Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unveiling the energetic potential of azahomocubane (AHC): a new class of potential propellants, explosives and oxidizers†‡
Sohan
Lal
 a,
Haixiang
Gao
a,
Haixiang
Gao
 b and
Jean'ne M.
Shreeve
b and
Jean'ne M.
Shreeve
 *a
*a
aDepartment of Chemistry, University of Idaho, Moscow, Idaho 83844-2343, USA. E-mail: jshreeve@uidaho.edu; Fax: +1-208-885-6173
bDepartment of Applied Chemistry, China Agricultural University, 100193, Beijing, China
First published on 30th January 2025
Abstract
Cage compounds are potential kinetic rocks and thermodynamic powerhouses. Their strain energy plays a crucial role. Hence, adamantane, cubane, homocubanes, and bishomocubane skeletons have become prominent recently. However, research on the design and development of azahomocubane-based energetic materials has yet to be explored. The aim of the present work is to illustrate the potential of azahomocubanes as next-generation propellants, explosives and oxidizers. The energetic potential of any new materials was determined using B3LYP/6-31+G**, G2, and MP2/6-311++G** levels at the Gaussian 03 suite of programs. The new azahomocubanes possess a density range of 1.33 g cm−3 to 2.14 g cm−3. Most of the azahomocubanes have significantly elevated high-positive heats of formation (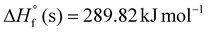 to 728.41 kJ mol−1). Compounds AHC-12–19 have superior potentials as solid propellants in rocket propulsion. Additionally, this study reveals that compounds AHC-20 and AHC-21 could be highly effective primary explosives (AHC-20, P = 44.46 GPa, D = 9706 m s−1; AHC-21, P = 45.64 GPa, D = 9708 m s−1) exceeding the performance of RDX, HMX and comparable to that of ONC and CL-20. Our finding suggests that azahomocubanes have great potential in the field of energetic materials.
to 728.41 kJ mol−1). Compounds AHC-12–19 have superior potentials as solid propellants in rocket propulsion. Additionally, this study reveals that compounds AHC-20 and AHC-21 could be highly effective primary explosives (AHC-20, P = 44.46 GPa, D = 9706 m s−1; AHC-21, P = 45.64 GPa, D = 9708 m s−1) exceeding the performance of RDX, HMX and comparable to that of ONC and CL-20. Our finding suggests that azahomocubanes have great potential in the field of energetic materials.
Introduction
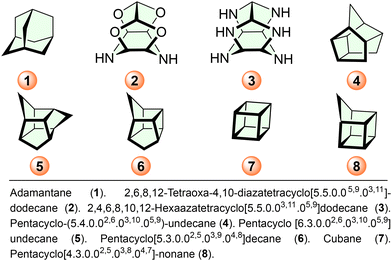
Homocubanes derived by the introduction of one methylene group (–CH2–) into the cubane core are homologues of cubane.1 Cubane became the most prevalent cage skeleton, which gained the scientific community's interest because of its potential applications in high energy density material (HEDM) and medicinal applications.2 In contrast, the synthesis, handling, and storage of cubane derivatives are problematic attributed to involving a multiple-step synthesis approach and production cost, and only a few data are available on such materials. The characteristic chemistry of these molecules, by their characteristic strain energy, has been extensively exploited recently.3,4 Strain with appropriate explosophoric groups, such as nitro (–NO2), nitrato (–ONO2), azido (–N3), and nitramine (–NHNO2), etc. yield novel potential high energy density materials.5 In the present era, computational-based design and prediction of emergent properties of new materials are prominent because these computational tools provide us with prospective properties before their actual synthesis, allowing us to design a new material cost-effectively.6 Recent reports on the synthesis and development of polycyclic cage compounds based on energetic material show that these materials have the potential to become future-generation fuel/propellants in solid rock propulsion.7 Very few reports also suggest that poly nitro-stained cage compounds possess superior explosive properties, which enhance their applications in civil, military, and space applications.7,8 Cage skeletons 1–7 based novel compounds are demonstrating prominence in their various applications. However, the homocubane core 8 despite its first synthesis in 1968, has not yet received comparable recognition in the scientific community (Schemes 1 and 2).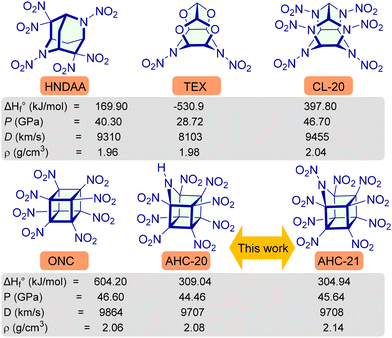 | ||
| Scheme 2 Highly dense top-performance energetic cage compounds.7,8 | ||
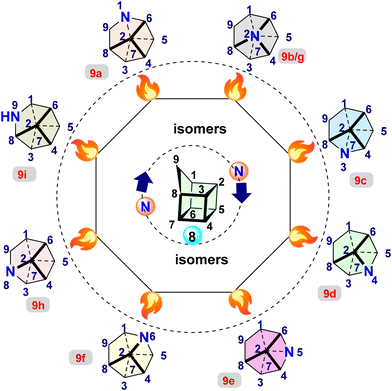
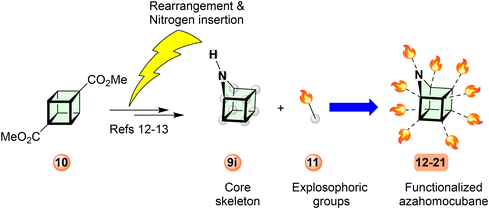
A homocubane was synthesized by Dunn et al.9 and Paquette et al.10 Subsequently, various research groups reported notable homocubanes derivatives.1,2,10,11 In contrast, an azahomocubane (AHC) is a relatively new compound synthesized by Eaton et al. by acid-catalyzed rearrangement of cubyl azides, giving 1-substituted 9-azahomocubanes.12 This transformation inspired Williams and co-workers to synthesize and characterize novel azahomocubane derivatives using dimethyl 1,4-cubanedicarboxylate 10.13 Subsequently, a new derivative of AHC, 1-azahomocubane, was reported.14 These recent reports demonstrate the feasibility of introducing nitrogen atoms into the homocubane skeleton. Based on the position of nitrogen, nine theoretical isomers are possible (Fig. 1).
Recent studies suggest that a secondary amine (9i, 9-azahomocubane) bearing isomer was the most stable skeleton compared to the other isomers with a tertiary amine.15 In contrast, 9-azahomocubane (9i, 9-AHC), rarely explored in the field of energetic materials, is a promising scaffold from the stereochemical perspective because it is effectively functionalized through the secondary amine moiety and the most stable skeleton over the other isomers, which allows the design of various novel energetic materials identified as an optimal cage skeleton for the present work (Scheme 3).
Computational method
The Density Functional Theory (DFT) based geometric optimization and frequency analyses of the new materials were determined using B3LYP/6-31+G** level, and single energy points were calculated at the MP2/6-311++G** level at Gaussian 03 (Revision D.01) suite of programs.16 The heats of formation for all ten compounds were obtained using an atomization approach using the G2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17ab initio method and the isodesmic method (Fig. S2, Tables S1 and S2‡). Furthermore, solid-state heats of formation of the new materials were calculated using gas-phase enthalpy and enthalpy of sublimation using eqn (S11) and (S12).‡
17ab initio method and the isodesmic method (Fig. S2, Tables S1 and S2‡). Furthermore, solid-state heats of formation of the new materials were calculated using gas-phase enthalpy and enthalpy of sublimation using eqn (S11) and (S12).‡
The densities of the title compounds were calculated using a newly developed modified Pulitzer method.18 Subsequently, based on their solid phase enthalpies of formation 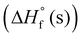 and calculated densities (Table 1), the corresponding propulsive (Isp and C*) and detonation performances (P and D) were evaluated using EXPLO5 V7.01 software.19
and calculated densities (Table 1), the corresponding propulsive (Isp and C*) and detonation performances (P and D) were evaluated using EXPLO5 V7.01 software.19
| Compound | Formulaa | FWb [g mol−1] | N + Oc [%] | OBCO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (%) d (%) |
ΔHfse [kJ mol−1] | ρ [g cm−3] | P [GPa] | D [m s−1] | h 50 [cm] |
|---|---|---|---|---|---|---|---|---|---|
| a Molecular formula. b Formula weight. c N + O contents in %. d CO2 based oxygen balance. e Calculated solid-phase standard enthalpy of formation. f Density calculated using the correlation of ρ = 1.0330 (M/V) + 0.001836 (σtot2ν) − (ν/6).18 g Detonation pressure calculated using EXPLO5 V 7.01. h Detonation velocity calculated using EXPLO5 V 7.01. i h 50 [cm] is the height from where 50% probability of the dropped materials resulted in an explosion calculated using equation S13. Then their corresponding impact energies h50[J] were calculated using a correlation h50[J] = mgh, where, m = 2.5 kg hammer weight, g = 9.81 m/s, and h = h50 [cm] values. | |||||||||
| AHC-12 | C8H9N | 119.16 | 11.76 | −275.25 | 390.22 | 1.33 | 12.52 | 5670.94 | 27.07 |
| AHC-13 | C8H8N2O2 | 164.16 | 36.55 | −175.44 | 402.36 | 1.53 | 15.88 | 6297.39 | 21.96 |
| AHC-14 | C8H9N3O2 | 179.18 | 41.31 | −165.20 | 454.36 | 1.53 | 15.66 | 6593.09 | 44.66 |
| AHC-15 | C9H10N2O2 | 178.19 | 33.68 | −188.57 | 356.02 | 1.47 | 14.67 | 6155.14 | 42.50 |
| AHC-16 | C9H10N2O3 | 194.19 | 39.15 | −164.79 | 289.82 | 1.51 | 15.57 | 6278.67 | 45.26 |
| AHC-17 | C9H10N4 | 174.21 | 32.16 | −211.25 | 728.41 | 1.40 | 14.41 | 6259.27 | 41.02 |
| AHC-18 | C9H8N4O6 | 268.19 | 56.68 | −95.46 | 345.45 | 1.71 | 21.40 | 7254.70 | 56.70 |
| AHC-19 | C10H10N4O6 | 282.21 | 53.86 | −107.72 | 322.45 | 1.66 | 19.20 | 7009.23 | 51.22 |
| AHC-20 | C8HN9O16 | 479.14 | 79.74 | 25.04 | 309.04 | 2.08 | 44.46 | 9706.85 | 6.96 |
| AHC-21 | C8N10O18 | 524.14 | 81.66 | 30.53 | 304.94 | 2.14 | 45.64 | 9708.21 | 6.98 |
| Cubane | C8H8 | 104.15 | 0.0 | −307.69 | 602.64 | 1.29 | 12.70 | 5990.00 | 24.14 |
| TNT | C7H5N3O6 | 227.13 | 60.76 | −73.96 | −59.30 | 1.65 | 18.56 | 6839.96 | 46.79 |
| RDX | C3H6N6O6 | 222.12 | 81.06 | −21.61 | 70.30 | 1.80 | 34.01 | 8858.00 | 26.14 |
| HMX | C4H8N8O8 | 296.16 | 81.06 | −21.61 | 74.80 | 1.91 | 38.44 | 9285.00 | 27.58 |
| CL-20 | C6H6N12O12 | 438.18 | 82.17 | −10.95 | 397.80 | 2.04 | 46.70 | 9455.00 | 9.25 |
| ONC | C8N8O16 | 464.13 | 79.29 | 0.0 | 604.20 | 2.06 | 46.60 | 9864.00 | 2.86 |
Additionally, the stability parameters of the new compounds, such as impact sensitivity (h50) (eqn (S13)‡), kinetic energy (ΔEHOMO–LUMO), and electrostatic potential (ESP) were predicted at the B3LYP/6-311++G (d,p) level of theory with the help of Multiwfn20 and VMD software21
Results and discussion
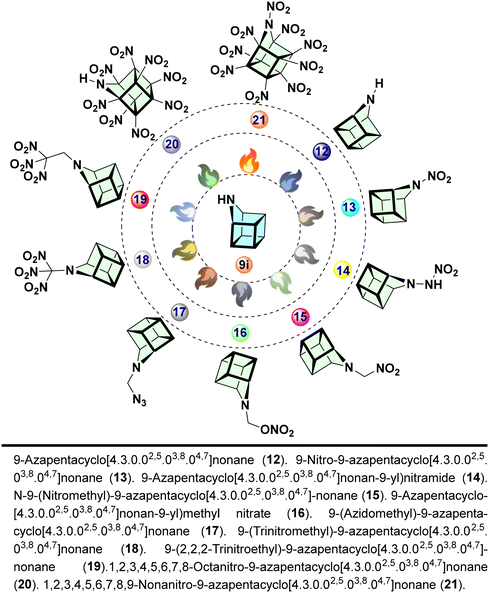
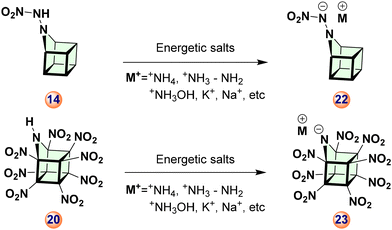
A proposed synthetic strategy for compounds AHC-12–21 is described in Scheme 3. Initially, 9-azahomocubane, 9i, can be prepared from compound 10 efficiently. Subsequently, using a multi-steps strategy, various explosophoric groups can be introduced. Azahomocubane (9i-AHC) with secondary amine (R2NH) enables us to introduce various functionalities by nucleophilic substitution/displacement of an N-proton. Based on the feasibility of the synthesis, we have designed ten derivatives of 9-AHC by introducing the most promising explosophoric groups, as listed in Fig. 2.Interestingly, derivatives AHC-14 and AHC-20 have vast potentials to generate energetic salts AHC-22–23, and that could be the future generation green solid propellants, explosives, and oxidizers (Scheme 4).
The computationally based design and studies of new high energy density materials (HEDMs) reveals the potential of new materials as an explosive and propellent in military and space industry applications. Thus, the efficiency of potential explosives can be measured in terms of their detonation performance (P and D), an oxidizer can be identified based on the combination of available oxygen for combustion, which is typically quantified in terms of oxygen balance (OB%) and specific impulse (Isp). High specific impulse (Isp) and density specific impulse (Isp) of a material, suggest their potential use as a propellant. In the present study, we evaluated all materials as solid materials, and their explosive and propulsive performances were calculated with different propellant formulations with hydroxylterminated polybutadiene (HTPB, a novel energetic binder), ammonium perchlorate (AP, oxidizer), and aluminium (Al, fuel supplement). Subsequently, their performance was compared with well-known energetic materials as listed in Tables 1 and 2.
| Compound | ΔEa,i [HOMO–LUMO] | Ispb,i [s] | ρIspc,i [s] | C*![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d,i [m s−1] d,i [m s−1] |
Ispe,i [s] | Ispf,i [s] | Ispg,i [s] | Isph,i [s] |
|---|---|---|---|---|---|---|---|---|
| a Energy difference between HOMO and LUMO orbitals in eV. b Isp = specific impulse of neat compound (monopropellant). c ρIsp = density specific impulse of neat compound (monopropellant). d Characteristic velocity. e Isp = specific impulse at 88% compound and 12% Al. f Isp = specific impulse at 78% compound, 12% Al (fuel additive) and 10% binder (HTPB). g Isp = specific impulse at 20% compound and 80% AP. h Isp = specific impulse at 80% compound and 20% HTPB. i Specific impulse calculated at an isobaric pressure of 70 bar and initial temperature of 3300 K using EXPLO5 V 7.01. | ||||||||
| AHC-12 | 6.080 | 167.55 | 223.52 | 990.40 | 178.30 | 173.79 | 246.08 | 158.48 |
| AHC-13 | 5.825 | 202.64 | 311.66 | 1226.70 | 267.98 | 248.30 | 258.38 | 185.10 |
| AHC-14 | 5.446 | 207.17 | 318.63 | 1257.60 | 270.04 | 253.04 | 258.98 | 188.72 |
| AHC-15 | 4.733 | 191.82 | 283.52 | 1155.60 | 242.20 | 226.34 | 256.28 | 177.16 |
| AHC-16 | 4.869 | 199.36 | 301.63 | 1208.50 | 223.30 | 227.87 | 257.79 | 182.92 |
| AHC-17 | 5.372 | 194.68 | 273.53 | 1166.10 | 203.03 | 195.66 | 255.71 | 179.49 |
| AHC-18 | 4.326 | 227.86 | 390.78 | 1415.80 | 240.91 | 232.06 | 243.45 | 201.99 |
| AHC-19 | 3.432 | 219.73 | 365.41 | 1358.50 | 236.95 | 229.91 | 247.79 | 196.94 |
| AHC-20 | 5.183 | 263.02 | 548.40 | 1563.40 | 266.40 | 267.44 | 194.66 | 232.91 |
| AHC-21 | 5.331 | 256.49 | 549.40 | 1525.40 | 261.51 | 273.27 | 189.49 | 237.40 |
| Cubane | 7.046 | 114.30 | 147.45 | 667.40 | 105.44 | 106.87 | 231.53 | 108.99 |
| TNT | 4.926 | 206.49 | 341.54 | 1283.5 | 232.00 | 225.11 | 186.98 | 200.55 |
| RDX | 5.991 | 266.91 | 480.45 | 1648.00 | 276.68 | 274.08 | 254.06 | 234.12 |
| AP | — | 156.63 | 306.15 | 977.00 | 232.00 | 262.11 | 156.63 | 227.37 |
Newly designed azahomocubanes AHC-12–21 possess high calculated densities (ρ = 1.33 g cm−3 to 2.14 g cm−3), associated with significantly positive solid-phase enthalpy of formation (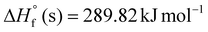 to 728.41 kJ mol−1), those are comparable to top performing energetic materials as listed in Table 1, which establish them as viable candidatesin the field of energetic materials. Compounds AHC-20–21 have high positive oxygen balances (AHC-20, OB% = 25.04 and AHC-21, OB% = 30.53) comparable to well-known oxidizers such as ammonium dinitramide (ADN, OB% = 25.80), ammonium perchlorate (AP, OB% = 27.23), 2,2,2-tetranitroacetimidic acid (TNAA, OB% = 30.12).
to 728.41 kJ mol−1), those are comparable to top performing energetic materials as listed in Table 1, which establish them as viable candidatesin the field of energetic materials. Compounds AHC-20–21 have high positive oxygen balances (AHC-20, OB% = 25.04 and AHC-21, OB% = 30.53) comparable to well-known oxidizers such as ammonium dinitramide (ADN, OB% = 25.80), ammonium perchlorate (AP, OB% = 27.23), 2,2,2-tetranitroacetimidic acid (TNAA, OB% = 30.12).
The detonation performance of azahomocubane was calculated using EXPLO5 V7.01 software as listed in Table 1. Compounds AHC-12–19 have an average detonation performance (P = 12.52 GPa to 21.40 GPa; D = 5670 m s−1 to 7254 m s−1) and which are comparable to cubane (P = 12.70 GPa; D = 5990 m s−1), and TNT (P = 18.56 GPa; D = 6839 m s−1). On the other hand, compound AHC-20–21 have detonation properties (AHC-20, P = 44.46 GPa, D = 9706 m s−1; AHC-21, P = 45.64 GPa, D = 9708 m s−1) which are better than those of top performing primary explosives RDX and HMX and comparable to ONC and CL-20.
The propulsive performance of azahomocubanes were predicted using EXPLO5 V7.01 software at five different propellent formulations and was examined (i) as a neat compound; (ii) at the ratio of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (azahomocubanes
12 (azahomocubanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al); (iii) at the ratio of 78
Al); (iii) at the ratio of 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (azahomocubanes
10 (azahomocubanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HTPB); (iv) at the ratio of 20
HTPB); (iv) at the ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 (azahomocubanes
80 (azahomocubanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AP); and (v) at the ratio of 80
AP); and (v) at the ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (azahomocubanes
20 (azahomocubanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HTPB) listed in Table 2 and Fig. 3. This indicates that the specific impulse (Isp, s) and density specific impulse (ρIsp, g cm−3 s) of compounds AHC-20 and AHC-21 are significantly higher than TNT, RDX and AP. On the other hand, most of the compounds possess high characteristic velocity, making them promising candidates for solid rocket propulsion. Interestingly, compounds AHC-13 and AHC-14 at the ratio of 88
HTPB) listed in Table 2 and Fig. 3. This indicates that the specific impulse (Isp, s) and density specific impulse (ρIsp, g cm−3 s) of compounds AHC-20 and AHC-21 are significantly higher than TNT, RDX and AP. On the other hand, most of the compounds possess high characteristic velocity, making them promising candidates for solid rocket propulsion. Interestingly, compounds AHC-13 and AHC-14 at the ratio of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (azahomocubanes
12 (azahomocubanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al) exhibit optimal specific impulse (AHC-13, Isp = 268 s; AHC-14, Isp = 270 s), revealing the best combination of strain (azahomocubanes core) and energy (–N–NO2 and –N–NH–NO2).
Al) exhibit optimal specific impulse (AHC-13, Isp = 268 s; AHC-14, Isp = 270 s), revealing the best combination of strain (azahomocubanes core) and energy (–N–NO2 and –N–NH–NO2).
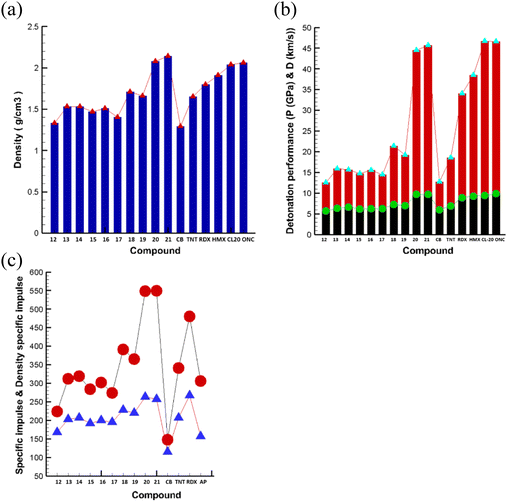 | ||
| Fig. 3 Comparison of physicochemical properties. (a) Densities (b) Detonation properties. (c) Propulsive properties. | ||
As for the kinetic stability, the energy gaps between HOMO and LUMO were estimated and are in the order of CB > AHC-12 > RDX > AHC-13 > AHC-14 > AHC-17 > AHC-21 > AHC-20 > TNT > AHC-16 > AHC-15 > AHC-18 > AHC-19, as listed in Table 2. Interestingly, the impact sensitivity of 20 and 21 is significantly higher (h50 (cm) = 6.96 and 6.98 respectively) compared to the impact sensitivity of RDX (26.14), HMX (27.58) and lower than that of ONC (2.86), making them promising primary explosive.
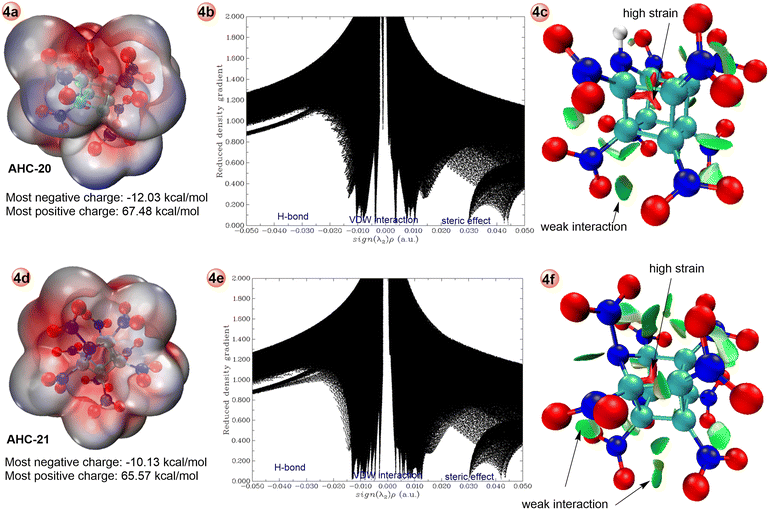
Additional analysis of stabilities of title compounds were evaluated with the help of electrostatic potential (ESP) measurements as shown in Fig. 4 and Fig. S14–S23.‡ Compounds 20 and 21 have comparable ESP maxima (+67.48 kcal mol−1 and +65.57 kcal mol−1, respectively) and global ESP minima (−12.03 kcal mol−1 and −10.13 kcal mol−1, respectively) to CL-20 (+66.61 kcal mol−1 and −15.74 kcal mol−1). For RDX (+50.01 kcal mol−1 and −20.81 kcal mol−1), and HMX (+53.74 kcal mol−1 and −24.80 kcal mol−1) see Fig. S3.‡ Furthermore, using scatter diagram and reduced density gradient (RDG), the high strain in the core structure and weak interaction between the substituents can be explained as shown in the Fig. 4b, 4c, 4d, 4e and Fig. S14–S23.‡
Conclusions
This study identified azahomocubanes as a new class of potential propellants and oxidizers. The densities were predicted to be 2.08 g cm−3 to 2.14 g cm−3 for compound AHC-20 and AHC-21, respectively, which are significantly higher than those of top energetic materials. In addition, the title compounds exhibit superior detonation performances with good propulsive performances and higher sensitivities than RDX, HMX, and CL-20, which suggests that AHC-20 and AHC-21 have a potential comparable to RDX, HMX and CL-20 for civil and military applications. Additionally, these azahomocubanes demonstrate significant potential as solid propellants in the form of their energetic salts 22 and 23.Author contributions
S. L. investigation, methodology, conceptualization and manuscript writing. H. G. manuscript writing – review and editing. J. M. S. conceptualization, manuscript writing – review and editing, supervision.Data availability
All data relevant to the work described here are available in the ESI‡ or from the current literature.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We are grateful for the support of the Fluorine-19 fund.References
- A. P. Marchand, Chem. Rev., 1989, 89, 1011–1033 CrossRef CAS.
- (a) K. F. Biegasiewicz, J. R. Griffiths, G. P. Savage, J. Tsanaktsidis and R. Priefer, Chem. Rev., 2015, 115, 6719–6745 CrossRef CAS PubMed; (b) T. A. Reekie, C. M. Williams, L. M. Rendina and M. Kassiou, J. Med. Chem., 2019, 62, 1078–1095 CrossRef CAS PubMed.
- (a) S. Lal, A. Bhattacharjee, A. Chowdhury, N. Kumbhakarna and I. N. N. Namboothiri, Chem. – Asian J., 2022, 17, e202200489 CrossRef CAS PubMed; (b) A. Bartonek, T. M. Klapötke and B. Krumm, Inorg. Chem., 2024, 63, 20870–20877 CrossRef CAS PubMed; (c) M. P. Wiesenfeldt, J. A. Rossi-Ashton, I. B. Perry, J. Diesel, O. L. Garry, F. Bartels, S. C. Coote, X. Ma, C. S. Yeung, D. J. Bennett and D. W. C. MacMillan, Nature, 2023, 618, 513–518 CrossRef CAS PubMed.
- (a) N. Kazi, M. C. Aublette, S. L. Allinson and S. C. Coote, Chem. Commun., 2023, 59, 7971–7973 RSC; (b) A. De Meijere, S. Redlich, D. Frank, J. Magull, A. Hofmeister, H. Menzel, B. König and J. Svoboda, Angew. Chem., Int. Ed., 2007, 46, 4574–4576 CrossRef CAS PubMed.
- (a) N. V. Muravyev, L. Fershtat and Q. Zhang, Chem. Eng. J., 2024, 486, 150410 CrossRef CAS; (b) D. M. Badgujar, M. B. Talawar, S. N. Asthana and P. P. Mahulikar, J. Hazard. Mater., 2008, 151, 289–305 CrossRef CAS PubMed; (c) S. Lal, L. Mallick, S. Rajkumar, O. P. Oommen, S. Reshmi, N. Kumbhakarna, A. Chowdhury and I. N. N. Namboothiri, J. Mater. Chem. A, 2015, 3, 22118–22128 RSC; (d) S. Lal, R. J. Staples and J. M. Shreeve, Chem. Eng. J., 2023, 468, 143737 CrossRef CAS.
- (a) J. Zhou, J. Zhang, B. Wang, L. Qiu, R. Xu and A. B. Sheremetev, FirePhysChem, 2022, 2, 83–139 CrossRef; (b) S. Lal, H. Gao and J. M. Shreeve, New J. Chem., 2022, 46, 16693–16701 RSC.
- (a) J. V. Viswanath, K. J. Venugopal, N. V. S. Rao and A. Venkataraman, Def. Technol., 2016, 12, 401–418 CrossRef; (b) M.-X. Zhang, P. E. Eaton and R. Gilardi, Angew. Chem., Int. Ed., 2000, 39, 401–404 CrossRef CAS; (c) P. E. Eaton, Angew. Chem., Int. Ed., 1992, 31, 1421–1436 CrossRef.
- (a) J. Zhang, T. Hou, L. Zhang and J. Luo, Org. Lett., 2018, 20, 7172–7176 CrossRef CAS PubMed; (b) E. C. Koch, Propellants, Explos., Pyrotech., 2015, 40, 374–387 CrossRef CAS.
- G. L. Dunn, V. J. DiPasquo and J. R. E. Hoover, J. Org. Chem., 1968, 33, 1454–1459 CrossRef CAS.
- L. A. Paquette and J. S. Ward, J. Org. Chem., 1972, 37, 3569 CrossRef CAS.
- (a) S. Lal, A. Chowdhury, N. Kumbhakarna, S. Nandagopal, A. Kumar and I. N. N. Namboothiri, Org. Chem. Front., 2021, 8, 531–548 RSC; (b) H. Takebe and S. Matsubara, Chem. – Eur. J., 2024, 30, e202303063 CrossRef CAS PubMed.
- P. E. Eaton, A. M. Fisher and R. E. Hormann, Synlett, 1990, 737–738 CrossRef CAS.
- T. Fahrenhorst-Jones, P. V. Bernhardt, G. P. Savage and C. M. Williams, Org. Lett., 2022, 24, 903–906 CrossRef CAS PubMed.
- T. Fahrenhorst-Jones, D. L. Marshall, J. M. Burns, G. K. Pierens, R. E. Hormann, A. M. Fisher, P. V. Bernhardt, S. J. Blanksby, G. P. Savage, P. E. Eaton and C. M. Williams, Chem. Sci., 2023, 14, 2821–2825 RSC.
- M. A. Fernández-Herrera, J. Barroso-Flores and G. Merino, RSC Adv., 2023, 13, 27672–27675 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. L. Caricato, X. H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Revision D.01 ed, Gaussian Inc.Wallingford CT, 2003 Search PubMed.
- (a) L. A. Curtiss, K. Raghavachari and J. A. Pople, J. Chem. Phys., 1993, 98, 1293–1298 CrossRef CAS; (b) L. A. Curtiss, K. Raghavachari, G. W. Trucks and J. A. Pople, J. Chem. Phys., 1991, 94, 7221–7230 CrossRef CAS.
- S. Lal, H. Gao and J. M. Shreeve, New J. Chem., 2024, 48, 17947–17952 RSC.
- EXPLO5; OZM Research s.r.o.: Hro̊chuv Tÿnec, Czech Republic. https://www.ozm.cz/explosives-performance-tests/thermochemi-cal-computer-code-explo5/.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Professor Eaton for his pioneer work in cubane chemistry. |
| ‡ Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt03457k |
| This journal is © The Royal Society of Chemistry 2025 |