Suppressed surface lattice vacancies and distortion through lattice anchoring for efficient FAPbI3 perovskite quantum dot solar cells†
Mingxu
Zhang
,
Xinyi
Mei
,
Guoliang
Wang
,
Junming
Qiu
,
Zhimei
Sun
 * and
Xiaoliang
Zhang
* and
Xiaoliang
Zhang
 *
*
School of Materials Science and Engineering, Beihang University, Beijing 100191, China. E-mail: zmsun@buaa.edu.cn; xiaoliang.zhang@buaa.edu.cn
First published on 12th November 2024
Abstract
Formamidinium lead triiodide perovskite quantum dots (FAPbI3 PQDs) exhibit outstanding optoelectronic characteristics for new-generation solar cells. However, PQDs seriously suffer from surface lattice vacancies and lattice distortion, resulting in serious energy losses and low operational stability of PQD solar cells (PQDSCs). Herein, a feasible surface lattice anchoring (SLA) strategy is reported to stabilize the surface lattice of PQDs using the multifunctional molecule tetrafluoroborate methylammonium (FABF4) for efficient solar cells. The results revealed that the FABF4 molecule could effectively occupy the surface lattice vacancies and partly substitute the oleylamine and oleic acid ligands at the PQD surface, which benefits the charge carrier transport in PQD solids with lowered energy losses induced by the trap-assisted nonradiative recombination. Meanwhile, the BF4− anion could also stabilize the surface lattice of PQDs to substantially ameliorate the surface lattice distortion of PQDs, leading to an improved crystal stability of PQDs. Consequently, the PQDSCs constructed using the SLA-PQDs show a high efficiency of up to 17.06%, which is the highest efficiency of FAPbI3 PQDSCs. This work provides important insights into the surface lattice modulation of PQDs for high-performance PQD optoelectronic devices.
Broader contextFormamidinium lead triiodide perovskite quantum dots (FAPbI3 PQDs) demonstrate outstanding optoelectronic properties including solution processability, high defect tolerance and good phase stability, which are beneficial for next-generation solar cells. Nonetheless, PQDs seriously suffer from surface lattice vacancies and lattice distortions during their purification, resulting in considerable energy losses and poor operational stability of PQD solar cells (PQDSCs). In this work, a feasible surface lattice anchoring strategy is reported, which could effectively repair the lattice vacancies and partially replace the long-chain oleic acid/oleylamine ligands at the surface of PQDs, substantially diminishing trap-assisted nonradiative recombination and facilitating charge carrier transport in the PQD solid films. Meanwhile, the stabilized surface lattice and alleviation of lattice distortion in PQDs contribute to the improved crystal phase stability of PQDs and good stability of PQDSCs. As a consequence, a power conversion efficiency of up to 17.06% was obtained in FAPbI3 PQDSCs, the highest efficiency of FAPbI3 PQDSCs. This work delineates the critical design criteria for the modulation of surface lattice in PQDs and offers a practical avenue for the construction of high-performing photovoltaic devices or other optoelectronic devices. |
1. Introduction
Compared with the conventional metal chalcogenide colloidal quantum dots (CQDs),1,2 metal halide perovskite quantum dots (PQDs) have attracted increasing scientific interest due to their exceptional optoelectronic characteristics, such as high defect tolerance,3,4 high photoluminescence quantum yields (PLQYs),5,6 and prolonged charge carrier lifetimes.7,8 Additionally, the ligand-dominated surface chemistry,9,10 size-dependent spectroscopic properties,11,12 and solution processability of PQDs13 make them highly promising optoelectronic materials for advancing new-generation solar cells,14 light-emitting diodes (LEDs),15–17 and photodetectors.18,19 Specifically, since the first inorganic CsPbI3 PQD solar cell (PQDSC) with a remarkable power conversion efficiency (PCE) of 10.77% was reported by Swarnkar et al. in 2016,20 tremendous progress has been attained in the photovoltaic performance and operational stability of PQDSCs, which were largely improved through the surface chemistry regulation of PQDs and device structure studies of PQDSCs.21 Very recently, the CsPbI3 PQDSC with a high efficiency of 16.64% was realized, which shows high potential for the development of next-generation photovoltaics.22Compared with the inorganic CsPbI3 PQDs, formamidinium lead triiodide (FAPbI3) PQD possesses an ideal Goldschmidt tolerance factor (∼1.03),23,24 favorable bandgap energy (Eg = ∼1.55 eV)25 and accelerated large polaron formation,26 which benefit the phase stability and near-infrared light absorption of the PQDs for the development of more stable and efficient solar cells.27,28 During the synthesis of FAPbI3 PQDs, the long alkyl chain oleylamine (OAm) and oleic acid (OA) were widely employed to regulate the growth of PQDs and maintain their good colloidal stability in non-polar solvents.29 However, the ionic bonding characteristic of halide perovskites and the relatively weak bonding of organic formamidinium cations (FA+) with the corner-sharing [PbI6]4− octahedral make FAPbI3 PQDs very sensitive to antisolvents, such as methyl acetate (MeOAC), ethyl acetate (EtOAC) and isopropanol (IPA).30,31 Especially, during the purification of the PQDs using antisolvents, part of the OAm/OA ligands are washed away from the PQD surface, which triggers the formation of the formamidinium cation (FA+)/iodide anion (I−) vacancies (VFA/VI) at the surface lattice of PQDs, leading to serious trap-assisted nonradiative recombination.32,33 Meanwhile, the VFA/VI at the surface lattice of PQDs also induced serious surface lattice distortions, which predominantly affect the crystal stability of the PQDs against moisture and oxygen erosion, ineluctably leading to phase transition or even the degradation of PQDs.22,34
To overcome the above issues, different feasible methods have been developed to restore the VFA/VI at the surface lattice of FAPbI3 PQDs. For instance, Ma et al. reported a novel surface reconfiguration methodology to modulate the surface chemistry of PQDs and manipulate the electronic coupling of PQDs, which largely enhanced the charge carrier transport in the PQD arrays, and subsequently, an efficiency of 14.47% was achieved in PQDSCs.32 Additionally, Zhang et al. reported a facile phase-transfer catalysis approach to fill the VFA/VI to suppress the formation of the lattice vacancies at the PQD surface and thus improve the optoelectronic properties and stability of PQDs, resulting in PQDSCs with a high efficiency of 16.29%.33 Through the above efforts, even though the photovoltaic performance of FAPbI3 PQDSCs has been largely improved, the efficiency of FAPbI3 PQDSCs still largely lags behind that of the PQDSCs constructed using other PQDs, such as CsPbI3 or FAxCs1−xPbI3 PQDs, with an efficiency of over 17%, which to a large extent results from serious energy losses within the FAPbI3 PQDSCs.35 Therefore, diminishing the energy losses of FAPbI3 PQDSCs induced by the surface lattice vacancies and lattice distortion is highly preferred to further improve the photovoltaic performance and operational stability of the PQDSCs. Meanwhile, a comprehensive understanding of the effects of the surface lattice on the optoelectronic properties and crystal stability of PQDs is of great importance to link the surface lattice of the PQDs with the device operation of PQDSCs, which will provide physical design principles for the surface lattice modulation of PQDs.
Herein, a facile surface lattice anchoring (SLA) strategy was reported to stabilize the surface lattice of PQDs, in which the functional molecule tetrafluoroborate methylammonium (FABF4) was applied as a lattice anchoring agent to stabilize the surface lattice of PQDs for efficient solar cells. Comprehensively, experimental studies and theoretical calculations were performed to fundamentally understand the surface lattice-dominated optoelectronic properties and crystal stability of PQDs. The results revealed that FABF4 could effectively occupy the surface lattice vacancies of the PQDs induced by the desorption of the OAm/OA ligands from the PQD surface, thus substantially diminishing the trap-assisted nonradiative-recombination of photoinduced charge carriers. Meanwhile, the FABF4 molecule could also partly substitute the OAm/OA ligands at the PQD surface, facilitating charge carrier transport in the PQD solids. Furthermore, the BF4− anion with a stable tetrahedral configuration can strongly bind with the uncoordinated Pb2+ cation through multi-active F atoms, which substantially restored the surface lattice distortion, leading to improved crystal stability of PQDs. As a consequence, compared with the control PQDSCs (14.52% efficiency), the photovoltaic performance of SLA-PQDSCs was largely improved and a high efficiency of up to 17.06% was obtained, which is the highest efficiency of FAPbI3 PQDSCs. Meanwhile, the stability of PQDSCs was also significantly enhanced due to the suppressed surface lattice distortion of PQDs. The improved performance in SLA-PQDSCs was attributed to the diminished surface lattice vacancies and stabilized surface lattices of PQDs, resulting in lowered energy losses in PQDSCs.
2. Results and discussion
Surface lattice anchoring and theoretical calculations
Fig. 1(a) schematically presents the SLA of FAPbI3 PQDs. The PQD with a size of ∼16.02 nm was synthesized using the conventional hot-injection method, which is described in the Experimental section of ESI.† Fig. S2 (ESI†) shows the light absorption and photoluminescence (PL) spectra of PQDs. 2-Octanol was used as an antisolvent to separate PQDs from the crude solution, during which partial OAm/OA ligands were detached from the PQD surface. The ionic bonding characteristic of perovskites and relatively weaker bonding between FA+ and the [PbI6]4− octahedral cage generally result in the formation of numerous VFA and VI at the surface lattice of PQDs. These vacancies inevitably lead to surface lattice stretching or distortion, which could become active sites for water or oxygen attack, causing the phase transition or even degradation of PQDs.36 Meanwhile, the remaining long-chain OAm/OA ligands significantly hinder charge carrier transfer between adjacent PQDs in the PQD solids. Therefore, in the second purification step, polar solvents are typically chosen to further remove part of the OAm/OA ligands from the PQD surface to facilitate charge carrier transport in the PQD solids.37To tackle the issues of surface lattice vacancies and lattice distortion, during the second purification of PQDs, FABF4 was used as a surface lattice anchoring agent for the PQD, which contains multifunctional active sites. The electrostatic potential distribution of FABF4 is illustrated in Fig. S3 (ESI†). The FA+ cation of FABF4 can fill the VFA at the surface lattice of the PQD formed due to the desorption of the OAm ligand from the PQD surface. The BF4− anion possessing spatial dimensions could effectively fill the VI at the surface lattice of the PQD induced by the desorption of the OA ligand, which can also restrain the surface lattice distortion caused by the VI. Additionally, the highly electronegative F atoms can strongly bind with the uncoordinated Pb2+ cation, firmly anchoring onto the PQD surface. It is worth noting that the hydrophobic F atoms may also prevent PQDs from water and oxygen erosion and thus enhance their environmental durability, which will be discussed in the next section.
First-principles density functional theory (DFT) calculations were first conducted to theoretically investigate the crucial role of the BF4− anion in stabilizing the surface lattice of PQDs.38,39 The surface of PQD is typically terminated by FA+ cations and I− anions.33 To investigate the influence of different ligands on the structures of the surface lattice, the (100) planes of the PQD surface with perfect surface lattice (P-PQD), I− vacancy (VI-PQD), VI filled by BF4− anion (VBF4-PQD) and VI filled by OA (VOA-PQD) were constructed. The front views of the optimized surface structures are displayed in Fig. 1(b)–(e), and the corresponding side views of these calculated surface structures are presented in Fig. S4 (ESI†). To evaluate the adsorption capability of these ligands on the PQD surface, the binding energies (Eb) of I−, BF4− and OA ligands on the surface of PQDs were also calculated. The calculated Eb of I− (EbI) and OA (EbOA) is 2.14 and 4.67 eV, respectively, which is much lower than that of the BF4− anion (EbBF4 = 7.26 eV), showing high potential to replace the I− anion and OA ligands by the BF4− anion at the PQD surface. Notably, BF4− anion forms two F–Pb bonds with the uncoordinated Pb2+ cation at the surface lattice of PQDs, which probably leads to a higher EbBF4 value.
As demonstrated above, these ligands may influence the surface lattice of PQDs, and therefore, the optimized PQDs surface configurations were analyzed in depth. For the P-PQD, the bond angle of I–Pb–I on the (100) crystal plane is 171.12°, which is decreased to 166.56° for the VI-PQD marked in Fig. 1(b) and (c), resulting in the surface lattice distortion of PQDs. After filling the VI using the BF4− anion, the bond angle of the I–Pb–I recovered to 170.31°, which is similar to that of P-PQD. It is notable that from the theoretical calculation, we also observed the surface lattice distortion in VOA-PQD with a bond angle of I–Pb–I of 160.52°, likely due to the large geometric configuration of the OA ligand. Therefore, the BF4− anion with a strong bonding with the PQD surface could ameliorate the surface lattice distortion caused by the VI, thereby improving the lattice stability of PQDs. The differential charge density maps can provide more insights into the interactions between these ligands and the PQD surface as well as details on charge transfer, which implies that the electron distribution is mainly concentrated around the BF4− anion with significant charge depletion around the Pb2+ cation and in the regions between the Pb2+ cation and BF4− anion (Fig. S5, ESI†). Additionally, the charge transfer between the BF4− anion and the surrounding four FA+ cations is also obvious, suggesting the interaction between the BF4− anion and FA+ cations, which may also inhibit the formation of VFA at the PQD surface. The Bader charge analysis presents a higher charge transfer of 0.59 electron from the PQD surface to the BF4− anion compared with that of the 0.49 electron from the PQD surface to the I− anion, which is favorable for enhancing the electronic coupling of PQDs and thus improves the charge transport between adjacent PQDs.
To fundamentally understand the impact of the ligands on the electronic structure of PQDs, the density of states (DOS) of the PQD anchored by different ligands was further calculated.40 The results show that compared with the P-PQD (Fig. 1(f)), shallow trap states emerged near the conduction band minimum (CBM) in the VI-PQD (Fig. 1(g)), which inevitably serve as nonradiative recombination centers for the photoinduced charge carriers and hinder charge transport in the PQD solids. However, after filling the VI using the BF4− anion, these unwanted trap states were effectively eliminated (Fig. 1(h)), thereby suppressing the trap-assisted nonradiative recombination. VOA-PQD also presents eliminated trap states in the PDOS curve (Fig. 1(i)), which could explain the VOA-PQD in the nonpolar solution, generally showing a high PLQY with diminished nonradiative recombination.41,42Fig. 1(j) and (k) show the 2D electron localization function (ELF) maps of VI- and VBF4-PQD, respectively, which could offer more insights into the mechanisms behind the generation and disappearance of these trap states.43 It is seen that the vacancy on the surface lattice of the VI-PQD results in non-bonding electrons on the uncoordinated Pb2+ cation, as visualized by the electron clouds in the ELF map (Fig. 1(j)), which may cause the formation of shallow trap states. However, after the introduction of the BF4− anion into the VI, the non-bonding electrons around the Pb2+ cation become highly localized around the F atoms, demonstrating the strong Coulombic interaction between Pb2+ and F atoms, which is responsible for the strong binding between the BF4− anion and PQD surface. This also results in the disappearance of defect states in the DOS diagram (Fig. 1(h)).
Therefore, based on the extensive theoretical calculations, FABF4 with multi-active sites can efficiently substitute long-chain OA ligands, thereby stabilizing the surface lattice of PQDs. The bonding between the BF4− anion and the uncoordinated Pb2+ cation at the PQD surface shows an ionic characteristic, which could eliminate shallow energy level defects stemming from the dangling bonds of Pb2+ cations, thus suppressing the nonradiative recombination of photogenerated charge carriers. Meanwhile, the BF4− anion can also repair the surface lattice distortion of PQDs caused by the VI, thereby enhancing the crystal stability of PQDs.
Characterization of PQDs
To verify the crucial role of the SLA strategy on the surface chemistry and optoelectric properties of PQDs, the PQD was systemically characterized. In our previous studies, was found that FAI can also effectively fill the VFA and VI at the PQD surface to improve the optoelectronic properties of PQDs.33 Therefore, FAI was also applied for the surface lattice engineering of PQDs for comparison, and the resulting PQD was named as the FAI-based PQD. The PQD without any SLA treatment was named as the control PQD and the PQD after the SLA treatment using the FABF4 was designated as FABF4-based PQD. Fig. 2(a) and Fig. S6 (ESI†) show the steady-state PL and light absorption spectra of the control, FAI- and FABF4-based PQDs, respectively. The light absorption edge and PL peak position of FAI-based PQDs both exhibit a slight red shift compared with the control and FABF4-based PQDs, which might be due to the weaker bonding of the I− anion to the PQD surface compared to OA and BF4− anions, leading to the fusion of PQDs. Among these samples, FABF4-based PQD demonstrates the highest PL emission intensity with the narrowest full width at half maximum (FWHM), which may result from the diminished surface defects and avoid the fusion of PQDs. The PLQYs of these PQDs were also tested, which shows that FABF4-based PQDs exhibit a PLQY value of 65.15%, largely enhanced than that of 45.42% and 46.17% for the control and FAI-based PQDs, respectively (Fig. 2(b)). Fig. S7 (ESI†) shows the effect of the FABF4 concentration on the PLQY of the PQDs, which reveals that the optimized concentration of the FABF4 for the SLA treatment of PQDs was 1 mg mL−1. The enhanced PLQY in FABF4-based PQDs could be attributed to the dangling bond passivation of the uncoordinated Pb2+ cation, thereby inhibiting nonradiative recombination induced by the surface lattice defects. However, when the concentration of FABF4 is excessively high, the equilibrium between ligand desorption and adsorption is disrupted, which could result in the formation of uncoordinated active sites on the PQD surface, consequently leading to the formation of numerous defects and thus lowering the optoelectronic properties of PQDs. To study the charge carrier dynamics induced by the surface lattice defects, the time-resolved photoluminescence spectra (TRPL) of these PQDs were recorded (Fig. 2(c)), and the fitted parameters are summarized in Table S1 (ESI†).44 After the SLA treatment using the FABF4, the average decay lifetime (τave) of PQDs was largely prolonged so that a τave of 38.65 ns was obtained in FABF4-based PQDs, whereas a τave of 17.42 and 23.76 ns was achieved for the control and FAI-based PQDs, respectively. Therefore, FAI can be also applied to effectively diminish the surface lattice defects of PQDs, thus lowering the nonradiative recombination. The significantly prolonged τave in FABF4-based PQDs can be assigned to the suppressed trap-assisted nonradiative recombination after repairing the surface lattice vacancies of the PQDs using the FABF4, which is in line with the steady-state PL spectra (Fig. 2(a)).To study the effect of the SLA treatment on the surface chemistry of PQDs, Fourier-transform infrared (FTIR) spectroscopy measurements of these PQDs were performed. As shown in Fig. 2(d), the vibrational bands associated with the –C–H bonds were observed at 2858 and 2925 cm−1, and the vibrational peak at 2923 cm−1 were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, originating from the OAm/OA ligands that remained on the PQD surface.45 Furthermore, the vibrational peak at 3160 cm−1 corresponds to the –NH3+ group, and the vibrational band encompassing the range of 3100–3480 cm−1 is attributed to the stretching vibrations of the –N–H bonds. It is worth noting that among these samples, the vibration peak intensity related to OA/OAm ligands is the lowest in the FABF4-based PQDs, and the N
C bonds, originating from the OAm/OA ligands that remained on the PQD surface.45 Furthermore, the vibrational peak at 3160 cm−1 corresponds to the –NH3+ group, and the vibrational band encompassing the range of 3100–3480 cm−1 is attributed to the stretching vibrations of the –N–H bonds. It is worth noting that among these samples, the vibration peak intensity related to OA/OAm ligands is the lowest in the FABF4-based PQDs, and the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration peak associated with the FA+ cations (located at 1710 cm−1) was the strongest, which suggests that the SLA strategy effectively facilitates the removal of OAm/OA ligands from the PQD surface, likely due to the strong binding affinity between the BF4− anion and the PQD surface.
C vibration peak associated with the FA+ cations (located at 1710 cm−1) was the strongest, which suggests that the SLA strategy effectively facilitates the removal of OAm/OA ligands from the PQD surface, likely due to the strong binding affinity between the BF4− anion and the PQD surface.
X-ray photoelectron spectroscopy (XPS) was further employed to gain more detailed information concerning the surface chemistry of the PQDs affected by the SLA treatment, and the full XPS spectra are presented in Fig. S8 (ESI†). The characteristic F 1s core level spectrum was clearly detected at 687.60 eV in FABF4-based PQDs, which could be evidence for the incorporation of BF4− anions onto the surface lattice of the PQDs during the SLA treatment (Fig. 2(e)). Additionally, the chemical stoichiometric ratio of X (X = I− or BF4−) to Pb in the PQDs was calculated to verify the crystal lattice anchoring effect of BF4− anions (Fig. 2(f)). For the control and FAI-treated PQDs, the chemical stoichiometric ratio of the X/Pb is for I and Pb, whereas for FABF4-based PQDs, X ions encompass both I− and BF4− anions (X = F/4 + I). The results show that compared to the control and FAI-treated PQDs, the X/Pb ratio of FABF4-based PQD was significantly increased, which could be attributed to construction of the I−/BF4−-rich surface lattice in FABF4-based PQDs, diminishing the VI at the surface lattice of PQDs.
The N 1s core level spectrum can be resolved into the protonated amine (–NH2+) peak of FA+ at 400.18 eV and the protonated amine (–NH3+) peak of OAm at 401.78 eV (Fig. 2(g)).46 Therefore, by comparing the relative areas of the –NH2+ and –NH3+ peaks, the relative content of OAm ligands to FA+ on the PQD surface (OAm/FA+) can be determined. Compared to the control and FAI-based PQDs, a slight shift of 0.4 eV of the –NH2+ peak towards higher binding energy was observed in FABF4-based PQDs, which may result from the interaction between the BF4− anion and FA+ cation, causing a decrease in the electron density around the N atoms. Meanwhile, the Pb 4f core level spectrum of FABF4-based PQDs was shifted by ∼0.07 eV towards higher binding energy compared to the control and FAI-based PQDs (Fig. 2(h)), which could be attributed to the strong ionic bonding between the highly electronegative F atom and Pb2+ cation, leading to a reduced charge density around the Pb2+ cation, as shown in the calculated results (Fig. 1(j) and (k)). As discussed in the above section, the desorption of OA ligands or I− anions from the PQD surface will lead to the formation of dangling bonds on the Pb2+ cation. Therefore, these uncoordinated Pb2+ cations were further analyzed, which manifest as low binding energy shoulder peaks near the main peak in the Pb 4f core level spectra, as shown in the filled color portions of Fig. 2(h). It can be seen that the feature signal of uncoordinated Pb2+ cations is substantially diminished in FABF4-based PQDs, indicating that most of the Pb2+ dangling bonds on the PQD surface were passivated by the BF4− anions. Meanwhile, after the SLA treatment, the I 3d core level shows a slight upward shift in the XPS spectrum (Fig. S9, ESI†), probably resulting from the introduction of BF4−. Therefore, these results verify that BF4− anions were successfully anchored on the PQD surface, and then I−/BF4− anions-rich surface lattice was constructed in FABF4-based PQDs, which could account for the improved optoelectronic properties of FABF4-based PQDs. Surface vacancies generally serve as active sites for water and oxygen attacks, and a reduction in the number of surface vacancies is beneficial for protecting PQDs from degradation due to water and oxygen erosion. As such, we conducted water contact angle tests on the PQD solid films (Fig. S10, ESI†). The water contact angles for the control and FAI-based PQDs were 82° and 95°, respectively. The hydrophobicity of the fluorine atoms and a significant reduction of surface vacancies led to an increased water contact angle of 102° for FABF4-based PQDs. Thus, compared to the control PQDs, the FABF4-based PQD demonstrated enhanced resistance to solvent erosion and thermal instability (Fig. S11 and S12, ESI†).
To investigate the effect of the SLA treatment on the crystal structures of PQDs, X-ray diffraction (XRD) characterization was carried out (Fig. 2(i)). The diffraction peaks at 14.28°, 28.46°, and 31.56° in the XRD patterns correspond to the (100), (200), and (210) crystal planes of α-phase FAPbI3 perovskites, respectively. FABF4-based PQDs exhibit a higher peak intensity corresponding to the (200) crystal plane, indicating that the SLA treatment may promote the crystallinity of PQDs. In addition, a distinct peak at 11.12° corresponding to the δ-phased FAPbI3 was detected in the control PQDs, whereas this signal was absent in the FABF4-based PQD. Since the δ-phased FAPbI3 is a non-photoactive material,47,48 the phase transition of the PQDs from the α-phase to the δ-phase could compromise the optoelectronic performance of PQDs, which will significantly deteriorate the performance of PQDSCs. Therefore, the SLA strategy can mitigate the surface lattice distortion of PQDs, leading to improved optoelectronic properties and phase stability.
Crystallographic identities of PQD solids
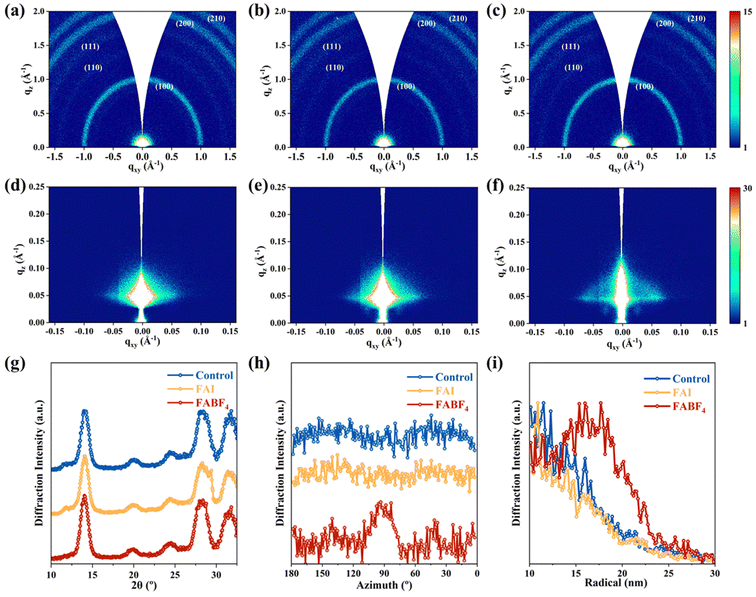
The above results demonstrated that the SLA strategy is beneficial for enhancing the optoelectronic properties and crystal stability of PQDs. However, the crystal orientation of PQDs and the PQD stacking in the PQD solids also play a critical role in charge carrier transport for efficient PQDSCs. Therefore, grazing-incidence wide-angle X-ray scattering (GIWAXS) and grazing-incidence small-angle X-ray scattering (GISAXS) measurements of the PQD solids constructed using different PQDs were carried out, which can be applied to qualitatively analyze the crystal lattice orientation of PQD solids.49Fig. 3(a)–(c) display the 2D GIWAXS patterns of the control, FAI- and FABF4-based PQD solids, respectively, in which the (100), (200), and (200) crystal planes of the perovskites with diffraction peaks at scattering vectors (q) of 1.00, 2.01, and 2.24 Å−1, respectively, can be clearly observed. For the control and FAI-based PQD solids, diffuse diffraction signals were observed close to the (100) crystal plane diffraction ring.50 To further investigate the crystal structure, the integrated 1D profiles of the GIWAXS patterns within the PQD solid films are compared in Fig. 3(g). It can be seen that the integrated curves of the control and FAI-based PQD solids exhibit a diffraction peak at about 11.78°, which corresponds to the characteristic peak of the δ-phased FAPbI3, in agreement with the XRD results (Fig. 2(i)). The absence of such a signal in the FABF4-based PQD solid further validates that the SLA strategy can inhibit the phase transition triggered by the surface lattice distortion of PQDs. By azimuthally integrating the diffraction intensity of the (100) crystal plane of PQD solids, the crystalline orientation of PQDs at different azimuthal angles can be determined, as shown in Fig. 3(h). A distinct crystal orientation at 90° relative to the substrate is observed in the FABF4-based PQD solid, whereas the control and FAI-based PQD solids show no distinct crystal orientations, which is unfavorable for charge carrier transport within the PQD solids.Extending the distance between the detector and the substrate can provide more detailed information regarding the arrangement and stacking of PQDs within the PQD solids. Fig. 3(d)–(f) show the 2D GISAXS patterns of the control, FAI- and FABF4-based PQD solids. The results reveal that the control and FAI-based PQD solids exhibit no distinct Bragg spots instead of diffuse ring-like features, suggesting that these PQD solids did not possess a long-range ordered periodic arrangement of PQDs. In contrast, the FABF4-based PQD solid exhibits pronounced primary diffraction Bragg spots in the 2D GISAXS patterns. The azimuthal integration of the diffraction pattern reveals a long-range ordered stacking structure with an average inter-dot spacing of 16.90 nm for the FABF4-based PQD solid, which is potentially attributed to enhanced surface lattice integrity (Fig. 3(i)).51
The orderly stacking of PQDs in the PQD solid with a preferential orientation is preferred for facilitating charge carrier transport within the PQD solids and thus improving the uniformity of the PQD solid film. To further examine the carrier dynamics in the PQD solids, we conducted 2D PL mapping measurement to observe the PL emission distribution crossing the PQD solid films, as shown in Fig. S13 (ESI†). Compared to the control and FAI-based PQD films, the FABF4-based PQD film exhibits the most uniform PL emission distribution with the highest PL emission intensity, which indicates an improvement in the optoelectronic uniformity after the SLA treatment. To explore the influence of the PQD crystal orientation and configuration on the surface morphology of PQD solid films, atomic force microscopy (AFM) images of these PQD solid films were recorded and the root mean square roughness (Rq) was calculated from the AFM images (Fig. S14, ESI†). Compared to the control PQD solid film (Rq = 6.33 nm) and the FAI-based PQD solid (Rq = 5.13 nm), the FABF4-based PQD solid exhibits the lowest Rq of 4.98 nm, displaying a smoother morphology over the solid film, which is desirable for efficient PQDSCs with lowered energy losses at the interface of PQDs and hole transport materials.52
Photovoltaic performance of PQDSCs
The above theoretical calculations and detailed experimental results show that the FABF4-based PQD is promising for the fabrication of high-performance solar cells. Thus, to study the FABF4-based PQD for photovoltaic applications, the PQDSC with a typical planar device structure of indium-doped tin oxide (ITO)/SnO2/PQDs/2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9′spirobifluorene(spiro-OMeTAD)/MoOx/Ag was fabricated, as schematically presented in Fig. 4(a). The surface chemistry of PQDs may influence their band structures and thus the ultraviolet photoemission spectra (UPS) of PQD solids were recorded to determine their energy levels (Fig. S15, ESI†).53 The energy levels of PQD solids, SnO2 and spiro-OMeTAD are summarized in Fig. 4(b). Compared to the control PQD solids, the conduction band minimum and valence band maximum of the FABF4-based PQD solid show a slightly upward shift, forming a more favorable energy level alignment with the charge transport materials, which could improve the charge cattier extraction at the interface of PQD/charge transport materials.54Fig. 4(c) shows the cross-sectional scanning electron microscopy (SEM) image of the PQDSC, and each functional layer can be clearly observed. The photocurrent density–voltage (J–V) curves of the control, FAI- and FABF4-based PQDSCs were recorded under AM 1.5G 100 mW cm−2 illumination, and the corresponding photovoltaic parameters are summarized in Fig. 4(d). The control PQDSC gave a moderate efficiency of 14.41% and after using the FAI to treat the PQDs, the FAI-based PQDSC showed an enhanced efficiency of 15.94%. After the concentration optimization of the FABF4, the FABF4-based PQDSC achieved a PCE of up to 17.06% (Fig. S16, ESI†). Compared to the control PQDSC, the open-circuit voltage (VOC) of FABF4-based PQDSC was significantly increased from 1.16 V to 1.20 V, which is attributed to reduced VOC deficit originated from the lowered surface defect-induced nonradiative recombination. Meanwhile, the short-circuit photocurrent density (JSC) and fill factor (FF) of FABF4-based PQDSCs were also effectively enhanced. When recording the J–V curves from different scanning directions (from JSC to VOC or from VOC to JSC), hysteresis in the J–V curves was observed, which may be attributed to ion migration during the device operation (Fig. S17, ESI†). However, the hysteresis in the FABF4-based PQDSC was notably reduced, which implies that the SLA approach may impede ion migration by anchoring the surface lattice of PQDs.55
Fig. 4(e) shows the incident photon-to-electron conversion efficiency (IPCE) spectrum of the FABF4-based PQDSC, and the IPCE spectrum of the control PQDSC is presented in Fig. S18 (ESI†), which reveals that these solar cells show good light absorption in the visible and near-infrared wavelength regions. The photocurrent density was also integrated from the IPCE spectra and the intergrade photocurrent density of 18.71 and 20.64 mA cm−2 was recorded for the control and FABF4-based PQDSCs, respectively. To validate the reproducibility of PQDSCs, 66 control, FAI- and FABF4-based PQDSC devices (22 for each) fabricated from different branches were applied for the statistical analysis of the photovoltaic parameters. Fig. 4(f) shows the statistical PCE of these PQDSCs and the other photovoltaic parameters are summarized in Fig. S19 (ESI†). The statistical results demonstrated that the FABF4-based PQDSCs show high reproducibility with a higher average PCE.
Fig. 4(g) illustrates the stable power output (SPO) of the control and FABF4-based PQDSCs. In comparison to a stable PCE of 14% for the control PQDSC, the FABF4-based PQDSC possesses a higher stable PCE of ∼16.60%. We also compared the photovoltaic performance of the demonstrated FABF4-based PQDSCs with the efficient FAPbI3 PQDSCs reported in the literature (Table S2, ESI†), and Fig. 4(h) summarizes the correlation between the PCE and VOC of these FAPbI3 PQDSCs. It can be seen that the FABF4-based PQDSC exhibits the highest PCE and VOC among these PQDSCs. The unencapsulated PQDSCs were stored under ambient conditions with a relative humidity (RH) of ∼40% to evaluate the device stability. As shown in Fig. 4(i), the FABF4-based PQDSC retained 85% of its initial PCE after storage for 480 hours, which exhibits superior stability compared to that of the control PQDSC, with only 58% of its initial efficiency retained. Additionally, the photostability of PQDSCs was also measured under continuous 100 mW cm−2 illumination, which shows that a large enhancement in photostability was also obtained in the FABF4-based PQDSC compared to the control PQDSC (Fig. S20, ESI†). Therefore, we could conclude here that the demonstrated SLA approach could not only improve the photovoltaic performance of PQDSCs but also enhance the device stability, which shows high potential for the fabrication of PQDSCs with higher performance.
Charge carrier extraction and recombination of PQDSCs
To fundamentally understand the causes leading to the improved performance of FABF4-based PQDSCs and link the surface properties of PQDs with the device operation of PQDSCs, the charge carrier dynamics in the solar cells were deeply studied. Since the photovoltaic performance of PQDSCs was largely improved after the SLA treatment of PQDs, we herein mainly focus on the control and FABF4-based PQDSCs. Transient photovoltage (TPV) of solar cells, showing the photovoltage decays with time, can provide detailed information on charge carrier extraction and recombination;56 thus, the TPV of the control and FABF4-based PQDSCs were measured (Fig. 5(a) and Table S3, ESI†).57 The fitted results show that the FABF4-based PQDSC has a longer charge carrier lifetime of 2.4 ms compared with that of 2.05 ms for the control PQDSC. The prolonged charge carrier lifetime in FABF4-based PQDSCs is primarily attributed to the surface lattice anchoring using the BF4− anions, which effectively suppresses the nonradiative recombination, thus lowering the VOC deficit in FABF4-based PQDSCs. Fig. 5(b) shows the transient photocurrent (TPC) curves of the control and FABF4-based PQDSCs, which can obtain more detailed information concerning the charge carrier transport within the solar cells. The TPC curve was fitted using a single exponential function, and the fitted results are summarized in Table S4 (ESI†).58 The results show that compared to the control PQDSC with a charge carrier transport time of 4.31 μs, the charge carrier transport time was largely shortened in the FABF4-based PQDSC, and a charge carrier transport time of 3.33 μs was achieved. The shortened charge carrier transport time in the FABF4-based PQDSC could be assigned to the improved crystallographic orientation of PQD solids, facilitating charge carrier transport in the PQD solids.To gain more insights into the charge carrier extraction and recombination in the PQDSCs, the light intensity-dependent VOC and JSC of PQDSCs were further investigated. As shown in Fig. 5(c), the VOC of PQDSC exhibits a logarithmic linear relationship with the light intensity, with the slope of nkT/q, where n, k, T and q correspond to the ideality factor, Boltzmann constant, the temperature in Kelvin, and elementary charge respectively.59 The calculated n values for control and FABF4-based PQDSCs were 1.28 and 1.15, respectively. The close-to-unity value of the n value for the FABF4-based PQDSC indicates the suppression of trap-assisted charge carrier recombination in the solar cells. We further explored the functional relationship between the JSC and light intensity of PQDSCs, which was fitted with the power law equation JSC ∝ Iα, as shown in Fig. S21 (ESI†). Compared to the control PQDSC, the α value of the FABF4-based PQDSC is closer to 1, suggesting enhanced charge extraction in the FABF4-based PQDSCs. The space charge-limited current (SCLC) measurement of PQD devices was further performed to quantify the trap density in the PQD solids. Hole-only PQD device with a structure of ITO/PEDOT:PSS/PQDs/spiro-OMeTAD/Ag was fabricated (Fig. 5(d)). The intersection of the trap-filling limit (TFL) region and the Ohmic region corresponds to the voltage known as the trap-filling limit voltage (VTFL). The trap density (Ntrap) in the PQD solids can be calculated using the following formula60
 | (1) |
As discussed in the above section, the surface lattice anchoring of PQDs benefits the charge carrier transport and extraction of PQDSCs. However, the surface chemistry of PQDs may also affect the built-in electronic field within the PQDSCs, which also plays an important role in the charge carrier extraction of PQDSCs. To this end, the Mott–Schottky test was conducted to study the built-in potential (Vbi) of PQDSCs (Fig. 5(e)), and the Vbi of the control and FABF4-based PQDSC was determined using the following equation1
 | (2) |
Based on the above extensive results, to link the surface properties of PQDs with the device operation of PQDSCs, we proposed a model to elucidate the role of the surface lattice anchoring of PQDs in enhancing the performance of PQDSCs. Generally, long-chain OA/OAm ligands are partly removed during PQD purification using the antisolvents, which leads to the production of numerous VFA and VI at the surface lattice of PQDs. These vacancies could serve as trap states for charge carriers and also become active sites for water/oxygen attacks, leading to poor photovoltaic performance and operational stability of PQDSCs (Fig. 5(g)).61 Meanwhile, the incompleteness of the surface lattice of PQDs also significantly influences the preferential orientation of PQD solids, which increases the roughness of PQD solids and thus hinders charge carrier transport. After the SLA treatment, the lattice pinning effect of BF4− anions can stabilize the surface lattice of PQDs, resulting in the PDQs exhibiting complete surface lattice coverage and demonstrating long-range ordered structures in the PQD solids (Fig. 5(h)). Consequently, the FABF4-based PQD solids effectively suppressed trap-assisted nonradiative recombination, and enhanced charge carrier transport was obtained in FABF4-based PQD solids, which led to improved photovoltaic performance in FABF4-based PQDSCs. Meanwhile, the reduction of surface lattice vacancies of PQDs and hydrophobicity of the fluorine atoms of BF4− anions substantially reduced the attacking channels of water and oxygen, thereby improving the stability of PQDSCs.
3. Conclusions
In summary, a facial surface lattice anchoring strategy was reported to stabilize the surface lattice of PQDs for efficient solar cells. The results revealed that the FABF4 can effectively repair the VFA and VI at the surface lattice of PQDs, substantially diminishing trap-assisted nonradiative recombination and thus leading to improved optoelectronic properties. Meanwhile, the FABF4 molecule could also substitute part of the OAm/OA ligands at the PQD surface, which greatly improved the electronic coupling of PQDs and thus facilitated charge carrier transport in the PQD solids. Moreover, the BF4− anion with a strong binding with the uncoordinated Pb2+ cation at the surface lattice of PQDs can stabilize the surface lattice of PQDs to effectively ameliorate the surface lattice distortion, resulting in high crystal stability of PQDs. As a consequence, FABF4-based PDQSCs delivered a record efficiency of up to 17.06%, which is largely enhanced compared with the 14.52% efficiency for the control PQDSCs. Meanwhile, after surface lattice anchoring, the operational stability of PQDSCs was also significantly enhanced. This work provides important insights into the physical design principles of the surface lattice of PQDs and its dominant effect on the optoelectronic properties and crystal stability of PQDs for high-performance solar cells or other optoelectronic devices.Author contributions
X. Zhang and M. Zhang designed the research and experiments. M. Zhang synthesized PQDs and conducted XRD, FTIR, TEM, SEM, light absorption and PL spectra measurements. M. Zhang, X. Mei and G. Wang fabricated solar cells and measured the solar cell performance. M. Zhang and J. Qiu conducted DFT calculations under the supervision of Z. Sun. M. Zhang wrote the first version of the manuscript. All authors contributed to discussions and commented on the manuscript, and all authors reviewed the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 52372169 and 51872014) and the National Key Research and Development Program of China (grant no. 2022YFB3807200). This work was also supported by the HPC of Beihang University.References
- X. Zhang, U. B. Cappel, D. Jia, Q. Zhou, J. Du, T. Sloboda, S. Svanström, F. O. L. Johansson, A. Lindblad, E. Giangrisostomi, R. Ovsyannikov, J. Liu, H. Rensmo, J. M. Gardner and E. M. J. Johansson, Chem. Mater., 2019, 31, 4081–4091 CrossRef CAS
.
- X. Zhang, J. Zhang, J. Liu and E. M. J. Johansson, Nanoscale, 2015, 7, 11520–11524 RSC
.
- Q. Zhao, A. Hazarika, X. Chen, S. P. Harvey, B. W. Larson, G. R. Teeter, J. Liu, T. Song, C. Xiao, L. Shaw, M. Zhang, G. Li, M. C. Beard and J. M. Luther, Nat. Commun., 2019, 10, 2842 CrossRef
.
- L. P. Maksym, V. Kovalenko and M. I. Bodnarchuk, Science, 2017, 358, 745–750 CrossRef
.
- R. Grisorio, F. Fasulo, A. B. Munoz-Garcia, M. Pavone, D. Conelli, E. Fanizza, M. Striccoli, I. Allegretta, R. Terzano, N. Margiotta, P. Vivo and G. P. Suranna, Nano Lett., 2022, 22, 4437–4444 CrossRef CAS PubMed
.
- F. Liu, Y. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase, T. Minemoto, K. Yoshino, S. Dai and Q. Shen, ACS Nano, 2017, 11, 10373–10383 CrossRef CAS
.
- T. Chiba, Y. Hayashi, H. Ebe, K. Hoshi, J. Sato, S. Sato, Y.-J. Pu, S. Ohisa and J. Kido, Nat. Photonics, 2018, 12, 681–687 CrossRef CAS
.
- Y. Gao, Y. Liu, F. Zhang, X. Bao, H. Xu, X. Bai, M. Lu, Y. Wu, Z. Wu, Y. Zhang, Q. Wang, X. Gao, Y. Wang, Z. Shi, J. Hu, W. W. Yu and Y. Zhang, Adv. Mater., 2022, 34, 2207445 CrossRef CAS
.
- L. M. Wheeler, E. M. Sanehira, A. R. Marshall, P. Schulz, M. Suri, N. C. Anderson, J. A. Christians, D. Nordlund, D. Sokaras, T. Kroll, S. P. Harvey, J. J. Berry, L. Y. Lin and J. M. Luther, J. Am. Chem. Soc., 2018, 140, 10504–10513 CrossRef CAS
.
- Y. Wang, J. Yuan, X. Zhang, X. Ling, B. W. Larson, Q. Zhao, Y. Yang, Y. Shi, J. M. Luther and W. Ma, Adv. Mater., 2020, 32, 2000449 CrossRef CAS
.
- Y. Qian, Y. Shi, G. Shi, G. Shi, X. Zhang, L. Yuan, Q. Zhong, Y. Liu, Y. Wang, X. Ling, F. Li, M. Cao, S. Li, Q. Zhang, Z. Liu and W. Ma, Sol. RRL, 2021, 5, 2100090 CrossRef CAS
.
- A. Hazarika, Q. Zhao, E. A. Gaulding, J. A. Christians, B. Dou, A. R. Marshall, T. Moot, J. J. Berry, J. C. Johnson and J. M. Luther, ACS Nano, 2018, 12, 10327–10337 CrossRef CAS
.
- J. W. Jo, Y. Kim, J. Choi, F. P. G. de Arquer, G. Walters, B. Sun, O. Ouellette, J. Kim, A. H. Proppe, R. Quintero-Bermudez, J. Fan, J. Xu, C. S. Tan, O. Voznyy and E. H. Sargent, Adv. Mater., 2017, 29, 1703627 CrossRef
.
- D. Jia, J. Chen, J. Qiu, H. Ma, M. Yu, J. Liu and X. Zhang, Joule, 2022, 6, 1632–1653 CrossRef CAS
.
- X. Mei, D. Jia, J. Chen, S. Zheng and X. Zhang, Nano Today, 2022, 43, 101449 CrossRef CAS
.
- Z. Qi, X. Mei, J. Wang, J. Qiu, W. Zheng, K. He, M. Zhang, X. Zhang and X. Zhang, Adv. Funct. Mater., 2024, 34, 2405679 CrossRef CAS
.
- C. Wang, C. Zhang, R. Li, Q. Chen, L. Qian and L. Chen, Acta Phys.-Chim. Sin., 2022, 38, 2104030 Search PubMed
.
- E. Yassitepe, Z. Yang, O. Voznyy, Y. Kim, G. Walters, J. A. Castañeda, P. Kanjanaboos, M. Yuan, X. Gong, F. Fan, J. Pan, S. Hoogland, R. Comin, O. M. Bakr, L. A. Padilha, A. F. Nogueira and E. H. Sargent, Adv. Funct. Mater., 2016, 26, 8757–8763 CrossRef CAS
.
- K. Chen, X. Zhang, P. A. Chen, J. Guo, M. He, Y. Chen, X. Qiu, Y. Liu, H. Chen, Z. Zeng, X. Wang, J. Yuan, W. Ma, L. Liao, T. Q. Nguyen and Y. Hu, Adv. Sci., 2022, 9, 2105856 CrossRef CAS PubMed
.
- A. Swarnkar, A. R. Marshall, E. M. Sanehira, B. D. Chernomordik, D. T. Moore, J. A. Christians, T. Chakrabarti and J. M. Luther, Science, 2016, 354, 92–95 CrossRef CAS
.
- J. Chen, D. Jia, E. M. J. Johansson, A. Hagfeldt and X. Zhang, Energy Environ. Sci., 2021, 14, 224–261 RSC
.
- D. Jia, J. Chen, R. Zhuang, Y. Hua and X. Zhang, Energy Environ. Sci., 2022, 15, 4201–4212 RSC
.
- L. Liu, A. Najar, K. Wang, M. Du and S. F. Liu, Adv. Sci., 2022, 9, 2104577 CrossRef CAS
.
- H. Huang, M. I. Bodnarchuk, S. V. Kershaw, M. V. Kovalenko and A. L. Rogach, ACS Energy Lett., 2017, 2, 2071–2083 CrossRef CAS PubMed
.
- J. Xue, J.-W. Lee, Z. Dai, R. Wang, S. Nuryyeva, M. E. Liao, S.-Y. Chang, L. Meng, D. Meng, P. Sun, O. Lin, M. S. Goorsky and Y. Yang, Joule, 2018, 2, 1866–1878 CrossRef CAS
.
- H. Zhu, K. Miyata, Y. Fu, J. Wang, P. P. Joshi, D. Niesner, K. W. Williams, S. Jin and X. Y. Zhu, Science, 2016, 353, 1409–1413 CrossRef CAS
.
- F. Li, S. Zhou, J. Yuan, C. Qin, Y. Yang, J. Shi, X. Ling, Y. Li and W. Ma, ACS Energy Lett., 2019, 4, 2571–2578 CrossRef CAS
.
- M. Suri, A. Hazarika, B. W. Larson, Q. Zhao, M. Vallés-Pelarda, T. D. Siegler, M. K. Abney, A. J. Ferguson, B. A. Korgel and J. M. Luther, ACS Energy Lett., 2019, 4, 1954–1960 CrossRef CAS
.
- S. Ding, M. Hao, C. Fu, T. Lin, A. Baktash, P. Chen, D. He, C. Zhang, W. Chen, A. K. Whittaker, Y. Bai and L. Wang, Adv. Sci., 2022, 9, 2204476 CrossRef CAS
.
- F. Li, X. Zhang, J. Shi, L. Jin, J. Qiao, J. Guo, H. Yin, Y. Li, J. Yuan and W. Ma, Adv. Funct. Mater., 2023, 33, 2302542 CrossRef CAS
.
- H. Aqoma, S.-H. Lee, I. F. Imran, J.-H. Hwang, S.-H. Lee and S.-Y. Jang, Nat. Energy, 2024, 9, 324–332 CrossRef CAS
.
- X. Zhang, H. Huang, L. Jin, C. Wen, Q. Zhao, C. Zhao, J. Guo, C. Cheng, H. Wang, L. Zhang, Y. Li, Y. M. Maung, J. Yuan and W. Ma, Angew. Chem., Int. Ed., 2022, 14, 241 Search PubMed
.
- M. Zhang, Q. Gao, X. Mei, J. Qiu, R. Zhuang, Y. Hua, Z. Sun and X. Zhang, Energy Environ. Sci., 2024, 17, 2145–2156 RSC
.
- Y. Han, W. Liang, X. Lin, Y. Li, F. Sun, F. Zhang, P. C. Sercel and K. Wu, Nat. Mater., 2022, 21, 1282–1289 CrossRef CAS
.
- M. Hao, Y. Bai, S. Zeiske, L. Ren, J. Liu, Y. Yuan, N. Zarrabi, N. Cheng, M. Ghasemi, P. Chen, M. Lyu, D. He, J.-H. Yun, Y. Du, Y. Wang, S. Ding, A. Armin, P. Meredith, G. Liu, H.-M. Cheng and L. Wang, Nat. Energy, 2020, 5, 79–88 CrossRef CAS
.
- S. Xiao, X. Mei and X. Zhang, Energy Environ. Sci., 2024, 17, 5756–5794 RSC
.
- L. Manna, O. M. Bakr, S. Brovelli and H. Li, Energy Mater. Adv., 2022, 2022, 9865891 Search PubMed
.
- J. Qiu, Q. Zhou, D. Jia, Y. Wang, S. Li and X. Zhang, J. Mater. Chem. A, 2022, 10, 1821–1830 RSC
.
- J. Qiu, Q. Zhou, M. Yu, J. Liu, R. Zhuang, Y. Hua, L. Ding and X. Zhang, SusMat, 2023, 3, 894–908 CrossRef CAS
.
- Y. Wang, X. Mei, J. Qiu, Q. Zhou, D. Jia, M. Yu, J. Liu and X. Zhang, J. Phys. Chem. Lett., 2021, 12, 11330–11338 CrossRef CAS PubMed
.
- I. Levchuk, A. Osvet, X. Tang, M. Brandl, J. D. Perea, F. Hoegl, G. J. Matt, R. Hock, M. Batentschuk and C. J. Brabec, Nano Lett., 2017, 17, 2765–2770 CrossRef CAS PubMed
.
- L. Protesescu, S. Yakunin, S. Kumar, J. Bar, F. Bertolotti, N. Masciocchi, A. Guagliardi, M. Grotevent, I. Shorubalko, M. I. Bodnarchuk, C. J. Shih and M. V. Kovalenko, ACS Nano, 2017, 11, 3119–3134 CrossRef CAS PubMed
.
- X. Mei, J. Wang, X. Zhang, R. Zhuang, Y. Hua, K. He, W. Zheng and X. Zhang, ACS Energy Lett., 2023, 8, 4386–4396 CrossRef CAS
.
- D. Jia, J. Chen, X. Mei, W. Fan, S. Luo, M. Yu, J. Liu and X. Zhang, Energy Environ. Sci., 2021, 14, 4599–4609 RSC
.
- D. Jia, J. Chen, M. Yu, J. Liu, E. M. J. Johansson, A. Hagfeldt and X. Zhang, Small, 2020, 16, 2001772 CrossRef CAS PubMed
.
- S. Ding, J. A. Steele, P. Chen, T. Lin, D. He, C. Zhang, X. Fan, E. Solano, A. K. Whittaker, M. Hao and L. Wang, Adv. Energy Mater., 2023, 23, 2301817 CrossRef
.
- Y. Zou, W. Yu, H. Guo, Q. Li, X. Li, L. Li, Y. Liu, H. Wang, Z. Tang, S. Yang, Y. Chen, B. Qu, Y. Gao, Z. Chen, S. Wang, D. Zhang, Y. Chen, Q. Chen, S. M. Zakeeruddin, Y. Peng, H. Zhou, Q. Gong, M. Wei, M. Gratzel and L. Xiao, Science, 2024, 385, 161–167 CrossRef CAS
.
- Q. Zhou, J. Qiu, Y. Wang, M. Yu, J. Liu and X. Zhang, ACS Energy Lett., 2021, 6, 1596–1606 CrossRef CAS
.
- J. Chen, D. Jia, R. Zhuang, Y. Hua and X. Zhang, Adv. Mater., 2022, 34, 2204259 CrossRef CAS
.
- D. Jia, J. Chen, S. Zheng, D. Phuyal, M. Yu, L. Tian, J. Liu, O. Karis, H. Rensmo, E. M. J. Johansson and X. Zhang, Adv. Energy Mater., 2019, 9, 1902809 CrossRef CAS
.
- M. Liu, O. Voznyy, R. Sabatini, F. P. Garcia de Arquer, R. Munir, A. H. Balawi, X. Lan, F. Fan, G. Walters, A. R. Kirmani, S. Hoogland, F. Laquai, A. Amassian and E. H. Sargent, Nat. Mater., 2017, 16, 258–263 CrossRef CAS
.
- J. Qiu, X. Mei, M. Zhang, G. Wang, S. Zou, L. Wen, J. Huang, Y. Hua and X. Zhang, Angew. Chem., Int. Ed., 2024, 63, 202401751 CrossRef
.
- Z. Xu, N. Li, X. Niu, H. Liu, G. Liu, Q. Chen and H. Zhou, Energy Mater. Adv., 2022, 2022, 9781073 Search PubMed
.
- J. Dou and Q. Chen, Energy Mater. Adv., 2022, 2022, 0002 CrossRef
.
- Q. Jiang, L. Zhang, H. Wang, X. Yang, J. Meng, H. Liu, Z. Yin, J. Wu, X. Zhang and J. You, Nat. Energy, 2016, 2, 16177 CrossRef
.
- Q. S. Zhou, J. M. Qiu, Y. F. Wang, S. Li, M. Yu, J. H. Liu and X. L. Zhang, Chem. Eng. J., 2022, 440, 135974 CrossRef CAS
.
- M. Zhang, Q. Zhou, X. Mei, J. Chen, J. Qiu, X. Li, S. Li, M. Yu, C. Qin and X. Zhang, Acta Phys.-Chim. Sin., 2022, 39, 2210002 Search PubMed
.
- Q. Zhou, J. Qiu, R. Zhuang, X. Mei, Y. Hua and X. Zhang, J. Mater. Chem. A, 2023, 11, 5199–5211 RSC
.
- J. Chen, S. Zheng, D. Jia, W. Liu, A. Andruszkiewicz, C. Qin, M. Yu, J. Liu, E. M. J. Johansson and X. Zhang, ACS Energy Lett., 2021, 6, 1970–1979 CrossRef CAS
.
- X. Mei, K. He, R. Zhuang, M. Yu, Y. Hua and X. Zhang, Chem. Eng. J., 2023, 453, 139909 CrossRef CAS
.
- J. Chen, D. Jia, R. Zhuang, Y. Hua and X. Zhang, Adv. Mater., 2024, 36, 2306854 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ee04112g |
| This journal is © The Royal Society of Chemistry 2025 |