High-entropy doping for high-performance zero-cobalt high-nickel layered cathode materials†
Jiahui
Zhou
ac,
Jiehui
Hu
a,
Xia
Zhou
a,
Zhen
Shang
a,
Yue
Yang
 *b and
Shengming
Xu
*b and
Shengming
Xu
 *a
*a
aInstitute of Nuclear and New Energy Technology, Tsinghua University, Beijing, 100084, China. E-mail: smxu@mail.tsinghua.edu.cn
bSchool of Minerals Processing & Bioengineering, Central South University, Hunan, China. E-mail: Eric1911@126.com
cPetrochina Shenzhen New Energy Research Institute Co.,LTD., Guangdong, China
First published on 12th November 2024
Abstract
Considering the high price and scarcity of cobalt resources, zero-cobalt, high-nickel layered cathode materials (LNMs) have been considered as the most promising material for next-generation high-energy-density lithium-ion batteries (LIBs). However, current LNMs face severe structural instability and poor electrochemical performance. Here, a high-entropy doping strategy has been developed to prepare high-performance LNMs by a typical co-precipitation method. Supported by transmission electron microscopy, in situ X-ray diffraction and X-ray absorption near edge structure analysis, the material exhibits small crystal size variations and no changes of (Ni, Mn)–O and (Ni, Mn)–Ni coordination distances, resulting in greatly reduced irreversible phase transformation and cracks. Formation energy and diffusion energy barrier analysis indicates that the material has a fast lithium-ion diffusion kinetics. Benefiting from these advantages, it exhibits excellent rate and cycling performance. This study provides a feasible high-entropy doping strategy to effectively achieve stable material circulation under a high capacity and gives more insights for developing new high-energy-density cathode materials.
Broader contextConsidering the scarcity of cobalt resources and the geopolitical constraints on cobalt mines, the high-energy-density lithium-ion battery industry urgently needs to move away from cobalt. Zero-cobalt high-nickel cathode materials are considered to be the next generation of lithium-ion battery cathode materials due to their high energy density and low cost. However, they face severe structural instability and poor electrochemical performance. In this study, a zero-cobalt high-nickel cathode material with high structural stability and high performance was prepared through a high-entropy doping strategy. High-entropy doping keeps zero-cobalt high-nickel cathode materials in a stable state during the charge and discharge process by suppressing surface irreversible phase transformation and reducing the expansion and contraction of the bulk unit cell parameters a and c. Not only that, the high-entropy zero-cobalt high-nickel cathode material also exhibits good electrical conductivity and a low lithium-ion diffusion energy barrier, resulting in excellent electrochemical performance. This work provides a feasible strategy to effectively solve the structural instability of zero-cobalt high-nickel cathode materials. |
Introduction
High-energy-density lithium-ion batteries (LIBs) play a key role in furthering the development of high-performance electric vehicles.1,2 Currently, ternary high nickel LiNi1−yMnxCoyO2 is the most widely applied cathode material for high-energy-density LIBs.3,4 Among the elements in ternary high nickel (Ni) materials, cobalt (Co) resource is very scarce.5 The world's cobalt recoverable reserves are only 15.9 Mt, and according to the current annual supply of Co of 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons, the Co resources are only enough to be mined for 113 years.6 Due to its scarcity, the price of Co has increased from US$26
000 tons, the Co resources are only enough to be mined for 113 years.6 Due to its scarcity, the price of Co has increased from US$26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton in 2019 to US$34
000 per ton in 2019 to US$34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton in 2023, leading to an increase in high-energy-density LIB prices.7 Therefore, getting rid of the dependence on Co and developing high-performance zero-cobalt, high-nickel cathode materials has been an urgent issue for the sustainable development of high-energy-density LIBs.8–13
000 per ton in 2023, leading to an increase in high-energy-density LIB prices.7 Therefore, getting rid of the dependence on Co and developing high-performance zero-cobalt, high-nickel cathode materials has been an urgent issue for the sustainable development of high-energy-density LIBs.8–13
Zero-cobalt, high-nickel cathode materials (LNM) for LIBs have been developed, but they are still trapped in insufficient electrochemical performances.1,14,15 On the one hand, the volume change caused by the unequal shrinkage and expansion along the a-axis and c-axis directions of high nickel cathode materials in charge and discharge processes easily lead to the formation of intergranular and intragranular cracking, resulting in poor cycling performance.7,16 On the other hand, Co is the most critical element in high-nickel cathode materials for stabilizing the structure and enhancing electrical conductivity.2,17–19 In the absence of Co, the Li/Ni cation mixing is intensified and the lithium ion diffusion is weakened, causing adverse rate performance.20–22 To improve the electrochemical performances of LNMs, approaches that trade-off between capacity and stability are always employed. Among various methods, element doping has been considered as one of the most successful approaches to suppress Li/Ni cation mixing and inhibit microcracks,23–26 but the substituted LNMs still show unsatisfactory cycling stability because a single substitution cannot significantly strengthen the interaction between transition metal–O and the Ni–O bond, so they still break easily to collapse the structure. Therefore, it is a significant challenge for the current LNMs to break the trade-off by inhibiting the structural degradation, enhancing lithium ion diffusion and reducing the volume change without sacrificing high capacity during the charge and discharge process.
The high-entropy (HE) strategy is a special doping method, usually by introducing more than three kinds of atoms to replace the original atoms.1,27 It is used to enhance the structural strength of alloy materials and improve the conductivity of solid electrolytes.28,29 It can greatly enhance the interaction between transition metal-O by regulating the maximum configuration entropy inside the crystal and forming a complex chemical bond network inside the material.27,28 In this study, we applied high-entropy strategies to LNM to improve structural strength and improve the lithium-ion diffusion kinetics. Six elements (Ni, Mn, Al, Mg, Nb and Mo) share transition metal sites. Ni is expected to be used to improve the reversible capacity and charge compensation, Mn can serve as a structure former due to its abundance, Al and Mg are used to improve the structural stability during the deintercalation process of lithium ions, and Nb and Mo are expected to be used to increase the average voltage. The specific content of other doping elements is naturally formed during the co-precipitation process under the condition of ensuring that the Ni content is greater than 90% and controlling the pH to be equal to 11. Based on previous explorations, we attempted to investigate the new electrochemical properties for LNM inspired by the HE concept. Herein, high-entropy zero-cobalt, high-nickel layered cathode material (LiNi0.915Mn0.0475Al0.0106Mg0.0106Nb0.0068Mo0.0095O2) (HE-LNM) has been developed by using this new doping strategy with a typical co-precipitation method. The HE-LNM shows structural stabilization by suppressing surface irreversible phase transformation, and reducing the expansion and contraction of the bulk unit cell parameters a and c. The reversible discharge capacity reaches 204.3 mA h g−1 at 0.1C, and the capacity retention rate reaches 81.56% after 350 cycles at 0.3C. Moreover, HE-LNM also exhibits good electrical conductivity and low lithium-ion diffusion energy barrier, resulting in an excellent rate performance. This study provides a feasible strategy to effectively achieve stable material circulation under high capacity and give more insights for developing new high-energy-density cathode materials.
Results and discussion
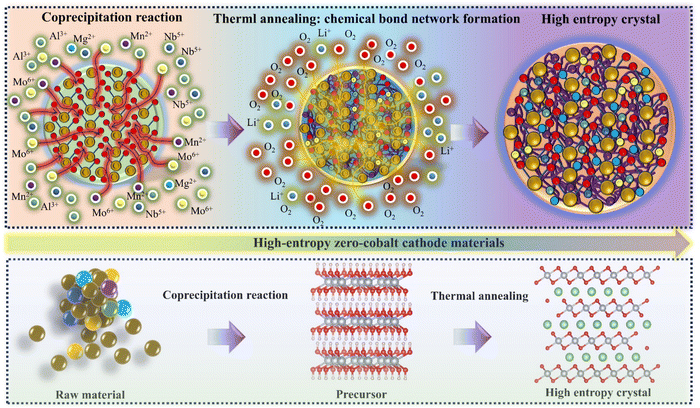
The high-entropy zero-cobalt, high-nickel cathode material (LiNi0.915Mn0.0475Al0.0106Mg0.0106Nb0.0068Mo0.0095O2) (HE-LNM) (Table S1, ESI†) is produced by a co-precipitation method combined with a thermal annealing process (Fig. 1). The co-precipitation is to evenly distribute the constituent elements in the form of hydroxides in the bulk phase, Al, Mg, Nb and Mo elements are introduced to replace Co in this process, which maximizes the configuration entropy of the material.1 The high entropy element can act as a rivet in the bulk phase, forming a complex chemical bond network with oxygen atoms.1,23 The thermal annealing process is to convert hydroxide into high-valent oxide and allow lithium ions to enter the oxide interlayer to obtain layered HE-LNM. Fig. 2 shows the morphology and element distribution of HE-LNM. The particle composed of primary nanoparticles exhibited a dense spherical morphology, with an average particle size of 6.46 μm (Fig. S1, ESI†). The HRTEM images of HE-LNM showed significant lattice fringes, suggesting a good layered structure (Fig. 2(c and d)). Meanwhile, Ni, Mn, Al, Mg, Nb, Mo and O are evenly distributed in the bulk phase (Fig. 2(e–l), suggesting that they are uniformly doped into the material.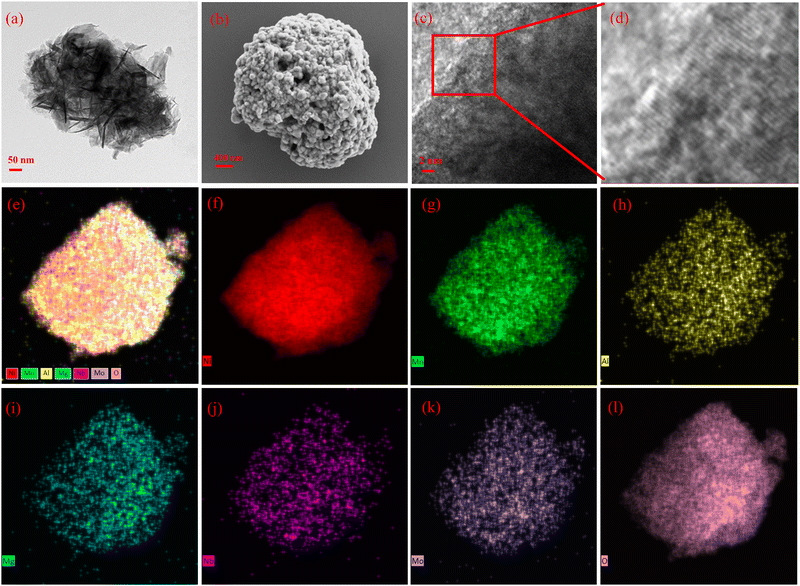 | ||
| Fig. 2 (a) SEM of the precursor, (b) SEM of HE-LNM, (c) HRTEM of HE-LNM, (d) magnification of the selected area in (c), and (e)–(l) mapping of HE-LNM. | ||
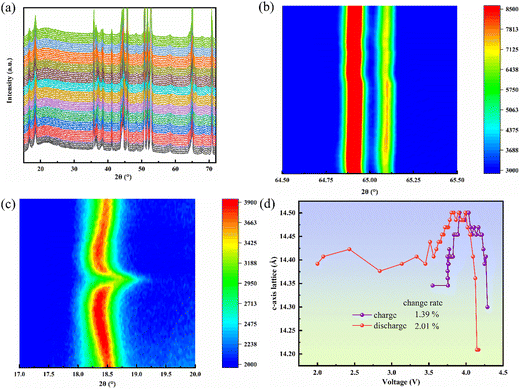
In order to further verify the structure of HE-LNM, in situ electrochemical XRD was performed. HE-LNM has a good correspondence with the standard diffraction peaks of LiNiO2 and no impurities exist. They have a layered structure of α-NaFeO2 type and belong to the R3m space group. In order to study the changes of HE-LNM crystals during the charge and discharge process, we analyzed the a- and c-axis dimensions of the crystals by in situ electrochemical XRD. During the charge and discharge process (Fig. 3(a-c)), the XRD diffraction peak corresponding to the a-axis direction of the crystal (2θ is between 64.5–65.5°) changed little, while the crystal size in the c-axis orientation (2θ is between 17–20°) shows fluctuations. The crystal size variation of HE-LNM in the c-axis direction was calculated according to eqn (S1) and (S2) (ESI†). This indicated that the maximal variation in the c-axis direction during the charge and discharge process is 2.01%. Compared with changes of commercial LiNi0.33Co0.33Mn0.33O2 and LiNi0.5Co0.2Mn0.3O2 cathode materials (for the commercial LiNi0.33Co0.33Mn0.33O2 cathode material, the maximal variations in the c-axis and a-axis directions were 5.1% and 1.4%, respectively;30 for the commercial LiNi0.5Co0.2Mn0.3O2 cathode material, the maximal variations in the c-axis and a-axis directions were 4.5% and 1.7%, respectively30) (Fig. 3d), the crystal size variations of HE-LNM in the a-axis and c-axis directions during the charge and discharge process were much smaller, indicating a more stable structure.
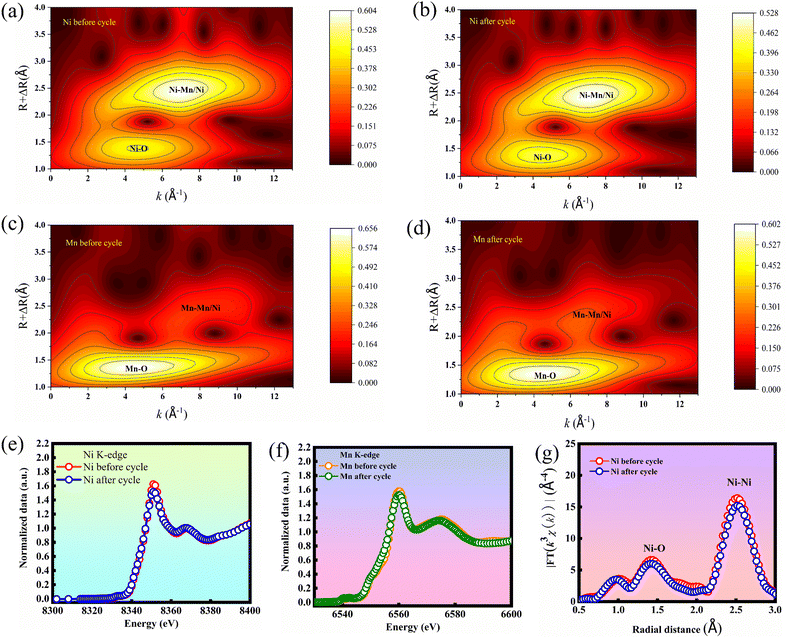
The in situ XRD result well agreed with the X-ray absorption near edge structure (XANES) analysis of Ni and Mn. The Wavelet Transform (WT) contour plots showed two maximum intensities (Fig. 4(a and b)), which are attributed to the Ni–O and Ni–Ni/Mn coordination paths. Simultaneously, the WT contour plots of Mn showed Mn–O and Mn–Mn/Ni coordination paths (Fig. 4(c and d)). The coordination paths of the samples before and after cycling are unchanged, implying the highly stable local coordination environments. The Ni-K and Mn-K edges of cycled HE-LNM nearly overlap with that of the pristine sample (Fig. 4(e and f)), indicating that the volume of HE-LNM changes very little before and after cycling. The changes of (Ni, Mn)–O and (Ni, Mn)–Ni coordination distances in HE-LNM are almost negligible after cycling, indicating that the lattice shrinkage and defect generation are suppressed.1 Thus, all of these results confirmed that high-entropy doping makes the HE-LNM have a highly stable structure.
Fig. 5 shows the electrochemical performance of HE-LNM. Its first discharge capacity reached 204.3 mA h g−1 with the initial coulombic efficiency of 88.86% (Fig. 5(a)). The charge and discharge platform in Fig. 5(a) is in accordance with the CV curve of HE-LNM (Fig. S2, ESI†). Significant redox peaks located at 3.94 V and 3.54 V were attributed to the oxidation peak and reduction peak, respectively. The voltage difference between the redox peaks is only 0.4 V and no displacement occurs as the number of cycles increases, indicating a good redox reversibility. When cycling under different current densities of 0.1, 0.3, 0.5, 0.7 and 1C, the discharge capacities are 200.9, 191, 181.5, 172.5 and 148 mA h g−1, respectively (Fig. 5(b)). When the current density returned to 0.1C, the discharge capacity returned to 199.3 mA h g−1. The cycling performance of HE-LNM is shown in Fig. 5(c-d). HE-LNM had a capacity retention efficiency of 81.56% after 350 cycles at 0.3C and a capacity retention efficiency of 85.75% after 100 cycles even at 1C. Moreover, the electron transfer impedance before cycling is 108.3 Ω, and the electron transfer impedance after cycling is 70.8 Ω (Fig. S3, ESI†), indicating a low impedance. All these results are better than those of commercial and reported related materials (Table S2, ESI†).
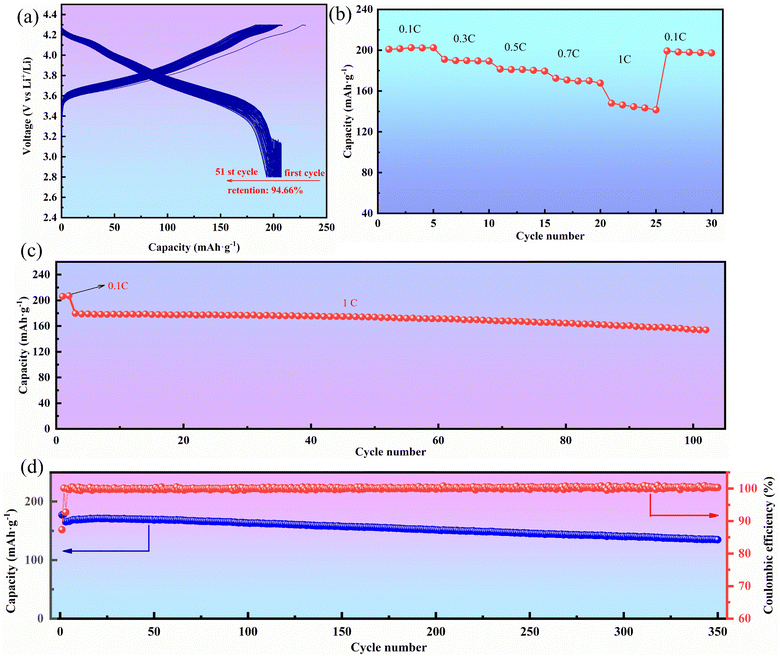 | ||
| Fig. 5 Electrochemical properties of HE-LNM: (a) 0.1C cycle curve, (b) rate performance, (c) 1C cycling performance, and (d) 0.3C cycling performance. | ||
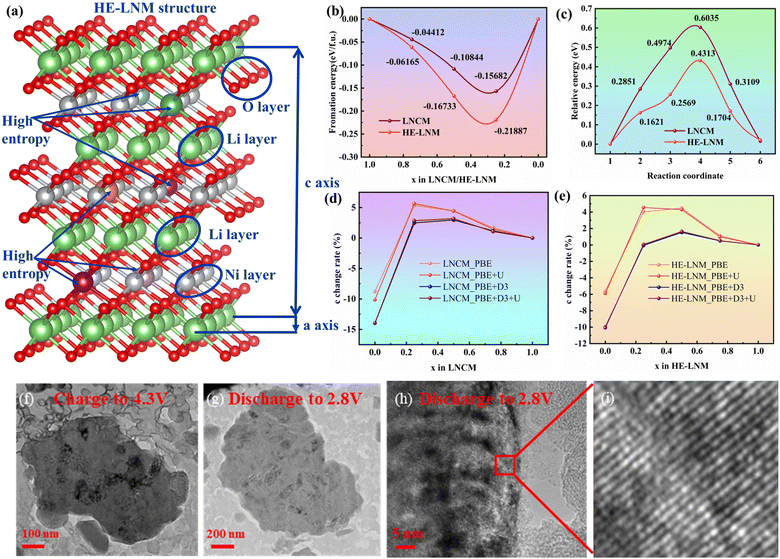
To further explain the reasons for the impressive electrochemical performance, the formation energy, diffusion energy barrier and lattice change of HE-LNM were investigated. According to the model (Fig. 6(a)) and calculation with eqn (S3) (ESI†), when the lithium content is 1, 0.75, 0.5 and 0.25, the formation energies of HE-LNM are 0, −0.06165, −0.16733 and −0.21887 eV, respectively, while the formation energies of undoped LNCM are 0, −0.04412, −0.10844 and −0.15682 eV respectively (Fig. 6(b)). The lower formation energy of HE-LNM meant that more lithium can be more easily lithiated and detached from HE-LNM, resulting in a higher capacity. Meanwhile, the diffusion energy barriers of undoped LNCM and HE-LNM are 0.6035 and 0.4313 eV, respectively (Fig. 6(c)). The diffusion energy barrier of HE-LNM is 0.1722 eV lower than that of undoped LNCM, indicating that HE-LNM has a fast lithium-ion diffusion kinetics, resulting in an excellent rate performance. In summary, high entropy enhances the chemical bond interaction between the transition metal and oxygen in the cathode material, weakens the interaction between lithium and oxygen, reduces the diffusion energy barrier of lithium ions, and lowers the free energy of the material, making it more stable. In addition, it is in good agreement with in situ XRD and XANES analysis that HE-LNM has a smaller crystal change rate under different pseudopotentials (Fig. 6(d and e)). More importantly, HE-LNM did not generate obvious cracks internally and maintained a layered structure after 100 cycles at 1C (Fig. 6(f)–(i)), showing a good structural stability.
Conclusions
A high-entropy zero-cobalt, high-nickel cathode material (Ni = 91.5%) (HE-LNM) is prepared through a co-precipitation method combined with a thermal annealing process. Benefitting from the introduction of high-entropy elements, the HE-LNM exhibits a stable and crack-free layered structure during the charge and discharge process. In addition, HE-LNM has high electronic conductivity, small changes in the c-axis direction, low formation energy and low lithium-ion diffusion energy barrier, resulting in high capacity, and impressive rate and cycling performances. The discharge capacities are 200.9, 191, 181.5, 172.5 and 148 mA h g−1 respectively under different current densities of 0.1, 0.3, 0.5, 0.7 and 1C. When the current density returned to 0.1C, the discharge capacity returned to 199.3 mA h g−1. Notably, HE-LNM had a capacity retention efficiency of 81.56% after 350 cycles at 0.3C and a capacity retention efficiency of 85.75% after 100 cycles even at 1C. This study offers a commercially viable cathode by a high-entropy doping strategy and guides the design of long-life, high-energy-density electrodes for next-generation LIBs.Author contributions
Jiahui Zhou completed the experimental design, experimental operation, data collection and paper writing; Jiehui Hu completed the purchase of chemical reagent, experimental operation and data collection; Xia Zhou completed the purchase of experimental consumables; Zhen Shang and Shengming Xu provided financial support and supervised this project; Yue Yang supervised this project and helped paper revision.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51834008) and the Guangxi Laboratory of New Energy Automobile Project (Guike AA 23062086).References
- R. Zhang, C. Wang, P. Zou, R. Lin, L. Ma, L. Yin, T. Li, W. Xu, H. Jia, Q. Li, S. Sainio, K. Kisslinger, S. E. Trask, S. N. Ehrlich, Y. Yang, A. M. Kiss, M. Ge, B. J. Polzin, S. J. Lee, W. Xu, Y. Ren and H. L. Xin, Nature, 2022, 610, 67–73 CrossRef CAS.
- W. E. Gent, G. M. Busse and K. Z. House, Nat. Energy, 2022, 7, 1132–1143 CrossRef CAS.
- K.-Y. Park, Y. Zhu, C. G. Torres-Castanedo, H. J. Jung, N. S. Luu, O. Kahvecioglu, Y. Yoo, J.-W. T. Seo, J. R. Downing, H.-D. Lim, M. J. Bedzyk, C. Wolverton and M. C. Hersam, Adv. Mater., 2022, 34, 2106402 CrossRef CAS PubMed.
- Z. Cui, Q. Xie and A. Manthiram, Adv. Energy Mater., 2021, 11, 2102421 Search PubMed.
- S. Lee and A. Manthiram, ACS Energy Lett., 2022, 7, 3058–3063 CrossRef CAS.
- G. M. Mudd, Z. Weng, S. M. Jowitt, I. D. Turnbull and T. E. Graedel, Ore Geol. Rev., 2013, 55, 87–98 CrossRef.
- G.-T. Park, B. Namkoong, S.-B. Kim, J. Liu, C. S. Yoon and Y.-K. Sun, Nat. Energy, 2022, 7, 946–954 CrossRef CAS.
- S. Chu, S. Guo and H. Zhou, Chem. Soc. Rev., 2021, 50, 13189–13235 RSC.
- Y. Song, Y. Chen, M. Xu, W. Wang, Y. Zhang, G. Yang, R. Ran, W. Zhou and Z. Shao, Adv. Mater., 2020, 32, 1906979 CrossRef CAS.
- R. Sim, L. Su and A. Manthiram, Adv. Energy Mater., 2023, 13, 2300096 Search PubMed.
- H. Zhao, W.-Y. A. Lam, L. Sheng, L. Wang, P. Bai, Y. Yang, D. Ren, H. Xu and X. He, Adv. Energy Mater., 2022, 12, 2103894 CrossRef CAS.
- L.-L. Zuo, Q. Ma, S.-C. Li, B.-C. Lin, M. Fan, Q.-H. Meng, X.-W. Wu, Y.-G. Guo and X.-X. Zeng, Adv. Energy Mater., 2021, 11, 2003285 CrossRef CAS.
- L. Su, E. Jo and A. Manthiram, ACS Energy Lett., 2022, 7, 2165–2172 CrossRef CAS.
- W. Guo, Y. Zhang, L. Lin, Y. Liu, M. Fan, G. Gao, S. Wang, B. Sa, J. Lin, Q. Luo, B. Qu, L. Wang, J. Shi, Q. Xie and D.-L. Peng, Small, 2023, 19, 2300175 CrossRef CAS.
- W. Yao, M. Chouchane, W. Li, S. Bai, Z. Liu, L. Li, A. X. Chen, B. Sayahpour, R. Shimizu, G. Raghavendran, M. A. Schroeder, Y.-T. Chen, D. H. S. Tan, B. Sreenarayanan, C. K. Waters, A. Sichler, B. Gould, D. J. Kountz, D. J. Lipomi, M. Zhang and Y. S. Meng, Energy Environ. Sci., 2023, 16, 1620–1630 RSC.
- Y. Kim, H. Kim, W. Shin, E. Jo and A. Manthiram, Adv. Energy Mater., 2023, 13, 2204054 Search PubMed.
- N. Voronina, Y.-K. Sun and S.-T. Myung, ACS Energy Lett., 2020, 5, 1814–1824 Search PubMed.
- C. Wang, L. Han, R. Zhang, H. Cheng, L. Mu, K. Kisslinger, P. Zou, Y. Ren, P. Cao, F. Lin and H. L. Xin, Matter, 2021, 4, 2013–2026 CrossRef CAS.
- Y.-h Luo, H.-x Wei, L.-b Tang, Y.-d Huang, Z.-y Wang, Z.-j He, C. Yan, J. Mao, K. Dai and J.-c Zheng, Energy Storage Mater., 2022, 50, 274–307 Search PubMed.
- W. Liu, J. Li, W. Li, H. Xu, C. Zhang and X. Qiu, Nat. Commun., 2020, 11, 3629 CrossRef CAS PubMed.
- S. Burke and J. F. Whitacre, Adv. Sci., 2023, 10, 2300068 CrossRef CAS.
- W. Li, S. Lee and A. Manthiram, Adv. Mater., 2020, 32, 2002718 CrossRef CAS PubMed.
- Y. Fan, E. Olsson, G. Liang, Z. Wang, A. M. D'Angelo, B. Johannessen, L. Thomsen, B. Cowie, J. Li, F. Zhang, Y. Zhao, W. K. Pang, Q. Cai and Z. Guo, Angew. Chem., Int. Ed., 2023, 62, e202213806 CrossRef CAS.
- N. Muralidharan, R. Essehli, R. P. Hermann, R. Amin, C. Jafta, J. Zhang, J. Liu, Z. Du, H. M. MeyerIII, E. Self, J. Nanda and I. Belharouak, Adv. Mater., 2020, 32, 2002960 CrossRef CAS PubMed.
- W. Jiang, C. Zhang, Y. Feng, B. Wei, L. Chen, R. Zhang, D. G. Ivey, P. Wang and W. Wei, Energy Storage Mater., 2020, 32, 37–45 CrossRef.
- L. Nie, Z. Wang, X. Zhao, S. Chen, Y. He, H. Zhao, T. Gao, Y. Zhang, L. Dong, F. Kim, Y. Yu and W. Liu, Nano Lett., 2021, 21, 8370–8377 CrossRef CAS.
- A. Bano, M. Noked and D. T. Major, Chem. Mater., 2023, 35, 8426–8439 CrossRef CAS.
- C. Zhao, F. Ding, Y. Lu, L. Chen and Y.-S. Hu, Angew. Chem., Int. Ed., 2020, 59, 264–269 CrossRef CAS PubMed.
- Z. Lun, B. Ouyang, D.-H. Kwon, Y. Ha, E. E. Foley, T.-Y. Huang, Z. Cai, H. Kim, M. Balasubramanian, Y. Sun, J. Huang, Y. Tian, H. Kim, B. D. McCloskey, W. Yang, R. J. Clément, H. Ji and G. Ceder, Nat. Mater., 2021, 20, 214–221 CrossRef CAS.
- W. Li, H. Y. Asl, Q. Xie and A. Manthiram, J. Am. Chem. Soc., 2019, 141, 5097–5101 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ee05020g |
| This journal is © The Royal Society of Chemistry 2025 |