EGFR) mutations. Han et al. constructed multifunctional ICG-MSN-Fe-CD nanoparticles for collaborative chemodynamic/photothermal/photodynamic therapy in vitro.232 The high heat generated by ICG under near-infrared light irradiation not only directly kills the cancer cells for PTT but also dissociates the capped β-CD, thus exposing the internal ferrocene. The exposed ferrocene can catalyze the generation of highly toxic –OH from intracellular H2O2 through a ferrocene-mediated Fenton reaction in tumour tissues, effectively killing cancer cells to achieve CDT. In addition, 1O2 generated by ICG can further synergistically kill cancer cells to perform PDT. The results of in vitro experiments showed that synergistic CDT/PTT/PDT had a significant inhibition rate on HeLa cells. A cell killing rate of up to 93% could be achieved using synergistic CDT/PTT/PDT, which was much higher than single CDT at 22% and dual PTT/PDT at 49%.
Currently, photo-immunotherapy (PIT), a novel therapeutic approach, is an ideal strategy for eliminating the primary tumour while triggering a systemic immune response to remove residual tumour cells and inhibit distant metastasis. It combines phototherapy and immunotherapy, whereby phototherapy effectively induces immunogenic cell death (ICD) in tumours by releasing tumour-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs), which may activate an immune response against antigens of the dead tumour cells. In contrast, phototherapy usually aims at directly killing the tumour cells and eradicating the tumour tissues, while simultaneously inducing an immune response that can either reduce or enhance immunomodulation. This process plays a crucial role in suppressing tumour recurrence and metastasis. Cyanine dyes have also been used in constructing photoimmunotherapy strategies due to their excellent phototherapy properties. Hao et al. developed a multimodal therapeutic platform (ICG-MOF-SS-AUNP12) based on the photothermic agent ICG.233 Under 808 nm near-infrared irradiation, ICG-MOF-SSSAUNP12 exhibited a potent photothermal effect that ablated the tumour cells while intelligently released PD-1 inhibitory peptide to promote DC cell maturation and activate the immune response. Effective treatment of melanoma is achieved through the synergistic effect of photothermal and immunotherapy.
Ma et al. developed endoplasmic reticulum-localised immunogenic cell death (ICD) photoinducers for the first time using thiopentamethylene cyanine dyes CY-104 to CY-106 to induce endoplasmic reticulum stress under mild near-infrared light irradiation (Fig. 31A).234 Among them, the poly-fluorinated dye CY-106 exhibited selective accumulation in the endoplasmic reticulum and superior reactive oxygen species generation under both normoxic and hypoxic conditions. Under NIR irradiation, cancer cells stained with the dye CY-106 resulted in endoplasmic reticulum stress and induced the release of many DAMPs, including up-regulation of GRP78, release of HMGB1, secretion of ATP, ecto-CRT, and ecto-HSP70. In vivo experiments demonstrated that by activating a synergistic combination of photodynamic effects and immune response activation, CY-106 greatly improved the therapeutic effect.
 | ||
| Fig. 31 (A) Molecular structures of CY-104 to CY-106. (B) Schematic of enhanced type I ROS production, photothermal conversion efficiency (PCE), and photostability of CY-107 analogues by introducing TPA. Adapted from ref. 235 with permission from the American Chemical Society, copyright 2024. | ||
Recently, Yu et al. introduced an intramolecular rotor TPA on the conjugated backbone of CY-107 to simultaneously enhance its reactive oxygen species generation, photothermal conversion and photostability (Fig. 31B).235 Experimental results and theoretical calculations showed that CY-108 not only generates O2˙−, but also has a high PCE with a η value of up to 45.6%. At lower temperatures where intramolecular motions are limited, reactive oxygen species generation dominates while photothermal effects are poor; however, at higher temperatures where intramolecular motions are promoted, significantly reduced reactive oxygen species generation occurs. This modulated PDT/PTT combination therapy maximizes reactive oxygen species and heat generation during phototherapy. More importantly, this phototherapy process effectively induces the generation and subsequent release of ICD-associated molecular patterns, which activate anti-tumour immunity leading to superior photoimmunotherapy.
5. Conclusions
This review provides a comprehensive summary of various photosensitizers for PDT and photothermic agents for PTT that have been developed based on cyanine dyes and their derivatives in recent years. Based on current research progress, effective tumour clearance requires a photothermic agent developed from cyanine dyes to achieve the following points: (1) improved generation of reactive oxygen species and photothermal conversion performance, (2) good thermal stability and resistance to photobleaching, (3) excellent biocompatibility, (4) efficient tumour targeting, and (5) no or minimal dark toxicity. Additionally, if the phototherapy reagent is intended for clinical diagnosis and treatment, it needs to exhibit strong absorption in the near infrared region to enable deep treatment. However, currently developed cyanine dye-based phototherapy reagents have drawbacks such as poor photosensitivity and photothermal performance, low water solubility, susceptibility to photobleaching, instability, and inadequate tumour targeting ability.Although combining with nanomaterials can improve some issues, they are not easily metabolized in vivo and may have potential long-term toxicity. Therefore, developing small molecules based on cyanine dyes with excellent performance remains the top priority of current research. To enhance the performance of cyanine phototherapy agents, several strategies can be employed: (1) regulating molecular absorption wavelengths, (2) expanding conjugation, (3) regulating molecular interactions, (4) introducing different electron donor/acceptor groups to modulate molecular energy levels, (5) forming aggregates, and (6) regulating molecular movements. In addition, due to the complexity and heterogeneity of the tumour pathological environment, single treatment methods struggle to achieve a satisfactory therapeutic effect. Based on this premise, effective tumour clearance is achieved by constructing small molecule multimodal diagnostic reagents through structural modification and fusion of multiple therapy agents.
Although cyanine dyes have been extensively developed in research fields such as the development of multifunctional phototherapy reagents and their applications, their tumour-targeting and biocompatibility mostly rely on nanomodification for improvement. Therefore, it is still necessary to develop an effective strategy for modifying their structural modification to further enhance their performance in tumour therapy. In addition, photoimmunotherapy, as an emerging therapeutic modality, is a current research hotspot in the field of therapy. However, only a few photoimmunotherapy reagents have been developed based on cyanine dyes. Hence, it is important to design molecular structures that can activate immune cells in the tumour microenvironment by introducing immunostimulants (e.g., CpG oligodeoxynucleotides) or activators of antigen-presenting cells (e.g., dendritic cell DCs). Moreover, designing new multifunctional photoimmunotherapy reagents that can exploit the PDT-induced immunogenic cell death (ICD) process and facilitate the release of tumour-associated antigens and inflammatory cytokines to activate the body's anti-tumour immune response is a direction for expanding cyanine dye-based phototherapy reagents. Consequently, future research should focus on improving existing diagnostic reagents developed based on cyanine dyes and developing more effective diagnostic reagents which will ultimately better serve tumour treatment. With the continuous advancement of research, we firmly believe that cyanine dye-based phototherapy reagents will continue to provide practical assistance for medical research and clinical treatment in the near future.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (U21A20314, 22304045, and 22378100), the Program for the Innovative Research Team (in Science and Technology) in the University of Henan Province (23IRTSTHN002), the Henan Province Central Leading Local Science and Technology Development Fund Project (Z20231811083), the Talent postdoctoral program for Henan province, the Key Scientific Research Project of Higher Education of Henan Province (23A150003), the Key Project of Science and Technology of Henan Province (222102310413), and the China Postdoctoral Science Foundation (2022M721051). We gratefully acknowledge the support of the Shenzhen University 2035 Program for Excellent Research (no. 00000225, X. P.). The authors acknowledge Tan Zhang and Yaxin Yang, students of the School of Chemistry and Chemical Engineering, Henan Normal University, for their contributions to the article typesetting and formatting check.Notes and references
- H. Huang, D. Huang, M. Li, Q. Yao, R. Tian, S. Long, J. Fan and X. Peng, Dyes Pigm., 2020, 177, 108284 CrossRef CAS.
- Y. Zhang, W. Shen, P. Zhang, L. Chen and C. Xiao, Chem. Commun., 2020, 56, 5645–5648 RSC.
- H. Liu, X. Hu, K. Li, Y. Liu, Q. Rong, L. Zhu, L. Yuan, F. Qu, X. Zhang and W. Tan, Chem. Sci., 2017, 8, 7689–7695 RSC.
- T. Qi, B. Chen, Z. Wang, H. Du, D. Liu, Q. Yin, B. Liu, Q. Zhang and Y. Wang, Biomaterials, 2019, 213, 119219 CrossRef CAS PubMed.
- F. Xue, P. Wei, X. Ge, Y. Zhong, C. Cao, D. Yu and T. Yi, Dyes Pigm., 2018, 156, 285–290 CrossRef CAS.
- L. Zhao, X. Zhang, X. Wang, X. Guan, W. Zhang and J. Ma, J. Nanobiotechnol., 2021, 19, 335 CrossRef CAS PubMed.
- D. Zhi, T. Yang, J. O’Hagan, S. Zhang and R. F. Donnelly, J. Controlled Release, 2020, 325, 52 CrossRef CAS.
- L. Fu, Y. Huang, J. Hou, M. Sun, L. Wang, X. Wang and L. Chen, J. Mater. Chem. B, 2022, 10, 8432–8442 RSC.
- P. C. A. Swamy, G. Sivaraman, R. N. Priyanka, S. O. Raja, K. Ponnuvel, J. Shanmugpriya and A. Gulyani, Coord. Chem. Rev., 2020, 411, 213233 CrossRef.
- D. Kobzev, O. Semenova, A. Tatarets, A. Bazylevich, G. Gellerman and L. Patsenker, Dyes Pigm., 2023, 212, 111101 CrossRef CAS.
- L. Yang, Y. Liu, X. Ren, R. Jia, L. Si, J. Bao, Y. Shi, J. Sun, Y. Zhong, P. Duan, X. Yang, R. Zhu, Y. Jia and F. Bai, ACS Nano, 2024, 18, 3161–3172 CrossRef CAS PubMed.
- O. Buyukcakir, O. A. Bozdemir, S. Kolemen, S. Erbas and E. U. Akkaya, Org. Lett., 2009, 11, 4644–4647 CrossRef CAS PubMed.
- J. Li and T. Chen, Coord. Chem. Rev., 2020, 418, 213355 CrossRef CAS.
- S. N. T. Koc, S. R. Benam, I. P. Aral, R. Shahbazi and K. Ulubayram, Int. J. Pharm., 2024, 655, 124057 CrossRef PubMed.
- L. Ke, F. Wei, L. Xie, J. Karges, Y. Chen, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2022, 61, e202205429 CrossRef CAS PubMed.
- A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100, 1973–2011 CrossRef CAS PubMed.
- H. A. Shindy, Mini-Rev. Org. Chem., 2012, 9, 352–360 CrossRef CAS.
- H. A. Shindy, Dyes Pigm., 2017, 145, 505–513 CrossRef CAS.
- G. S. Gopika, P. M. H. Prasad, A. G. Lekshmi, S. Lekshmypriya, S. Sreesaila, C. Arunima, M. S. Kumar, A. Anil, A. Sreekumar and Z. S. Pillai, Mater. Today Proc., 2021, 46, 3102–3108 CrossRef CAS.
- V. W. König, Angew. Chem., Int. Ed. Engl., 1925, 38, 743–748 CrossRef.
- W. Sun, S. Guo, C. Hu, J. Fan and X. Peng, Chem. Rev., 2016, 116, 7768–7817 CrossRef CAS PubMed.
- L. A. Galindo, O. P. Gomes, C. F. O. Graeff and A. Batagin-Neto, Comput. Theor. Chem., 2021, 1199, 113197 CrossRef CAS.
- K. Bilici, S. Cetin, E. Celikbas, H. Y. Acar and S. Kolemen, Front. Chem., 2021, 9, 707876 CrossRef CAS PubMed.
- G. Jiang, H. Liu, H. Liu, G. Ke, T. Ren, B. Xiong, X. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2024, 63, e202315217 CrossRef CAS PubMed.
- Y. Li, Y. Zhou, X. Yue and Z. Dai, Bioact. Mater., 2021, 6, 794–809 CAS.
- Z. Li, P. Liang, T. Ren, L. Yuan and X. Zhang, Angew. Chem., Int. Ed., 2023, e202305742 CAS.
- Z. Li, L. Xu, J. Li, L. Lei, P. Liang, Q. Wu, F. Yang, T. Ren, X. Yin, L. Yuan and X. Zhang, J. Am. Chem. Soc., 2023, 145, 26736–26746 CrossRef CAS PubMed.
- D. Ma, H. Bian, M. Gu, L. Wang, X. Chen and X. Peng, Coord. Chem. Rev., 2024, 505, 215677 CrossRef CAS.
- D. Mejlachowicz, P. Lassiaz, M. Zola, B. Leclercq, E. Gélizé, S. Achiedo, M. Zhao, A. Rousseau and F. Behar-Cohen, Invest. Ophthalmol. Visual Sci., 2024, 65, 25 CrossRef CAS PubMed.
- J. Lin, J. Zhang, N. Wei, A. Wu, L. Wang, F. Teng, Y. Xian and R. Han, Front. Oncol., 2024, 14, 1345288 CrossRef CAS PubMed.
- N. Russolillo, S. Langella, S. Perotti, R. L. Tesoriere, F. Forchino and A. Ferrero, Int. J. Surg., 2016, 31, 80–85 CrossRef PubMed.
- S. Xu, Y. Yuan, X. Cai, C. Zhang, F. Hu, J. Liang, G. Zhang, D. Zhang and B. Liu, Chem. Sci., 2015, 6, 5824–5830 RSC.
- W. Yin, Y. Ma, S. Chen, L. Xing, S. Ji, J. W. Y. Lam, R. T. K. Kwok, Y. Huo and B. Z. Tang, Adv. Opt. Mater., 2024, 12, 2302197 CrossRef CAS.
- H. Lu, Y. Wang, S. K. Hill, H. Jiang, Y. Ke, S. Huang, D. Zheng, S. Perrier and Q. Song, Angew. Chem., Int. Ed., 2023, 62, e202311224 CrossRef CAS.
- P. Bhattarai and Z. Dai, Adv. Healthcare Mater., 2017, 6, 1700262 CrossRef.
- E. P. Porcu, A. Salis, E. Gavini, G. Rassu, M. Maestri and P. Giunchedi, Biotechnol. Adv., 2016, 34, 768–789 CrossRef CAS PubMed.
- C. Shao, C. Liao, P. Hu, C. Chu, L. Zhang, M. H. T. Bui, C. S. Ng, D. Y. Josephson, B. Knudsen, M. Tighiouart, H. L. Kim, H. E. Zhau, L. W. K. Chung, R. Wang and E. M. Posadas, PLoS One, 2014, 9, e88967 CrossRef PubMed.
- X. Zhao, Y. Ma, J. Di, Y. Qiao, J. Yu, Y. Yin, R. Xi and M. Meng, ACS Appl. Mater. Interfaces, 2024, 16, 12310–12320 CrossRef CAS PubMed.
- H. Ma, S. Long, J. Cao, F. Xu, P. Zhou, X. Zhou, C. Shi, W. Sun, J. Du, K. Han, G. Zeng, J. Fan and X. Peng, Chem. Sci., 2021, 12, 13809–13816 RSC.
- X. Zhao, Q. Yao, S. Long, W. Chi, Y. Yang, D. Tan, X. Liu, H. Huang, W. Sun, J. Du, J. Fan and X. Peng, J. Am. Chem. Soc., 2021, 143, 12345–12354 CrossRef CAS PubMed.
- H. Yang, F. Li, S. Jin, S. Chen, L. Sun, L. Wei, G. Xu, S. Cao, W. Song, X. Zeng, W. Zhong and W. Sun, Chem. Eng. J., 2024, 494, 153089 CrossRef CAS.
- X. Zhao, S. He, W. Chi, X. Liu, P. Chen, W. Sun, J. Du, J. Fan and X. Peng, Adv. Sci., 2022, 9, 2202885 CrossRef CAS PubMed.
- W. Liu, Y. Hou, W. Liu, R. Wang, S. He, X. Xia, C. Lv, H. Gu, Q. Yao, Q. Pan, Z. Su, D. Zhou, W. Sun, J. Fan and X. Peng, Chin. Chem. Lett., 2024, 35, 109631 CrossRef CAS.
- H. Huang, D. Ma, Q. Liu, D. Huang, X. Zhao, Q. Yao, T. Xiong, S. Long, J. Du, J. Fan and X. Peng, CCS Chem., 2022, 4, 3627–3636 CrossRef CAS.
- O. Semenova, D. Kobzev, I. Hovor, M. Atrash, F. Nakonechny, O. Kulyk, A. Bazylevich, G. Gellerman and L. Patsenker, Pharmaceutics, 2023, 15, 247 CrossRef CAS PubMed.
- M. Zhang, R. Zhang, Y. Dong, J. Liu, Z. Gao, X. Zhou and J. Cao, Chem. Eng. J., 2023, 459, 141484 CrossRef CAS.
- R. Tovtik, E. Muchová, L. Štacková, P. Slavícěk and P. Klán, J. Org. Chem., 2023, 88, 6716–6728 CrossRef CAS PubMed.
- L. Wu, X. Meng, Q. Zhang, X. Han, F. Yang, Q. Wang, H. Hu and N. Xing, Chin. Chem. Lett., 2024, 35, 108663 CrossRef CAS.
- J. Cao, J. Chi, J. Xia, Y. Zhang, S. Han and Y. Sun, ACS Appl. Mater. Interfaces, 2019, 11, 25720–25729 CrossRef CAS PubMed.
- J. Atchison, S. Kamila, H. Nesbitt, K. A. Logan, D. M. Nicholas, C. Fowley, J. Davis, B. Callan, A. P. McHale and J. F. Callan, Chem. Commun., 2017, 53, 2009–2012 RSC.
- O. Semenova, D. Kobzev, F. Yazbak, F. Nakonechny, O. Kolosova, A. Tatarets, G. Gellerman and L. Patsenker, Dyes Pigm., 2021, 195, 109745 CrossRef CAS.
- K. Mitra, C. E. Lyons and M. C. T. Hartman, Angew. Chem., Int. Ed., 2018, 57, 10263–10267 CrossRef CAS PubMed.
- A. V. Prakash, F. Yazabak, I. Hovor, F. Nakonechny, O. Kulyk, O. Semenova, A. Bazylevich, G. Gellerman and L. Patsenker, Dyes Pigm., 2023, 211, 111053 CrossRef CAS.
- J. Sun, E. Feng, Y. Shao, F. Lv, Y. Wu, J. Tian, H. Sun and F. Song, ChemBioChem, 2022, 23, e202200421 CrossRef CAS.
- H. Liu, J. Yin, E. Xing, Y. Du, Y. Su, Y. Feng and S. Meng, Dyes Pigm., 2021, 190, 109327 CrossRef CAS.
- I. Noh, D. Y. Lee, H. Kim, C. U. Jeong, Y. Lee, J. O. Ahn, H. Hyun, J. H. Park and Y. C. Kim, Adv. Sci., 2018, 5, 1700481 CrossRef.
- W. Liu, S. He, X. Ma, C. Lv, H. Gu, J. Cao, J. Du, W. Sun, J. Fan and X. Peng, Angew. Chem., Int. Ed., 2024, e202411802 CAS.
- Y. Dong, L. Zhou, Z. Shen, Q. Ma, Y. Zhao, Y. Sun and J. Cao, Drug Delivery, 2022, 29, 238–253 CrossRef CAS PubMed.
- L. Jiao, F. Song, J. Cui and X. Peng, Chem. Commun., 2018, 54, 9198–9201 RSC.
- X. Hao, Y. Tang, R. Zhang, Z. Wang, M. Gao, R. Wei, Y. Zhao, X. Mu, Y. Lu and X. Zhou, Acta. Biomater., 2024, 178, 287–295 CrossRef CAS PubMed.
- S. Kolemen, M. Işık, G. M. Kim, D. Kim, H. Geng, M. Buyuktemiz, T. Karatas, X. Zhang, Y. Dede, J. Yoon and E. U. Akkaya, Angew. Chem., Int. Ed., 2015, 54, 5340–5344 CrossRef CAS PubMed.
- Y. Cakmak, S. Kolemen, S. Duman, Y. Dede, Y. Dolen, B. Kilic, Z. Kostereli, L. T. Yildirim, A. L. Dogan, D. Guc and E. U. Akkaya, Angew. Chem., Int. Ed., 2011, 50, 11937–11941 CrossRef CAS PubMed.
- X. Zhou, H. Li, C. Shi, F. Xua, Z. Zhang, Q. Yao, H. Ma, W. Sun, K. Shao, J. Du, S. Long, J. Fan, J. Wang and X. Peng, Biomaterials, 2020, 253, 120089 CrossRef CAS.
- J. T. Buck, A. M. Boudreau, A. DeCarmine, R. W. Wilson, J. Hampsey and T. Mani, Chem, 2019, 5, 138–155 CAS.
- X. Zhou, C. Shi, S. Long, Q. Yao, H. Ma, K. Chen, J. Du, W. Sun, J. Fan, B. Liu, L. Wang, X. Chen, L. Sui, K. Yuan and X. Peng, ACS Cent. Sci., 2023, 9, 1679–1691 CrossRef CAS PubMed.
- Y. Chen, C. Li, Z. Zeng, W. Wang, X. Wang and B. Zhang, J. Mater. Chem., 2005, 15, 1654–1661 RSC.
- E. Stathatos, P. Lianos, A. Laschewsky, O. Ouari and P. V. Cleuvenbergen, Chem. Mater., 2001, 13, 3888–3892 CrossRef CAS.
- P. Fromherz and C. O. Müller, Biochim. Biophys. Acta, Biomembr., 1993, 1150, 111–122 CrossRef CAS.
- L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510–13523 CrossRef CAS PubMed.
- L. Yuan, W. Lin, Y. Yang and H. Chen, J. Am. Chem. Soc., 2012, 134, 1200–1211 CrossRef CAS PubMed.
- F. Xu, H. Li, Q. Yao, H. Ge, J. Fan, W. Sun, J. Wang and X. Peng, Chem. Sci., 2019, 10, 10586–10594 RSC.
- X. Zhou, H. Li, C. Shi, F. Xu, Z. Zhang, Q. Yao, H. Ma, W. Sun, K. Shao, J. Du, S. Long, J. Fan, J. Wang and X. Peng, Biomaterials, 2020, 253, 120089 CrossRef CAS PubMed.
- Y. Chen, X. Zhao, T. Xiong, J. Du, W. Sun, J. Fan and X. Peng, Sci. China: Chem., 2021, 64, 808–816 CrossRef CAS.
- Z. Cheng, S. Benson, L. M. Tapia, E. Nestoros, C. Lochenie, D. Seah, K. Y. Chang, Y. Feng and M. Vendrell, Angew. Chem., Int. Ed., 2024, 63, e202404587 CrossRef CAS PubMed.
- Y. Wang, C. Zhang, L. Hao, X. Huo, L. Dou, Y. Xie, J. Wang, X. Peng and H. Li, Dyes Pigm., 2024, 224, 112026 CrossRef CAS.
- Y. Liu, Q. Su, X. Zou, M. Chen, W. Feng, Y. Shi and F. Li, Chem. Commun., 2016, 52, 7466–7469 RSC.
- R. Tian, W. Sun, M. Li, S. Long, M. Li, J. Fan, L. Guo and X. Peng, Chem. Sci., 2019, 10, 10106–10112 RSC.
- Y. Li, G. Li, Q. Zhang, Y. Li, Q. Jia, W. Zhang, X. Feng, W. Xu and J. Liu, Chem. Phys. Lett., 2021, 784, 139091 CrossRef CAS.
- S. Lee, H. Wu, S. Yeo, W. K. Lee and I. Yoon, Inorg. Chem. Commun., 2023, 152, 110693 CrossRef CAS.
- J. Ribes, Y. Bourdeau, E. Rascol, I. Bestel and E. Badarau, Bioorg. Med. Chem., 2023, 81, 117210 CrossRef CAS.
- Y. Zhang, M. Zhao, J. Miao, W. Gu, J. Zhu, B. Cheng, Q. Li and Q. Miao, ACS Mater. Lett., 2023, 5, 3058–3067 CrossRef CAS.
- M. Zhang, S. Wang, Y. Bai, D. Wang, Y. Fu, Z. Su, G. Zhang, M. Meng, F. Yu, B. Wang, H. Jin and W. Zhao, Adv. Healthcare Mater., 2024, 13, 2303432 CrossRef CAS.
- B. T. Makowski, B. Valle, K. D. Singerb and C. Weder, J. Mater. Chem., 2012, 22, 2848–2850 RSC.
- G. Chen, H. Sasabe, T. Igarashi, Z. Hong and J. Kidoa, J. Mater. Chem. A, 2015, 3, 14517–14534 RSC.
- M. S. T. Gonçalves, Chem. Rev., 2009, 109, 190–212 CrossRef.
- D. Ramaiah, A. Joy, N. Chandrasekhar, N. V. Eldho, S. Das and M. V. George, Photochem. Photobiol., 1997, 65, 783–790 CrossRef CAS.
- D. Ramaiah, I. Eckert, K. T. Arun, L. Weidenfeller and B. Epe, Photochem. Photobiol., 2004, 79, 99–104 CrossRef CAS.
- L. Serpe, S. Ellena, N. Barbero, F. Foglietta, F. Prandini, M. P. Gallo, R. Levi, C. Barolo, R. Canaparo and S. Visentin, Eur. J. Med. Chem., 2016, 113, 187–197 CrossRef CAS PubMed.
- N. Bordignon, M. Köber, G. Chinigò, C. Pontremoli, E. Sansone, G. Vargas-Nadal, M. J. M. Plata, A. F. Pla, N. Barbero, J. Morla-Folch and N. Ventosa, Pharmaceutics, 2023, 15, 902 CrossRef CAS PubMed.
- T. C. D. Fernandes, E. Lima, R. E. Boto, D. Ferreira, J. R. Fernandes, P. Almeidac, L. F. V. Ferreira, A. M. Silva and L. V. Reis, Photodiagn. Photodyn. Ther., 2020, 31, 101844 CrossRef CAS PubMed.
- A. F. Magalhães, V. C. Graça, R. C. Calhelha, I. L. F. Machado, L. F. V. Ferreira, I. C. F. R. Ferreira and P. F. Santos, Photochem. Photobiol. Sci., 2019, 18, 336–342 CrossRef PubMed.
- P. F. Santos, L. V. Reis, P. Almeida, J. P. Serrano, A. S. Oliveira and L. F. V. Ferreira, J. Photochem., 2004, 163, 267–269 CAS.
- S. Webster, D. Peceli, H. Hu, L. A. Padilha, O. V. Przhonska, A. E. Masunov, A. O. Gerasov, A. D. Kachkovski, Y. L. Slominsky, A. I. Tolmachev, V. V. Kurdyukov, O. O. Viniychuk, E. Barrasso, R. Lepkowicz, D. J. Hagan and E. W. V. Stryland, J. Phys. Chem. Lett., 2010, 1, 2354–2360 CrossRef CAS.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- H. Qian, Q. Cheng, Y. Tian, H. Dang, C. Teng and L. Yan, J. Mater. Chem. B, 2021, 9, 2688–2696 RSC.
- X. Mu, Y. Lu, F. Wu, Y. Wei, H. Ma, Y. Zhao, J. Sun, S. Liu, X. Zhou and Z. Li, Adv. Mater., 2020, 32, 1906711 CrossRef CAS.
- P. Sun, Q. Wu, X. Sun, H. Miao, W. Deng, W. Zhang, Q. Fan and W. Huang, Chem. Commun., 2018, 54, 13395–13398 RSC.
- F. Wu, Y. Lu, X. Mu, Z. Chen, S. Liu, X. Zhou, S. Liu and Z. Li, ACS Appl. Mater. Interfaces, 2020, 12, 32388–32396 CrossRef CAS.
- X. Mu, Y. Lu, F. Wu, Y. Wei, H. Ma, Y. Zhao, J. Sun, S. Liu, X. Zhou and Z. Li, Adv. Mater., 2020, 32, 1906711 CrossRef CAS.
- J. Yu, J. Li and M. Li, ACS Sens., 2024, 9, 3581–3593 CrossRef CAS PubMed.
- Y. Zou, W. Liu, W. Sun, J. Du, J. Fan and X. Peng, Adv. Funct. Mater., 2022, 32, 2111853 CrossRef CAS.
- J. Pan, J. Du, Q. Hu, Y. Liu, X. Zhang, X. Li, D. Zhou, Q. Yao, S. Long, J. Fan and X. Peng, Adv. Healthcare Mater., 2023, 12, 2301091 CrossRef CAS PubMed.
- X. Zhao, H. Zhao, S. Wang, Z. Fan, Y. Ma, Y. Yin, W. Wang, R. Xi and M. Meng, J. Am. Chem. Soc., 2021, 143, 20828–20836 CrossRef CAS PubMed.
- V. G. Bandi, M. P. Luciano, M. Saccomano, N. L. Patel, T. S. Bischof, J. G. P. Lingg, P. T. Tsrunchev, M. N. Nix, B. Ruehle, C. Sanders, L. Riffle, C. M. Robinson, S. Difilippantonio, J. D. Kalen, U. Resch-Genger, J. Ivanic, O. T. Bruns and M. J. Schnermann, Nat. Methods, 2022, 19, 353–358 CrossRef CAS PubMed.
- J. Xue, Q. Liang, R. Wang, J. Hou, W. Li, Q. Peng, Z. Shuai and J. Qiao, Adv. Mater., 2019, 31, 1808242 CrossRef PubMed.
- L. Zhao, H. Zhu, Y. Duo, Z. Wang, D. Pang and S. Liu, Adv. Healthcare Mater., 2024, 2304421 CrossRef CAS.
- D. M. Coles, A. J. H. M. Meijer, W. C. Tsoi, M. D. B. Charlton, J. S. Kim and D. G. Lidzey, J. Phys. Chem. A, 2010, 114, 11920 CrossRef CAS.
- M. Shakiba, K. K. Ng, E. Huynh, H. Chan, D. M. Charron, J. Chen, N. Muhanna, F. S. Foster, B. C. Wilson and G. Zheng, Nanoscale, 2016, 8, 12618–12625 RSC.
- E. E. Jelley, Nature, 1936, 138, 1009–1010 CrossRef CAS.
- X. Song, H. Gong, T. Liu, L. Cheng, C. Wang, X. Sun, C. Liang and Z. Liu, Small, 2014, 10, 4362–4370 CrossRef CAS PubMed.
- R. Liu, J. Tang, Y. Xu, Y. Zhou and Z. Dai, Nanotheranostics, 2017, 1, 430–439 CrossRef PubMed.
- M. Millard, Y. Bernhard, N. Canilho, S. Grandemange, S. Parant, M. Mourer, H. P. Lassalle and A. Pasc, Colloids Surf., B, 2023, 230, 113516 CrossRef CAS PubMed.
- Z. Dai, W. Tian, X. Yue, Z. Zheng, J. Qi, N. Tamai and J. I. Kikuchi, Chem. Commun., 2009, 2032–2034 RSC.
- F. Wu, Y. Lu, X. Mu, Z. Chen, S. Liu, X. Zhou, S. Liu and Z. Li, ACS Appl. Mater. Interfaces, 2020, 12, 32388–32396 CrossRef CAS PubMed.
- X. Li, M. Yang, J. Cao, H. Gu, W. Liu, T. Xia, W. Sun, J. Fan and X. Peng, ACS Mater. Lett., 2022, 4, 724–732 CrossRef CAS.
- X. Mu, Y. Lu, F. Wu, Y. Wei, H. Ma, Y. Zhao, J. Sun, S. Liu, X. Zhou and Z. Li, Adv. Mater., 2020, 32, 1906711 CrossRef CAS PubMed.
- G. Feng, G. Zhang and D. Ding, Chem. Soc. Rev., 2020, 49, 8179–8234 RSC.
- Y. Zhu, H. Lai, H. Guo, D. Peng, L. Han, Y. Gu, Z. Wei, D. Zhao, N. Zheng, D. Hu, L. Xi, F. He and L. Tian, Angew. Chem., Int. Ed., 2022, 61, e202117433 CrossRef CAS PubMed.
- S. Liu, X. Zhou, H. Zhang, H. Ou, J. W. Y. Lam, Y. Liu, L. Shi, D. Ding and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 5359–5368 CrossRef CAS PubMed.
- N. B. Teran, G. S. He, A. Baev, Y. Shi, M. T. Swihart, P. N. Prasad, T. J. Marks and J. R. Reynolds, J. Am. Chem. Soc., 2016, 138, 6975–6984 CrossRef CAS PubMed.
- S. Sasaki, G. P. C. Drummen and G. I. Konishi, J. Mater. Chem. C, 2016, 4, 2731–2743 RSC.
- R. Ghosh and D. K. Palit, J. Phys. Chem. A, 2015, 119, 11128–11137 CrossRef CAS PubMed.
- W. Chi, Q. Qiao, R. Lee, W. Liu, Y. S. Teo, D. Gu, M. J. Lang, Y. T. Chang, Z. Xu and X. Liu, Angew. Chem., Int. Ed., 2019, 58, 7073–7077 CrossRef CAS PubMed.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4032 CrossRef PubMed.
- C. Wang, W. Chi, Q. Qiao, D. Tan, Z. Xu and X. Liu, Chem. Soc. Rev., 2021, 50, 12656–12678 RSC.
- S. Ma, L. Zhou, Y. Ma, H. Zhao, L. Li, M. Wang, H. Diao, X. Li, C. Zhang and W. Liu, Spectrochim., 2023, 302, 123132 CrossRef CAS PubMed.
- S. Yao, Y. Chen, H. Xu, F. Qi, Y. Zhang, T. Yang, Y. Wu, H. Fang, W. He and Z. Guo, Dyes Pigm., 2022, 206, 110583 CrossRef CAS.
- J. Wu, A. Xing, Y. Yuan, Y. Hao, P. Pan, S. He, J. Yuan and D. Zeng, Dyes Pigm., 2024, 221, 111797 CrossRef CAS.
- S. Xu, F. Guo, Z. Xu, Y. Wang and T. D. James, Sens. Actuators, B, 2023, 383, 133510 CrossRef CAS.
- X. Wang, H. Wang, J. Duan, Q. Sun, C. Zhang, L. Xua and Z. Liu, Sens. Actuators, B, 2024, 407, 135453 CrossRef CAS.
- Y. Shu, C. Huang, H. Liu, F. Hu, H. Wen, J. Liu, X. Wang, C. Shan and W. Li, Spectrochim., 2022, 281, 121529 CrossRef CAS PubMed.
- L. Zhao, H. Zhu, Y. Duo, D. Pang, Z. Wang and S. Liu, Adv. Healthcare Mater., 2023, 12, 2301584 CrossRef CAS PubMed.
- L. Zhang, M. Wang, F. Wu, L. Liu, X. Ren and Z. Hai, Adv. Healthcare Mater., 2023, 12, 2202676 CrossRef CAS PubMed.
- S. H. Lee, C. S. Park, K. K. Lee, T. H. Han, H. S. Ban and C. S. Lee, Int. J. Mol. Sci., 2023, 24, 6074 CrossRef CAS PubMed.
- Y. Liu, L. Teng, X. Lou, X. Zhang and G. Song, J. Am. Chem. Soc., 2023, 145, 5134–5144 CrossRef CAS PubMed.
- L. Zhao, H. Zhu, Y. Duo, D. Pang, Z. Wang and S. Liu, Adv. Healthcare Mater., 2023, 12, 2301584 CrossRef CAS PubMed.
- H. Gu, W. Liu, W. Sun, J. Du, J. Fan and X. Peng, Chem. Sci., 2022, 13, 9719–9726 RSC.
- B. Zhou, Y. Li, G. Niu, M. Lan, Q. Jia and Q. Liang, ACS Appl. Mater. Interfaces, 2016, 8, 29899–29905 CrossRef CAS PubMed.
- W. Cao, Y. Zhu, F. Wu, Y. Tian, Z. Chen, W. Xu, S. Liu, T. Liu and H. Xiong, Small, 2022, 18, 2204851 CrossRef CAS.
- J. He, Y. J. Jo, X. Sun, W. Qiao, J. Ok, T. Kim and Z. Li, Adv. Funct. Mater., 2021, 31, 2008201 CrossRef CAS.
- K. Ilina, W. M. MacCuaig, M. Laramie, J. N. Jeouty, L. R. McNally and M. Henary, Bioconjugate Chem., 2020, 31, 194–213 CrossRef CAS PubMed.
- W. Zhang, W. Deng, H. Zhang, X. Sun, T. Huang, W. Wang, P. Sun, Q. Fan and W. Huang, Biomaterials, 2020, 243, 119934 CrossRef CAS PubMed.
- W. Qiao, T. Ma, S. Wang, L. Li, M. Liu, H. Jiang, Y. Wu, J. Zhu and Z. Li, Adv. Funct. Mater., 2021, 31, 2105452 CrossRef CAS.
- J. H. Kim, A. Liess, M. Stolte, A. M. Krause, V. Stepanenko, C. Zhong, D. Bialas, F. Spano and F. Würthner, Adv. Mater., 2021, 33, 2100582 CrossRef CAS PubMed.
- Y. Yao, Y. Zhang, J. Zhang, X. Yang, D. Ding, Y. Shi, H. Xu and X. Gao, ACS Appl. Mater. Interfaces, 2022, 14, 19192–19203 CrossRef CAS PubMed.
- D. Yao, Y. Wang, R. Zou, K. Bian, P. Liu, S. Shen, W. Yang, B. Zhang and D. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 4276–4284 CrossRef CAS PubMed.
- N. J. Hestand and F. C. Spano, Chem. Rev., 2018, 118, 7069–7163 CrossRef CAS PubMed.
- Z. Gu, X. Tian, S. Guang, G. Wei, Y. Mao and H. Xu, Spectrochim., 2024, 306, 123576 CrossRef CAS.
- J. Lim, D. Kai and C. K. Lee, New J. Chem., 2023, 47, 550–553 RSC.
- Y. Wang, G. Xia, M. Tan, M. Wang, Y. Li and H. Wang, Adv. Funct. Mater., 2022, 32, 2113098 CrossRef CAS.
- S. Cai, L. Wu, Y. Li, S. Samanta, J. Wang, B. Liu, F. Wu, K. Lai, Y. Liu, J. Qu and Z. Yang, Chin. Chem. Lett., 2024, 35, 108599 CrossRef CAS.
- M. J. Thun, J. O. DeLancey, M. M. Center, A. Jemal and E. M. Ward, Carcinogenesis, 2010, 31, 100–110 CrossRef CAS PubMed.
- Y. Fan, D. Yu, D. Li and X. Wang, Lasers Surg. Med., 2020, 52, 682–691 CrossRef PubMed.
- F. A. Begum, M. A. Rahman, H. Rabbi, G. Mostofa and Q. Chowdhury, Gastrointest. Tumors, 2019, 6, 36–42 CrossRef CAS PubMed.
- S. Yi, M. Wei and L. Zhao, Crit. Rev. Oncol., 2024, 196, 104313 CrossRef PubMed.
- Q. Shang, Y. Dong, Y. Su, F. Leslie, M. Sun and F. Wang, Adv. Drug Delivery, 2022, 185, 114308 CrossRef CAS PubMed.
- Q. Liu, Y. Duo, J. Fu, M. Qiu, Z. Sun, D. Adah, J. Kang, Z. Xie, T. Fan, S. Bao, H. Zhang, L. Liu and Y. Cao, Nano Today, 2021, 36, 101023 CrossRef CAS.
- Z. Jiang, W. Xiao and Q. Fu, J. Controlled Release, 2023, 361, 547–567 CrossRef CAS PubMed.
- J. Zhu, A. Ouyang, Z. Shen, Z. Pan, S. Banerjee, Q. Zhang, Y. Chen and P. Zhang, Chin. Chem. Lett., 2022, 33, 1907–1912 CrossRef CAS.
- Y. Zhang, Y. Yang, Y. Feng, X. Gao, L. Pei, X. Li, B. Gao, L. Liu, C. Wang and S. Gao, J. Pharm. Anal., 2024, 14, 100909 CrossRef PubMed.
- Y. Li, X. Liu, X. Zhang, W. Pan, N. Li and B. Tang, Adv. Funct. Mater., 2021, 31, 2107540 CrossRef CAS.
- C. Hu, B. Hou and S. Xie, RSC Adv., 2022, 12, 22722 RSC.
- Y. Huang, W. Ouyang, Z. Lai, G. Qiu, Z. Bu, X. Zhu, Q. Wang, Y. Yu and J. Liu, Nanoscale Adv., 2024, 6, 1974–1991 RSC.
- A. Steven, S. A. Fisher and B. W. Robinson, Respirology., 2016, 21, 821–833 CrossRef PubMed.
- T. Shen, Y. Zhang, S. Zhou, S. Lin, X. Zhang and G. Zhu, ACS Appl. Bio. Mater., 2020, 3, 2838–2849 CrossRef CAS PubMed.
- S. Sun, D. Wang, R. Yin, P. Zhang, R. Jiang and C. Xiao, Small, 2022, 18, 2202558 CrossRef CAS PubMed.
- X. Cao, M. Li, Q. Liu, J. Zhao, X. Lu and J. Wang, Small, 2023, 19, 2303195 CrossRef CAS.
- A. D. Levinson, Science, 2010, 328, 137 CrossRef CAS PubMed.
- J. Woodcock, J. P. Griffin and R. E. Behrman, N. Engl. J. Med., 2011, 364, 985–987 CrossRef CAS PubMed.
- Y. Li, J. Wu, X. Qiu, S. Dong, J. He, J. Liu, W. Xu, S. Huang, X. Hu and D. Xiang, Bioact. Mater., 2023, 20, 548–560 CAS.
- Y. Dai, L. Zhu, X. Li, F. Zhang, K. Chen, G. Jiao, Y. Liu, Z. Yang, Z. Guo, B. Zhang, Q. Shen and Q. Zhao, Biomaterials, 2024, 305, 122455 CrossRef CAS PubMed.
- Q. Liu, J. Tian, Y. Tian, Q. Sun, D. Sun, D. Liu, F. Wang, H. Xu, G. Ying, J. Wang, A. K. Yetisen and N. Jiang, Acta. Biomater., 2021, 127, 287–297 CrossRef CAS PubMed.
- S. Chen, X. Wang, M. Lin, Y. Hou, M. Ding, D. Kong, H. Sun, Q. Zhang, J. Li and Q. Zhou, Appl. Mater. Today, 2021, 25, 101258 CrossRef.
- S. Liang, J. Yao, D. Liu, L. Rao, X. Chen and Z. Wang, Adv. Mater., 2023, 35, 2211130 CrossRef CAS PubMed.
- D. Wei, Y. Huang, B. Wang, L. Ma, J. Karges and H. Xiao, Angew. Chem., Int. Ed., 2022, 61, e202201486 CrossRef CAS PubMed.
- W. Du, W. Chen, J. Wang, H. Zhang, L. Song, Y. Hu and X. Ma, J. Mater. Chem. B, 2023, 11, 5185–5194 RSC.
- J. Xu, Q. Zheng, X. Cheng, S. Hu, C. Zhang, X. Zhou, P. Sun, W. Wang, Z. Su, T. Zou, Z. Song, Y. Xia, X. Yi and Y. Gao, J. Nanobiotechnol., 2021, 19, 355 CrossRef CAS PubMed.
- S. Guo, D. Gu, Y. Yang, J. Tian and X. Chen, J. Nanobiotechnol., 2023, 21, 348 CrossRef CAS PubMed.
- G. Shi, Y. Cui, J. Zhao, J. Liu, Y. Wang, Y. Yang, J. Han, X. Cheng, L. Chen, Y. Yuan and P. Mi, ACS Nano, 2023, 17, 6193–6207 CrossRef CAS PubMed.
- H. Kim, Y. Baek, T. Ha, D. Choi, W. J. Lee, Y. Cho, J. Park, S. Kim and J. Doh, Small, 2023, 19, 2301377 CrossRef CAS PubMed.
- X. Yu, N. Han, Z. Dong, Y. Dang, Q. Zhang, W. Hu, C. Wang, S. Du and Y. Lu, ACS Appl. Mater. Interfaces, 2022, 14, 42988–43009 CrossRef CAS PubMed.
- S. Zhou, X. Hu, R. Xia, S. Liu, Q. Pei, G. Chen, Z. Xie and X. Jing, Angew. Chem., Int. Ed., 2020, 59, 23198–23205 CrossRef CAS PubMed.
- Y. Wang, Y. Xie, J. Li, Z. Peng, Y. Sheinin, J. Zhou and D. Oupický, ACS Nano, 2017, 11, 2227–2238 CrossRef CAS PubMed.
- S. Yang, Z. Tang, C. Hu, D. Zhang, N. Shen, H. Yu and X. Chen, Adv. Mater., 2019, 31, 1805955 CrossRef PubMed.
- N. Kwon, H. Kim, X. Li and J. Yoon, Chem. Sci., 2021, 12, 7248–7268 RSC.
- Z. Sheng, D. Hu, M. Zheng, P. Zhao, H. Liu, D. Gao, P. Gong, G. Gao, P. Zhang, Y. Ma and L. Cai, ACS Nano, 2014, 8, 12310–12322 CrossRef CAS PubMed.
- X. Liu, N. Xu, X. Pu, J. Wang, X. Liao, Z. Huang and G. Yin, J. Mater. Chem. B, 2022, 10, 4605–4614 RSC.
- D. Thankachan, R. Anbazhagan, H. C. Tsai, V. T. T. Dinh, H. T. Gebrie, S. L. Kitaw, Y. W. Ahmed, B. E. Anley, Y. Liao, W. Chen and J. Chen, Dyes Pigm., 2024, 221, 111812 CrossRef CAS.
- X. Wang, H. Peng, W. Yang, Z. Ren, X. Liu and Y. Liu, J. Mater. Chem. B, 2017, 5, 1856–1862 RSC.
- W. Tang, J. Kang, L. Yang, J. Lin, J. Song, D. Zhou and F. Ye, Colloids Surf., B, 2023, 226, 113317 CrossRef CAS PubMed.
- Y. Chen, Y. Li, J. Liu, Q. Zhu, J. Ma and X. Zhu, J. Controlled Release, 2021, 335, 345–358 CrossRef CAS PubMed.
- B. Gong, Y. Shen, H. Li, X. Li, X. Huan, J. Zhou, Y. Chen, J. Wu and W. Li, J. Nanobiotechnol., 2021, 19, 41 CrossRef CAS PubMed.
- Y. Chien, T. Wang, P. Liao, W. Wu and C. Chen, Macromol. Biosci., 2018, 18, 1700409 CrossRef.
- Y. Prince, N. Hiremath and R. Vankayala, Biomater. Sci., 2023, 11, 7759–7767 RSC.
- S. Ye, W. Zhang, Y. Shen, S. Han, H. Hu, Y. Liang, Z. Lin, Y. Jin, T. Lawson, Y. Liu and Z. Cai, Macromol. Rapid Commun., 2023, 44, 2300298 CrossRef CAS PubMed.
- S. Zhu, S. Wang, C. Liu, M. Lyu and Q. Huang, Front. Oncol., 2022, 12, 918416 CrossRef CAS PubMed.
- E. Pang, X. Li, S. Zhao, Y. Tang, X. Xing, Q. Wang, K. Yang, B. Wang, S. Jin, X. Song and M. Lan, J. Mater. Chem. B, 2024, 12, 1846–1853 RSC.
- Y. Liu, J. Zhang, X. Zhou, Y. Wang, S. Lei, G. Feng, D. Wang, P. Huang and J. Lin, Angew. Chem., Int. Ed., 2024, e202408064 CAS.
- X. Wei, C. Zhang, S. He, J. Huang, J. Huang, S. S. Liew, Z. Zeng and K. Pu, Angew. Chem., Int. Ed., 2022, 61, e202202966 CrossRef CAS PubMed.
- B. Guo, M. Wu, Q. Shi, T. Dai, S. Xu, J. Jiang and B. Liu, Chem. Mater., 2020, 32, 4681–4691 CrossRef CAS.
- L. Hua, Z. Cao, L. Ma, Z. Liu, G. Liao, J. Wang, S. Shen, D. Li and X. Yang, Biomaterials, 2019, 223, 119469 CrossRef PubMed.
- L. Zhang, Y. Gao, S. Sun, Z. Li, A. Wu and L. Zeng, J. Mater. Chem. B, 2020, 8, 1739–1747 RSC.
- M. Yu, R. Cao, Z. Ma and M. Zhu, J. Mater. Chem. B, 2023, 11, 1416–1433 RSC.
- L. Zhang, J. Wang, Y. Zhang, L. Ke, X. Lin, Z. Li, H. Chen and Y. Gao, Dyes Pigm., 2019, 170, 107588 CrossRef CAS.
- G. Hou, J. Qian, M. Guo, W. Xu, J. Wang, Y. Wang and A. Suo, Carbohydr. Polym., 2022, 276, 118810 CrossRef CAS PubMed.
- A. Chaudhuri, Y. Venkatesh, J. Das, K. K. Behara, S. Mandal, T. K. Maiti and N. D. P. Singh, ACS Appl. Nano Mater., 2018, 1, 6312–6319 CrossRef CAS.
- J. Xiong, X. Wang, J. Kim, J. Gong, Z. Mao, J. S. Kim and Z. Liu, Adv. Funct. Mater., 2024, 34, 2312590 CrossRef CAS.
- J. Yuan, Q. Zhou, S. Xu, Q. Zuo, W. Li, X. Zhang, T. Ren, L. Yuan and X. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202206169 CrossRef CAS.
- H. H. Mansour, M. Eid and M. B. El-Arnaouty, Hum. Exp. Toxicol., 2018, 37, 38–50 CrossRef CAS PubMed.
- G. Minckwitz, M. Rezai, H. Tesch, J. Huober, B. Gerber, D. M. Zahm, J. Hilfrich, S. D. Costa, P. Dubsky, J. U. Blohmer, C. Denkert, C. Hanusch, C. Jackisch, S. Kümmel, P. A. Fasching, A. Schneeweiss, S. Paepke, M. Untch, N. Burchardi, K. Mehta and S. Loibl, Eur. J. Cancer, 2016, 64, 12–21 CrossRef PubMed.
- C. L. A. Yeung, N. N. Co, T. Tsuruga, T. L. Yeung, S. Y. Kwan, C. S. Leung, Y. Li, E. S. Lu, K. Kwan, K. K. Wong, R. Schmandt, K. H. Lu and S. C. Mok, Nat. Commun., 2016, 7, 11150 CrossRef CAS PubMed.
- W. Gao, L. Li, X. Zhang, L. Luo, Y. He, C. Cong and D. Gao, Nanomedicine, 2020, 15, 871–890 CrossRef CAS PubMed.
- Y. Li, H. Qi, Y. Geng, L. Li and X. Cai, Colloids Surf., B, 2024, 234, 113743 CrossRef CAS PubMed.
- B. Li, H. Shao, L. Gao, H. Li, H. Sheng and L. Zhu, Drug Delivery, 2022, 29, 2130–2161 CrossRef CAS PubMed.
- R. H. Wijdeven, B. Pang, Y. G. Assaraf and J. Neefjes, Drug Resistance Updates, 2016, 28, 65–81 CrossRef PubMed.
- K. Dong, Z. Liu, Z. Li, J. Ren and X. Qu, Adv. Mater., 2013, 25, 4452–4458 CrossRef CAS PubMed.
- S. Peng, Y. He, M. Er, Y. Sheng, Y. Gu and H. Chen, Biomater. Sci., 2017, 5, 475–484 RSC.
- G. Sheng, Y. Chen, L. Han, Y. Huang, X. Liu, L. Li and Z. Mao, Acta Biomater., 2016, 43, 251–261 CrossRef CAS PubMed.
- W. Huang, Y. Huang, Y. You, T. Nie and T. Chen, Adv. Funct. Mater., 2017, 27, 1701388 CrossRef.
- S. Su, Y. Tian, Y. Li, Y. Ding, T. Ji, M. Wu, Y. Wu and G. Nie, ACS Nano, 2015, 9, 1367–1378 CrossRef CAS PubMed.
- F. Liu, Y. Chen, Y. Li, Y. Guo, Y. Cao, P. Li, Z. Wang, Y. Gong and H. Ran, Int. J. Nanomed., 2018, 13, 5139–5158 CrossRef CAS PubMed.
- R. Ma, N. Alifu, Z. Du, S. Chen, Y. Heng, J. Wang, L. Zhu, C. Ma and X. Zhang, Int. J. Nanomed., 2021, 16, 4847–4861 CrossRef.
- X. Chen, J. Zou, K. Zhang, J. Zhu, Y. Zhang, Z. Zhu, H. Zheng, F. Li and J. Piao, Acta Pharm. Sin. B, 2021, 11, 271–282 CrossRef CAS PubMed.
- J. Yu, L. Wang, X. Xie, W. Zhu, Z. Lei, L. Lv, H. Yu, J. Xu and J. Ren, Int. J. Nanomed., 2023, 18, 323–337 CrossRef CAS PubMed.
- Y. Zou, D. Huang, S. He, X. Song, W. Liu, W. Sun, J. Du, J. Fan and X. Peng, Chem. Sci., 2023, 14, 1010–1017 RSC.
- Z. Lu, F. Huang, R. Cao, G. Tan, G. Yi, N. He, L. Xu and L. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 26028–26038 CrossRef CAS PubMed.
- Y. Tian, Z. Chen, S. Liu, F. Wu, W. Cao, D. Pang and H. Xiong, Angew. Chem., Int. Ed., 2023, 62, e202309768 CrossRef CAS PubMed.
- X. Zhang, Z. Yan, Z. Meng, N. Li, Q. Jia, Y. Shen and Y. Ji, Front. Bioeng. Biotechnol., 2022, 12, 889284 CAS.
- N. Wang, Z. Deng, Q. Zhu, J. Zhao, K. Xie, P. Shi, Z. Wang, X. Chen, F. Wang, J. Shi and G. Zhu, Chem. Sci., 2021, 12, 14353–14362 RSC.
- W. Zhou, S. Chen, Y. Ouyang, B. Huang, H. Zhang, W. Zhang and J. Tian, Chem. Sci., 2023, 14, 11481–11489 RSC.
- Z. Li, L. Zhu, W. Liu, Y. Zheng, X. Li, J. Ye, B. Li, H. Chen and Y. Gao, Acta Biomater., 2020, 107, 242–259 CrossRef CAS PubMed.
- R. Han, S. Wu, Y. Yan, W. Chen and K. Tang, Mater. Today Commun., 2021, 26, 101842 CrossRef CAS.
- Y. Hao, T. Liu, H. Zhou, J. Peng, K. Li and Y. Chen, Front. Bioeng. Biotechnol., 2023, 11, 1294074 CrossRef PubMed.
- H. Ma, Y. Lu, Z. Huang, S. Long, J. Cao, Z. Zhang, X. Zhou, C. Shi, W. Sun, J. Du, J. Fan and X. Peng, J. Am. Chem. Soc., 2022, 144, 3477–3486 CrossRef CAS PubMed.
- Y. Yu, H. Wang, Z. Zhuang, C. Ji, L. Zhang, Y. Li, Z. Zhao, D. Ding, G. Feng and B. Z. Tang, ACS Nano, 2024, 18, 13019–13034 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |