Metal–organic framework-based hybrids with photon upconversion
Xiaokai
Chen
,
Xiaodong
Zhang
 and
Yanli
Zhao
and
Yanli
Zhao
 *
*
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang link, Singapore, 637371, Singapore. E-mail: zhaoyanli@ntu.edu.sg
First published on 14th November 2024
Abstract
Upconversion materials (UCMs) featuring an anti-Stokes type emission establish them as an important category of photoluminescent materials. Metal–organic frameworks (MOFs) are rapidly gaining prominence as a class of versatile materials with favourable physical and chemical properties, including high porosity, controllable pore size, flexible design, and diverse functional sites. To endow MOFs with upconversion capability and improve the properties and performance of UCMs, the hybrids integrating UCMs and MOFs are proven to be successful. This review focuses on the research advancements of upconverting MOF-based hybrids, encompassing classifications, luminescence mechanisms, designs, properties, and applications in energy, catalysis, and biomedical fields. The analyses on the functions of upconversion and MOFs, as well as the advantages and disadvantages of various upconverting MOF-based hybrids, are included. Future research directions spanning from properties and performance to applications are explored. This review will be valuable in highlighting the research accomplishments, inspiring more ideas, facilitating deeper investigations in diverse avenues, and further advancing the research field.
1. Introduction
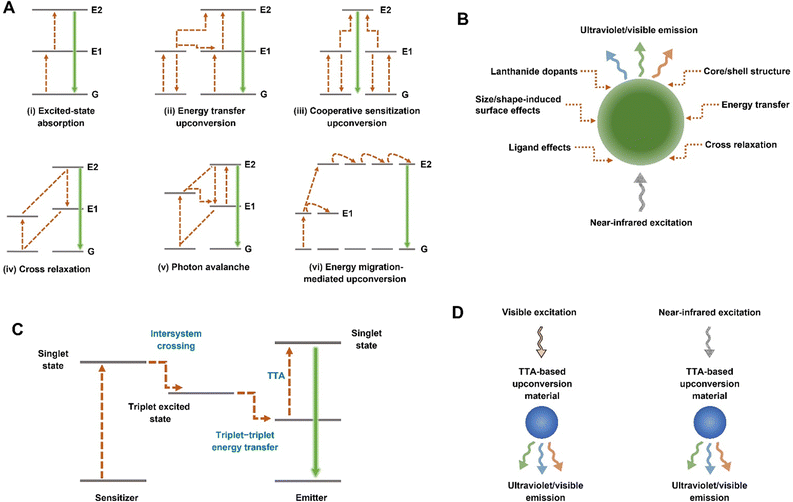
Photoluminescent materials, often standing at the forefront of materials science and engineering, are capable of absorbing and emitting photons, undergoing a fascinating transformation in the process.1–9 Of particular interest to scientists are upconversion materials (UCMs), renowned for their capability to convert long-wavelength light to short-wavelength light, yielding a high signal-to-noise ratio and enabling deep penetration.2,10–15 To date, UCMs have consistently showcased their versatility and efficacy in a wide array of applications, spanning optoelectronic devices, anti-counterfeiting technology, photocatalysis, luminescence imaging, sensing/bioassay, optogenetics, photo-switching, 3D printing, and disease treatments.2,3,5,10,15–28 Generally, there are two common categories of UCMs, including rare-earth-based UCMs and triplet–triplet annihilation (TTA)-based UCMs.2–4,10,11,15,21,23,27,29–34 In comparison to TTA-based UCMs, rare-earth-based UCMs usually manifest a set of distinctive and noteworthy characteristics including sharp emission bands, prolonged luminescence lifetimes, substantial Stokes shifts, and notable resistance to photobleaching,11,23,35 which collectively render rare-earth-based UCMs particularly noteworthy in the field of luminescence research. The underlying upconversion mechanism of rare-earth-based UCMs relies upon doped lanthanide ions that showcase abundant electronic transitions within the 4f electron shell.10,22,23 As depicted in Fig. 1A, the upconversion process of rare-earth-based UCMs can be categorized into six distinct mechanisms: (i) excited-state absorption, (ii) energy transfer upconversion, (iii) cooperative sensitization upconversion, (iv) cross-relaxation, (v) photon avalanche and (vi) energy migration-mediated upconversion.10,30 The emission of rare-earth-based UCMs can typically be adjusted from ultraviolet (UV) to visible light when subjected to a near-infrared (NIR) excitation wavelength (700–2500 nm),36 as shown in Fig. 1B. This tunability is influenced by various factors, including the type, concentration, and ratio of lanthanide dopants; the size and shape; surface ligands; the implementation of core/shell architectures; and the establishment of an energy transfer system between rare-earth-based UCMs and coupled fluorescent materials.3,10,30,37,38In addition to rare-earth dopants, researchers have delved into an alternative avenue for upconversion known as TTA-based upconversion. In contrast to rare-earth-based UCMs, TTA-based UCMs exhibit elevated upconversion luminescence quantum efficiency, robust light absorption capability, and reduced power excitation density.4,15,39 Different from the lanthanide ion-dependent pathway, the upconversion process inherent to TTA-based UCMs transpires between two distinct chromophores, serving as the sensitizer and the annihilator (emitter), respectively (Fig. 1C).15,33 Upon the absorption of low-energy photons, the sensitizer undergoes a transition to the singlet excited state, then progresses to the triplet excited state through an intersystem crossing process. Subsequently, the transfer of triplet–triplet energy from the sensitizer to the emitter occurs, paving the way for the TTA process and giving rise to the emission of high-energy photons.11,27,31 Consequently, the excitation and emission wavelengths characterizing TTA-based UCMs are dictated by the properties of the sensitizer and the emitter, respectively. At present, TTA-based UCMs have achieved photon upconversion from visible/NIR excitation to UV/visible emission (Fig. 1D).4,15,26,33,34,40 To construct TTA-based upconversion systems, several platforms such as silica nanoparticles, liposomes, and polymer matrixes have been harnessed to effectively integrate the sensitizer and emitter into a cohesive system.4,11,32
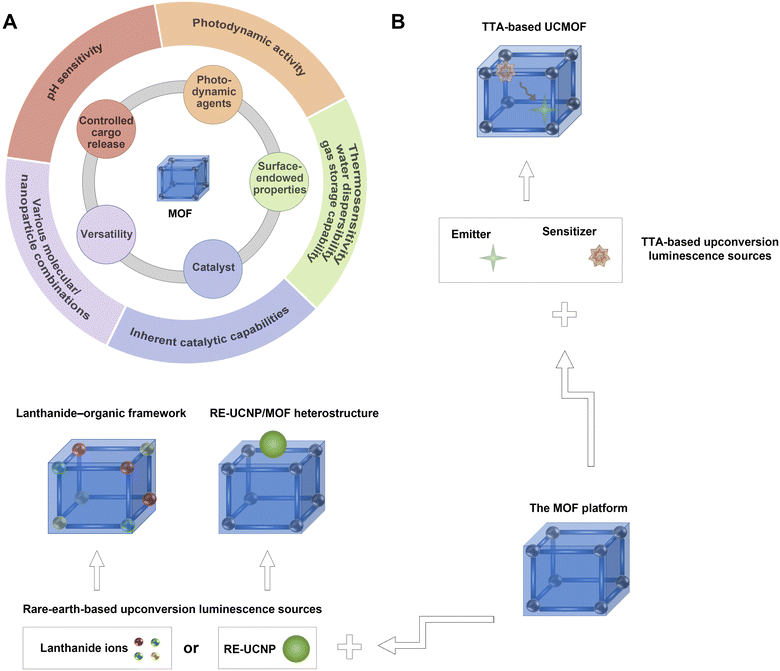
Metal–organic frameworks (MOFs) represent a distinctive class of coordination networks built from the metal ions/clusters coordinated with organic ligands. On account of their unique chemical nature, porous architecture, adjustable porosity, surface modifiability, and biodegradability, MOFs have risen to prominence as highly advantageous systems for a variety of applications, including catalysis, biosensing, water harvesting, gas storage/separation, and disease theranostics.41–45 To date, a large number of MOFs, each possessing distinctive characteristics, have been synthesized to fulfil a wide array of requisites (Fig. 2A). (1) Some MOFs, such as zeolitic imidazolate framework-8, manifest stimulus responsiveness, rendering them viable systems for regulating cargo release mechanisms.46 (2) The network architecture of MOFs, exemplified by UiO-66, NU-1000, MOF-808, Al-PMOF, MMPF-2, and NU-601, with detailed definitions provided in Table 1, allows densely distributed active catalytic sites with substrate accessibility and ordered tailorable cavities/channels with a hydrophobic confined environment, enabling intrinsic catalytic capabilities.43,44 (3) For porphyrinic MOFs including DBP-UiO, DBC-UiO, HKUST-I-Zn, and PCN-224, each accompanied by detailed definitions available in Table 1, the meticulous arrangement of photosensitizing porphyrin and metalloporphyrin ligands within the intricate framework architecture serves to avert issues like self-quenching and undesirable aggregation, thereby enhancing the overall photodynamic efficiency.45 (4) The performance of MOFs can be improved through surface modifications, such as adding poly(N-isopropylacrylamide) for thermosensitivity,47 methoxy polyethylene glycol phosphate for colloidal stability and water dispersibility,48 and amide groups for enhanced gas storage.42 (5) Incorporating MOFs with diverse molecules or nanoparticles confers them with multiple functions. The incorporation of various molecules, such as chemotherapeutics, photosensitizers, antimicrobials, catalysts, and sensitizers/emitters, imbues MOFs with an array of capabilities including antitumour, photodynamic, antimicrobial, catalytic, and upconversion functionalities. Moreover, the integration of MOFs and nanoparticles synergistically harnesses their respective advantages, thereby engendering an innovative category of multifunctional composites.49–58
| MOFs | Metal ions/clusters | Organic ligands |
|---|---|---|
| UiO-66 | [Zr6O4(OH)4] | 1,4-Benzene dicarboxylate (BDC) |
| NU-1000 | Zr6(μ3-O)4(μ3-OH)4(H2O)4(OH)4 | Tetratopic 1,3,6,8(p-benzoate)pyrene (TBAPy) |
| MOF-808 | Zr6O4(OH)4 | Benzene-1,3,5-tricarboxylate (BTC) |
| Al-PMOF | Al3+ | Tetrakis(4-carboxyphenyl)porphyrin (TCPP) |
| MMPF-2 | Co2+ | Tetrakis(3,5-dicarboxyphenyl)porphine (TDCPP) |
| NU-601 | Zn2+ | 4,4′-Bipyridine (BIPY) |
| DBP-UiO | Hf4+ | 5,15-Di(p-benzoato)porphyrin (DBP) |
| DBC-UiO | Hf4+ | 5,15-Di(p-benzoato)chlorin (DBC) |
| HKUST-I-Zn | Zn2+ | Tetrakis(1-methylpyridinium-4-yl)porphyrin (TMPyP) |
| UiO-66 | Zr6O4(OH)4 | Benzenedicarboxylate (BDC) |
| PCN-224 | Zr4+ | Tetrakis(4-carboxyphenyl)porphyrin (TCPP) |
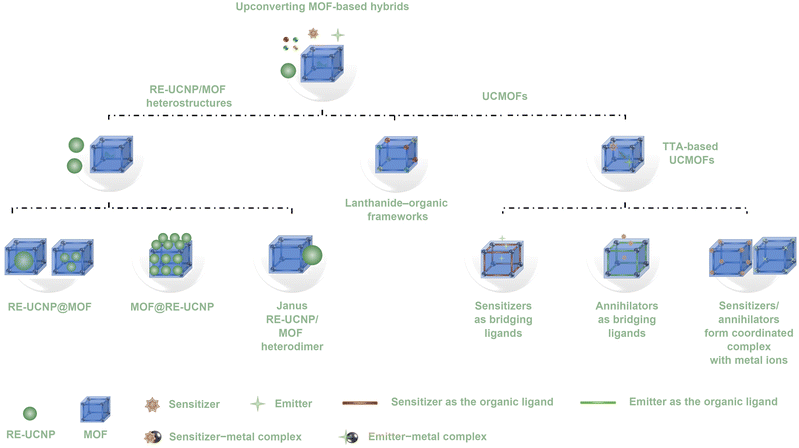
This review focuses upon a rapidly burgeoning realm of a research field, upconverting MOF-based hybrids, which include TTA-based upconversion MOFs (TTA-based UCMOFs), lanthanide–organic frameworks, and heterostructures composed of MOFs and rare-earth-doped upconversion nanoparticles (RE-UNCP/MOF heterostructures). TTA-based UCMOFs are a subset of TTA-based UCMs, while lanthanide–organic frameworks and RE-UNCP/MOF heterostructures belong to rare-earth-based UCMs (Fig. 2B). This review offers a comprehensive and in-depth exploration of the luminescence mechanism and properties inherent to MOF-based hybrids featuring photon-upconversion capabilities. Furthermore, it provides an exhaustive discourse surrounding the conception, synthesis, and categorization of diverse MOF-based hybrids with upconversion. In-depth analysis is conducted on both the upconversion and MOF functionalities, critically evaluating the merits and demerits of various heterogeneous systems. The review also encapsulates recent strides achieved in the realm of MOF-based hybrids with upconversion across a spectrum of domains including biosensing/bioimaging, solar energy conversion, catalysis, cancer therapy, and bacterial treatment. Lastly, we underscore prospective avenues of research within the ambit of MOF-based hybrids with upconversion, which encompasses delving into their intrinsic properties and performance nuances, alongside the exploration of novel applications. The aspiration is that this review will not only furnish methodologies and innovative cues for the evolution of avant-garde MOF-based hybrids with upconversion but also galvanize the broader integration of MOF-based hybrids with photon upconversion across diverse scientific domains.
2. Upconverting MOF-based hybrids
Upconverting MOF-based hybrids represent a promising area of research that combines the distinctive properties of MOFs with the capabilities of UCMs. These hybrids have garnered increasing attention due to their exceptional upconversion luminescence, tunable photoluminescence, favourable photostability, flexible design, diverse functional sites, high porosity, and controllable pore structures. The amalgamation of UCMs with MOFs has surfaced as a propitious trajectory for enhancing upconversion capabilities and ameliorating the intrinsic properties and functionalities of MOFs. Over the past decade, the development of upconverting MOF-based hybrids, achieved through the incorporation of MOFs with triplet sensitizers/emitters, lanthanide ions, or rare-earth-based upconversion nanoparticles (RE-UCNPs), has garnered significant attention due to their ability to synergistically combine the advantages of both UCMs and MOFs. These MOF-based hybrids not only possess the capability to convert long-wavelength excitation into short-wavelength emission, but they also retain the distinctive benefits of MOFs, such as a high surface area with inherent modifiability, well-defined pores with tuneable pore size, and coordinatively unsaturated metal sites, all of which contribute to their multifaceted functionality.43,59–64The development of upconverting MOF-based hybrids presents a viable solution to surmount the inherent limitations exhibited by standalone UCMs and individual MOFs: (1) MOFs can serve as a robust and versatile platform for loading UCMs, including lanthanide ions,65–68 sensitizers/emitters,69–72 and RE-UCNPs,49,51,52,54,73,74 as well as other small molecules or nanoparticles, endowing the hybrid systems with diverse activities ranging from catalytic, anticancer, photodynamic to antimicrobial, complementing its inherent photon-upconversion capability.43,59,68,70,75–79 This intrinsic flexibility empowers upconverting MOF-based hybrids with the capacity to concurrently offer a spectrum of functions, thereby further broadening their utility in various scientific and technological domains; (2) due to their distinctive physical and chemical characteristics, MOFs enable an appropriate distance between the donors (e.g., sensitizers lanthanide ions, or RE-UCNPs) and the acceptors (e.g., annihilators/emitters, lanthanide dopants, or coupled fluorescent materials). This deliberate spatial arrangement significantly bolsters the energy transfer efficiency between the donors and acceptors, thereby facilitating meticulous control over emission colour or a remarkable amplification in photoluminescence efficiency;65,66,70–72,78,80 (3) naked RE-UCNPs and primitive sensitizers/emitters exhibit constrained water solubility. The amalgamation with MOFs offers a viable avenue for augmenting both their stability and solubility within aqueous mediums;70,74,81,82 (4) the precisely defined network architecture of MOFs aids in mitigating the undesirable self-quenching and aggregation of lanthanide dopants or organic sensitizers/emitters, thus contributing to improving photoluminescence performance;4,65,70,71 (5) functional MOFs can bestow the system with additional functions. For instance, the integration of porphyrinic MOFs can facilitate the application of photodynamic therapy,76,83,84 the utilization of primitive MOFs exhibiting catalyst-like behaviour augments catalytic activities,64,85 while the incorporation of stimulus-responsive MOFs enables precise control over cargo release mechanisms;60,79,86 (6) a key advantage lies in the modifiability of the MOF surface. Through targeted surface modifications, the properties, performances, and functions of MOF-based hybrids with photon upconversion can be further refined and enhanced.49,61,73,75,78,87,88 For instance, the surface coating of TiO2 nanoparticles imparts upconverting MOF-based hybrids with photodynamic effects suitable for photodynamic therapy.87 Surface modifications involving targeting agents, such as biotin,61 glucose,73 and folic acid,75,88 enhance the tumour-targeting capability of upconverting MOF-based hybrids. The surface modification of triphenylphosphine enables the mitochondrial-targeting capability of upconverting MOF-based hybrids.78 The surface coating of chiral NiSx nanoparticles endows upconverting MOF-based hybrids with the function of selectively detecting reactive oxygen species;49 (7) the introduction of constituents such as sensitizers/emitters, lanthanide ions, and RE-UCNPs, impacts upconversion luminescence attributes to MOFs, consequently extending their applicability across a diverse array of photoluminescence-related fields.49,50,52,73,75,78,88,89
According to the structure, upconverting MOF-based hybrids are distinctly categorized into two primary classes, namely upconversion metal–organic frameworks (UCMOFs) and heterostructures composed of RE-UCNPs and MOFs (Fig. 3). The first category, UCMOFs, contain MOFs that have intrinsic upconversion properties, typically comprising MOF architectures with chromophores (sensitizers/emitters) or lanthanide ions. The synthesis methods of UCMOFs adhere to well-established methodologies for MOF synthesis. These methodologies encompass conventional approaches, such as solvothermal, hydrothermal, and microwave-assisted techniques, alongside other advanced methods, including nanoprecipitation, reverse microemulsion, and coordination modulation.2–65,79,90–92 UCMOFs can be further categorized into two types according to their photon-upconversion mechanisms: (1) TTA-based UCMOFs containing a pair of chromophores, including sensitizers and annihilators (emitters); (2) lanthanide–organic frameworks that consist of organic bridging ligands and lanthanide ions.65–68,79,90,91 The subtype of TTA-based UCMOFs typically comprises MOF architectures with a pair of chromophores, and can be further categorized into four types: (i) sensitizers as bridging ligands in conjunction with metal ions to generate MOF architectures. In this formulation, emitters can be encapsulated/loaded into the MOF systems, thus generating upconversion luminescence. (ii) Emitters as bridging ligands in combination with metal ions for the construction of MOF architectures. In this formulation, sensitizers can be encapsulated/loaded into the MOF systems, thus generating upconversion luminescence. (iii) Sensitizer-coordinated metal ion complexes with organic linkers to generate MOF architectures. In this formulation, emitters can act as the organic linkers or be encapsulated/loaded into the MOF systems, thus contributing to upconversion luminescence. (iv) Emitter-coordinated metal ion complexes with organic linkers to generate MOF architectures. In this formulation, sensitizers can act as the organic linkers or be encapsulated/loaded into the MOF systems, thus contributing to upconversion luminescence. Additionally, Table 2 summarises the used sensitizers and emitters of as-developed TTA-based UCMOFs. In the subtype of lanthanide–organic frameworks, upconversion photoluminescence arises from lanthanide dopants. The sensitizers commonly encompass lanthanide ions, such as Yb3+ and Nd3+, while the emitters typically also consist of lanthanide ions such as Er3+, Tm3+, Eu3+, and Gd3+. Table 3 summarizes the components and key parameters of as-reported lanthanide–organic frameworks, including the used rare-earth ions, organic linkers, preparation methods, and upconversion mechanisms.
| Sensitizers | Emitters | MOFs with building blocks | MOF functions | Ref. |
|---|---|---|---|---|
| Pd(II)-meso-tetrakis(4-carboxyphenyl)porphyrin | 4,4′-(Anthracene-9,10-diyl)dibenzoic acid | UiO-68 consisting of Zr6 clusters and 4,4′-(anthracene-9,10-diyl)dibenzoic acid | (1) Tuning sensitizer/emitter concentrations; (2) Controlling the distance and orientation of sensitizers/emitters; (3) Improving water dispersibility; (4) Acting as emitters | 70 |
| Pd(II) mesoporphyrin IX | 9,10-Anthracenedicarboxylic acid | 9,10-MOF consisting of Zr4+ and 9,10-anthracenedicarboxylic acid | (1) Regulating chromophore distance; (2) Acting as emitters | 93 |
| Platinum octaetylporphyrin | 4,4′-(Anthracene-9,10-diyl)dibenzoic acid | MOFs consisting of Zn2+ and 4,4′-(anthracene-9,10-diyl)dibenzoic acid | (1) Controlling the distance between emitters; (2) Acting as emitters | 72 |
| Pd(II) 5,15-diphenyl-10,20-di(4-carboxyphenyl) porphyrin | 4,4′-(Anthracene-9,10-diyl)dibenzoic acid | The “A” MOFs consisting of Zn2+ and 4,4′-(anthracene-9,10-diyl) dibenzoic acid; The “B” MOFs consisting of Zn2+ and Pd(II) 5,15-diphenyl-10,20-di(4-carboxyphenyl) porphyrin | (1) Providing the A–B–A heterostructure for sufficient electron exchanges; (2) Acting as emitters | 69 |
| Tetrakis(4-carboxyphenyl)porphyrin | 2,5-Di(pyridin-4-yl)thiazolo[5,4-d]thiazole | TzPMOFs consisting of Zn2+ and tetrakis(4-carboxyphenyl)porphyrin | (1) Providing an organized array of axially coordinated sensitizers and emitters; (2) Acting as sensitizers | 71 |
| Platinum octaethylporphyrin | Tetrakis(p-benzoic-acid)pyrene | NU-1000 consisting of Zr6 clusters and tetrakis(p-benzoic-acid)pyrene | (1) Promoting triplet fusion; (2) Acting as emitters | 94 |
| Platinum octaethylporphyrin | Perylene-3,9-dicarboxylic acid | SURMOFs consisting of Zn2+ and perylene-3,9-dicarboxylic acid | Acting as emitters | 92 |
| Rare-earth ions | Organic linkers | Preparation methods | Upconversion mechanisms | Ref. |
|---|---|---|---|---|
| Er3+ and Y3+ | 1,3,5-Benzenetricarboxylic acid | Solvothermal method | Excited state absorption | 66 |
| Er3+, Yb3+ and Y3+ | 1,3,5-Benzenetricarboxylic acid | Solvothermal method | Cross-relaxation | 67 |
| Nd3+ | 2,6-Naphthalene dicarboxylic acid | Solvothermal method | Excited-state absorption, and energy transfer upconversion | 68 |
| Er3+ | 1,4-Dicarboxybenzene and biphenyl-2,5,2′,5′-tetracarboxylic acid | Solvothermal method | Excited-state absorption | 90 |
| Y3+, Er3+, and Yb3+ | 1,4-Dicarboxybenzene and biphenyl-2,5,2′,5′-tetracarboxylic acid | Solvothermal method | Excited-state absorption | 90 |
| La3+ and Tm3+ | 1,3,5-Benzenetricarboxylic acid | Solvothermal method | Energy transfer upconversion | 79 |
| Er3+ and Yb3+ | 1,3,5-Benzenetricarboxylic acid | Hydrothermal method | Energy transfer upconversion | 80 |
| Ho3+ and Yb3+ | 1,3,5-Benzenetricarboxylic acid | Hydrothermal method | Energy transfer upconversion | 80 |
| Tb3+ and Yb3+ | 1,3,5-Benzenetricarboxylic acid | Hydrothermal method | Cooperative sensitization upconversion | 80 |
| Eu3+ and Yb3+ | 1,3,5-Benzenetricarboxylic acid | Hydrothermal method | Cooperative sensitization upconversion | 80 |
The second category contains heterostructures composed of RE-UCNPs and MOFs. RE-UCNPs refer to the inclusion of rare-earth ions (such as erbium, ytterbium, thulium, and neodymium) within the crystal lattice of the nanoparticles. Analogous to lanthanide–organic frameworks, their upconversion photoluminescence is attributed to lanthanide dopants. The sensitizers commonly involve lanthanide ions, such as Yb3+ and Nd3+, while the emitters typically encompass lanthanide ions such as Er3+, Tm3+, Eu3+, and Gd3+. RE-UCNP/MOF heterostructures have three distinct formulations, i.e., (1) rare-earth-doped upconversion nanoparticle@metal–organic framework (RE-UCNP@MOF) configurations,49,54,89,91,95 (2) core–satellite metal–organic framework@rare-earth-doped upconversion nanoparticle (MOF@RE-UCNP) configurations,74,81,96 and (3) Janus-type heterodimers composed of rare-earth-doped upconversion nanoparticles and metal–organic frameworks (Janus RE-UCNP/MOF heterodimers).51,54 The fabrication of RE-UCNP/MOF heterostructures necessitates not solely the synthesis of MOFs but also involves the synthesis of RE-UCNPs. The controllable synthesis of diverse RE-UCNP/MOF heterostructures can be realized through pathways mediated by surface ligands, driven by electrostatic interactions, or guided by desoxyribonucleic acid (DNA).49,54,74,96Table 4 summarizes the chemical compositions and structures of as-developed diverse MOF/RE-UCNP heterostructures, including the used metal ions/clusters in MOFs, organic linkers in MOFs, RE-UCNP components and structures, and MOF/RE-UCNP heterostructure formulations.
| Metal ions/clusters in MOFs | Organic linkers in MOFs | RE-UCNP components and structures | Formulations of MOF/RE-UCNP heterostructures | Ref. |
|---|---|---|---|---|
| Zn2+ | 2-Methylimidazole | NaYF4:Yb3+/Er3+ | RE-UCNP@MOF | 49 |
| Zn2+ | 2-Methylimidazole | NaYF4 (30%Gd, 18%Yb, 2%Er) and NaYF4 (20%Yb, 0.2%Tm) | RE-UCNP@MOF | 54 |
| Zn2+ | 2-Methylimidazole | NaYF4:Yb,Tm@NaYF4 | RE-UCNP@MOF | 89 |
| Fe3+ | Terephthalic acid | NaYF4:Yb,Tm | RE-UCNP@MOF | 91 |
| Fe3+ | 1,3,5-Trimesic acid | NaGdF4:Yb,Tm and NaGdF4:Yb,Tm@NaGdF4:Yb,Nd | RE-UCNP@MOF | 95 |
| Zr4+ | 2-Aminoterephthalic acid | NaYF4:Yb/Er | MOF@RE-UCNP | 74 |
| Zr4+ | Aminotriphenyl dicarboxylic acid | NaGdF4:Yb,Er@NaGdF4:Nd,Yb | MOF@RE-UCNP | 81 |
| Zr4+ | Tetrakis(4-carboxyphenyl) porphyrin | NaYF4:Yb,Er | MOF@RE-UCNP | 96 |
| Zn2+ | Imidazole-2-carboxaldehyde | NaYF4:Yb/Er@NaGdF4 | Janus MOF/RE-UCNP heterodimers | 51 |
| Zr6 cluster | 5,10,15,20-Tetrakis (4-carboxyphenyl) porphyrin | NaGdF4:Yb,Er@NaGdF4 | Janus MOF/RE-UCNP heterodimers and RE-UCNP@MOF | 52 |
3. Upconversion metal–organic frameworks (UCMOFs)
3.1 Triplet–triplet annihilation-based upconversion metal–organic frameworks (TTA-based UCMOFs)
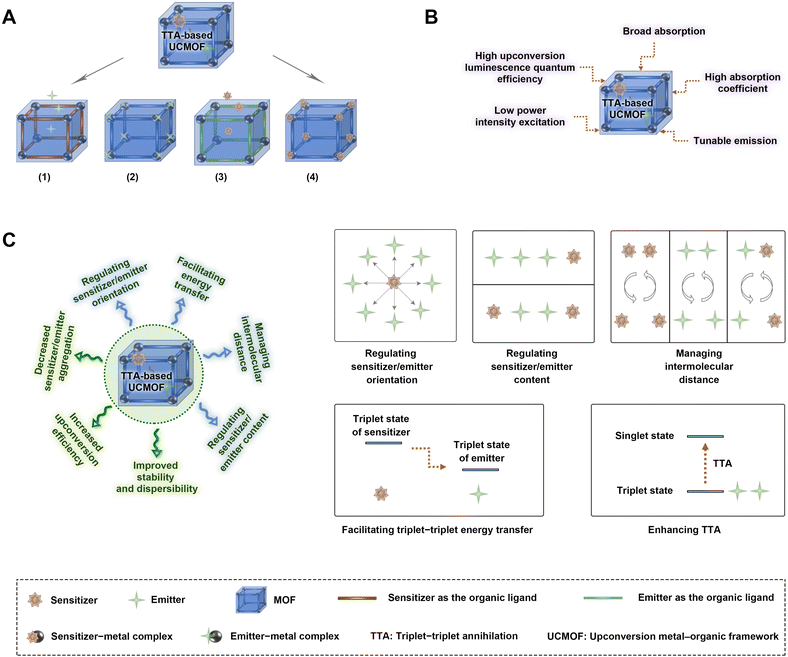
TTA-based UCMOFs typically comprise MOF architectures with a pair of chromophores including sensitizers and annihilators (emitters). In TTA-based upconversion systems, sensitizers should have strong absorption and efficient intersystem crossing to facilitate the transition from singlet to triplet states. Organic molecules with large conjugated systems (such as porphyrin derivatives) and heavy atoms (such as Pd and Pt) are frequently preferred due to their pronounced efficacy in mediating intersystem crossing.69–72,92–94 It should be considered that the triplet state energy of the sensitizers is required to be higher than that of emitters, enabling energy transfer from the sensitizers to the emitter molecules. Regarding the design of emitters, it necessitates the selection of molecules characterized by a long-lived triplet state to accumulate the requisite exciton density essential for the TTA process. A paramount criterion for emitters is the high photoluminescence quantum yield with reduced nonradiative decay pathways. Thus, polycyclic aromatic molecules with rigid structures, such as the derivatives of anthracene, pyrene and perylene, are predominantly opted for as emitters.69–72,92–94 Additionally, it is crucial for both sensitizers and emitters to possess two or more coordinating functionalities, mainly carboxyl groups, to facilitate the formation of MOF structures through coordination with metal ions or clusters.69–72,92–94Based on their distinct structural configurations, TTA-based UCMOFs can be categorized into four types (Fig. 4A): (1) the first category entails the utilization of sensitizers as bridging ligands in conjunction with metal ions to generate TTA-UCMOF architectures.71 (2) The second category employs annihilators as bridging ligands in combination with metal ions for the construction of TTA-UCMOF architectures.69,70,72,92–94 (3) The third category integrates sensitizer-coordinated metal ion complexes with organic linkers to form TTA-UCMOFs.70 (4) The fourth category incorporates annihilator-coordinated metal ion complexes with organic linkers to obtain TTA-UCMOFs.69 TTA-based UCMOFs inherit the merits inherent to TTA-based UCMs, which encompass high upconversion luminescence quantum efficiency, low power intensity excitation requirements, broad absorption spectra, high absorption coefficients, and tuneable emission luminescence (Fig. 4B).93 Thanks to their large pores, MOFs facilitate the loading of sensitizers or emitters without significant change in their structures,94 thereby preserving the inherent properties of the MOF.
Furthermore, TTA-based UCMOFs offer distinct advantages over other TTA-based upconversion systems, such as TTA-based upconversion micelles and liposomes. The latter systems are hindered by the limited mobility of sensitizers/emitters and pose challenges in regulating ligand orientation and intermolecular distances. In contrast, TTA-based UCMOFs excel in fine-tuning the orientation of chromophores, mitigating chromophore aggregation, enhancing chromophore stability, augmenting chromophore dispersibility, and thus upregulating upconversion efficiency. These enhancements can be ascribed to the unique properties of MOFs, which serve as a versatile platform facilitating the management of intermolecular distances, precise regulation of sensitizer/emitter orientation, optimization of sensitizer/emitter content, and enhancement of triplet–triplet energy transfer as well as TTA (Fig. 4C).69–72,92,93 It is found that oxygen can strongly affect the TTA-based upconversion systems, because it can quench the triplet state of the sensitizers through the energy transfer process, thus significantly impacting the efficiency of TTA-based upconversion emission.33 To overcome oxygen-induced quenching of TTA-based upconversion, MOFs can form a hydrophobic environment in their inner cavity, thus protecting the sensitizers from being quenched by oxygen.70Table 2 presents a comprehensive overview of the components and key parameters characterizing the TTA-based UCMOFs developed thus far. Additionally, it delineates the diverse functionalities exhibited by MOFs within distinct TTA-based UCMOF systems.
Various TTA-based UCMOFs have been developed to enhance upconversion efficiency across a spectrum of conditions, such as the amelioration of upconversion efficiency in both liquid-state and solid-state environments. As an illustration, Park et al. developed a water-stabilized TTA-based UCMOF, wherein sensitizers were in situ coordinated with the metal clusters inherent to the emitter-based MOFs.92 This distinctive structural arrangement ensured that the sensitizers were entirely enveloped by the closely-aligned emitter to realize a long-range three-dimensional triplet diffusion spanning 1.6 μm, enhancing the probability of the TTA process. Furthermore, the MOF-based systems enabled the precise adjustment of the emitter-to-sensitizer ratio, representing a viable approach to screen the ratio with high upconversion efficiency.
In another work, Oldenburg et al. realized solid-state upconversion by designing a dual-MOF-based TTA system.69 Compared to liquid-state upconversion, solid-state upconversion is typically difficult to control molecular orientation and distance, resulting in low upconversion efficiency. To overcome this problem, a dual-MOF-based TTA upconversion system composed of MOF “A” and MOF “B” has been designed. Notably, MOF “A” and MOF “B” exhibited unit cell sizes of 2.3 and 2.4 nm, respectively. MOF “A” acted as the sensitizer, while MOF “B” served as the emitter (Fig. 5A). They found that triplet transfer was available across the sensitizer layer–emitter layer heterojunction and the upconversion threshold could be lowered by using a thicker sensitizer and thinner emitter layer to concentrate the triplets in the emitter layer. Based on the UCMOF heterojunctions, the solid-state upconversion threshold can be lower than 1 mW cm−2.
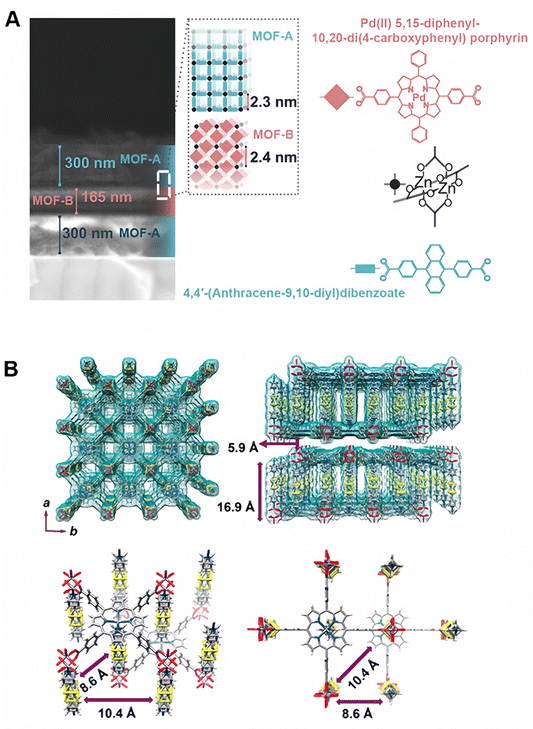 | ||
| Fig. 5 Upconverting MOF structures. (A) Scheme illustrating the compositions, structures, and unit cell sizes of the dual-MOF-based TTA system composed of MOF A and MOF B. The unit cell sizes are 2.3 and 2.3 nm for MOF A and MOF B, respectively. Figure reproduced with permission from ref. 69, Wiley-VCH. (B) Scheme illustrating the distance of 16.9 Å between the top and bottom two-dimensional sheets composed of the Zn-metalated sensitizers and Zn2 nodes, the distance of 5.9 Å apart from each two-dimensional sheet, the distance of 8.6 Å between two adjacent annihilators along diagonals, the distance of 10.4 Å between two adjacent annihilators with 8.6 and 10.4 Å along the sides, and the distribution of sensitizers and annihilators within the system, wherein each sensitizer is tethered to five annihilators. Figure reproduced with permission from ref. 71, American Chemical Society. | ||
Moreover, new strategies have been devised to enhance the performance of solid-state TTA-based UCMOF systems, affording precise control over the relative orientations of chromophores and interchromophoric distances, in order to maximize the efficacy of energy transfer and hence establish TTA-based upconversion systems that can operate under low-power excitation conditions. Notably, Roy and co-workers recently reported an instance involving a pillared-paddlewheel MOF constructed from Zn-metalated sensitizers and an annihilator, dipyridyl thiazolothiazole (Table 2).71 The MOF-based system was meticulously designed, aiming to facilitate efficient TTA-based upconversion processes. Within this system, the distances between two adjacent annihilators were 8.6 and 10.4 Å along diagonals and sides, respectively (Fig. 5B). The Zn-metalated sensitizers are coordinated to Zn2 nodes in a paddlewheel configuration, giving rise to the formation of two-dimensional sheets, connected by the emitter linkers, with a distance of 16.9 Å between their top and bottom and a distance of 5.9 Å apart from each other. These two-dimensional sheets are subsequently interconnected with annihilators, resulting in each sensitizer being tethered to five annihilators. This precise spatial arrangement of sensitizers in relation to annihilators, coupled with a high annihilator-to-sensitizer ratio, actively promotes Dexter energy transfer,69,71,97 thereby maximizing solid-state energy transfer efficiency. As a result, the TTA-based UCMOF system achieves a high upconversion efficiency of 1.95% at an excitation power density of 25 mW cm−2.
3.2 Lanthanide–organic frameworks
Lanthanide–organic frameworks are a class of MOFs that incorporate lanthanide ions as the metal centres. Lanthanide–organic frameworks are created by linking lanthanide ions with organic ligands, which are molecules that can form multiple bonds to the metal ions, creating a highly porous, three-dimensional structure. In contrast to TTA-based UCMOFs, whose photon upconversion hinges upon organic chromophores or molecules capable of undergoing TTA, the upconversion capacity of the lanthanide–organic framework is rooted in the characteristics of rare-earth ions. Lanthanides are recognized for their unique luminescence properties, attributed to their distinct energy-level structures that facilitate emissions across a wide spectral range from UV to NIR regions.80 The luminescence emission of lanthanides is underpinned by the presence of electrons in the 4f orbital, which are effectively shielded by the 5s and 5p orbitals. This shielding effect gives rise to sharply defined electronic energy levels that remain largely unaffected by environmental changes. These discrete energy levels are essential for efficient absorption and emission processes involved in photon upconversion.The large ionic radii of lanthanides and their ability to adopt a variety of coordination geometries render them highly suitable as metal nodes in the construction of MOFs. These MOFs, namely lanthanide–organic frameworks, are formed through the combination of lanthanides with organic bridging ligands, leveraging the synergistic properties of both components. The as-employed lanthanide ions for the formation of lanthanide–organic frameworks involve Y3+, Eu3+, Er3+, Yb3+, Gd3+, Lu3+, Ho3+, Tb3+, Tm3+, Pr3+, Sm3+, Dy3+, Nd3+, La3+, etc. Meanwhile, the organic linkers adopted to form lanthanide–organic frameworks include 1,4-dicarboxybenzene, 1,3,5-benzenetricarboxylic acid, biphenyl-2,5,2′,5′-tetracarboxylic acid, 2,6-naphthalene dicarboxylic acid, and so forth (Fig. 6A).66–68,79,80,90 Lanthanide–organic frameworks possess the distinctive structural attributes intrinsic to MOFs, such as mesoporous architecture, adjustable porosity, and surface modifiability, while also featuring the inherent optical attributes of lanthanide ions, including well-defined emission bands, extended luminescent lifetimes, large Stokes shifts, and remarkable resistance to photobleaching.
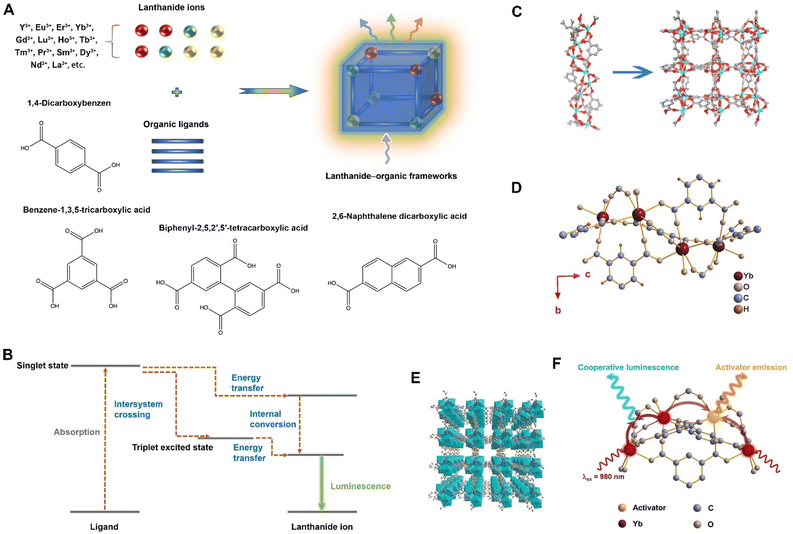 | ||
| Fig. 6 Lanthanide–organic frameworks. (A) Scheme illustrating the synthetic process of lanthanide–organic frameworks with a specific presentation of the lanthanide ions and organic linkers as-employed in generating lanthanide–organic frameworks. (B) Scheme illustrating the function of ligands in facilitating the upconversion process within lanthanide–organic frameworks. (C) One-dimensional helical chain along the [001] direction and the three-dimensional structures of the lanthanide–organic frameworks. Figure reproduced with permission from ref. 66, American Chemical Society. (D) Scheme illustrating the structure in which seven organic linkers were coordinated to one mental centre of the lanthanide–organic frameworks. (E) Schematic illustration of the three-dimensional crystallized architecture of the lanthanide–organic frameworks. (F) Schematic upconversion process within the lanthanide–organic frameworks. Figure reproduced with permission from ref. 80, Wiley-VCH. | ||
The organic ligands within lanthanide–organic frameworks commonly serve as antennae. Their role involves absorbing incident light and subsequently facilitating efficient energy transfer to the lanthanide ions, thereby triggering emission (Fig. 6B).65 The amplification of upconversion luminescence in lanthanide–organic frameworks can be realized through the deliberate selection of organic ligands characterized by robust absorption properties, effectively sensitizing the lanthanide ions to augment the emission process. Besides, when lanthanide ions are incorporated into MOFs, they are protected by the organic framework, which can increase their chemical and thermal stability.
To fabricate lanthanide–organic frameworks, several synthetic strategies have been developed, including the nanoprecipitation method, the reverse microemulsion approach, the coordination modulation pathway, solvothermal/hydrothermal treatment, and microwave-assisted treatment.65–68,79,90,91 As an illustration, Er3+-doped lanthanide–organic frameworks were successfully synthesized via a one-step solvothermal treatment of lanthanide ions and 1,3,5-benzenetricarboxylic acid.66 In this instance, 1,3,5-benzenetricarboxylic acid functioned as the organic linker, while the lanthanide ion mixture comprised Er3+, along with other possible rare-earth ions such as Y3+, Lu3+, Gd3+, or Yb3+. The as-prepared lanthanide–organic frameworks exhibited three-dimensional porous framework crystallization (Fig. 6C) and had characteristic upconversion emissions of Er3+ at 520, 540, and 651 nm under excitation at 980 nm. Notably, the upconversion mechanism of the lanthanide–organic frameworks was attributed to the excited state absorption of Er3+. In another case, one-dimensional microrod-shaped lanthanide–organic frameworks composed of Yb3+ and 1,3,5-benzenetricarboxylic acid (BTC) were synthesized using the hydrothermal method.80 Within the crystalized three-dimensional architecture of lanthanide–organic frameworks, seven BTC linkers were coordinated to one metal centre (Fig. 6D and E). Upon doping with other lanthanide ions such as Er3+, Ho3+, Tb3+, or Eu3+ during MOF formation, the microrod-shaped lanthanide–organic frameworks presented characteristic upconverted luminescence of these ions when subjected to 980 nm excitation. The authors ascribed the upconversion mechanisms to the energy transfer between the sensitizer (Yb3+) and the emitter (Er3+ or Ho3+), as well as the cooperative sensitization of emitters (Tb3+ or Eu3+) by the excited state of Yb3+ (Fig. 6F), providing valuable insights for exploring lanthanide–organic frameworks with upconversion properties. Additional examples of recently developed lanthanide–organic frameworks are provided in Table 3.
4. Heterostructures of rare-earth-based upconversion nanoparticles (RE-UCNPs)/MOFs
Another strategy to obtain upconverting MOF-based hybrids is the combination of MOFs and traditional RE-UCNPs. RE-UCNPs typically consist of a host lattice based on fluoride, chloride, bromide, or oxides, doped with lanthanide ions. Similar to lanthanide–organic frameworks, the upconversion mechanism of RE-UCNPs originates from the doped lanthanides. Besides forming the crystal lattice that supports the lanthanide dopants, the host materials can optimize the interactions between the sensitizer and activator ions and minimize non-radiative relaxation processes, enhancing the upconversion efficiency.RE-UCNP/MOF heterostructures can be categorized into three distinct formulations: (1) rare-earth-doped upconversion nanoparticle@metal–organic framework (RE-UCNP@MOF) configurations, (2) core–satellite metal–organic framework@rare-earth-doped upconversion nanoparticle (MOF@RE-UCNP) configurations, and (3) Janus-type heterodimers composed of rare-earth-doped upconversion nanoparticles and metal–organic frameworks (Janus RE-UCNP/MOF heterodimers).
4.1 RE-UCNP@MOF formulations
To attain core–shell structured formulations of RE-UCNP@MOF, the process involves doping of RE-UCNPs during the MOF formation stage. It is important to note the existence of two distinct subtypes within the category of RE-UCNP@MOF formulations. The initial subtype pertains to a singular RE-UCNP that is modified with a MOF layer on its surface, exemplifying a one-to-one configuration (Fig. 7A type (i) and Fig. 7B).49,95 Conversely, the second subtype encompasses scenarios wherein a MOF particle is encapsulated by multiple RE-UCNPs, signifying a many-to-one configuration (Fig. 7A type (ii) and Fig. 7C).54,89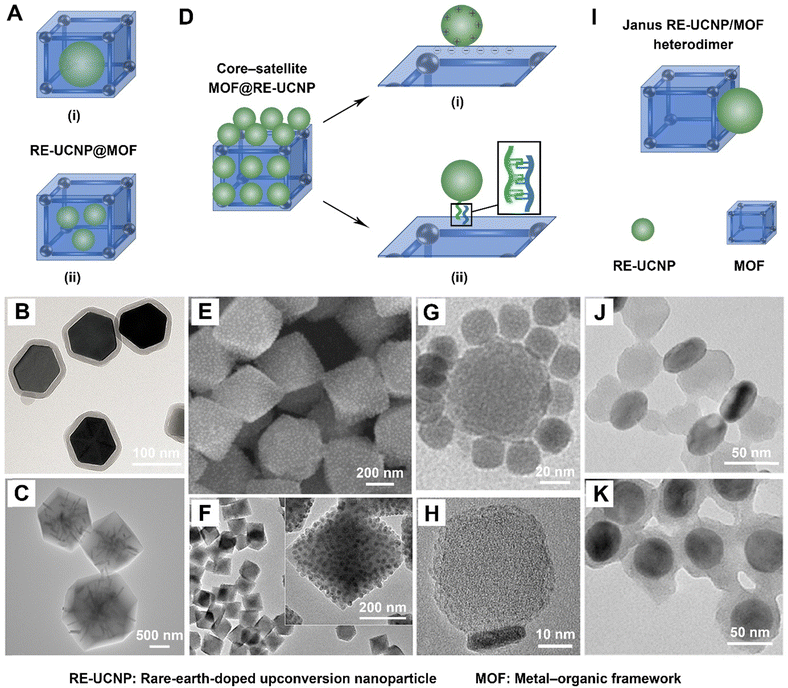 | ||
| Fig. 7 Current strategies for fabricating rare-earth-doped upconverting MOF-based hybrids with different structures. (A) Scheme illustrating the structure of RE-UCNP@MOF composites. (B) Transmission electron microscopy image of single RE-UCNP-encapsulated MOFs. Figure reproduced with permission from ref. 49, American Chemical Society. (C) Transmission electron microscopy image of multiple RE-UCNP-encapsulated MOFs. Figure reproduced with permission from ref. 54, Nature Publishing Group. (D) Scheme illustrating the structure of MOF@RE-UCNP composites. (E) and (F) Scanning electron microscopy image (E) and transmission electron microscopy image (F) of MOF@RE-UCNP composites formed via electrostatic interactions. Figure reproduced with permission from ref. 74, American Chemical Society. (G) Transmission electron microscopy image of MOF@RE-UCNP composites formed by complementary base pairing. Figure reproduced with permission from ref. 96, Wiley-VCH. (H) Transmission electron microscopy image of Janus RE-UCNP/MOF heterodimers. Figure reproduced with permission from ref. 51, American Chemical Society. (I) Scheme illustrating the structure of Janus RE-UCNP/MOF heterodimers. (J) and (K) Transmission electron microscopy images of Janus (J) and core–shell structured (K) RE-UCNP@MOF composites prepared from polyvinylpyrrolidone- and citrate acid-modified RE-UCNPs, respectively. Figure reproduced with permission from ref. 52, American Chemical Society. RE-UCNP: rare-earth-doped upconversion nanoparticle. MOF: metal–organic framework. | ||
To fabricate single RE-UCNP-encapsulated MOFs, diverse approaches have been developed, including the surface ligand-mediated method,49 the layer-by-layer growing technique,91 and the one-pot liquid–solid-solution strategy.95 Specifically, hydrophilic core–shell structured nanostructures, denoted as RE-UCNP@ZIF-8, were meticulously synthesized through the surface ligand-mediated approach.49 Initially, the hydrophobic oleic acid (OA)-capped surface of RE-UCNPs was eliminated and subsequently capped with the hydrophilic ligand polyvinylpyrrolidone (PVP). The decoration of PVP served a dual purpose by conferring water solubility to the RE-UCNPs and augmenting the growth of ZIF-8 onto the surface of RE-UCNPs due to the interaction between Zn2+ and the amines of PVP. Consequently, a ZIF-8 layer, comprising Zn2+ and 2-methylimidazole, was successfully coated onto the PVP-capped RE-UCNPs, resulting in the generation of hydrophilic RE-UCNP@ZIF-8 nanostructures. Furthermore, the layer-by-layer growth technique has been refined based on the surface ligand-mediated strategy to enhance the precision of forming RE-UCNP@MOF structures.91 This method involves the repetitive deposition of MOF layers onto the PVP-functionalized RE-UCNPs, culminating in the gradual construction of the MOF shell. Additionally, a one-pot liquid–solid-solution method has been innovatively developed for the fabrication of RE-UCNP@MOF composites, specifically designated as UCNP@MIL-100(Fe).95 First, oleic acid (OA)-coated RE-UCNPs and OA-coated Fe(III) oleate clusters were dispersed into the mixture solution (1) comprising OA and dimethyl sulfoxide (DMSO). Subsequently, the introduction of a mixture solution (2), containing water and DMSO along with 1,3,5-trimesic acid (H3BTC) ligands, was carried out in order to generate an oil/water interface. At the interfaces of DMSO-OA (liquid) and water-DMSO solutions (solution), the coordination reaction between the Fe(III) oleate clusters (solid) and the H3BTC ligands was initiated through an anion exchange of OA and H3BTC ligands as the temperature was elevated to 120 °C, consequently leading to the formation of the core–shell UCNPs@MIL-100(Fe) nanostructures (Fig. 8). The chemical compositions and structures of diverse RE-UCNP@MOF formulations are listed in Table 4.
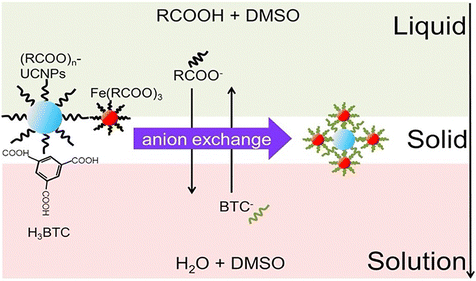 | ||
| Fig. 8 Schematic illustration for the formation of UCNPs@MIL-100(Fe) nanostructures. Figure reproduced with permission from ref. 95, ELSEVIER. | ||
Multiple RE-UCNPs, encapsulated within a metal–organic framework (MOF) matrix, can be synthesized utilizing the surface ligand-mediated and electrostatic interaction-driven methodologies.54,89 To exemplify, the surface of rod-shaped RE-UCNPs underwent modification with the amphiphilic polymer PVP, which enables successive adsorption of RE-UCNPs onto the evolving surface of zeolitic imidazolate framework-8 (ZIF-8) crystals, culminating in the formation of composite structures denoted as RE-UCNP-encapsulated ZIF-8 composites.54 For an illustration involving the electrostatic interaction-driven synthesis of multiple RE-UCNP-encapsulated MOFs, researchers adopted zinc hydroxide nano-strands as zinc ion precursors to react with 2-methylimidazole to form ZIF-8 structures.89 Consequently, RE-UCNPs were firmly confined within the MOF architecture due to the electrostatic interaction occurring between the negatively charged RE-UCNPs and the positively charged zinc hydroxide nano-strands.
Both subtypes of RE-UCNP@MOF formulations hold respective advantages and disadvantages. (1) In contrast to multiple RE-UCNP-encapsulated MOFs that may have a photoluminescence quenching effect caused by the collision/aggregation of RE-UCNPs, single RE-UCNP-encapsulated MOFs exhibit superiorities in photoluminescence efficiency. (2) Configurations involving multiple RE-UCNP-encapsulated MOFs exhibit a heightened capacity to absorb light, enabling their excitation at lower power levels compared to their singular RE-UCNP counterparts within MOFs. (3) Single RE-UCNP-encapsulated MOFs exhibit greater amenability for size manipulation through controlled MOF growth in comparison with their multiple RE-UCNP-encapsulated counterparts. (4) Multiple RE-UCNP-encapsulated MOFs often exceed dimensions of >300 nm,54,89 and singular RE-UCNP-encapsulated MOFs typically feature smaller dimensions of less than 200 nm,49,91,95 rendering them particularly suitable for applications in biomedicine.
4.2 MOF@RE-UCNP formulations
The preparation process of MOF@RE-UCNP formulations differs from that of RE-UCNP@MOF structures. The former entails the interaction between RE-UCNPs and MOF particles, whereas the latter relies on the interaction of RE-UCNPs with precursor materials for MOF formation. Two distinct methods have been devised for the fabrication of MOF@RE-UCNP formulations (Fig. 7D). The first method involves the in situ attachment of RE-UCNPs onto the surface of MOFs through electrostatic interactions (Fig. 7D(i), E, and F).74,81 This approach entails the fabrication of negatively charged MOFs and positively charged RE-UCNPs, and the latter can be achieved by removing the OA-capped surface and subsequently treating them with hydrochloric acid. This electrostatic interaction-driven method has been demonstrated to successfully pave various MOFs with RE-UCNPs. Notable examples include UiO-66-NH2, composed of zirconium(IV) and 2-aminoterephthalic acid; UiO-66, composed of zirconium(IV) and 1,4-benzodicarboxylic acid; MOF-801, composed of zirconium(IV) and fumaric acid; and PCN-223, composed of zirconium(IV) and tetrakis(4-carboxyphenyl) porphyrin.74Alternatively, a DNA-assisted method is proposed to create MOF@RE-UCNP superstructures by exploiting the interaction between single-stranded-DNA-modified RE-UCNPs and MOFs decorated with complementary DNA (Fig. 7D(ii) and G).96 In this approach, the surfaces of MOFs and RE-UCNPs are adorned with DNA1 and DNA2 strands, respectively. The core–satellite MOF@RE-UCNP superstructure is generated through the annealing of DNA1-modified MOFs with DNA2-modified RE-UCNPs at a temperature of 60 °C for 10 minutes. By increasing the ratio of DNA2-modified RE-UCNPs to DNA1-modified MOFs, the quantity of RE-UCNPs on the surface of MOFs can be enhanced. Specifically, when the MOF to RE-UCNP ratio reaches 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, a well-defined core–satellite structure is generated, where a solitary MOF acts as the central core, orbited by multiple RE-UCNPs serving as satellites. The chemical compositions of various MOF@RE-UCNP formulations are delineated in Table 4.
12, a well-defined core–satellite structure is generated, where a solitary MOF acts as the central core, orbited by multiple RE-UCNPs serving as satellites. The chemical compositions of various MOF@RE-UCNP formulations are delineated in Table 4.
4.3 Janus RE-UCNP/MOF heterodimers
Janus heterodimers, comprised of RE-UCNPs and MOFs, have recently garnered research interest, augmenting the aforementioned formulations of RE-UCNP@MOF and MOF@RE-UCNP.51 Thus far, researchers have successfully devised an efficient method for synthesizing heterostructural RE-UCNP/MOF dimers. In this process, Zr-based MOF growth onto RE-UCNPs occurs in a selective anisotropic manner, which is effectively facilitated by the presence of polyvinylpyrrolidone (PVP) residing on the surface of RE-UCNPs. Specifically, the facet-selective binding of PVP assumes pivotal significance in achieving the anisotropic growth pattern, and the interaction between Zr and PVP serves as the driving force for the preferential nucleation and growth of MOFs on the top (001) facets of RE-UCNPs. As a result, the attainment of Janus RE-UCNP/MOF heterodimers in substantial yields has been realized (Fig. 7H and I).51 Compared to isotropic systems, Janus heterodimers present an enhanced capacity to furnish anisotropic surface platforms and facilitate technological potential for site-specific functionalization, establishment of electrical connections, and programmable assembly of nanoparticles, thereby enabling a broad spectrum of applications spanning solar energy conversion, catalysis, and cancer therapy. Additionally, researchers have reported that the morphology of RE-UCNP/MOF composites is intimately tied to the surface engineering of RE-UCNPs, where the surface ligands of RE-UCNPs play a pivotal role in this mechanism.52 Specifically, when polyvinylpyrrolidone serves as the surface ligand for RE-UCNPs, it leads to the formation of a Janus RE-UCNP/MOF heterodimeric configuration (Fig. 7J). In contrast, a shift in the surface ligand of RE-UCNPs to citric acid yields a core–shell structured RE-UCNP@MOF composite (Fig. 7K). The chemical compositions of various Janus RE-UCNP/MOF heterostructures are demonstrated in Table 4.5. Applications of upconverting MOF-based hybrids
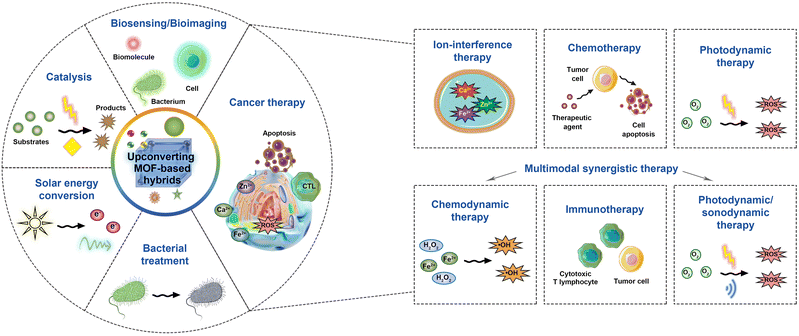
In recent years, upconverting MOF-based hybrids have experienced burgeoning utilization in various applications due to their advantageous physical, chemical, and optical properties. These applications encompass biosensing and bioimaging, solar energy conversion, catalysis, as well as disease treatment, notably in the realms of cancer therapy and bacterial treatment (Fig. 9).5.1 Upconverting MOF-based hybrids for bioimaging and biosensing
When biological tissues are subjected to visible light, they often manifest autofluorescence, thereby posing a potential challenge to imaging signal clarity. In contrast, NIR light possesses a superior ability to permeate biological tissues. The process of upconversion, utilizing NIR light, mitigates the likelihood of inducing autofluorescence, thereby resulting in heightened signal-to-noise ratios and superior image quality. Furthermore, the flexibility of UCMs enables the intentional emission of distinct colours of light in response to varying wavelengths of NIR, which allows for the detection of multiple targets and underscores the versatility of upconversion in biomedical imaging applications. Upconverting MOF-based hybrids exhibit enhanced upconversion luminescence properties while preserving the intrinsic characteristics of MOF materials, including surface modifiability and stimulus responsivity. Consequently, these upconverting MOF-based hybrids enable both in vitro and in vivo photoluminescence imaging of biomolecules, such as octopamine (Fig. 10A)82 and reactive oxygen species (Fig. 10B),49 as well as lymph nodes (Fig. 10C),70 tumour cells (Fig. 10D),75 and bacteria (Fig. 10E).59 As an illustration, Park et al. developed a water-stable TTA-based UCMOF system consisting of Zr, a porphyrinic sensitizer, and an anthracene emitter.70 Unlike conventional TTA-based upconversion systems that have low efficiency in aqueous media, the TTA-based UCMOFs exhibited remarkable upconversion luminescence in water. This is because closely aligned chromophores in the MOF facilitate a long-range three-dimensional triplet diffusion of 1.6 μm allowing efficient energy migration in water. The tunable ratio between the sensitizer and the emitter also allows optimization of the system for maximized TTA-based upconversion efficiency with a quantum efficiency of up to 1.28%. As a result, the TTA-based UCMOFs could realize real-time in vivo imaging of the lymph node under a low excitation power density of 5 mW cm−2 with a high signal-to-noise ratio of ∼31, wherein the threshold of excitation power density was as low as 2.5 mW cm−2. Additionally, upconverting MOF-based hybrids have showcased their multifaceted utility, serving not only as agents for upconversion luminescence imaging but also as proficient contributors to magnetic resonance imaging, achieved through the strategic incorporation of metal sources, such as Gd and Fe ions, during the preparation of MOFs or RE-UCNPs.75,98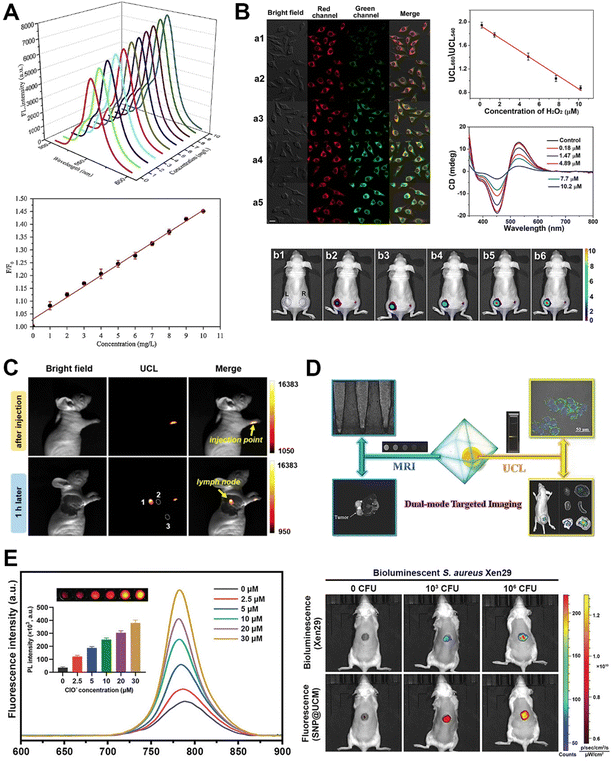 | ||
| Fig. 10 Applications of upconverting MOF-based hybrids in bioimaging and biosensing. (A) In vitro sensing of the biomolecule octopamine using upconverting MOF-based hybrids. Figure reproduced with permission from ref. 82, Elsevier. (B) In vitro and in vivo upconversion luminescence imaging of reactive oxygen species based on upconverting MOF-based hybrids. Figure reproduced with permission from ref. 49, American Chemical Society. (C) In vivo upconversion luminescence imaging of lymph nodes using upconverting MOF-based hybrids. Figure reproduced with permission from ref. 70, American Chemical Society. (D) Schematic illustration and images demonstrating the applications of upconverting MOF-based hybrids for magnetic resonance and upconversion luminescence imaging of tumour cells. Figure reproduced with permission from ref. 75, Wiley-VCH. (E) In vivo luminescence imaging of bacteria based on upconverting MOF-based hybrids. Figure reproduced with permission from ref. 59, Wiley-VCH. | ||
5.2 Upconverting MOF-based hybrids for solar energy conversion
Light-harvesting materials are of substantial interest for the design of energy conversion devices such as solar cells, among which upconverting MOF-based hybrids stand out as promising candidates. Integrating UCMs into MOFs or MOF-sensitized solar cells demonstrates much promise for the solar cell design for enhanced harvesting of NIR photons and would generate a new kind of highly efficient solar cells owing to their enhanced light-harvesting properties that can realize the utilization of the solar light energy in a broad spectrum from the UV to the NIR region. Meanwhile, the MOF-based platforms facilitate conversion efficiency through the features of well-established crystal structures, and adjustable chromophore distances. Meinardi et al. engineered a TTA-based UCMOF nanocomposite to function as a photonic energy harvester.72 This approach markedly enhanced the efficiency of solar energy conversion devices, specifically by harnessing low-energy photons. In this instance, thresholdless upconversion was achieved with a 100% yield, attributable to the fact that the size of the MOFs (26.2 nm) was smaller than the exciton diffusion length. Such a high-performance TTA-based upconversion made the nanoscale MOF suitable as triplet collectors to receive the sensitizer-harvested energy and transfer the absorbed energy (Fig. 11A and B). Ahmad and co-workers effectively established a photoelectrochemical cell by implementing a TTA-based UCMOF system.92 This system featured a Zn-perylene surface-supported MOF grown on the TiO2 substrate, serving as the “emitter.” Concurrently, a platinum octaethylporphyrin was employed as the “sensitizer” in acetonitrile solution. The authors have demonstrated that the photocurrent can be enhanced relative to an epitaxial Zn-perylene surface-supported MOF due to the TTA-based upconversion mechanism (Fig. 11C and D). This initial finding shows promise for applications in UCMOF-based solar energy conversion devices.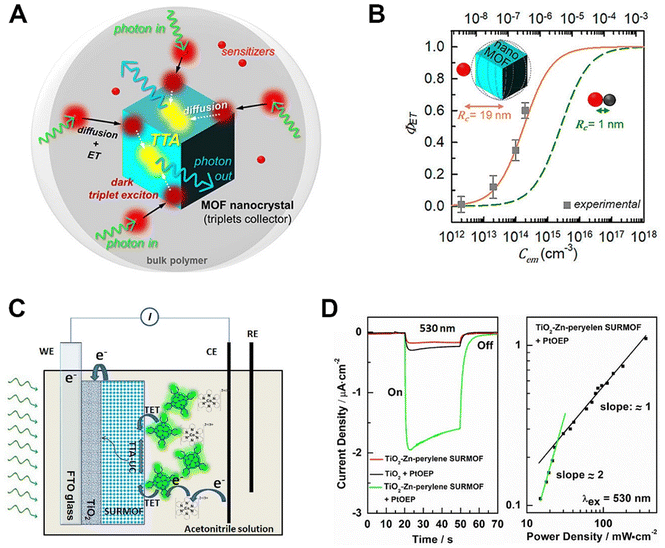 | ||
| Fig. 11 Applications of upconverting MOF-based hybrids in solar energy conversion. (A) Scheme illustrating the energy transfer mechanism of the upconverting MOF-based hybrids. (B) Energy transfer yield of the upconverting MOF-based hybrids. Figure reproduced with permission from ref. 72, American Chemical Society. (C) Schematic illustration of the photoelectrochemical cell mechanism based on the upconverting MOF-based hybrid system. (D) Transient photocurrents generated from the photoelectrochemical cell. Figure reproduced with permission from ref. 92, American Chemical Society. | ||
5.3 Upconverting MOF-based hybrids for catalysis
Upconverting MOF-based hybrids have found two principal applications within the field of catalysis: photocatalysis and biocatalysis. In the field of photocatalysis, upconverting MOF-based hybrids achieved through synergistic integration of UCMs and MOFs represent a promising class of efficient photocatalysts due to the features of broadband light absorption spanning from the UV to NIR region, thus harvesting the solar spectrum as broadly as possible. Meanwhile, high catalysis efficiency can be achieved as a result of the distinctive network architecture featuring highly ordered and tailorable cavities/channels, allowing for the dense distribution of active substrate-accessible catalytic sites. As an exemplification, Li et al. designed core–shell structured upconverting MOF-based hybrids, named UCNPs-Pt@MOF/Au (Fig. 12A), to facilitate photocatalytic hydrogen production.50 The engineered design of upconverting MOF-based hybrids enables the conversion of NIR light into UV and visible light through the unique optical properties of RE-UCNPs (Fig. 12B). Subsequently, the upconverted UV emission is absorbed by the MOF shell, inducing the generation of excited electrons. These electrons are then efficiently transferred to Pt sites, facilitating the subsequent reduction of protons. Simultaneously, the upconverted visible emission is effectively harnessed by Au nanoparticles via surface plasmon resonance, leading to the generation of hot electrons. These hot electrons are injected into the lowest unoccupied molecular orbital of the MOF shell, culminating in their eventual transfer to the Pt sites for enhancing their photocatalytic activity. The well-designed upconverting MOF-based hybrids, adorned with Pt and Au nanoparticles, demonstrate impressive photocatalytic activity when subjected to NIR and solar light. Additionally, they exhibited good recyclability, further emphasizing their potential for sustainable and efficient photocatalytic applications.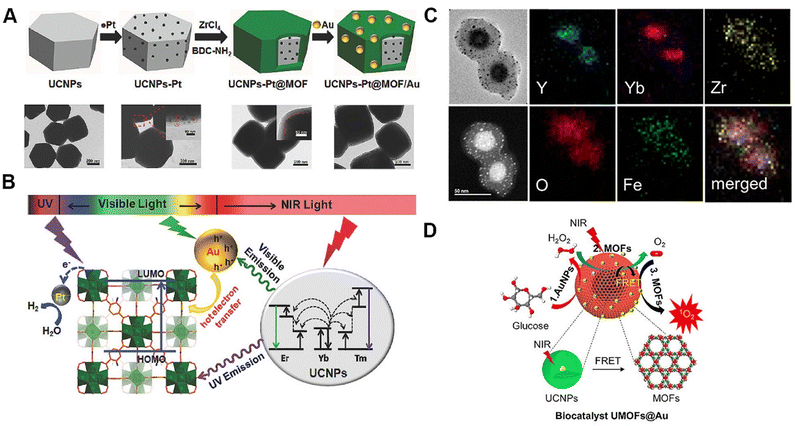 | ||
| Fig. 12 Photocatalysis and biocatalysis based on upconverting MOF-based hybrids. (A) Schematic illustration and transmission electron microscopy images illustrating the morphology and structure of UCNP-Pt@MOF/Au. (B) Schematic illustration of the mechanism for photocatalysis using the UCNP-Pt@MOF/Au composites. Figure reproduced with permission from ref. 50, Wiley-VCH. (C) Transmission electron microscopy and element mapping images illustrating the morphology and structure of biocatalysts predicated on upconverting MOF-based hybrids incorporating Au nanoparticles. (D) Schematic illustration of the mechanism for biocatalysis using the upconverting MOF-based hybrids incorporating Au nanoparticles. Figure reproduced with permission from ref. 73, American Chemical Society. | ||
To demonstrate the practical application within the field of biocatalysis, He et al. introduced a biocatalyst based on upconverting MOF-based hybrids incorporating Au nanoparticles (Fig. 12C).73 Within this configuration, the Au nanoparticles assume the role of catalysts in the generation of hydrogen peroxide through the consumption of glucose, while the MOF shell operates as a biomimetic catalyst for the subsequent decomposition of hydrogen peroxide into oxygen (Fig. 12D). Furthermore, RE-UCNPs function as NIR light harvesters, facilitating the energy transfer to iron-porphyrinic MOFs, thereby enabling the conversion of oxygen into singlet oxygen. Consequently, this intricately designed system facilitates cascade biocatalytic reactions, thereby facilitating the continuous production of singlet oxygen when subjected to NIR laser irradiation.
5.4 Upconverting MOF-based hybrids for disease treatment
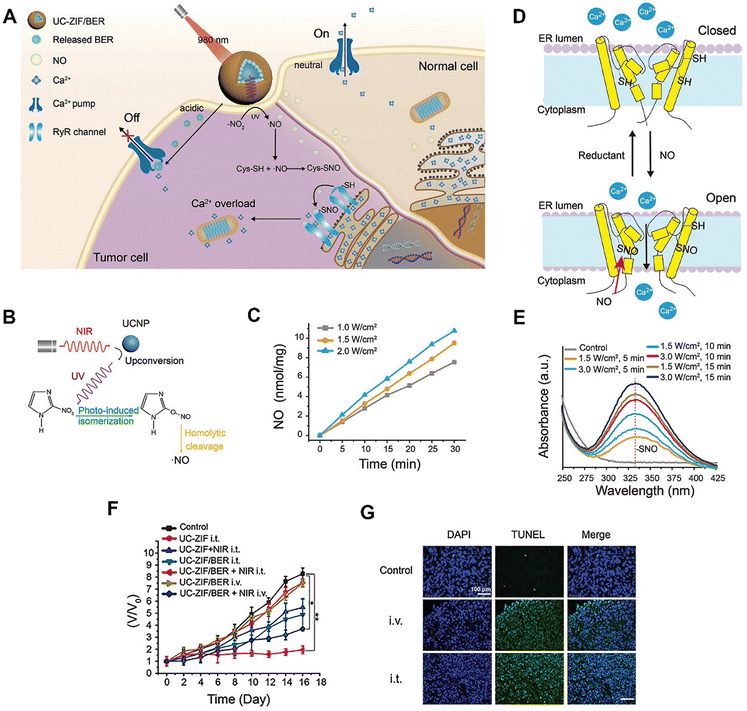 | ||
| Fig. 13 Applications of upconverting MOF-based hybrids in cancer therapy. (A) Scheme illustrating the rationally designed upconverting MOF-based hybrids (UC-ZIF/BER) for calcium ion-initiated cancer therapy. (B) Schematic illustration of NIR light-triggered NO generation. (C) NO generation profiles of the upconverting MOF-based hybrids under 980 nm laser irradiation. (D) Scheme illustrating the release of calcium ions from the endoplasmic reticulum (ER) through the nitrosothiol (–SNO)-mediated pathway. (E) UV–vis absorption spectra indicating the generation of –SNO in the mixture containing cysteine and upconverting MOF-based hybrids under 980 nm laser irradiation. (F) Relative tumour volume changes of 4T1 tumour-bearing mice after various treatments. (G) Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)-stained images of tumour tissues after various treatments. Figure reproduced with permission from ref. 60, Wiley-VCH. | ||
Lately, Yang et al. employed upconverting MOF-based hybrids as an intelligent nitric oxide nanogenerator for wound healing of infectious diabetic ulcers.59 The nitric oxide nanogenerator consisted of a RE-UCNP core, an inner MOF shell, sodium nitroprusside (a nitric oxide donor), and an outer ROS-responsive shell ssPDA (Fig. 14A). A large amount of nitric oxide was released from the upconverting MOF-based nanogenerator under NIR excitation due to the degradation of sodium nitroprusside triggered by the upconversion of visible light from RE-UCNPs. Then, the generated nitric oxide reacted with bacterial infection-induced ROS to produce ONOO−, thereby inactivating bacteria by disrupting bacterial membrane integrity. The released nitric oxide also inhibited the degradation of hypoxia-inducible factor-1α. Therefore, the upconverting MOF-based nanogenerator combined with NIR light irradiation could promote angiogenesis at bacteria-infected wound sites by upregulating the expression of hypoxia-inducible factor-1α and enhancing the secretion of vascular endothelial growth factor in endothelial cells. Ultimately, the treatment of UCMOF plus NIR light irradiation significantly accelerated the healing of bacteria-infected wounds with dramatically reduced wound area, improved wound closure, and higher antibacterial activity (Fig. 14B–D).
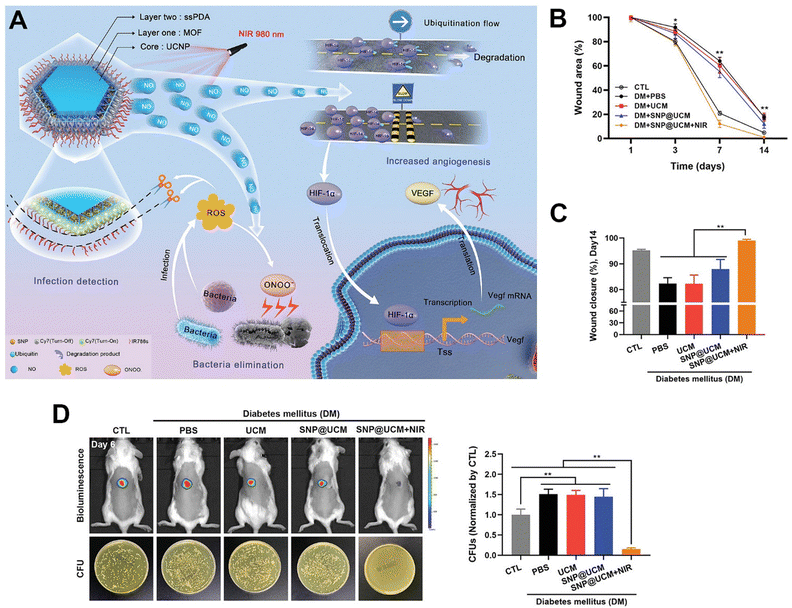 | ||
| Fig. 14 Applications of upconverting MOF-based hybrids in wound healing. (A) Scheme illustrating the applications of the upconverting MOF-based hybrids (SNP@UCM) in theranostics of bacteria-infected wounds. (B) and (C) Relative wound areas (B) and wound closures (C) after various treatments. (D) Bioluminescence images and bacterial amounts of bacteria-infected wounds after various treatments. Figure reproduced with permission from ref. 59, Wiley-VCH. | ||
6. Discussions
6.1 Merits of MOFs in photon upconversion within various upconverting MOF-based hybrids
Overall, MOFs show various advantages over other nanoparticles and porous materials for photon upconversion. (1) In TTA-based UCMOFs, the structural configuration permits precise adjustment of both the orientation and intermolecular distances of sensitizers/emitters. The unique structure of MOFs helps to improve the stability and dispersibility of the sensitizers/emitters within the composite matrix. These capabilities, as well as the feasibility of tuning the loading contents/ratios of sensitizers/emitters with the MOFs, collectively contribute to their high upconversion efficiency. In addition, the MOFs are capable of creating a hydrophobic environment within their internal cavities, which plays a crucial role in shielding the sensitizer molecules from oxygen-induced quenching. (2) In comparison to other lanthanide ion-based UCMs, lanthanide–organic frameworks can be easily synthesized on a large scale through coordination interactions between lanthanide ions and organic linkers. The stable and orderly aligned structures of lanthanide–organic frameworks provide an appropriate distance between the lanthanide ions, mitigating the undesirable self-quenching and aggregation of lanthanides. The organic links in lanthanide–organic frameworks can help to absorb light and transfer energy to the lanthanides. Moreover, using or doping different lanthanide ions, and changing the proportion of lanthanide ions can obtain lanthanide–organic frameworks with the same structure, but with tunable upconversion emission properties, when utilizing the same organic linker. In addition, similar to other MOFs, lanthanide–organic frameworks have high surface areas and porosities, making them have potential as a versatile platform for diverse applications. (3) Regarding RE-UCNP/MOF heterostructures, compared with other RE-UCNP-based nanocomposites, they exhibit a strong capacity for loading other molecules or nanoparticles thanks to the high porosity and large surface area of MOFs. Besides, MOFs can possess some other fascinating properties, such as surface modifiability, stimulus responsiveness, photodynamic functionality, and catalytic capability, allowing UCNP/MOF heterostructures to be customized for different applications.6.2 Advantages and limitations of various upconverting MOF-based hybrids
Different types of upconverting MOF-based hybrids exhibit their own advantages and limitations. (1) As rare-earth-based UCMs, lanthanide–organic frameworks and RE-UCNP/MOF heterostructures offer similar advantages over TTA-based UCMOFs including a broad absorption range, sharp emission peaks and anti-photobleaching. Moreover, they can absorb and be excited by NIR light (Table 5), allowing for the use of NIR light in sunlight and deeper tissue penetration with minimal damage and autofluorescence. Multiple photoluminescence emissions were observed in most lanthanide–organic frameworks and RE-UCNP/MOF heterostructures, allowing for the simultaneous imaging of multiple targets or processes within a biological system. (2) TTA-based UCMOFs have advantages over lanthanide–organic framework and RE-UCNP/MOF heterostructures, including strong light-absorption capabilities, high upconversion quantum yields, and low excitation power intensity (Table 5). However, they are usually sensitive to oxygen, which can quench the triplet state and reduce upconversion efficiency. This requires the use of an oxygen-free environment for optimal performance, which can be a limitation in some applications. (3) The synthesis of a lanthanide–organic framework is the simplest, followed by that of TTA-based UCMOFs, while the synthesis of RE-UCNP/MOF heterostructures stands out as the most complex among the three routes.| Name | Type | Power density (W cm−2) | Excitation wavelength (nm) | Emission wavelength (nm) | Quantum yield (%) | Ref. |
|---|---|---|---|---|---|---|
| a The upconversion quantum yields are the values in the original literature divided by 2, resulting in a theoretical maximum efficiency of 50%. | ||||||
| Pd-TCPP/DCDPA | TTA-based UCMOF | 0.0025–0.02 | 532 | ∼450 | 0.64a | 70 |
| Pd@9,10-MOF | TTA-based UCMOF | 0.104 | 532 | 475 | 0.23a | 93 |
| nMOFs/PtOEP | TTA-based UCMOF | 0.01–0.1 | 532 | 435 | 6 | 72 |
| Pd-DCP SURMOF | TTA-based UCMOF | 0.001–0.1 | 532 | 450–500 | <0.1 | 69 |
| NU-1000:PtOEP | TTA-based UCMOF | 0.035 | 532 | ∼480 | 2.1 | 94 |
| TzPMOF | TTA-based UCMOF | 0.0025–0.065 | 565/602 | 465 | 1.95 | 71 |
| Up-MOFs-Y0.94/Er0.06 | Lanthanide–organic framework | — | 980 | 520, 540, 651 | 0.1312 | 66 |
| Yb-BTC MOFs | Lanthanide–organic framework | 7.3–13 | 980 | 497 | — | 80 |
| Er-doped Yb-BTC MOFs | Lanthanide–organic framework | 10 | 980 | 497, 525, 545, 655, 1532 | — | 80 |
| Ho-doped Yb-BTC MOFs | Lanthanide–organic framework | — | 980 | 497, 660 | — | 80 |
| Tb-doped Yb-BTC MOFs | Lanthanide–organic framework | — | 980 | 497, 545 | — | 80 |
| Eu-doped Yb-BTC MOFs | Lanthanide–organic framework | — | 980 | 497, 615 | — | 80 |
| UCNP@ZIF-NiSx | RE-UCNP@MOF | — | 980 | 540, 660 | — | 49 |
| UCNPs@MIL-100(Fe) | RE-UCNP@MOF | 0.5 | 808 | 450, 475, 650 | — | 95 |
| UCNP/DCM/C6@ZIF-8 | RE-UCNP@MOF | 1.45–7.8 | 980 | 450, 475, 646 | 0.0125 | 89 |
| UiO-66-NH2@NaYF4:Yb/Er | MOF@RE-UCNP | — | 980 | 550, 650 | — | 74 |
| CR@MUP | MOF@RE-UCNP | 1 | 808 | 540, 660 | — | 81 |
| Core–satellite MOF–UCNP | MOF@RE-UCNP | 15.9 | 980 | 550, 660 | — | 96 |
| UCMOF heterodimers | RE-UCNP/MOF heterodimers | 1.2–3.2 | 808/980 | 522, 541, 654 | 0.2 | 51 |
6.3 Design of upconverting MOF-based hybrids for various applications
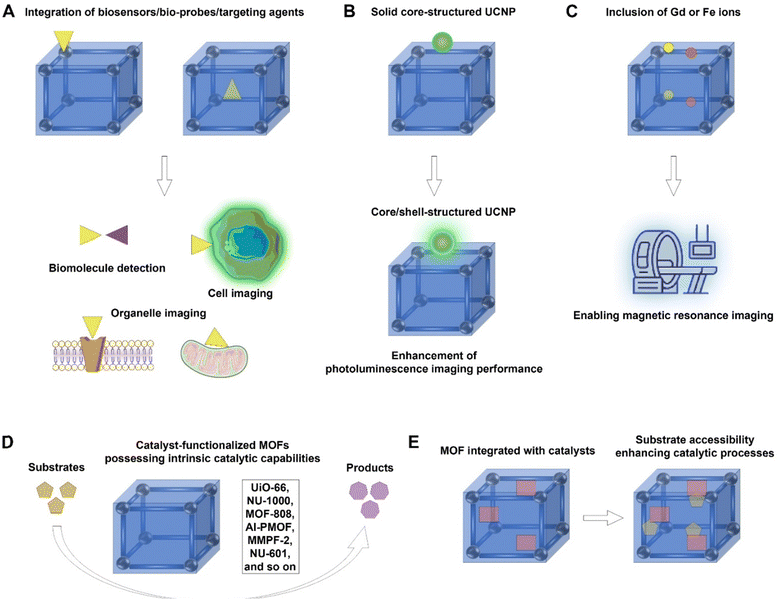
To fully unlock the potential of MOF-based hybrids for different applications, a more strategic and detailed approach is essential. Below, we elaborate on some of the key strategies, supported by schematic diagrams and figures to demonstrate these concepts.To facilitate the utilization of MOF-based hybrids in bioimaging and biosensing, several strategies can be considered. Firstly, the inherent characteristics of MOFs, such as high porosity, tunable pore size, and diverse functional sites, can be exploited to integrate biosensors or bioprobes within the MOFs (Fig. 15A). This approach enables selective detection and imaging of specific biomolecules. By incorporating functional elements such as fluorophores or recognition elements into the MOF structure, targeted biomolecules can be detected with high precision. Secondly, the modifiability of the surface of MOF-based hybrids allows for the conjugation of targeting agents, enabling specific sensing/imaging applications such as tumour diagnosis/imaging and organelle imaging (Fig. 15A). Thirdly, to enhance photoluminescence imaging performance, it is recommended to opt for core–shell structured upconversion nanoparticles instead of solid core structures (Fig. 15B). Fourthly, diverse imaging techniques can be realized, including photoluminescence imaging through the incorporation of photoluminescent agents/materials and magnetic resonance imaging through the inclusion of Gd or Fe ions (Fig. 15C).
For solar energy conversion, MOF-based hybrids have shown great promise due to their tunable light-harvesting properties. Here are some strategies for improving their efficiency. Firstly, it is advisable to synthesize MOFs with intrinsic light-harvesting capabilities by integrating light-absorbable agents during their fabrication. This process may involve the incorporation of Ru(II)-bipyridine building blocks characterized by robust visible light absorption and long-lived triple metal-to-ligand charge transfer (3MLCT) excited states, as well as the inclusion of light-harvestable pyrene-based ligands and chromophores (e.g., porphyrin derivatives). Secondly, to augment the light-harvesting proficiency of MOF-based hybrids, the integration of upconversion materials is recommended, which can enhance conversion efficiency across a wide spectrum ranging from the UV to the NIR region. For further refinement of light-harvesting capabilities, it is advisable to opt for core–shell structured upconversion nanoparticles, which may exhibit enhanced upconverting capability over solid core structured upconversion nanoparticles. This strategic selection contributes to a more efficient enhancement of MOF-based hybrid solar cells.
To enable the application of MOF-based hybrids in catalysis, two pathways can be pursued. One avenue entails the utilization of catalyst-functionalized MOFs that possess intrinsic catalytic capabilities. Noteworthy examples of such MOFs include UiO-66, NU-1000, MOF-808, Al-PMOF, MMPF-2, and NU-601, among others (Fig. 15D). Alternatively, catalysts can be integrated by harnessing the advantageous physical and chemical properties inherent in MOFs, which include densely distributed active catalytic sites ensuring substrate accessibility, as well as ordered tailorable cavities with a hydrophobic confined environment that further enhances catalytic processes (Fig. 15E).
To enhance the applicability of MOF-based hybrids in disease treatments, such as cancer therapy and bacterial treatment, researchers can consider various pathways, including photodynamic therapy, chemotherapy, chemodynamic therapy, gas therapy, and immunotherapy. For photodynamic therapy, it can be considered to load photosensitizing molecules/nanoparticles or utilize porphyrinic MOFs in upconversion–MOF hybrids. Concerning chemotherapy, in addition to loading chemotherapeutics, it is advisable to endow MOF-based hybrids with targeted accumulation through modifiable surfaces and stimulus responsiveness for intelligent/controllable drug release. These stimuli may include pH, light, and miRNA, among others. For chemodynamic therapy, MOF-based hybrids containing iron or copper ions can be designed to engage in Fenton or Fenton-like reactions. In the realm of gas therapy, MOF-based hybrids can be incorporated with gas generators, such as the NO generator, thereby enabling gas-mediated treatment of diseases. For cancer immunotherapy, two pathways can be considered to activate the immune response. One involves the incorporation of exogenous vaccines in MOF-based hybrids, while the other entails designing functional MOF-based hybrids that act as in situ vaccines by inducing immunogenic cell death in tumour cells. For bacterial immunotherapy, it is considered to design functional MOF-based hybrids that can target immune checkpoints (such as the PD-1/PD-L1 pathway), modulate cytokines (such as TNF-α, which activates macrophages and other myeloid cells to enhance their bactericidal activity), or trigger macrophages adoptively transferred to monocyte-derived macrophages for combating bacteria.
7. Conclusions and outlook
In conclusion, this review provides a comprehensive summary of the various types, upconversion luminescence mechanisms, preparation methods, and applications of MOF-based hybrids with photon upconversion. By combining UCMs with MOFs, these hybrids possess several advantageous features, including flexible design, diverse functional sites, high porosity, controllable pores, upconversion luminescence properties, tuneable photoluminescence, and favourable photostability. To date, significant progress has been achieved in the development of MOF-based hybrids with photon upconversion, specifically rare-earth-doped upconverting MOF-based hybrids and TTA-based UCMOFs. These advancements have been particularly notable in interdisciplinary research areas, such as biosensing/bioimaging, solar energy conversion, catalysis, and disease treatment. Importantly, these achievements serve as a solid foundation for further advancements in the field of upconverting MOF-based hybrids and have inspired advanced studies in related fields.Despite these achievements, some challenges should be considered in this field. (1) Lanthanide–organic frameworks often exhibit a low upconversion efficiency, typically remaining below 1%. The high concentration of lanthanide dopants has been demonstrated to facilitate upconversion luminescence by yielding a high density of optical centres to harvest and sustain the energy of excitation light. Nevertheless, self-quenching induced by increasing lanthanide dopant concentration and the non-radiative relaxation processes within the MOF structure represent two obstacles to the development of bright lanthanide–organic frameworks. Therefore, greater efforts should be devoted to improving the upconversion efficiency of lanthanide–organic frameworks, such as optimizing the concentration/ratio of lanthanide dopants and choosing suitable organic linkers. (2) Regardless of the large anti-Stokes shifts, rare-earth-doped upconverting MOF-based hybrids, including lanthanide–organic frameworks and RE-UCNP/MOF heterostructures, exhibit poor light absorption characteristics due to their limited capacity to absorb light within a narrow wavelength spectrum and their inherent weak light-absorption capabilities. (3) The synthesis of RE-UCNP/MOF heterostructures usually involves multiple steps, including the preparation of RE-UCNPs and MOFs separately and surface modification before combining them. This process is complex and time-consuming, restricting their scalability for industrial applications. (4) TTA-based UCMs exhibit large absorption cross-sections and high upconversion efficiencies, yet they are burdened with the shortcomings of small anti-Stokes shifts and poor stability. Recently, the development of TTA-based upconversion inorganic–organic hybrid systems, such as coupling organic molecules to inorganic lanthanide-doped nanoparticles,102 PbS nanoparticles,103,104 and InP-based quantum dots,105 has emerged as an effective strategy to overcome the inherent limitations of organic molecules. MOFs show great potential in controlling triplet dynamics by integrating organic molecules and inorganic nanoparticles, and it is encouraged to construct inorganic–organic upconverting MOF-based hybrid systems in the future. (5) In order to form a MOF structure, the selection of sensitizer/emitter pairs crucially impacts the feasibility of preparing TTA-based UCMOFs, with only a limited subset demonstrating compatibility. In contrast, most sensitizer/emitter pairs pose challenges in forming MOFs with metal ions. Consequently, the universality of MOFs as a platform for all TTA-based sensitizer/emitter pairs is constrained. (6) Although some TTA-based upconversion systems have achieved NIR light excitation,34,106 the present TTA-based UCMOFs exclusively respond to visible light. There is still a lack of TTA-based UCMOF MOFs that can be excited by NIR light, which limits their penetration depth and applications. (7) The MOF moieties in the systems can be sensitive to moisture and the environment, leading to the degradation of the framework, and a consequent decrease in upconversion efficiency over time. Moreover, upconverting MOF-based hybrids may raise biosafety concerns because the unstable MOF moieties have the potential to release toxic metal ions107 and organic molecules108 under specific conditions. (8) Although upconverting MOF-based hybrids have shown great potential as solar energy conversion devices, more efforts are still needed to overcome the challenges in material and optical design, as well as address the longevity of upconversion units. (9) The potential application of TTA-based UCMOFs in cancer therapy or bacterial treatment should be explored. Developing biocompatible TTA-based UCMOFs for more biomedical uses should be a research focus in this area. (10) To foster the development of upconverting MOF-based hybrids in the field of catalysis, the mature technology of MOF-based catalysts should be fully utilized to design functional upconverting MOF-based hybrids with enhanced catalytic activities. (11) When utilizing upconverting MOF-based hybrids as drug delivery vehicles in chemotherapy, the release of encapsulated drugs currently hinges upon pH- or miRNA-responsive mechanisms. Subsequent endeavours directed at the design of more sophisticated upconverting MOF-based hybrids as precise drug delivery systems are strongly advocated, such as the design of light-, biomolecule-, or redox stress-responsive upconverting MOF-based hybrids, which hold significant promise for enhancing drug delivery control. (12) Regarding photodynamic therapy based on upconverting MOF-based hybrids, the therapeutic efficiency can be negatively affected by energy loss during energy transfer between RE-UCNP/TTA-based upconversion formulations and photosensitizers. Hence, more endeavours are needed to construct effective strategies to address this obstacle. (13) Notwithstanding the demonstrated feasibility of multimodal synergistic cancer therapy in living mouse models using MOF-based hybrids with photon upconversion, many factors need systematic exploration for future clinical applications. These include the optimal combination of different treatment modalities, treatment sequence-induced effects, long-term systemic toxicity, potential biological reactions, and more. (14) There is an urgent need for the development of more versatile upconverting MOF-based hybrids to overcome various challenges posed by the complex tumour microenvironment, such as hypoxia conditions, immunosuppressive environments, and drug penetration barriers. (15) The research on functional upconverting MOF-based hybrids for bacterial theranostics is an emerging and promising field, to which more efforts should be devoted. (16) The second NIR (NIR-II, 1100–1350 nm) window exhibits superiority over the first NIR (NIR-I, 700–950 nm) window in the context of in vivo imaging, primarily attributable to its capacity for achieving further reductions in scattering, absorption, and tissue autofluorescence. UCMs have been demonstrated a success in NIR-I excitation, with some advancements in the development of rare-earth-doped UCMs capable of being excited within the NIR-II range.12–14,109 However, the conceptualization and realization of upconverting MOF-based hybrids excited in the NIR-II range remain unexplored and is an uncharted territory. (17) Compared with other photoluminescent materials and MOFs, the current applications of upconverting MOF-based hybrids are still rather limited. For example, they lack applications in optogenetics, gas storage, and gas separation. These unsettled issues represent the next frontiers for the further development of MOF-based hybrids with photon upconversion.
Author contributions
Conceptualization: X. C. and Y. Z.; investigation: X. C.; writing – original draft: X. C. and X. Z.; writing – review & editing: X. C., X. Z., and Y. Zhao.; funding acquisition: Y. Z.; supervision: Y. Z.Data availability
This review article does not contain any primary data. All data discussed in this manuscript are available within the cited literature and publicly accessible sources. No new datasets were generated or analysed during the preparation of this manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the National Research Foundation Singapore under Its Competitive Research Programme (NRF-CRP26-2021-0002).References
- W. R. Algar, M. Massey, K. Rees, R. Higgins, K. D. Krause, G. H. Darwish, W. J. Peveler, Z. Xiao, H. Y. Tsai, R. Gupta, K. Lix, M. V. Tran and H. Kim, Chem. Rev., 2021, 121, 9243–9358 CrossRef CAS PubMed.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS.
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC.
- Q. Dou, L. Jiang, D. Kai, C. Owh and X. J. Loh, Drug Discov. Today, 2017, 22, 1400–1411 CrossRef CAS PubMed.
- X. Zhu, J. Zhang, J. Liu and Y. Zhang, Adv. Sci., 2019, 6, 1901358 CrossRef CAS.
- X. D. Zhang, X. K. Chen, J. J. Yang, H. R. Jia, Y. H. Li, Z. Chen and F. G. Wu, Adv. Funct. Mater., 2016, 26, 5958–5970 CrossRef CAS.
- X. K. Chen, X. D. Zhang, L. Y. Xia, H. Y. Wang, Z. Chen and F. G. Wu, Nano Lett., 2018, 18, 1159–1167 CrossRef CAS PubMed.
- X. D. Zhang, X. K. Chen, Y. X. Guo, H. R. Jia, Y. W. Jiang and F. G. Wu, Nanoscale Horiz., 2020, 5, 481–487 RSC.
- X. K. Chen, X. D. Zhang and F. G. Wu, Chin. Chem. Lett., 2021, 32, 3048–3052 CrossRef CAS.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- F. Wang, R. Deng, J. Wang, Q. Wang, Y. Han, H. Zhu, X. Chen and X. Liu, Nat. Mater., 2011, 10, 968–973 CrossRef CAS PubMed.
- M. Zhao, B. Li, P. Wang, L. Lu, Z. Zhang, L. Liu, S. Wang, D. Li, R. Wang and F. Zhang, Adv. Mater., 2018, 30, 1804982 CrossRef.
- B. Zhou, L. Yan, J. Huang, X. Liu, L. Tao and Q. Zhang, Nat. Photonics, 2020, 14, 760–766 CrossRef CAS.
- L. Liu, S. Wang, B. Zhao, P. Pei, Y. Fan, X. Li and F. Zhang, Angew. Chem., Int. Ed., 2018, 57, 7518–7522 CrossRef CAS PubMed.
- P. Bharmoria, H. Bildirir and K. Moth-Poulsen, Chem. Soc. Rev., 2020, 49, 6529–6554 RSC.
- B. Zhou, B. Shi, D. Jin and X. Liu, Nat. Nanotechnol., 2015, 10, 924–936 CrossRef CAS.
- Z. Li, Y. Zhang and S. Jiang, Adv. Mater., 2008, 20, 4765–4769 CrossRef CAS.
- X. Chen, Y. Zhang, X. Zhang, Z. Zhang and Y. Zhang, Microchim. Acta, 2021, 188, 349 CrossRef CAS PubMed.
- Q. Mei, A. Bansal, M. K. G. Jayakumar, Z. Zhang, J. Zhang, H. Huang, D. Yu, C. J. A. Ramachandra, D. J. Hausenloy, T. W. Soong and Y. Zhang, Nat. Commun., 2019, 10, 4416 CrossRef.
- Z. Zhang, M. K. G. Jayakumar, X. Zheng, S. Shikha, Y. Zhang, A. Bansal, D. J. J. Poon, P. L. Chu, E. L. L. Yeo, M. L. K. Chua, S. K. Chee and Y. Zhang, Nat. Commun., 2019, 10, 4586 CrossRef.
- N. M. Idris, M. K. Gnanasammandhan, J. Zhang, P. C. Ho, R. Mahendran and Y. Zhang, Nat. Med., 2012, 18, 1580–1585 CrossRef CAS PubMed.
- A. A. Ansari, V. K. Thakur and G. Chen, Coord. Chem. Rev., 2021, 436, 213821 CrossRef CAS.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS.
- Y. Yang, Q. Shao, R. Deng, C. Wang, X. Teng, K. Cheng, Z. Cheng, L. Huang, Z. Liu, X. Liu and B. Xing, Angew. Chem., Int. Ed., 2012, 51, 3125–3129 CrossRef CAS.
- T. Schloemer, P. Narayanan, Q. Zhou, E. Belliveau, M. Seitz and D. N. Congreve, ACS Nano, 2023, 17, 3259–3288 CrossRef CAS.
- C. Gao, W. W. H. Wong, Z. Qin, S. C. Lo, E. B. Namdas, H. Dong and W. Hu, Adv. Mater., 2021, 33, 2100704 CrossRef CAS.
- W. Wang, Q. Liu, C. Zhan, A. Barhoumi, T. Yang, R. G. Wylie, P. A. Armstrong and D. S. Kohane, Nano Lett., 2015, 15, 6332–6338 CrossRef CAS.
- F. Wang and X. G. Liu, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS.
- Q. Su, S. Han, X. Xie, H. Zhu, H. Chen, C. K. Chen, R. S. Liu, X. Chen, F. Wang and X. Liu, J. Am. Chem. Soc., 2012, 134, 20849–20857 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- S. H. Askes, A. Bahreman and S. Bonnet, Angew. Chem., Int. Ed., 2014, 53, 1029–1033 CrossRef CAS.
- L. Zeng, L. Huang, J. Han and G. Han, Acc. Chem. Res., 2022, 55, 2604–2615 CrossRef CAS.
- L. Huang, W. Wu, Y. Li, K. Huang, L. Zeng, W. Lin and G. Han, J. Am. Chem. Soc., 2020, 142, 18460–18470 CrossRef CAS PubMed.
- K. Zheng, K. Y. Loh, Y. Wang, Q. Chen, J. Fan, T. Jung, S. H. Nam, Y. D. Suh and X. Liu, Nano Today, 2019, 29, 100797 CrossRef CAS.
- M. Vasilopoulou, A. Fakharuddin, F. P. García de Arquer, D. G. Georgiadou, H. Kim, A. R. B. Mohd Yusoff, F. Gao, M. K. Nazeeruddin, H. J. Bolink and E. H. Sargent, Nat. Photonics, 2021, 15, 656–669 CrossRef CAS.
- W. Zou, C. Visser, J. A. Maduro, M. S. Pshenichnikov and J. C. Hummelen, Nat. Photonics, 2012, 6, 560–564 CrossRef CAS.
- S. Wen, J. Zhou, P. J. Schuck, Y. D. Suh, T. W. Schmidt and D. Jin, Nat. Photonics, 2019, 13, 828–838 CrossRef CAS.
- D. J. Garfield, N. J. Borys, S. M. Hamed, N. A. Torquato, C. A. Tajon, B. Tian, B. Shevitski, E. S. Barnard, Y. D. Suh, S. Aloni, J. B. Neaton, E. M. Chan, B. E. Cohen and P. J. Schuck, Nat. Photonics, 2018, 12, 402–407 CrossRef CAS.
- J. K. Li, M. Y. Zhang, L. Zeng, L. Huang and X. Y. Wang, Angew. Chem., Int. Ed., 2023, 62, e202303093 CrossRef CAS.
- W. Xu and O. M. Yaghi, ACS Cent. Sci., 2020, 6, 1348–1354 CrossRef CAS.
- D. X. Xue, Q. Wang and J. Bai, Coord. Chem. Rev., 2019, 378, 2–16 CrossRef CAS.
- D. D. Wang, D. Jana and Y. L. Zhao, Acc. Chem. Res., 2020, 53, 1389–1400 CrossRef CAS.
- I. Nath, J. Chakraborty and F. Verpoort, Chem. Soc. Rev., 2016, 45, 4127–4170 RSC.
- M. Lismont, L. Dreesen and S. Wuttke, Adv. Funct. Mater., 2017, 27, 1606314 CrossRef.
- H. Ren, L. Zhang, J. An, T. Wang, L. Li, X. Si, L. He, X. Wu, C. Wang and Z. Su, Chem. Commun., 2014, 50, 1000–1002 RSC.
- S. Nagata, K. Kokado and K. Sada, Chem. Commun., 2015, 51, 8614–8617 RSC.
- X. Chen, Y. Zhuang, N. Rampal, R. Hewitt, G. Divitini, C. A. O'Keefe, X. Liu, D. J. Whitaker, J. W. Wills, R. Jugdaohsingh, J. J. Powell, H. Yu, C. P. Grey, O. A. Scherman and D. Fairen-Jimenez, J. Am. Chem. Soc., 2021, 143, 13557–13572 CrossRef CAS PubMed.
- C. Hao, X. Wu, M. Sun, H. Zhang, A. Yuan, L. Xu, C. Xu and H. Kuang, J. Am. Chem. Soc., 2019, 141, 19373–19378 CrossRef CAS PubMed.
- D. Li, S. H. Yu and H. L. Jiang, Adv. Mater., 2018, 30, 1707377 CrossRef.
- Y. Li, Z. Di, J. Gao, P. Cheng, C. Di, G. Zhang, B. Liu, X. Shi, L. D. Sun, L. Li and C. H. Yan, J. Am. Chem. Soc., 2017, 139, 13804–13810 CrossRef CAS PubMed.
- Y. Shao, B. Liu, Z. Di, G. Zhang, L. D. Sun, L. Li and C. H. Yan, J. Am. Chem. Soc., 2020, 142, 3939–3946 CrossRef CAS PubMed.
- X. Sun, A. I. O. Suarez, M. Meijerink, T. van Deelen, S. Ould-Chikh, J. Zecevic, K. P. de Jong, F. Kapteijn and J. Gascon, Nat. Commun., 2017, 8, 1680 CrossRef.
- G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. DuChene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T. Hupp and F. Huo, Nat. Chem., 2012, 4, 310–316 CrossRef CAS PubMed.
- S. Saha, G. Das, J. Thote and R. Banerjee, J. Am. Chem. Soc., 2014, 136, 14845–14851 CrossRef CAS PubMed.
- H. Han, F. Karlicky, S. Pitchaimuthu, S. H. R. Shin and A. Chen, Small, 2019, 15, 1902771 CrossRef.
- J. Wang, B. Song, J. Tang, G. Hu, J. Wang, M. Cui and Y. He, Nano Res., 2020, 13, 1614–1619 CrossRef CAS.
- A. R. Chowdhuri, T. Singh, S. K. Ghosh and S. K. Sahu, ACS Appl. Mater. Interfaces, 2016, 8, 16573–16583 CrossRef CAS PubMed.
- Y. Yang, K. Huang, M. Wang, Q. Wang, H. Chang, Y. Liang, Q. Wang, J. Zhao, T. Tang and S. Yang, Adv. Mater., 2021, 33, 2103593 CrossRef CAS.
- X. Chu, X. Jiang, Y. Liu, S. Zhai, Y. Jiang, Y. Chen, J. Wu, Y. Wang, Y. Wu, X. Tao, X. He and W. Bu, Adv. Funct. Mater., 2021, 31, 2008507 CrossRef CAS.
- Z. Wang, B. Liu, Q. Sun, L. Feng, F. He, P. Yang, S. Gai, Z. Quan and J. Lin, ACS Nano, 2021, 15, 12342–12357 CrossRef CAS.
- J. Liu, J. Huang, L. Zhang and J. Lei, Chem. Soc. Rev., 2021, 50, 1188–1218 RSC.
- H. S. Wang, Coord. Chem. Rev., 2017, 349, 139–155 CrossRef CAS.
- L. Zhu, X. Q. Liu, H. L. Jiang and L. B. Sun, Chem. Rev., 2017, 117, 8129–8176 CrossRef CAS.
- X. Li, S. Lu, D. Tu, W. Zheng and X. Chen, Nanoscale, 2020, 12, 15021–15035 RSC.
- X. Zhang, B. Li, H. Ma, L. Zhang and H. Zhao, ACS Appl. Mater. Interfaces, 2016, 8, 17389–17394 CrossRef CAS.
- L. Xu, Y. Li, Q. Pan, D. Wang, S. Li, G. Wang, Y. Chen, P. Zhu and W. Qin, ACS Appl. Mater. Interfaces, 2020, 12, 18934–18943 CrossRef CAS.
- P. Parnicka, W. Lisowski, T. Klimczuk, J. Łuczak, A. Żak and A. Zaleska-Medynska, Appl. Catal., B, 2021, 291, 120056 CrossRef CAS.
- M. Oldenburg, A. Turshatov, D. Busko, S. Wollgarten, M. Adams, N. Baroni, A. Welle, E. Redel, C. Woll, B. S. Richards and I. A. Howard, Adv. Mater., 2016, 28, 8477–8482 CrossRef CAS PubMed.
- J. Park, M. Xu, F. Li and H. C. Zhou, J. Am. Chem. Soc., 2018, 140, 5493–5499 CrossRef CAS.
- I. Roy, S. Goswami, R. M. Young, I. Schlesinger, M. R. Mian, A. E. Enciso, X. Zhang, J. E. Hornick, O. K. Farha, M. R. Wasielewski, J. T. Hupp and J. F. Stoddart, J. Am. Chem. Soc., 2021, 143, 5053–5059 CrossRef CAS.
- F. Meinardi, M. Ballabio, N. Yanai, N. Kimizuka, A. Bianchi, M. Mauri, R. Simonutti, A. Ronchi, M. Campione and A. Monguzzi, Nano Lett., 2019, 19, 2169–2177 CrossRef CAS PubMed.
- L. He, Q. Ni, J. Mu, W. Fan, L. Liu, Z. Wang, L. Li, W. Tang, Y. Liu, Y. Cheng, L. Tang, Z. Yang, Y. Liu, J. Zou, W. Yang, O. Jacobson, F. Zhang, P. Huang and X. Chen, J. Am. Chem. Soc., 2020, 142, 6822–6832 CrossRef CAS PubMed.
- Z. Yuan, L. Zhang, S. Li, W. Zhang, M. Lu, Y. Pan, X. Xie, L. Huang and W. Huang, J. Am. Chem. Soc., 2018, 140, 15507–15515 CrossRef CAS.
- Y. Li, J. Tang, L. He, Y. Liu, Y. Liu, C. Chen and Z. Tang, Adv. Mater., 2015, 27, 4075–4080 CrossRef CAS.
- M. X. Wu and Y. W. Yang, Adv. Mater., 2017, 29, 1606134 CrossRef.
- R. Li, T. Chen and X. Pan, ACS Nano, 2021, 15, 3808–3848 CrossRef CAS PubMed.
- C. Liu, B. Liu, J. Zhao, Z. Di, D. Chen, Z. Gu, L. Li and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 2634–2638 CrossRef CAS.
- F. Tian, C. Xu, M. Xu, H. Gao, Z. Xiao, L. Li and Y. Wang, RSC Adv., 2020, 10, 33894–33902 RSC.
- Y. Xie, G. Sun, G. A. Mandl, S. L. Maurizio, J. Chen, J. A. Capobianco and L. Sun, Angew. Chem., Int. Ed., 2023, 62, e202216269 CrossRef CAS PubMed.
- Z. Li, X. Qiao, G. He, X. Sun, D. Feng, L. Hu, H. Xu, H. B. Xu, S. Ma and J. Tian, Nano Res., 2020, 13, 3377–3386 CrossRef CAS.
- Y. Cao, X. Hu, T. Zhao, Y. Mao, G. Fang and S. Wang, Sens. Actuators, B, 2021, 326, 128838 CrossRef CAS.
- J. Y. Zeng, X. S. Wang, W. F. Song, H. Cheng and X. Z. Zhang, Adv. Ther., 2019, 2, 1800100 CrossRef.
- D. D. Wang, H. H. Wu, W. Q. Lim, S. Z. F. Phua, P. P. Xu, Q. W. Chen, Z. Guo and Y. L. Zhao, Adv. Mater., 2019, 31, 1901893 CrossRef.
- P. Z. Li, X. J. Wang, J. Liu, J. S. Lim, R. Zou and Y. Zhao, J. Am. Chem. Soc., 2016, 138, 2142–2145 CrossRef CAS PubMed.
- C. Zuo, Y. Guo, J. Li, Z. Peng, S. Bai, S. Yang, D. Wang, H. Chen and G. Xie, RSC Adv., 2021, 11, 8871–8878 RSC.
- Z. Shi, K. Zhang, S. Zada, C. Zhang, X. Meng, Z. Yang and H. Dong, ACS Appl. Mater. Interfaces, 2020, 12, 12600–12608 CrossRef CAS.
- A. R. Chowdhuri, D. Laha, S. Chandra, P. Karmakar and S. K. Sahu, Chem. Eng. J., 2017, 319, 200–211 CrossRef CAS.
- X. Wang, C. Li, Z. Li, X. Ma, D. Chen, X. Wan, Z. Deng, R. Deng and X. Peng, Chem. Eng. J., 2021, 409, 128220 CrossRef CAS.
- M. Li, S. Gul, D. Tian, E. Zhou, Y. Wang, Y. Han, L. Yin and L. Huang, Dalton Trans., 2018, 47, 12868–12872 RSC.
- M. Li, Z. Zheng, Y. Zheng, C. Cui, C. Li and Z. Li, ACS Appl. Mater. Interfaces, 2017, 9, 2899–2905 CrossRef CAS.
- S. Ahmad, J. Liu, C. Gong, J. Zhao and L. Sun, ACS Appl. Energy Mater., 2018, 1, 249–253 CrossRef CAS.
- J. M. Rowe, J. Zhu, E. M. Soderstrom, W. Xu, A. Yakovenko and A. J. Morris, Chem. Commun., 2018, 54, 7798–7801 RSC.
- D. G. Ha, R. Wan, C. A. Kim, T. A. Lin, L. Yang, T. Van Voorhis, M. A. Baldo and M. Dinca, Nat. Mater., 2022, 21, 1275–1281 CrossRef CAS PubMed.
- D. Yang, J. Xu, G. Yang, Y. Zhou, H. Ji, H. Bi, S. Gai, F. He and P. Yang, Chem. Eng. J., 2018, 344, 363–374 CrossRef CAS.
- L. He, M. Brasino, C. Mao, S. Cho, W. Park, A. P. Goodwin and J. N. Cha, Small, 2017, 13, 1700504 CrossRef PubMed.
- S. S. Skourtis, C. Liu, P. Antoniou, A. M. Virshup and D. N. Beratan, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 8115–8120 CrossRef CAS PubMed.
- D. Ling, H. Li, W. Xi, Z. Wang, A. Bednarkiewicz, S. T. Dibaba, L. Shi and L. Sun, J. Mater. Chem. B, 2020, 8, 1316–1325 RSC.
- H. J. Cai, T. T. Shen, J. Zhang, C. F. Shan, J. G. Jia, X. Li, W. S. Liu and Y. Tang, J. Mater. Chem. B, 2017, 5, 2390–2394 RSC.
- J. Lu, X. Zhu, M. Li, C. Fu, Y. Li, J. Zhang, J. Liu and Y. Zhang, ACS Appl. Bio Mater., 2021, 4, 6316–6325 CrossRef CAS PubMed.
- K. Deng, Z. Hou, X. Li, C. Li, Y. Zhang, X. Deng, Z. Cheng and J. Lin, Sci. Rep., 2015, 5, 7851 CrossRef CAS PubMed.
- S. Han, R. Deng, Q. Gu, L. Ni, U. Huynh, J. Zhang, Z. Yi, B. Zhao, H. Tamura, A. Pershin, H. Xu, Z. Huang, S. Ahmad, M. Abdi-Jalebi, A. Sadhanala, M. L. Tang, A. Bakulin, D. Beljonne, X. Liu and A. Rao, Nature, 2020, 587, 594–599 CrossRef CAS PubMed.
- E. M. Gholizadeh, S. K. K. Prasad, Z. L. Teh, T. Ishwara, S. Norman, A. J. Petty, J. H. Cole, S. Cheong, R. D. Tilley, J. E. Anthony, S. Huang and T. W. Schmidt, Nat. Photonics, 2020, 14, 585–590 CrossRef CAS.
- Z. Huang, Z. Xu, M. Mahboub, Z. Liang, P. Jaimes, P. Xia, K. R. Graham, M. L. Tang and T. Lian, J. Am. Chem. Soc., 2019, 141, 9769–9772 CrossRef CAS PubMed.
- R. Lai, Y. Sang, Y. Zhao and K. Wu, J. Am. Chem. Soc., 2020, 142, 19825–19829 CrossRef CAS.
- L. Huang, T. Le, K. Huang and G. Han, Nat. Commun., 2021, 12, 1898 CrossRef.
- C. Tamames-Tabar, D. Cunha, E. Imbuluzqueta, F. Ragon, C. Serre, M. J. Blanco-Prieto and P. Horcajada, J. Mater. Chem. B, 2014, 2, 262–271 RSC.
- S. E. Seo, H. S. Choe, H. Cho, H. I. Kim, J. H. Kim and O. S. Kwon, J. Mater. Chem. C, 2022, 10, 4483–4496 RSC.
- X. Yin, W. Xu, G. Zhu, Y. Ji, Q. Xiao, X. Dong, M. He, B. Cao, N. Zhou, X. Luo, L. Guo and B. Dong, Nat. Commun., 2022, 13, 6549 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |