Open Access Article
Open Access ArticleAdvances in self-assembled monolayer-engineered organic solar cells
Abdul
Azeez
 *a,
Yexiao
Huang
*a,
Yexiao
Huang
 a,
Lorreta
Stanly
a,
Lorreta
Stanly
 a,
Zhipeng
Kan
a,
Zhipeng
Kan
 b and
Safakath
Karuthedath
b and
Safakath
Karuthedath
 *ac
*ac
aInstitute of Materials Research, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen, 518055, China. E-mail: azeez@sz.tsinghua.edu.cn; safakath@sz.tsinghua.edu.cn
bCenter on Nanoenergy Research, Institute of Science and Technology for Carbon Peak & Neutrality, School of Physical Science & Technology, Guangxi University, Nanning 530004, China
cShenzhen Key Laboratory of Advanced Layered Materials for Value-added Applications, Institute of Materials Research, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen 518055, P. R. China
First published on 14th May 2025
Abstract
The emergence of high-performing donor and acceptor materials, along with tailored interfacial layers, has enabled efficient and stable organic solar cells (OSCs) that are comparable to other state-of-the-art technologies. With power conversion efficiencies (PCEs) now exceeding 20%, further advancements via interfacial and morphology engineering are critical for improving performance metrics, which are crucial for the commercialization of OSC technology. The remarkable developments in self-assembled monolayer (SAM)-engineered OSCs over the past few years call for a new assessment of their progress. The potential of SAMs in advancing the PCE of single-material OSCs also appears promising. In this review, we explore the diverse roles of SAM in OSCs, highlighting their impact on charge generation, transport, and extraction, particularly in high-efficiency, non-fullerene acceptor (NFA)-based devices. We also examine the stability challenges associated with SAM-engineered OSCs and the trade-offs between efficiency and durability. Finally, we discuss future directions, including scalability considerations for SAM-integrated OSCs. Given the recent surge of interest in SAM-engineered OSCs, this article provides valuable insights for the research community.
Broader contextThe rapidly growing energy demand necessitates a paradigmatic transition towards sustainable green energy technologies, wherein solar cells play a vital role. Organic solar cells (OSCs) are an attractive option in this context, owing to their distinctive features, including bandgap tunability, semitransparency, facile fabrication, and low payback time. Recently, OSCs have experienced a resurgence, with power conversion efficiencies (PCEs) surpassing the benchmark value of 20%. This progress can be attributed to the development of novel non-fullerene acceptor (NFA) molecules, coupled with renewed insights into photophysical processes. Notably, the recent integration of self-assembled monolayers (SAMs) in OSCs has significantly improved efficiency and stability. SAMs have emerged as a viable alternative to conventional electron and hole transport layers, such as ZnO and PEDOT:PSS, for high-performance OSCs. The charge transport barrier at the metal–organic interface can be modulated through interfacial dipoles formed by the SAM molecule to facilitate barrierless charge carrier extraction. Furthermore, owing to their facile processability, SAMs are expected to potentially replace conventional charge transport layers in large-area fabrication processes. This review systematically discusses the diverse roles of SAMs in OSCs, highlighting their impact on charge generation, transport, and extraction. We also examine the stability aspects of SAM-integrated OSCs and provide valuable insights for future research. |
1. Introduction
Global energy consumption demands a paradigm shift towards green energy technologies for sustainability. Solar cells are a viable and reliable option that can meet this demand due to their minimal environmental impact.1 Organic solar cells (OSCs) are an excellent choice in this regard owing to their key features of bandgap tunability, semitransparency, facile fabrication capability, flexibility, and low payback time.2 In recent years, OSCs have experienced a resurgence, with power conversion efficiencies (PCEs) now exceeding 20%.3 This progress is largely driven by the development of novel non-fullerene acceptor molecules, and due to better photophysical understanding.4,5 Device optimization via interfacial and morphology engineering also played a critical role in improving performance metrics.6–12 These optimizations, based on insights gained from the study of charge generation, transport, and recombination dynamics, are crucial for extracting the full potential of a given material system.5,13–16The device optimization of OSCs necessitates a meticulous selection and tuning of the optoelectronic properties of various layers and interfaces. The basic device architecture of a bulk heterojunction (BHJ) OSC consists of an active layer sandwiched between metal electrodes, a cathode, and an anode, in which one is transparent for light illumination.17 Here, the active layer in which photoabsorption and charge generation occur is a blend of donor and acceptor materials.18,19 In this case, the open circuit voltage is limited by the work function difference of the electrodes, while the back diffusion of the mobile carriers can contribute to a significant energy loss.6 To overcome this, an electron transport layer (ETL) and a hole transport layer (HTL) are introduced at the cathode-active layer and anode-active layer interfaces, respectively.20 This type of interfacial engineering for an efficient and unipolar charge carrier extraction from the active layer to the electrodes is crucial for optimal device performance in thin film OSCs.21 This modification primarily helps to selectively extract one type of carrier (electron or hole) and prevents the other from reaching the electrode, thereby suppressing significant recombination loss at the electrode interfaces (Fig. 1a).6 This type of device architecture improves the open circuit voltage and consequently the efficiency.22 Beyond improving open-circuit voltage and efficiency, these interlayers also help protect the active layer from rapid degradation by preventing direct contact with the metal electrodes.23–25
Currently, the standard device architecture adopted in laboratories consists of charge transport layers (CTLs) in addition to counter electrodes. Over the last few decades, research efforts have resulted in the development of an excellent set of electron transport layers (ETL) and hole transport layers (HTL), which are now commonly employed in standard device architectures. The most commonly used efficient ETLs include zinc oxide (ZnO), TiO2, PFN derivatives, and PDIN derivatives.20,26–28 The efficient HTLs are relatively fewer in number, including PEDOT:PSS and MoOx.29,30 These are among the most commonly used transport layers in OSCs, and each of which carries its shortcomings in terms of degradation of the device.31 Additional modifications of these CTLs via various methods or by introducing even more CTLs in conjunction are beneficial for unipolar carrier extraction and reduced recombination.6 This includes additional interlayers using self-assembled monolayers (SAMs) and polyelectrolytes, as well as the addition of plasmonic nanoparticles and optical spacer layers.32–37 Herein, we focus on the interfacial engineering of OSCs using SAMs and comprehend all utilities offered to improve the device performance. Several comprehensive reviews and perspective articles about self-assembled monolayers (SAMs) in optoelectronic devices have been published.33,38–41 While several reviews have discussed SAMs in optoelectronic devices, most have focused on hybrid perovskite solar cells.42–45 In contrast, fewer studies have examined their role in OSCs.46–48 Given the recent surge of interest in SAM-engineered OSCs, this article provides timely insights for the research community.
Self-assembled monolayers (SAMs) are an important class of nanomaterials, providing a unique means to tailor interfacial electronic and chemical properties. They feature a facile film formation, yielding crystalline domains with long-range order.33,49 These organic molecules possessing amphiphilic characteristics form a spontaneous molecular assembly on a surface of interest via chemisorption and align vertically with an angle to the surface normal.33,38 The structure of a SAM molecule generally consists of an anchoring, spacer, and a terminal group, with the anchoring group binding to the surface (Fig. 1b).38 The critical component of the SAM is the terminal group, which electronically couples with the overlayer to influence the morphology and determine the energetic alignment at the interface.50 The spacer group, also known as the linker, is usually an alkyl chain or aromatic group with their length governing the electronic isolation of the interconnecting layers. They control the lateral interaction between the molecules during the formation via van der Waals interaction and are responsible for the molecular arrangement in the film phase. The advantage of spontaneous self-assembly of SAMs, either from a solution or vapor phase provides a facile strategy to introduce high-quality interlayers on devices at different scales.50 Beyond organic solar cells, SAMs have found applications in hybrid perovskite solar cells, field-effect transistors, and biosensors, demonstrating their versatility in advanced electronic and optoelectronic systems.38,43,51–53
2. Progress and performance of SAM-engineered OSCs
The development of viable, robust, and efficient alternate charge transport layers remains an important exploration in the field of OSCs, with SAMs serving as excellent candidates for this role.11 Apart from the ease of processing, the bulk properties of CTLs are not a concern when SAMs are employed as CTLs in OSCs owing to the ultra-low thickness. As previously mentioned, the molecular structure of a SAM typically comprises an anchoring group, a spacer or linker, and a terminal group, with the anchoring group binding to the surface. The commonly used anchoring groups include carboxylic acids, boronic acids, benzoic acids, phosphonic acids, and silyl trialkoxide[–Si(OR)3].33 They are primarily utilized on top of metal oxide layers such as ITO, FTO, and ZnO and are also incorporated into the BHJ to form the active layer.54–56 From a device fabrication perspective, SAMs can be incorporated at various positions within a standard architecture, where the active layer is sandwiched between buffer layers and counter electrodes (Fig. 2).41,55,57,582.1. SAM as a charge transport layer between the metal electrode and the active layer
Appropriate HTL and ETL materials are imperative in OSCs to enable efficient and unipolar charge extraction. SAMs can be used as interfacial modifiers to effectively adjust the WF of the ITO electrode, thereby improving unipolar charge carrier extraction.6 SAMs can form dipoles at the metal–organic interface, facilitating unipolar charge carrier extraction.33Table 1 summarizes various SAMs utilized in OSCs to enhance device performance, along with the active layer components employed in the studies. Fig. 3 presents the chemical structures of the HTL-SAM molecules, while Fig. 4 illustrates the chemical structures of the ETL-SAM molecules that have been successfully employed in OSCs.| SAMs | Device architecture | w/o SAM (%) | with SAM (%) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | |||
| a HTL. b Ternary component. c ETL. d Comparison with bare ITO. e Combined effects of SAM and graphene oxide (GO) layers. | ||||||||||
| F8 | ITO/SAM/P3HT:PCBM/Al | 0.31 | 8.32 | 45 | 1.18 | 0.58 | 8.75 | 52 | 2.61 | 59 |
| CF3 | ITO/SAM/P3HT:PC61BM/Al | 0.36 | 5.38 | 35 | 0.75 | 0.60 | 13.87 | 38 | 3.15 | 60 |
| CBA | ITO/SAM/ClAlPc/C60/BCP/Al | 0.47 | 5.47 | 52 | 1.32 | 0.79 | 6.84 | 59 | 3.25 | 61 |
| CBSA | ITO/SAM/PTB7-Th:PC71BM/PFN/Al | 0.6 | 15.8 | 59 | 5.5 | 0.8 | 16.4 | 70 | 9.2 | 62 |
| CBS | ITO/SAM/CuPc/C60/BCP/Al | 0.48 | 1.27 | 26 | 0.16 | 0.45 | 5.88 | 48 | 1.27 | 63 |
| B4C | ITO/SAM/PEDOT:PSS/CuPC/C60/BCP/Al | 0.48 | 4.11 | 49 | 1.0 | 0.50 | 4.41 | 52 | 1.14 | 64 |
| B6C | ITO/SAM/PEDOT:PSS/CuPC/C60/BCP/Al | 0.48 | 4.11 | 49 | 1.0 | 0.49 | 4.21 | 50 | 1.02 | 64 |
| 3-BPIC | ITO/SAM/PM6:L8-BO:BTP-eC9/PNDIT-F3N/Ag | 0.86 | 27.69 | 78.59 | 18.74 | 0.871 | 27.89 | 79.61 | 19.34 | 65 |
| 3-BPIC-F | ITO/SAM/PM6:L8-BO:BTP-eC9/PNDIT-F3N/Ag | 0.86 | 27.69 | 78.59 | 18.74 | 0.872 | 28.12 | 80.43 | 19.71 | 65 |
| Br-2EPO | ITO/SAM/PM6:BTP-eC11/Phen-NaDPO/Al | 0.854 | 26.57 | 71.08 | 16.13 | 0.853 | 27.42 | 73.65 | 17.21 | 66 |
| Br-2EPT | ITO/SAM/PM6:BTP-eC11/Phen-NaDPO/Al | 0.854 | 26.57 | 71.08 | 16.13 | 0.853 | 27.62 | 74.22 | 17.46 | 66 |
| Br-2EPSe | ITO/SAM/PM6:BTP-eC11/Phen-NaDPO/Al | 0.854 | 26.57 | 71.08 | 16.13 | 0.855 | 27.87 | 75.12 | 17.91 | 66 |
| Cbz-2Ph | ITO/SAM/PM6:BTP-eC9/PNDIT-F3N/Ag | 0.846 | 27.86 | 72.64 | 17.12 | 0.859 | 28.62 | 76.83 | 18.89 | 67 |
| 4Cl-PACz | ITO/SAM/PM6:Y6/PDINN/Ag | 0.89 | 25.3 | 74.6 | 16.8 | 0.9 | 25.6 | 78.8 | 18.2 | 68 |
| 2PACz | ITO/SAM/PM6:N3/PFN-Br/Ag | 0.515 | 23.32 | 53.7 | 6.45 | 0.840 | 26.53 | 74.5 | 16.6 | 69 |
| F-2PACz | ITO/SAM/PM6:PM7-Si:BTP-eC9/PNDIT-F3N/Ag | 0.862 | 26.39 | 76.8 | 17.5 | 0.846 | 26.80 | 78.2 | 17.7 | 70 |
| I-2PACz | ITO/SAM/PM6:PM7-Si:BTP-eC9/PNDIT-F3N/Ag | 0.862 | 26.39 | 76.8 | 17.5 | 0.858 | 26.85 | 78.8 | 18.2 | 70 |
| 2PACz:PyCA-3F | ITO/SAM/PM1:PTQ10:m-BTP-PhC6/PFN-Br-MA/Ag | 0.878 | 26.65 | 81 | 18.95 | 0.876 | 27.57 | 80.8 | 19.51 | 71 |
| Br-2PACz | ITO/SAM/PM6:BTP-eC9:PC71BM/PFN-Br/Ag | 0.842 | 26.37 | 77.7 | 17.5 | 0.864 | 27.10 | 78.6 | 18.4 | 54 |
| 3,6-Cl-2PACz | ITO/SAM/PM6:BTP-eC9/PNDIT-F3N/Ag | 0.836 | 27.61 | 74.9 | 17.29 | 0.840 | 28.42 | 76 | 18.17 | 72 |
| 4,5-Cl-2PACz | ITO/SAM/PM6:BTP-eC9/PNDIT-F3N/Ag | 0.836 | 27.61 | 74.9 | 17.29 | 0.842 | 28.69 | 77.17 | 18.67 | 72 |
| 3PACz | ITO/SAM/PM6:BTP-eC9/PFN-Br/Ag | 0.86 | 24.9 | 75 | 16.1 | 0.86 | 25.3 | 78 | 17 | 73 |
| 4PACz | ITO/SAM/PM6:BTP-eC9/PFN-Br/Ag | 0.86 | 24.9 | 75 | 16.1 | 0.86 | 25.3 | 75 | 16.3 | 73 |
| Me-4PACz | ITO/SAM/PM6:Y6/PNDIT-F3N/Ag | 0.836 | 25.17 | 66.96 | 14.09 | 0.826 | 27.08 | 76.26 | 17.06 | 74 |
| F5BnPA | ITO/SAM/PM6:BO-4Cl/PFN-Br/Ag | 0.84 | 27.6 | 74.8 | 17.4 | 0.837 | 28.4 | 75.1 | 17.8 | 75 |
| BnPA/F5BnPA | ITO/SAM/PM6:BO-4Cl/PFN-Br/Ag | 0.84 | 27.6 | 74.8 | 17.4 | 0.845 | 28.7 | 74.4 | 18 | 75 |
| HMDS | ITO/SAM/P3HT:PC61BM/LiF/Al | 0.44 | 7.7 | 35.9 | 1.21d | 0.46 | 10.1 | 37.1 | 1.7 | 76 |
| Br-PA:HPWO | ITO/SAM/PM6:BTP-eC9:L8-F/PDINOH/Ag | 0.862 | 27.5 | 75.7 | 17.9 | 0.869 | 28.2 | 77.5 | 19.1 | 77 |
| JJ25 | ITO/SAM/PM6:BTP-eC9:dT9TBO/PNDIT-F3N/Ag | 0.847 | 28.18 | 77.10 | 18.41 | 0.856 | 28.84 | 77.15 | 19.05 | 78 |
| JJ26 | ITO/SAM/PM6:BTP-eC9:dT9TBO/PNDIT-F3N/Ag | 0.847 | 28.18 | 77.10 | 18.41 | 0.862 | 28.8 | 77.63 | 19.27 | 78 |
| CF3BA | ITO/SAM/ZnO/P3HT:PC61BM/MoO3/Al | 0.61 | 9.05 | 58.46 | 3.24 | 0.61 | 9.40 | 58.6 | 3.38 | 79 |
| OCF3BA | ITO/SAM/ZnO/P3HT:PC61BM/MoO3/Al | 0.61 | 9.05 | 58.46 | 3.24 | 0.62 | 9.44 | 59.85 | 3.48 | 79 |
| BBA | ITO/ZnO/SAM/P3HT:PCBM/WO3/Al | 0.60 | 7.83 | 53.0 | 2.49 | 0.62 | 7.88 | 60.4 | 2.94 | 56 |
| MBA | ITO/ZnO/SAM/P3HT:PCBM/WO3/Al | 0.60 | 7.83 | 53.0 | 2.49 | 0.63 | 8.77 | 60.5 | 3.34 | 56 |
| APTES | ITO/SAM/PBDTTT-C:PCBM/Ag | 0.30 | 8.65 | 24.0 | 0.64d | 0.76 | 12.42 | 50 | 4.83 | 80 |
| Serine | ITO/SAM/P3HT:PC61BM/MoO3/Al | 0.3 | 8.5 | 25.0 | 0.63 | 0.55 | 12.3 | 62 | 4.17 | 58 |
| C60-SAM | ITO/TiO2/SAM/P3HT:PC61BM/PEDOT:PSS/Ag | 0.61 | 9.80 | 46.9 | 2.8 | 0.62 | 10.6 | 57.2 | 3.8 | 81 |
| C60-SAM | ITO/ZnONPs/SAM/P3HT:PC61BM/PEDOT:PSS/Ag | 0.63 | 11.2 | 56.3 | 3.93 | 0.63 | 12.6 | 62.3 | 4.94 | 82 |
| C60-SAM | ITO/ZnO NPs/SAM/P3HT:PC61BM/PEDOT:PSS/ZnONPs/SAM/P3HT:PC61BM/PEDOT:PSS/Ag | 0.86 | 2.23 | 48.6 | 0.9 | 1.24 | 4.26 | 55 | 2.9 | 83 |
| C60-SAM | ITO/ZnO/D18:Y6: C60-SAM/MoO3/Ag | 0.81 | 25.0 | 71.0 | 14.4 | 0.81 | 25.9 | 72 | 15 | 55 |
| C3 | ITO/ZnO/SAM/PBDB-T:ITIC/MoO3/Ag | 0.90 | 15.55 | 72.58 | 10.03 | 0.89 | 16.76 | 72.89 | 10.9 | 57 |
| CT17 | ITO/TiO2/SAM/P3HT:PC61BM/MoO3/Ag | 0.57 | 5.30 | 65.70 | 2.00 | 0.60 | 5.35 | 68.9 | 2.21 | 84 |
| CT19 | ITO/TiO2/SAM/P3HT:PC61BM/MoO3/Ag | 0.57 | 5.30 | 65.70 | 2.00 | 0.61 | 5.82 | 68.4 | 2.43 | 84 |
| CT21 | ITO/TiO2/SAM/P3HT:PC61BM/MoO3/Ag | 0.60 | 6.63 | 66.60 | 2.65 | 0.61 | 6.68 | 66.7 | 2.72 | 85 |
| CT23 | ITO/TiO2/SAM/P3HT:PC61BM/MoO3/Ag | 0.60 | 6.63 | 66.60 | 2.65 | 0.61 | 6.93 | 69 | 2.91 | 85 |
| 1-OMe | FTO/c-TiO2/SAM/P3HT:PCBM/PEDOT:PSS/Ag | 0.478 | 9.42 | 48.64 | 2.19 | 0.474 | 10.60 | 44.92 | 2.26 | 86 |
| 2-OMe | FTO/c-TiO2/SAM/P3HT:PCBM/PEDOT:PSS/Ag | 0.478 | 9.42 | 48.64 | 2.19 | 0.510 | 11.15 | 41.19 | 2.34 | 86 |
| 3-OMe | FTO/c-TiO2/SAM/P3HT:PCBM/PEDOT:PSS/Ag | 0.478 | 9.42 | 48.64 | 2.19 | 0.512 | 11.51 | 46.87 | 2.76 | 86 |
| 1-F | ITO/TiO2/SAM/P3HT:PC61BM/MoO3/Ag | 0.53 | 10.55 | 41.57 | 2.33 | 0.56 | 12.48 | 46.26 | 3.23 | 87 |
| 2-F | ITO/TiO2/SAM/P3HT:PC61BM/MoO3/Ag | 0.53 | 10.55 | 41.57 | 2.33 | 0.57 | 16.51 | 45.67 | 4.35 | 87 |
| 3-F | ITO/TiO2/SAM/P3HT:PC61BM/MoO3/Ag | 0.53 | 10.55 | 41.57 | 2.33 | 0.57 | 11.37 | 51.83 | 3.36 | 87 |
| PCBM-COOHe | ITO/ZnO/SAM/PIDTTDFBT-TT:PC71BM/GO/MoO3/Ag | 0.73 | 9.5 | 44.3 | 3.08 | 0.97 | 11.6 | 64.5 | 7.29e | 88 |
| C60-SAM | ITO/ZnO/SAM/PTB7-Th:IEICO-4F/MoO3/Ag | 0.71 | 22.47 | 59.67 | 9.46 | 0.71 | 22.92 | 61.18 | 10 | 89 |
| Open-PCBM | ITO/ZnO/SAM/PTB7-Th:PC71BM/MoO3/Ag | 0.81 | 17.10 | 69.0 | 9.51 | 0.81 | 17.32 | 74 | 10.3 | 90 |
| MPPA | ITO/ZnO/PCE10:PC71BM/MoO3/Ag | 0.79 | 16.33 | 61.2 | 8.0 | 0.80 | 17.11 | 64.1 | 8.8 | 91 |
| ITO/ZnO/SAM/PBDB-T:ITIC/MoO3/Ag | 0.88 | 17.31 | 62.4 | 9.5 | 0.89 | 18.82 | 65.6 | 11 | ||
| MUA | ITO/PEDOT:PSS/P3HT:PC61BM/ZnO/SAM/Al | 0.63 | 8 | 48 | 2.4 | 0.65 | 11.1 | 63 | 4.6 | 92 |
| NPC60-OH | ITO/ZnO-C60/SAM/p-DTS(FBTTh2)2:PC71BM/MoO3/Ag | 0.780 | 14.32 | 73.1 | 8.30 | 0.78 | 15.02 | 73.5 | 8.76 | 93 |
| NPC70-OH | ITO/ZnO-C60/SAM/p-DTS(FBTTh2)2:PC71BM/MoO3/Ag | 0.780 | 14.32 | 73.1 | 8.30 | 0.79 | 15.22 | 73.7 | 9.14 | 93 |
| Catechol C60 | ITO/TiOx/SAM/P3HT:PC61BM/PEDOT:PSS/Au | 0.6 | 8.32 | 49 | 2.46 | 0.6 | 8.4 | 56 | 2.81 | 94 |
| BA derivative SAM1 | ITO/ZnO/SAM/PBDB-T:ITIC/MoO3/Ag | 0.89 | 15.53 | 72 | 9.87 | 0.89 | 16.52 | 72 | 10.59 | 95 |
| BA derivative SAM2 | ITO/ZnO/SAM/PBDB-T:ITIC/MoO3/Ag | 0.89 | 15.53 | 72 | 9.87 | 0.88 | 16.14 | 72 | 10.23 | 95 |
 | ||
| Fig. 4 Representative electron transport layer SAMs. Chemical structures of various self-assembled monolayers used as an electron transport layer in organic solar cells. | ||
Carbazole-based self-assembled monolayers (SAMs) bearing phosphonic acid anchoring groups—commonly referred to as 2PACz ([2-(9H-carbazol-9-yl)ethyl]phosphonic acid)—have emerged as key molecular components in recent high-performance non-fullerene acceptor (NFA)-based organic solar cells (OSCs) due to their efficacy in hole transport. Originally introduced by Al-Ashouri et al.96 for use in hybrid perovskite solar cells, 2PACz-SAMs demonstrated the ability to surpass the 20% power conversion efficiency (PCE) threshold without the inclusion of polymeric hole-transport layers (HTLs) such as PTAA.96 The synthesis of 2PACz is relatively straightforward and cost-effective, requiring no metal-based catalysts. Detailed procedures for its preparation were first reported by A. Magomedov et al.97 The spin-coating-driven self-assembly of 2PACz results in strong and stable binding of the phosphonic acid group to the indium tin oxide (ITO) surface.96,97 Furthermore, π–π interactions between adjacent carbazole moieties promote intrinsic stabilization and ordering within the monolayer. This structural integrity facilitates efficient hole extraction with minimal material consumption and reduced parasitic absorption.69 In 2020, Lin et al. demonstrated superior device performance in PM6:N3 BHJ OSCs utilizing 2PACz as compared to those using PEDOT:PSS as the HTL material.69 During spin coating, 2PACz SAM molecules attach to the ITO surface through the coordination of phosphoryl oxygen with the metal Lewis acid, forming an ultra-thin layer. The 2PACz SAM-functionalized ITO exhibits an increased work function, a minimal hole injection barrier, and efficient hole transfer, leading to an improved JSC.69 This finding underscores the potential for developing high-efficiency OSCs with a conventional device architecture, eliminating the need for the acidic and hygroscopic PEDOT:PSS layer.98 The significant stability implications of these PEDOT:PSS-free OSCs are discussed in a later section.
Various modifications to the 2PACz SAM molecule with different functional groups, such as F-2PACz, Cl-2PACz, Br-2PACz, I-2PACz, and MeO-2PACz, were also employed in OSCs to fine-tune the ITO work function.70 The presence of halogenated 2PACz-SAM atop ITO effectively increases its work function, yielding a better ohmic contact and efficient hole extraction compared to PEDOT:PSS HTL devices. Notably, the chlorinated 2PACZ provides optimal WF of anode for the current state-of-the-art high-performing donors such as PM6. Additionally, Wang et al. modified the position of Cl atoms in carbazole close to the molecular axis to improve the dipole moment of the Cl-2PACz molecule.72 They demonstrated that the modified 4,5-Cl-2PACz could improve the J–V parameters in PM6:BTP-eC9 OSCs with PCE close to 19%.72 Further, this molecular re-design can significantly improve the photostability of OSCs owing to the better morphological stability induced by the 4,5-Cl-2PACz SAM molecule. Similarly, Jen et al. further studied these hole-selective SAMs based on carbazole with a phosphonic acid anchor group by tuning alkyl linker length and halogen substitution.33 They employed SAMs with a range of substitutions and linker lengths (Cz-X-4 and Cz-I-n) in PM6:Y6 OSCs and found that Cz-I-2 (I-2PACz) could enhance the SAM packing regularity and density with optimal intramolecular interactions.33
Another promising HTL-SAM design for replacing PEDOT:PSS involves increasing the phosphonic acid anchoring groups to modulate the molecular dipole moment. Liu et al. tuned the dipole moment of bi-phosphonic acid-based SAMs such as 3-BPIC(i), 3-BPIC, and 3-BPIC-F, and achieved a champion PCE as high as 19.71% in PM6:L8-BO:BTP-ec9 ternary blend OSCs.65 The fluorinated SAM: 3-BPIC-F exhibits uniform distribution and coverage with short interfacial spacing. Furthermore, the deep HOMO level achieved due to the increased dipole moment of 3-BPIC-F, along with reduced interfacial resistance and a high fill factor (FF), contributed to enhanced device performance.65
2.2. SAM as an additional interfacial modifier
The introduction of SAM atop HTL or ETL can simultaneously modify the morphology of CTL and the active layer, favoring enhanced charge generation and transport. For instance, in a recent study from our group, Zhao et al. demonstrated the dual role of the MPA2FPh-BT-BA SAM (2F-SAM) molecule in PM6:Y6 OSCs.36 Here, the 2F-SAM molecule is mixed with PEDOT:PSS in the solution phase to achieve a SAM-doped PEDOT:PSS layer as HTL in the device. Incorporating the 2F-SAM molecule simultaneously improved the aggregation morphology of both PEDOT:PSS and the active layer, resulting in a PCE exceeding 17%, accompanied by a significant enhancement in the JSC. Furthermore, the 2F-SAM substantially improved the thickness tolerance of the HTL, increasing it from 30 nm to 170 nm.36The interfacial dipole formed by the 2F-SAM molecule at the anode significantly enhances the electrical conductivity of the HTL and modifies the work function of ITO, thereby promoting efficient hole extraction in the device. The benzoic acid anchoring group exhibits a strong binding affinity to oxygen vacancies on the ITO surface, resulting in a 2F-SAM-enriched bottom interface. The disparity in surface energy drives the migration of 2F-SAM molecules toward the bottom of the HTL during film formation, where they chemisorb onto the ITO surface. This interaction induces a pronounced electric dipole at the ITO/PEDOT:PSS interface, shifting the ITO work function from 5.1 eV to 5.4 eV and enabling efficient unipolar charge carrier extraction.36 Hole mobility measurements conducted on both control and 2F-SAM-doped devices demonstrate a notable enhancement, increasing from 4.96 × 10−4 cm2 V−1 s−1 to 1.35 × 10−3 cm2 V−1 s−1, thus confirming the improved hole extraction facilitated by the SAM.36 Furthermore, morphological analysis reveals that 2F-SAM doping promotes π–π stacking among conductive PEDOT chains while suppressing π–π interactions within the non-conductive PSS segments. Fig. 5 presents the J–V characteristics and corresponding external quantum efficiency (EQE) spectra of a representative PM6:Y6 OSC with and without the incorporation of 2F-SAM. These results highlight the effectiveness of SAM engineering in enhancing device performance and advancing the commercial viability of OSCs.36 The modification of PEDOT:PSS through the introduction of SAMs has been shown to significantly improve the performance of OSCs.99
Fullerene-based SAMs have demonstrated the capability to tune the work function of ZnO to match the LUMO level of the acceptor molecule.57 The net dipole moment of the SAM molecule can simultaneously shift the work function of ZnO to reduce the energy barrier and segregate the acceptor components near the electron transport layer.95 In addition to this, the photocatalytic reaction of the ZnO layer can damage the active layer components thereby accelerating the device degradation.24 The introduction of the SAM atop ZnO could suppress this reaction, consequently improving the photostability. Xu et al. demonstrated the utility of five structurally similar SAMs to improve the thin film homogeneity of the ZnO layer in PM6:Y6 inverted OSCs.100 Liu et al. developed three different SAMs based on the benzoic acid anchoring group to modify the WF of the ZnO layer for efficient electron extraction in PBDB-T:ITIC and PBDB-T-SF:ITIC-4F OSCs.95 The SAM synthesized by the condensation reaction of 4-formylbenzoic acid and electron-withdrawing group 1,3-indanedione (named SAM-1) could efficiently induce the vertical segregation of acceptor components near the cathode interface. Further, this SAM could also prevent the photocatalytic reaction of the ZnO layer thereby protecting the active layer from rapid degradation.95
2.3. SAM as a third component in the donor–acceptor blend
The sufficient solubility of certain SAM-polar organic molecules in solvents commonly used for donor–acceptor (D–A) materials offers advantages in designing SAM-based ternary blend systems with optimal phase segregation. Jeong et al. has demonstrated that C60-SAM can be incorporated as a third component in D18:Y6 BHJ OSCs to tune the active layer morphology that can improve the charge carrier lifetime and minimize recombination losses.55 In this ternary system, the vertical self-assembly of C60 SAM at the surface of the ZnO layer creates a pseudo-ZnO/SAM/BHJ configuration and simultaneously acts as a passivation layer. At the same time, C60 SAM induces molecular orientation of BHJ components from edge-on to face-on orientation, consequently enabling improved charge carrier lifetime and mobility. This results in improved JSC and FF in D18:Y6 OSCs and this strategy has also been demonstrated in PM6:Y6 and PTQ10:Y6 BHJ combinations.552.4. SAM as a hole extraction layer in single-material OSCs
Single-material devices are vital for increasing the reliability and commercial viability of OSCs.101–103 The efforts to realize high-performing single-material OSCs owing to their enhanced thermodynamic stability are of keen interest to the research community. The progress in this regard, particularly after developing high-efficiency small molecule NFAs and polymer donors, is promising.104 Block copolymer (BCP) based OSCs, in which the donor and acceptor components are chemically linked to facilitate efficient intra-chain charge generation and transport, have recently achieved significant advancements in PCE.104 The BCP material PM6-b-PYIT exhibits PCE beyond 14% with improved device lifetime.105 However, the synthetic complexity of the single material with desired optoelectronic properties remains a challenge.The development of the Y6 acceptor molecule opened up novel insights into charge generation processes in OSCs.106 Unlike fullerene derivatives and other widely used acceptors, such as the ITIC family, spectroscopic evidence indicates that Y6 can intrinsically generate charge-transfer (CT) states without requiring a donor material.107,108 The relatively high dielectric constant (∼5.3) and low exciton binding energy (∼100–250 meV) of the Y6 molecule can aid the formation of an ‘intra-moiety’ intermediate state with a CT state-like character.107 It is believed that the morphology and energetic cascade resulting from the aggregation of Y6 may play a role in this ultrafast exciton dissociation.109 The demonstration of moderately efficient Y6-based devices sensitized by the copper(I) thiocyanate (CuSCN) HTL is optimistic.110–112 In this regard, efficient and stable SAM-HTLs are excellent choices for intrinsically charge-generating single-material OSCs. The tunability of ITO WF by modifying the functional groups in carbazole-based SAMs with phosphonic acid anchoring groups (2PACz) could serve as an effective strategy to separate and extract charges from ‘Y6-like’ acceptor materials. More investigations are needed in this direction to examine the possibility of high-performing single-material OSCs using intrinsically charge-generating materials.
3. Various roles of SAM in OSCs
The addition of SAMs can impact the device performance through energy level alignment for better contact selectivity, optimization of the active layer morphology, and passivation of charge trap sites at the interfaces.55,57,69 Further, the SAM layer can protect the active layer components from direct contact with metal electrodes and/or other reactive layers to mitigate the degradation.69,72 In this section, we examine the role of SAMs in optimizing OSCs to enhance charge carrier extraction, thereby improving PCE.3.1. Energy level alignment using SAM
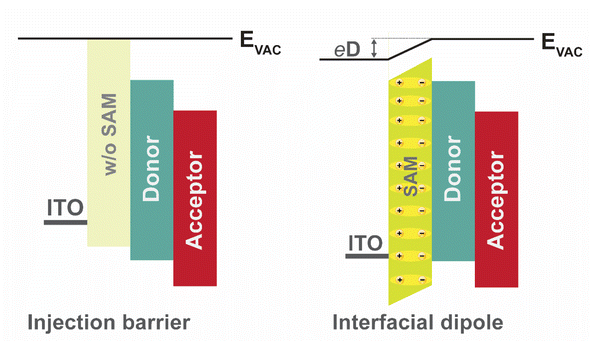
At its fundamental level, the device architecture of an OSC comprises a photoactive layer sandwiched between a high WF anode and a low WF cathode. However, in a typical OSC stack, the HTL (ETL) is employed primarily to suppress the electron (hole) transport to the anode (cathode) by introducing a high LUMO (HOMO) offset.6,113 In this way, interfacial charge recombination losses can be significantly minimized, thereby maximizing charge collection efficiency (Fig. 1a). Additionally, the transparent CTLs serve as exciton reflectors, preventing exciton quenching at the metal electrode interface, and function as optical spacers to maximize photon absorption, thereby mitigating the thickness constraints of the active layer.113 The interfacial energy level alignment of the stack must be tuned to facilitate efficient charge transport and collection to achieve high JSC, FF, and VOC values.113–115 The hole (electron) injection barrier at the metal–organic interface can be tuned via interfacial dipoles essentially altering the metal work function for a barrierless charge carrier extraction.116 Charge transport interlayers composed of self-assembled monolayer (SAM) materials or polyelectrolytes, such as polyethyleneimine ethoxylated (PEIE), are widely employed to suppress transport barriers.54,117,118The net dipole moment of the SAM molecule can effectively modify the work function of ITO by introducing the desired anchoring and terminal groups of the SAM.119,120 The work function of SAM-modified ITO changes according to the orientation of the dipoles in the SAM, enabling a tailored energy level alignment favorable for efficient carrier extraction (Fig. 6). The high WF of ITO, achieved through modification by SAM interfacial dipoles, induces Fermi-level pinning near the hole transport level, thereby facilitating efficient charge transport across the interface.54 Additionally, in contrast to traditional CTLs such as ZnO or PEDDOT:PSS, the ultra-thin nature of SAM (∼1–3 nm) could facilitate tunnelling of the separated charge carriers (when a Schottky contact is formed at the interface).121,122 A comparison study of bare ITO and ITO/CBS-SAM, Khodabakhsh et al. shows that the PCE of CuPc:C60 OSCs is highest with CBS SAM due to improved JSC and FF.63 The improved device performance was mainly attributed to a reduction in the hole injection barrier resulting in an enhanced charge carrier extraction.63 In a recent study, Lin et al. measured the WF of ITO (calculated WF of bare ITO ≈ 5.02 eV) as 5.81 eV and 4.58 eV when treated with Br-2PACz and MeO-2PACz SAMs, respectively.54 In this case, the calculated dipole moment per molecule is 3.56 D for Br-2PACz, and −0.94 D to −1.57 D for MeO-PACz SAMs, respectively.54 The charge transport across an ultra-thin SAM in an OSC device can be explained via different mechanisms, and a consensus in this regard requires detailed studies in the future.
3.2. Morphological modification of the photoactive layer using SAM
The morphology of the photoactive layer plays a critical role in determining the performance of an OSC.10 To ensure efficient exciton dissociation and charge transport, the microstructure and morphology of the active layer components must be carefully optimized.9 The morphology control in OSCs is achieved in two ways using SAM molecules. Firstly, the incorporation of a SAM on top of the electrode or transport layer prior to the deposition of the active layer can influence the thin-film formation kinetics of the active layer components. In this case, the SAM acts as a template to modify the morphology of the photoactive layer.81 In addition, the application of an ultra-thin SAM on ITO before the deposition of the organic layer can modify the wettability due to the replacement –OH terminal groups of the ITO surface.63,123 This interlayer strategy can influence the morphology of subsequent layers in the device.124 Secondly, the incorporation of the SAM molecule as a third component in the active layer blend can influence the morphology, affect phase segregation, and modify molecular orientation, thereby offering opportunities to further optimize device performance.55The halogen-substituted 2PACz-SAM, specifically 3,6-Cl-2PACz, has been demonstrated to be an effective HTL material in a variety of NFA-based OSCs. Wang et al. further enhanced the molecular dipole moment of 3,6-Cl-2PACz by introducing regio-specific chlorine substitutions near the molecular axis, resulting in the formation of 4,5-Cl-2PACz.72 This derivative potentially promotes a more robust and stable vertical distribution of the active layer components, thereby enabling high PCE and improved photostability in PM6:BTP-eC9-based OSCs. The pronounced influence of 4,5-Cl-2PACz in stabilizing active layer morphology is supported by time-of-flight secondary ion mass spectrometry (ToF-SIMS) analyses of both pristine and aged films (Fig. 7a–(d)).72 The fresh films exhibit a donor-rich (PM6) distribution proximal to the SAM layer, which is comparatively higher than that observed with PEDOT:PSS. Notably, after photodegradation, the vertical composition of the active layer remains largely unchanged in devices incorporating the 4,5-Cl-2PACz SAM. In contrast, devices employing 3,6-Cl-2PACz SAM show a significant redistribution of PM6, characterized by a decreased concentration near the HTL interface (Fig. 7d). These morphological differences account for the enhanced photostability observed in PM6:BTP-eC9 OSCs utilizing the 4,5-Cl-2PACz SAM relative to those incorporating 3,6-Cl-2PACz.72
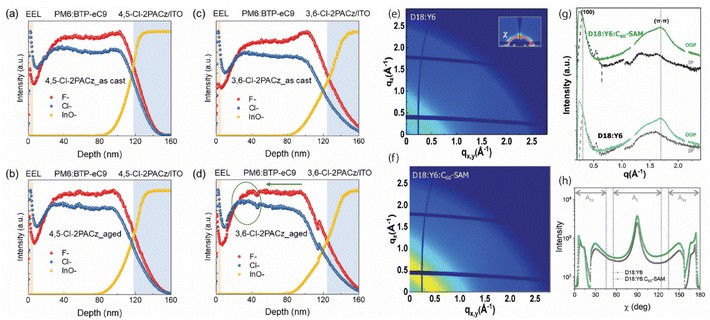 | ||
| Fig. 7 Optimization of photoactive layer morphology in organic solar cells through the implementation of self-assembled monolayers. (a–d) Show ToF-SIMS profiles for (a) fresh and (b) aged PM6:BTP-eC9 BHJ films coated on SAM 4,5-Cl-2PACz, and (c) fresh and (d) aged films coated on SAM 3,6-Cl-2PACz (note that PNDIT-F3N is the electron transport layer used in the device fabrication and were also spin-coated on the top of PM6:BTP-eC9 layer), respectively (reproduced from ref. 72 with permission from Wiley-VCH GmbH, copyright 2023) and (e–h) Grazing incidence wide-angle X-ray scattering (GIWAXS) pattern of D18:Y6 film showing the impact of C60-SAM as a third component in the photoactive layer blend. The inset of (e) is the polar angle (χ) range. (g) In-plane (black) and out-of-plane (green) line-cut profiles of the D18:Y6 and D18:Y6:C60-SAM films. (f) GIWAXS pattern of D18:Y6:C60-SAM ternary film. (h) Pole figure analysis of the blend films extracted from the lamellar diffraction82 showing an increased ratio of face-on to edge-on orientation (Axy/Az) in the ternary blend (reproduced from ref. 55 with permission from Wiley-VCH GmbH, copyright 2023).55,72 | ||
The introduction of C60-SAM atop ITO/TiO2 surface in a P3HT:PCBM BHJ-OSCs shows a clear modification of crystallinity, morphology, and phase separation of the active layer favorable for enhanced charge generation.81 In this study, Jen et al. show an increase in surface roughness of the SAM-based BHJ layer due to the preferable ordering of P3HT chains in the blend. Further, X-ray diffraction measurements reveal a clear indication of increased crystallinity of the active layer in the presence of SAM in the stack.81 Similarly, in a more recent study by Jeong et al., the C60-SAM blended with D18 and Y6 to form a ternary shows a favorable morphological tunability induced by the SAM (Fig. 7e–h).55 Pole figure analysis of the lamellar diffraction in both the binary and C60-SAM-based ternary systems reveals an increased ratio of face-on to edge-on orientation (Axy/Az) in the ternary blend (Fig. 7h). The incorporation of trace amounts of C60-SAM promotes the face-on alignment of the D18:Y6 film, thereby enhancing charge carrier mobility and charge collection efficiency, resulting in the enhancement of JSC and FF in the ternary blend OSCs.55
The control of the processing conditions of SAM can act as an excellent route to tune the active layer morphology in OSCs. For instance, Du et al. show that processing Cbz-2Ph HTL from an azeotropic solvent of isopropyl alcohol (IPA) and toluene can optimize the active layer morphology deposited atop the Cbz-2Ph SAM.125 As compared with IPA-processed SAM, the azeotropic solvent strategy resulted in a pinhole-free active layer formation with a more robust morphology.125
3.3. Trap passivation using SAM
The widespread use of SAMs in hybrid perovskite solar cells is primarily attributed to their exceptional ability to passivate traps.126,127 The crystallinity of the hybrid perovskite layer and the trap density are critical parameters for optimal device performance.42 The high density of deep trap states in the transport layer can significantly impact the charge transport and increase the non-radiative voltage losses.128 SAMs have demonstrated a superior ability to passivate defects originating from oxygen vacancies in metal oxide transport layer materials and also in the hybrid perovskite layer.126 In OSCs, the charge transport mechanism differs significantly from that of hybrid perovskite systems, exhibiting a relatively lower impact from trap passivation effects when using SAMs.129 However, recent high-performance NFA-based OSCs have demonstrated promising potential for trap passivation through SAMs.130 In addition to tuning the work function of ITO, SAMs can effectively passivate surface defects in the ITO and CTLs. For instance, Jen et al. demonstrated the utility of fullerene-based SAM (C60-SAM) to passivate the surface defects of the ZnO layer commonly used in inverted OSCs.81 The low-temperature processed ZnO ETL suffers from surface defects and can expedite the photocatalytic reaction degradation of the active layer.1313.4. Charge generation
The key distinction of OSCs from inorganic systems is that photoabsorption generates strongly bound excitons, which migrate to the D-A interface and undergo femtosecond-scale quenching. This process forms an interfacial charge transfer (CT) state, leading to exciton dissociation and the generation of spatially separated charge carriers. This process requires an optimal energetic offset exceeding the exciton binding energy in the LUMO for electrons and/or HOMO for holes.132,133 However, efficient charge generation has been observed in D–A systems without stringent energetic offset requirements and even in small-molecule NFAs such as Y6 without a donor component.5 The mechanism of charge generation in intrinsically charge-generating materials such as Y6 and similar NFAs is still at a rudimentary level.107,110The hole-selective SAMs with tuneable energy levels can serve as ‘donor-like’ components, facilitating exciton dissociation in small molecule acceptor-based single-material devices. Moreover, we anticipate that utilizing SAM as a sensitizer could enhance the tolerance of the acceptor layer thickness. In this regard, the report on single material Y6-OSCs with CuSCN-HTL with a PCE of about 4.4% is exhilarating.110 Likewise, CuSCN/PC70BM devices have demonstrated a PCE exceeding 1%, attributed to the dual functionality of the CuSCN hole transport layer.111 This suggests that the development of more robust SAM-HTLs is highly advantageous for simultaneously promoting charge generation and hole extraction in these devices, with the added potential for large-area fabrication. This finding aligns with the superior performance metrics demonstrated by SAM-based printed OSCs compared to conventional transport layers.125 However, more investigations are necessary in this direction to unlock the potential of SAMs in single-material OSCs.
4. Stability aspects of SAM-engineered OSCs
To establish OSCs as a reliable and mature technology, it is essential to mitigate rapid performance degradation. Various extrinsic factors, including photothermal stress, oxygen, and moisture, trigger chemical processes that ultimately lead to the deterioration of performance parameters.134,135 Additionally, intrinsic factors, such as chemical interactions between the charge CTL and the photoactive layer, can further accelerate degradation by impairing charge transport.131 The conventional hole transport layer PEDOT:PSS used in the standard device architecture is known to induce several degradation mechanisms due to its acidic and hygroscopic nature, effectively lowering the cell lifetime.136 Several strategies have been implemented to enhance device stability by mitigating specific degradation processes, this includes replacing CTLs with intrinsically stable and chemically inert alternatives compatible with the active layer.137–139 While various SAMs have shown promise in this context, comprehensive degradation studies remain limited.77,125,140Table 2 shows a survey of stability improvements achieved in OSCs using SAMs along with the specific improvements. For clarity, a parameter Tn is used, which indicates the time taken to reduce the PCE of the device to n% of its initial value. This is similar to the common standard of T80 lifetime reports used widely in the literature and included in the table whenever available. The stability of SAM-employed devices relies on many aspects, such as the chemical structure, layer formation kinetics, post-processing methods, reactivity with the adjacent layers, and intrinsic stability or evolution of the layer during long-term operation. This section discusses different aspects and case studies to summarize the stability of OSCs employing SAMs.| SAMs | Device structure | Role of SAM | Stability improvements with SAM | Test condition | Ref. |
|---|---|---|---|---|---|
| 2PACz | ITO/SAM/PM6:N3/PFN-Br/Ag | HTL | 120 hours for T74 (control devices reached T20 in 50 hours) | White light (LED) soaking (200 mW cm−2) | 69 |
| BA derivative SAM1 | ITO/ZnO/SAM/PBDB-T:ITIC/MoO3/Ag | ETL | 180 hours for T70 (control devices reached T30 in 180 hours) | Simulated 1-Sun illumination | 95 |
| C60-SAM | ITO/ZnO/D18:Y6:SAM/MoO3/Ag | Ternary component | More than 8 hours for T90 (control devices reached T50 within 0.33 hours) | Simulated 1-Sun illumination in N2 environment | 55 |
| M-BT | ITO/ZnO/PM6:Y6:SAM/MoO3/Ag | Ternary component | More than 1000 hours for T80 | 85 °C in N2 environment | 141 |
| APTES | ITO/SAM/PBDTTT-C:PCBM/Ag | ETL | More than 30 days for T80 (control devices with bare ITO reached T10 within 10 days) | Unencapsulated devices exposed to ambient conditions | 80 |
| B4C | ITO/SAM/PEDOT:PSS/CuPC/C60/BCP/Al | HTL | More than 98 days for T50 (control devices reached T50 within 49 days) | Shelf-life test | 64 |
| B6C | ITO/SAM/PEDOT:PSS/CuPC/C60/BCP/Al | HTL | More than 98 days for T50 (control devices reached T50 within 49 days) | Shelf-life test | 64 |
| Br-2PACz | ITO/ZnO/PFN-Br/PM6:PM7-Si:BTP-eC9/Br-2PACz/MoO3/Ag | HTL | 615 hours for T80 (297 h for device w/o SAM) | AM1.5 G light soaking (100 mW cm−2) in N2 environment | 130 |
| Br-2PACz | ITO/ZnO/PFN-Br/PM6:PM7-Si:BTP-eC9/Br-2PACz/MoO3/Ag | HTL | 1064 hours for T80 (731 h for device w/o SAM) | 80 °C in N2 environment | 130 |
| Br-2PACz/POM | ITO/SAM/POM/PM6:BTP-eC9:L8-F/PFN-Br/Ag | C-HTL | More than 1000 hours for T90 | AM1.5 G (LED) soaking (100 mW cm−2) | 77 |
| Br-2PACz/POM | ITO/SAM/POM/PM6:BTP-eC9:L8-F/PFN-Br/Ag | C-HTL | More than 1000 hours for T80 | 65 °C in N2 environment | 77 |
The thermodynamic-driven growth processes of ultra-thin SAM formation involve intermolecular interactions and anchoring to the substrate, which evolves until equilibrium is reached and a uniform layer is formed.33 In OSCs, the SAM-based CTLs are normally cast via the spin-coating method onto hydrophilic ITO substrate or interlayers. This kinetic process can be modulated via different strategies to control the properties of the amphiphilic SAM formation.49 In this case, the micelle formation can be detrimental to an optimal SAM formation particularly when high concentrations of SAM are present in the processing solvent.142 Processes, including preheating of the substrate and solvent washing of the SAM layer, can minimize this effect and improve the quality of the layer.143 A recent study by Du et al. demonstrates that an azeotrope solvent strategy to deposit Cbz-2Ph SAM as an HTL could significantly minimize the micelle formation and improve stability.125 The azeotropic solvent of IPA and toluene results in extraordinary dispersion of Cbz-2Ph SAM and a homogeneous film that is stable for more than 45 days under ambient storage.125 Another interesting strategy reported by Fan et al. to improve the stability of Br-2PACz-HTL-based OSCs is to combine the SAM with a polyoxometalate (POM – H3PW12O40) via sequential deposition on ITO.77 The resulting composite HTL employed PM6:BTP-eC9:L8-F ternary OSCs exhibits improved PCE (∼19.1%) and remarkably high thermal and photostability (T80 lifetime above 1000 hours for accelerated aging).77
Similar to processing conditions, post-processing methods, such as thermal annealing or solvent washing, can influence the morphology of the SAM and, consequently, the morphology of the active layer. Processing conditions, kinetics, and post-processing methods dictate the molecular orientation, surface morphology, molecular packing, and surface binding strength and can impact the device performance and stability.49,144–146 For instance, thermal annealing of the SAM CTL can enhance the binding to the substrate and mitigate the rapid degradation of the devices.33,147 It must be noted that the molecular orientation and binding strength can modify the ITO work function effectively providing a route to improve charge extraction in the devices.119
In several recent studies, it has been demonstrated that the ITO/SAM electrode is exceptionally stable in comparison with the ITO/PEDOT:PSS configuration due to the relatively inert behavior of the SAM to the active layer.69,77 In the 2PACz family of HTLs, the phosphonic acid linkers bind to the oxygen sites of the ITO substrate, thereby reducing the detrimental reactions to the active layer components. For instance, the Cl-substituted 2PACz-SAM has been demonstrated to enhance the photostability of PM6:BTP-eC9 OSCs, attributable to its inherently higher photostability compared to PEDOT:PSS. Notably, replacing PEDOT:PSS with 4,5-Cl-2PACz resulted in a significant improvement in photostability, increasing from approximately 100 hours to over 800 hours (Fig. 8e–h). However, some reports show that these highly efficient SAM-HTLs are also prone to induce photodegradation and can react with active layer components.140,148 To suppress these degradation issues, Han et al. demonstrated that combining NiOx with SAM could be beneficial.140 This necessitates a reassessment of degradation processes induced by interlayers in OSCs, as well as a comprehensive investigation of degradation mechanisms involving SAMs.
 | ||
| Fig. 8 Stability improvements demonstrated in organic solar cells by introducing self-assembled monolayers (a–d) show normalized decay of PCE, VOC, JSC, and FF, indicating the photostability improvements by introducing C60-SAM as a third component in D18:Y6 bulk heterojunction organic solar cells, respectively,55 and (e–h) show normalized decay of PCE, VOC, FF, and JSC, demonstrating the photostability improvements by introducing Cl-2PACz SAM as a hole transport layer instead of PEDOT:PSS in PM6:BTP-eC9 organic solar cells, respectively72 (Reproduced from ref. 55 with permission from Wiley-VCH GmbH, copyright 2023, and ref. 72 with permission from Wiley-VCH GmbH, copyright 2023). | ||
Although OSCs with an inverted architecture (Anode deposited on top of the stack) utilizing a ZnO electron transport layer exhibit relatively high stability, several factors may still impact overall device durability.149,150 Notably, the photocatalytic reactions of ZnO with active layer components can disrupt conjugation, thereby impairing charge transport properties.151 This is mainly driven by the formation of OH free radicals due to large surface defects in the ZnO layer and the presence of UV lights in the illumination.131,150,152 It has been reported that the incorporation of C60-SAM as a third component in D18:Y6 BHJ OSCs significantly enhances their photostability (Fig. 8a–d). The vertical self-assembly of C60-SAM near the cathode interface effectively passivates surface traps on the ZnO layer, thereby suppressing undesirable photocatalytic reactions.55 However, relatively few efficient and stable CTLs are available for inverted OSCs, highlighting the need for the design and synthesis of additional SAM-ETLs that surpass commonly used ZnO in performance to enhance device stability.
5. Outlook
SAMs represent an exemplary class of materials for modifying OSC interfaces via a low-cost and facile process. By serving as an interlayer, SAMs can reduce the energy barrier for charge transport. As an active layer component, they can directly modulate morphology and carrier extraction, enhancing device performance. Notably, SAMs have demonstrated potential as alternatives to conventional hole and electron transport layers, such as PEDOT:PSS and ZnO, in high-performing NFAs-based OSCs. Despite their promise, new SAMs must be explored to capitalize on their facile processability and rich molecular design possibilities. A deeper understanding of charge carrier dynamics in SAM-engineered devices is still needed. Moreover, SAMs can concurrently enhance charge transport and stability in state-of-the-art OSCs, although the stability aspect has received limited attention. The trap passivation capabilities of SAMs can improve chemical stability and device lifetime, and the emerging trends of slow degradation mechanisms in SAM-engineered devices warrant further investigation. Below listed are the major challenges and exciting prospects in the field, providing a roadmap for future research and development.(1) Designing functional molecules: the development of design rules for functional groups in SAM molecules that interact with specific active layer components will be crucial in optimizing the efficiency and stability of OSCs. This can bring additional advantages of tuning active layer composition in addition to electrode work function.
(2) Overcoming solubility and film uniformity challenges: addressing solubility issues and achieving uniform layer deposition will be essential for realizing the full potential of SAM-engineered OSCs.
(3) Exploiting SAMs as active layer components: exploring the use of SAMs as active layer components could lead to breakthroughs in device architecture and performance metrics. Given the critical role of active layer morphology in both bulk and planar heterojunction devices, SAMs can be utilized as a versatile tool for optimizing morphology in diverse active layer architectures of OSCs.
(4) Investigating charge carrier dynamics: studying the charge carrier dynamics in SAM-engineered devices will provide a deeper understanding of their operational mechanisms. Comprehensive investigations employing a combination of time- and frequency-domain experimental techniques can offer valuable insights into charge-transfer state dissociation and charge-transport mechanisms in SAM-incorporated OSCs. These findings can help in designing novel SAM molecules with tailored functionalities, thereby enabling further optimization of OSC performance.
(5) Mitigating recombination losses: developing SAMs for modulating optoelectronic properties and trap passivation of commonly employed CTLs could be further explored as a strategy to suppress the recombination losses and further improve J–V parameters.
(6) Elucidating degradation mechanisms: investigating the degradation mechanisms in SAM-engineered OSCs will provide valuable insights into enhancing the stability and commercial viability of OSCs. The primary focus should be directed toward isolating and analyzing the detrimental effects of SAM molecules on the active layer, as well as elucidating the evolution of these effects under various extrinsic factors, including light, thermal stress, oxygen, and moisture.
6. Summary
In summary, we have presented the different roles of self-assembled monolayers in state-of-the-art organic solar cells. SAMs have exhibited the potential as an alternative to commonly used electron and hole transport layers such as ZnO and PEDOT:PSS for high-performing organic solar cells. However, considering the facile processability and richness in molecular design, new SAMs need to be synthesized and explored. A detailed understanding of the charge carrier dynamics in SAM-engineered devices is still lacking. They function to simultaneously improve the charge transport and the stability of OSCs, even though the stability aspect is seldom explored. It is anticipated that SAMs could replace the conventionally used charge transport layer in the large-area fabrication. The feasibility of commercialization relies on the facile processing methods and stability. The passivation capability of SAMs could improve the chemical stability and device lifetime, and the early trends of slow degradation in SAM-engineered organic solar cells need to be exploited in the future.Data availability
The data supporting this article have been included within the article. No new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. K acknowledges financial support from the National Natural Science Foundation of China (W2432044), Scientific Research Start-up fund (Grant No. QD2023005C), Shenzhen Science and Technology Program (JCYJ20240813112055071 and ZDSYS20230626091100001), and Guangdong General Funding (2025A1515011578). Z. K. acknowledges the financial support from the National Natural Science Foundation of China (62275057) and the Guangxi Natural Science Foundation (2023GXNSFFA026004 and 2022GXNSFDA035066).References
- M. Hasanuzzaman, U. S. Zubir, N. I. Ilham and H. Seng Che, Global electricity demand, generation, grid system, and renewable energy polices: a review, Wiley Interdiscip. Rev.:Energy Environ., 2017, 6(3), e222 Search PubMed.
- M. Riede, D. Spoltore and K. Leo, Organic solar cells—the path to commercial success, Adv. Energy Mater., 2021, 11(1), 2002653 CrossRef CAS.
- L. Zhu, M. Zhang, G. Zhou, Z. Wang, W. Zhong and J. Zhuang, et al., Achieving 20.8% organic solar cells via additive-assisted layer-by-layer fabrication with bulk pin structure and improved optical management, Joule, 2024, 8(11), 3153–3168 CrossRef CAS.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip and T.-K. Lau, et al., Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core, Joule, 2019, 3(4), 1140–1151 CrossRef CAS.
- S. Karuthedath, J. Gorenflot, Y. Firdaus, N. Chaturvedi, C. S. De Castro and G. T. Harrison, et al., Intrinsic efficiency limits in low-bandgap non-fullerene acceptor organic solar cells, Nat. Mater., 2021, 20(3), 378–384 CrossRef CAS PubMed.
- B. Walker, H. Choi and J. Y. Kim, Interfacial engineering for highly efficient organic solar cells, Curr. Appl. Phys., 2017, 17(3), 370–391 CrossRef.
- A. Azeez and K. Narayan, Enhanced device performance via interfacial engineering in non-fullerene acceptor based organic solar cells, Appl. Phys. Lett., 2020, 117(4), 043302 CrossRef CAS.
- Z. Zhao, S. Chung, Y. Y. Kim, M. Jeong, X. Li and J. Zhao, et al., Room-temperature-modulated polymorphism of nonfullerene acceptors enables efficient bilayer organic solar cells, Energy Environ. Sci., 2024, 17(15), 5666–5678 RSC.
- F. Zhao, C. Wang and X. Zhan, Morphology control in organic solar cells, Adv. Energy Mater., 2018, 8(28), 1703147 CrossRef.
- R. Vijayan, A. Azeez and K. Narayan, Toward reliable high performing organic solar cells: Molecules, processing, and monitoring, APL Mater., 2020, 8(4), 040908 CrossRef CAS.
- H. Tang, Y. Bai, H. Zhao, X. Qin, Z. Hu and C. Zhou, et al., Interface engineering for highly efficient organic solar cells, Adv. Mater., 2024, 36(16), 2212236 CrossRef CAS.
- L. Tian, Q. Xue, Z. Hu and F. Huang, Recent advances of interface engineering for non-fullerene organic solar cells, Org. Electron., 2021, 93, 106141 CrossRef CAS.
- J. Wang, Y. Xie, K. Chen, H. Wu, J. M. Hodgkiss and X. Zhan, Physical insights into non-fullerene organic photovoltaics, Nat. Rev. Phys., 2024, 1–17 Search PubMed.
- L. Perdigón-Toro, H. Zhang, A. Markina, J. Yuan, S. M. Hosseini and C. M. Wolff, et al., Barrierless free charge generation in the high-performance PM6: Y6 bulk heterojunction non-fullerene solar cell, Adv. Mater., 2020, 32(9), 1906763 CrossRef PubMed.
- L. J. Hart, J. Grüne, W. Liu, T. Lau, J. Luke and Y. C. Chin, et al., Understanding the Role of Triplet-Triplet Annihilation in Non-Fullerene Acceptor Organic Solar Cells, Adv. Energy Mater., 2023, 13(36), 2301357 CrossRef CAS.
- B. Sun, N. Tokmoldin, O. Alqahtani, A. Patterson, C. S. De Castro and D. B. Riley, et al., Toward more efficient organic solar cells: a detailed study of loss pathway and its impact on overall device performance in Low-Offset organic solar cells, Adv. Energy Mater., 2023, 13(26), 2300980 CrossRef CAS.
- A. Azeez, and K. Narayan, Solution-Processed Photovoltaics, Energy Materials: World Scientific, 2023, pp. 1–33 Search PubMed.
- A. J. Heeger, 25th anniversary article: bulk heterojunction solar cells: understanding the mechanism of operation, Adv. Mater., 2014, 26(1), 10–28 CrossRef CAS.
- Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Bulk heterojunction solar cells: morphology and performance relationships, Chem. Rev., 2014, 114(14), 7006–7043 CrossRef CAS PubMed.
- Z. Liang, Q. Zhang, L. Jiang and G. Cao, ZnO cathode buffer layers for inverted polymer solar cells, Energy Environ. Sci., 2015, 8(12), 3442–3476 RSC.
- Q. Liao, Q. Kang, Y. Yang, C. An, B. Xu and J. Hou, Tailoring and modifying an organic electron acceptor toward the cathode interlayer for highly efficient organic solar cells, Adv. Mater., 2020, 32(7), 1906557 CrossRef CAS PubMed.
- J. Luo, H. Wu, C. He, A. Li, W. Yang and Y. Cao, Enhanced open-circuit voltage in polymer solar cells, Appl. Phys. Lett., 2009, 95(4), 043301 CrossRef.
- Y. Li, X. Huang, K. Ding, Jr H. K. Sheriff, L. Ye and H. Liu, et al., Non-fullerene acceptor organic photovoltaics with intrinsic operational lifetimes over 30 years, Nat. Commun., 2021, 12(1), 5419 CrossRef CAS PubMed.
- Y. Jiang, L. Sun, F. Jiang, C. Xie, L. Hu and X. Dong, et al., Photocatalytic effect of ZnO on the stability of nonfullerene acceptors and its mitigation by SnO 2 for nonfullerene organic solar cells, Mater. Horiz., 2019, 6(7), 1438–1443 RSC.
- M. T. Lloyd, D. C. Olson, P. Lu, E. Fang, D. L. Moore and M. S. White, et al., Impact of contact evolution on the shelf life of organic solar cells, J. Mater. Chem., 2009, 19(41), 7638–7642 RSC.
- C. Sun, Z. Wu, Z. Hu, J. Xiao, W. Zhao and H.-W. Li, et al., Interface design for high-efficiency non-fullerene polymer solar cells, Energy Environ. Sci., 2017, 10(8), 1784–1791 RSC.
- H. Zhang, W. Zhou, C. Yu, J. Guo and F. Li, Revealing working mechanisms of PFN as a cathode interlayer in conventional and inverted polymer solar cells, Phys. Chem. Chem. Phys., 2019, 21(36), 20065–20072 RSC.
- J. Yao, B. Qiu, Z.-G. Zhang, L. Xue, R. Wang and C. Zhang, et al., Cathode engineering with perylene-diimide interlayer enabling over 17% efficiency single-junction organic solar cells, Nat. Commun., 2020, 11(1), 2726 CrossRef CAS PubMed.
- J. Meyer, S. Hamwi, M. Kröger, W. Kowalsky, T. Riedl and A. Kahn, Transition metal oxides for organic electronics: energetics, device physics and applications, Adv. Mater., 2012, 24(40), 5408–5427 CrossRef CAS.
- Y. Lin, B. Adilbekova, Y. Firdaus, E. Yengel, H. Faber and M. Sajjad, et al., 17% efficient organic solar cells based on liquid exfoliated WS2 as a replacement for PEDOT: PSS, Adv. Mater., 2019, 31(46), 1902965 CrossRef CAS PubMed.
- J. Cameron and P. J. Skabara, The damaging effects of the acidity in PEDOT: PSS on semiconductor device performance and solutions based on non-acidic alternatives, Mater. Horiz., 2020, 7(7), 1759–1772 RSC.
- L. Yan, Y. Song, Y. Zhou, B. Song and Y. Li, Effect of PEI cathode interlayer on work function and interface resistance of ITO electrode in the inverted polymer solar cells, Org. Electron., 2015, 17, 94–101 CrossRef CAS.
- M. Li, M. Liu, F. Qi, F. R. Lin and A. K.-Y. Jen, Self-assembled monolayers for interfacial engineering in solution-processed thin-film electronic devices: design, fabrication, and applications, Chem. Rev., 2024, 124(5), 2138–2204 CrossRef CAS PubMed.
- J. Y. Kim, S. H. Kim, H. H. Lee, K. Lee, W. Ma and X. Gong, et al., New architecture for high-efficiency polymer photovoltaic cells using solution-based titanium oxide as an optical spacer, Adv. Mater., 2006, 18(5), 572–576 CrossRef CAS.
- A. Uddin and X. Yang, Surface plasmonic effects on organic solar cells, J. Nanosci. Nanotechnol., 2014, 14(2), 1099–1119 CrossRef CAS.
- B. Zhao, X. Huang, S. Chung, M. Zhang, Y. Zhong and A. Liang, et al., Hole-selective-molecule doping improves the layer thickness tolerance of PEDOT: PSS for efficient organic solar cells, eScience, 2024, 100305 Search PubMed.
- B. A. Courtright and S. A. Jenekhe, Polyethylenimine interfacial layers in inverted organic photovoltaic devices: Effects of ethoxylation and molecular weight on efficiency and temporal stability, ACS Appl. Mater. Interfaces, 2015, 7(47), 26167–26175 CrossRef CAS.
- M. Singh, N. Kaur and E. Comini, The role of self-assembled monolayers in electronic devices, J. Mater. Chem. C, 2020, 8(12), 3938–3955 RSC.
- S. Casalini, C. A. Bortolotti, F. Leonardi and F. Biscarini, Self-assembled monolayers in organic electronics, Chem. Soc. Rev., 2017, 46(1), 40–71 RSC.
- M. Halik, and A. Hirsch, The Potential of Molecular Self-Assembled Monolayers in Organic Electronic Devices, Wiley Online Library, 2011 Search PubMed.
- M. Li, Y. Xie, F. R. Lin, Z. Li, S. Yang and A. K.-Y. Jen, Self-assembled monolayers as emerging hole-selective layers enable high-performance thin-film solar cells, Innovation, 2023, 4(1), 100369 CAS.
- K. Choi, H. Choi, J. Min, T. Kim, D. Kim and S. Y. Son, et al., A short review on interface engineering of perovskite solar cells: a self-assembled monolayer and its roles, Sol. RRL, 2020, 4(2), 1900251 CrossRef.
- S. Y. Kim, S. J. Cho, S. E. Byeon, X. He and H. J. Yoon, Self-assembled monolayers as interface engineering nanomaterials in perovskite solar cells, Adv. Energy Mater., 2020, 10(44), 2002606 CrossRef CAS.
- F. Ali, C. Roldán-Carmona, M. Sohail and M. K. Nazeeruddin, Applications of self-assembled monolayers for perovskite solar cells interface engineering to address efficiency and stability, Adv. Energy Mater., 2020, 10(48), 2002989 CrossRef CAS.
- B. Li, Y. Chen, Z. Liang, D. Gao and W. Huang, Interfacial engineering by using self-assembled monolayer in mesoporous perovskite solar cell, RSC Adv., 2015, 5(114), 94290–94295 RSC.
- J. Hu, W. Fu, X. Yang and H. Chen, Self-assembled monolayers for interface engineering in polymer solar cells, J. Polym. Sci., 2022, 60(15), 2175–2190 CrossRef CAS.
- H. Ma, H. L. Yip, F. Huang and A. K. Y. Jen, Interface engineering for organic electronics, Adv. Funct. Mater., 2010, 20(9), 1371–1388 CrossRef CAS.
- E. D. Gomez and Y.-L. Loo, Engineering the organic semiconductor-electrode interface in polymer solar cells, J. Mater. Chem., 2010, 20(32), 6604–6611 RSC.
- A. Ulman, Formation and structure of self-assembled monolayers, Chem. Rev., 1996, 96(4), 1533–1554 CrossRef CAS.
- C. Nicosia and J. Huskens, Reactive self-assembled monolayers: from surface functionalization to gradient formation, Mater. Horiz., 2014, 1(1), 32–45 RSC.
- J. Collet, O. Tharaud, A. Chapoton and D. Vuillaume, Low-voltage, 30 nm channel length, organic transistors with a self-assembled monolayer as gate insulating films, Appl. Phys. Lett., 2000, 76(14), 1941–1943 CrossRef CAS.
- H.-F. Ji, E. Finot, R. Dabestani, T. Thundat, G. M. Brown and P. F. Britt, A novel self-assembled monolayer (SAM) coated microcantilever for low level caesium detection, Chem. Commun., 2000,(6), 457–458 RSC.
- A. S. Sizov, E. V. Agina and S. A. Ponomarenko, Self-assembled semiconducting monolayers in organic electronics, Russ. Chem. Rev., 2018, 87(12), 1226 CrossRef CAS.
- Y. Lin, A. Magomedov, Y. Firdaus, D. Kaltsas, A. El-Labban and H. Faber, et al., 18.4% organic solar cells using a high ionization energy self-assembled monolayer as hole-extraction interlayer, ChemSusChem, 2021, 14(17), 3569–3578 CrossRef CAS.
- S. Jeong, A. Rana, J. H. Kim, D. Qian, K. Park and J. H. Jang, et al., New Ternary Blend Strategy Based on a Vertically Self-Assembled Passivation Layer Enabling Efficient and Photostable Inverted Organic Solar Cells, Adv. Sci., 2023, 2206802 CrossRef CAS.
- Y. E. Ha, M. Y. Jo, J. Park, Y.-C. Kang, S. I. Yoo and J. H. Kim, Inverted type polymer solar cells with self-assembled monolayer treated ZnO, J. Phys. Chem. C, 2013, 117(6), 2646–2652 CrossRef CAS.
- S. Zhang, L. Zhan, S. Li, C.-Z. Li and H. Chen, Enhanced performance of inverted non-fullerene organic solar cells through modifying zinc oxide surface with self-assembled monolayers, Org. Electron., 2018, 63, 143–148 CrossRef CAS.
- A. Li, R. Nie, X. Deng, H. Wei, S. Zheng and Y. Li, et al., Highly efficient inverted organic solar cells using amino acid modified indium tin oxide as cathode, Appl. Phys. Lett., 2014, 104(12), 123303 CrossRef.
- H. Wang, E. D. Gomez, Z. Guan, C. Jaye, M. F. Toney and D. A. Fischer, et al., Tuning contact recombination and open-circuit voltage in polymer solar cells via self-assembled monolayer adsorption at the organic–metal oxide interface, J. Phys. Chem. C, 2013, 117(40), 20474–20484 CrossRef CAS.
- J. S. Kim, J. H. Park, J. H. Lee, J. Jo, D.-Y. Kim and K. Cho, Control of the electrode work function and active layer morphology via surface modification of indium tin oxide for high efficiency organic photovoltaics, Appl. Phys. Lett., 2007, 91(11), 112111 CrossRef.
- N. Beaumont, I. Hancox, P. Sullivan, R. A. Hatton and T. S. Jones, Increased efficiency in small molecule organic photovoltaic cells through electrode modification with self-assembled monolayers, Energy Environ. Sci., 2011, 4(5), 1708–1711 RSC.
- L. Huang, L. Chen, P. Huang, F. Wu, L. Tan and S. Xiao, et al., Triple dipole effect from self-assembled small-molecules for high performance organic photovoltaics, Adv. Mater., 2016, 28(24), 4852–4860 CrossRef CAS PubMed.
- S. Khodabakhsh, B. M. Sanderson, J. Nelson and T. S. Jones, Using self-assembling dipole molecules to improve charge collection in molecular solar cells, Adv. Funct. Mater., 2006, 16(1), 95–100 CrossRef CAS.
- M.-C. Chen, Y.-S. Chiou, J.-M. Chiu, A. Tedla and Y. Tai, Marked improvement in the stability of small molecule organic photovoltaics by interfacial modification using self-assembled monolayers to prevent indium diffusion into the active layer, J. Mater. Chem. A, 2013, 1(11), 3680–3687 RSC.
- H. Liu, Y. Xin, Z. Suo, L. Yang, Y. Zou and X. Cao, et al., Dipole moments regulation of Biphosphonic acid molecules for self-assembled monolayers boosts the efficiency of organic solar cells exceeding 19.7%, J. Am. Chem. Soc., 2024, 146(20), 14287–14296 CrossRef CAS PubMed.
- A. Ullah, K. H. Park, Y. Lee, S. Park, A. B. Faheem and H. D. Nguyen, et al., Versatile hole selective molecules containing a series of heteroatoms as self-assembled monolayers for efficient p-i-n perovskite and organic solar cells, Adv. Funct. Mater., 2022, 32(49), 2208793 CrossRef CAS.
- G. Du, W. Jiang, N. Zhang, Y. Wang, M. Liu and T. Lei, et al., Efficient organic solar cells with a printed p–i–n stack enabled by an azeotrope-processed self-assembled monolayer, Energy Environ. Sci., 2025, 18(2), 799–806 RSC.
- R. Geng, P. Liu, R. Pan, H. Xu, S. Gao and Z. Zhang, et al., Self-assembled monolayers featuring multi-chlorinated carbazole unit for improving hole extraction efficiency in organic photoelectronic device, Chem. Eng. J., 2023, 454, 140138 CrossRef CAS.
- Y. Lin, Y. Firdaus, F. H. Isikgor, M. I. Nugraha, E. Yengel and G. T. Harrison, et al., Self-assembled monolayer enables hole transport layer-free organic solar cells with 18% efficiency and improved operational stability, ACS Energy Lett., 2020, 5(9), 2935–2944 CrossRef CAS.
- Y. Lin, Y. Zhang, J. Zhang, M. Marcinskas, T. Malinauskas and A. Magomedov, et al., 18.9% Efficient Organic Solar Cells Based on n-Doped Bulk-Heterojunction and Halogen-Substituted Self-Assembled Monolayers as Hole Extracting Interlayers, Adv. Energy Mater., 2022, 12(45), 2202503 CrossRef CAS.
- D. Li, Q. Lian, T. Du, R. Ma, H. Liu and Q. Liang, et al., Co-adsorbed self-assembled monolayer enables high-performance perovskite and organic solar cells, Nat. Commun., 2024, 15(1), 7605 CrossRef CAS.
- Y. Wang, W. Jiang, S. C. Liu, C. T. Lin, B. Fan and Y. Li, et al., Durable Organic Photovoltaics Enabled by a Morphology-Stabilizing Hole-Selective Self-Assembled Monolayer, Adv. Energy Mater., 2024, 14(5), 2303354 CrossRef CAS.
- H. Bin, K. Datta, J. Wang, T. P. van der Pol, J. Li and M. M. Wienk, et al., Finetuning hole-extracting monolayers for efficient organic solar cells, ACS Appl. Mater. Interfaces, 2022, 14(14), 16497–16504 CrossRef CAS.
- Y. Han, J. Chu, R. Zhang, L. Zhang, L. Sun and Y. Zhang, Enhanced efficiency and stability of PM6: Y6 organic solar cells using hydrophobic self-assembled monolayers, Appl. Phys. Lett., 2024, 125(5), 053504 CrossRef CAS.
- J. Hu, C. He, X. Zheng, Y. Li, X. Yang and W. Wang, et al., Tailoring Self-Assembled Monolayers for High-Performance Polymer Solar Cells with Improved Stability, Sol. RRL, 2023, 7(6), 2201106 CrossRef CAS.
- S. Das, J. Joslin and T. Alford, Self-assembled monolayer modified ITO in P3HT: PC61BM organic solar cells with improved efficiency, Sol. Energy Mater. Sol. Cells, 2014, 124, 98–102 CrossRef CAS.
- B. Fan, H. Gao, Y. Li, Y. Wang, C. Zhao and F. R. Lin, et al., Integration of polyoxometalate clusters with self-assembled monolayer for efficient and robust organic solar cells, Joule, 2024, 8(5), 1443–1456 CrossRef CAS.
- W. Jiang, Y. Li, H. Gao, L. Kong, C.-T. Wong and X. Yang, et al., Regiospecific Halogenation Modulates Molecular Dipoles in Self-Assembled Monolayers for High-Performance Organic Solar Cells, Angew. Chem., Int. Ed. Engl., 2025, e202502215 Search PubMed.
- P.-J. Hsu, A. Tedla, M. Zharnikov and Y. Tai, Dual interfacial modifications of an organic solar cell by self-assembled monolayers, J. Photochem. Photobiol., A, 2022, 422, 113554 CrossRef CAS.
- M. Song, J.-W. Kang, D.-H. Kim, J.-D. Kwon, S.-G. Park and S. Nam, et al., Self-assembled monolayer as an interfacial modification material for highly efficient and air-stable inverted organic solar cells, Appl. Phys. Lett., 2013, 102(14), 143303 CrossRef.
- S. K. Hau, H.-L. Yip, O. Acton, N. S. Baek, H. Ma and A. K.-Y. Jen, Interfacial modification to improve inverted polymer solar cells, J. Mater. Chem., 2008, 18(42), 5113–5119 RSC.
- S. K. Hau, H.-L. Yip, H. Ma and A. K.-Y. Jen, High performance ambient processed inverted polymer solar cells through interfacial modification with a fullerene self-assembled monolayer, Appl. Phys. Lett., 2008, 93(23), 233304 CrossRef.
- S. K. Hau, H.-L. Yip, K.-S. Chen, J. Zou and A. K.-Y. Jen, Solution processed inverted tandem polymer solar cells with self-assembled monolayer modified interfacial layers, Appl. Phys. Lett., 2010, 97(25), 253307 CrossRef.
- C. Tozlu, A. Mutlu, M. Can, A. K. Havare, S. Demic and S. Icli, Effect of TiO2 modification with amino-based self-assembled monolayer on inverted organic solar cell, Appl. Surf. Sci., 2017, 422, 1129–1138 CrossRef CAS.
- A. Mutlu, M. Can and C. Tozlu, Performance improvement of organic solar cell via incorporation of donor type self-assembled interfacial monolayer, Thin Solid Films, 2019, 685, 88–96 CrossRef CAS.
- Ç. Kırbıyık, D. A. Kara, K. Kara, S. Büyükçelebi, M. Z. Yiğit and M. Can, et al., Improving the performance of inverted polymer solar cells through modification of compact TiO2 layer by different boronic acid functionalized self-assembled monolayers, Appl. Surf. Sci., 2019, 479, 177–184 CrossRef.
- Ç. Kırbıyık, M. Can and M. Kuş, Interfacial modification via boronic acid functionalized self-assembled monolayers for efficient inverted polymer solar cells, Mater. Sci. Semicond. Process., 2020, 107, 104860 CrossRef.
- J. J. Intemann, K. Yao, Y. X. Li, H. L. Yip, Y. X. Xu and P. W. Liang, et al., Highly Efficient Inverted Organic Solar Cells Through Material and Interfacial Engineering of Indacenodithieno [3, 2-b] thiophene-Based Polymers and Devices, Adv. Funct. Mater., 2014, 24(10), 1465–1473 CrossRef CAS.
- X. Xu, J. Xiao, G. Zhang, L. Wei, X. Jiao and H.-L. Yip, et al., Interface-enhanced organic solar cells with extrapolated T80 lifetimes of over 20 years, Sci. Bull., 2020, 65(3), 208–216 CrossRef CAS.
- C. Z. Li, J. Huang, H. Ju, Y. Zang, J. Zhang and J. Zhu, et al., Modulate Organic-Metal Oxide Heterojunction via [1, 6] Azafulleroid for Highly Efficient Organic Solar Cells, Adv. Mater., 2016, 28(33), 7269–7275 CrossRef CAS.
- T. Hu, X. Lv, X. Cheng, L. Huang, L. Zhang and W. Zhou, et al., Self-assembly monolayers manipulate the power conversion processes in organic photovoltaics, J. Power Sources, 2019, 409, 66–75 CrossRef CAS.
- H.-L. Yip, S. K. Hau, N. S. Baek and A. K.-Y. Jen, Self-assembled monolayer modified ZnO/metal bilayer cathodes for polymer/fullerene bulk-heterojunction solar cells, Appl. Phys. Lett., 2008, 92(19), 193313 CrossRef.
- S.-H. Liao, H.-J. Jhuo, Y.-S. Cheng, V. Gupta and S.-A. Chen, A high performance inverted organic solar cell with a low band gap small molecule (p-DTS (FBTTh 2) 2) using a fullerene derivative-doped zinc oxide nano-film modified with a fullerene-based self-assembled monolayer as the cathode, J. Mater. Chem. A, 2015, 3(45), 22599–22604 RSC.
- I. Jeon, K. Ogumi, T. Nakagawa and Y. Matsuo, Enhancement of fill factor in air-processed inverted organic solar cells using self-assembled monolayer of fullerene catechol, Jpn. J. Appl. Phys., 2016, 55(8), 082301 CrossRef.
- H. Liu, Z. X. Liu, S. Wang, J. Huang, H. Ju and Q. Chen, et al., Boosting organic–metal oxide heterojunction via conjugated small molecules for efficient and stable nonfullerene polymer solar cells, Adv. Energy Mater., 2019, 9(34), 1900887 CrossRef.
- A. Al-Ashouri, A. Magomedov, M. Roß, M. Jošt, M. Talaikis and G. Chistiakova, et al., Conformal monolayer contacts with lossless interfaces for perovskite single junction and monolithic tandem solar cells, Energy Environ. Sci., 2019, 12(11), 3356–3369 RSC.
- A. Magomedov, A. Al-Ashouri, E. Kasparavičius, S. Strazdaite, G. Niaura and M. Jošt, et al., Self-assembled hole transporting monolayer for highly efficient perovskite solar cells, Adv. Energy Mater., 2018, 8(32), 1801892 CrossRef.
- J. Jing, S. Dong, K. Zhang, B. Xie, J. Zhang and Y. Song, et al., In-situ self-organized anode interlayer enables organic solar cells with simultaneously simplified processing and greatly improved efficiency to 17.8%, Nano Energy, 2022, 93, 106814 CrossRef CAS.
- Y. R. Kim, O. J. Sandberg, S. Zeiske, G. Burwell, D. B. Riley and P. Meredith, et al., Mitigating Detrimental Effect of Self-Doping Near the Anode in Highly Efficient Organic Solar Cells, Adv. Funct. Mater., 2023, 33(16), 2300147 CrossRef CAS.
- Z. Xu, R. Meitzner, A. Anand, A. S. Djoumessi, S. Stumpf and C. Neumann, et al., Dual-Use Self-Assembled Monolayer Controlling Charge Carrier Extraction in Organic Solar Cells, Small Methods, 2024, 8(9), 2301451 CrossRef CAS PubMed.
- J. Roncali and I. Grosu, The dawn of single material organic solar cells, Adv. Sci., 2019, 6(1), 1801026 CrossRef PubMed.
- A. Sharma, N. Gasparini, A. Markina, S. Karuthedath, J. Gorenflot and H. Xu, et al., Semitransparent Organic Photovoltaics Utilizing Intrinsic Charge Generation in Non-Fullerene Acceptors, Adv. Mater., 2024, 36(9), 2305367 CrossRef CAS.
- Y. Firdaus, V. M. Le Corre, S. Karuthedath, W. Liu, A. Markina and W. Huang, et al., Long-range exciton diffusion in molecular non-fullerene acceptors, Nat. Commun., 2020, 11(1), 5220 CrossRef CAS.
- B. Li, X. Yang, S. Li and J. Yuan, Stable block copolymer single-material organic solar cells: progress and perspective, Energy Environ. Sci., 2023, 16(3), 723–744 RSC.
- Y. Wu, Q. Fan, B. Fan, F. Qi, Z. Wu and F. R. Lin, et al., Non-fullerene acceptor doped block copolymer for efficient and stable organic solar cells, ACS Energy Lett., 2022, 7(7), 2196–2202 CrossRef CAS.
- M. B. Price, P. A. Hume, A. Ilina, I. Wagner, R. R. Tamming and K. E. Thorn, et al., Free charge photogeneration in a single component high photovoltaic efficiency organic semiconductor, Nat. Commun., 2022, 13(1), 2827 CrossRef CAS PubMed.
- P. A. Hume, M. B. Price and J. M. Hodgkiss, New Avenues for Organic Solar Cells Using Intrinsically Charge-Generating Materials, JACS Au, 2024, 4(4), 1295–1302 CrossRef CAS PubMed.
- P. Wan, X. Chen, Q. Liu, S. Mahadevan, M. Guo and J. Qiu, et al., Direct observation of the charge transfer states from a non-fullerene organic solar cell with a small driving force, J. Phys. Chem. Lett., 2021, 12(43), 10595–10602 CrossRef CAS PubMed.
- Y. Fu, T. H. Lee, Y.-C. Chin, R. A. Pacalaj, C. Labanti and S. Y. Park, et al., Molecular orientation-dependent energetic shifts in solution-processed non-fullerene acceptors and their impact on organic photovoltaic performance, Nat. Commun., 2023, 14(1), 1870 CrossRef CAS.
- E. Sağlamkaya, A. Musiienko, M. S. Shadabroo, B. Sun, S. Chandrabose and O. Shargaieva, et al., What is special about Y6; the working mechanism of neat Y6 organic solar cells, Mater. Horiz., 2023, 10(5), 1825–1834 RSC.
- S. Karuthedath, J. Gorenflot, Y. Firdaus, W. Y. Sit, F. Eisner and A. Seitkhan, et al., Charge and triplet exciton generation in neat PC70BM films and hybrid CuSCN: PC70BM solar cells, Adv. Energy Mater., 2019, 9(1), 1802476 CrossRef.
- Y. Firdaus, A. Seitkhan, F. Eisner, W. Y. Sit, Z. Kan and N. Wehbe, et al., Charge Photogeneration and Recombination in Mesostructured CuSCN-Nanowire/PC70BM Solar Cells, Sol. RRL, 2018, 2(8), 1800095 CrossRef.
- W. Tress. Device Physics of Organic Solar Cells. 2011 Search PubMed.
- J.-K. Tan, R.-Q. Png, C. Zhao and P. K. Ho, Ohmic transition at contacts key to maximizing fill factor and performance of organic solar cells, Nat. Commun., 2018, 9(1), 3269 CrossRef.
- W. Tress and O. Inganäs, Simple experimental test to distinguish extraction and injection barriers at the electrodes of (organic) solar cells with S-shaped current–voltage characteristics, Sol. Energy Mater. Sol. Cells, 2013, 117, 599–603 CrossRef CAS.
- C. J. Brabec, S. E. Shaheen, C. Winder, N. S. Sariciftci and P. Denk, Effect of LiF/metal electrodes on the performance of plastic solar cells, Appl. Phys. Lett., 2002, 80(7), 1288–1290 CrossRef CAS.
- Y. Zhou, C. Fuentes-Hernandez, J. Shim, J. Meyer, A. J. Giordano and H. Li, et al., A universal method to produce low–work function electrodes for organic electronics, Science, 2012, 336(6079), 327–332 CrossRef CAS.
- T. Yang, M. Wang, C. Duan, X. Hu, L. Huang and J. Peng, et al., Inverted polymer solar cells with 8.4% efficiency by conjugated polyelectrolyte, Energy Environ. Sci., 2012, 5(8), 8208–8214 RSC.
- R. Grzibovskis, A. Aizstrauts, A. Pidluzhna, M. Marcinskas, A. Magomedov and S. Karazhanov, et al., Energy-Level Interpretation of Carbazole Derivatives in Self-Assembling Monolayer, Molecules, 2024, 29(9), 1910 CrossRef CAS PubMed.
- K. Demirkan, A. Mathew, C. Weiland, Y. Yao, A. Rawlett and J. Tour, et al., Energy level alignment at organic semiconductor/metal interfaces: Effect of polar self-assembled monolayers at the interface, J. Chem. Phys., 2008, 128(7), 074705 CrossRef CAS.
- J. G. Simmons, Generalized formula for the electric tunnel effect between similar electrodes separated by a thin insulating film, J. Appl. Phys., 1963, 34(6), 1793–1803 CrossRef.
- G. D. Kong, S. E. Byeon, S. Park, H. Song, S. Y. Kim and H. J. Yoon, Mixed molecular electronics: tunneling behaviors and applications of mixed self-assembled monolayers, Adv. Electron. Mater., 2020, 6(2), 1901157 CrossRef CAS.
- T. Yılmaz and Ç. Kırbıyık Kurukavak, Comparison of self-assembled monolayers with long alkyl chains on ITO for enhanced surface properties and photovoltaic performance, Opt. Quantum Electron., 2021, 53(4), 170 CrossRef.
- N. R. Armstrong, C. Carter, C. Donley, A. Simmonds, P. Lee and M. Brumbach, et al., Interface modification of ITO thin films: organic photovoltaic cells, Thin Solid Films, 2003, 445(2), 342–352 CrossRef CAS.
- G. Du, W. Jiang, N. Zhang, Y. Wang, M. Liu and T. Lei, et al., Efficient organic solar cells with a printed p–i–n stack enabled by an azeotrope-processed self-assembled monolayer, Energy Environ. Sci., 2025, 18(2), 799–806 RSC.
- J. Suo, B. Yang, D. Bogachuk, G. Boschloo and A. Hagfeldt, The dual use of SAM molecules for efficient and stable perovskite solar cells, Adv. Energy Mater., 2025, 15(2), 2400205 CrossRef CAS.
- J. Han, H. Kwon, E. Kim, D.-W. Kim, H. J. Son and D. H. Kim, Interfacial engineering of a ZnO electron transporting layer using self-assembled monolayers for high performance and stable perovskite solar cells, J. Mater. Chem. A, 2020, 8(4), 2105–2113 RSC.
- D. Luo, R. Su, W. Zhang, Q. Gong and R. Zhu, Minimizing non-radiative recombination losses in perovskite solar cells, Nat. Rev. Mater., 2020, 5(1), 44–60 CrossRef CAS.
- J. Wu, H. Cha, T. Du, Y. Dong, W. Xu and C. T. Lin, et al., A comparison of charge carrier dynamics in organic and perovskite solar cells, Adv. Mater., 2022, 34(2), 2101833 CrossRef CAS PubMed.
- Y. Lin, Y. Zhang, A. Magomedov, E. Gkogkosi, J. Zhang and X. Zheng, et al., 18.73% efficient and stable inverted organic photovoltaics featuring a hybrid hole-extraction layer, Mater. Horiz., 2023, 10(4), 1292–1300 RSC.
- A. Azeez and K. Narayan, Dominant Effect of UV-Light-Induced “Burn-in” Degradation in Non-Fullerene Acceptor Based Organic Solar Cells, J. Phys. Chem. C, 2021, 125(23), 12531–12540 CrossRef CAS.
- S. Few, J. M. Frost and J. Nelson, Models of charge pair generation in organic solar cells, Phys. Chem. Chem. Phys., 2015, 17(4), 2311–2325 RSC.
- R. Shivanna, S. Rajaram and K. Narayan, Role of Charge-Transfer State in Perylene-Based Organic Solar Cells, ChemistrySelect, 2018, 3(32), 9204–9210 CrossRef CAS.
- W. R. Mateker and M. D. McGehee, Progress in understanding degradation mechanisms and improving stability in organic photovoltaics, Adv. Mater., 2017, 29(10), 1603940 CrossRef.
- M. Wang, F. Xie, J. Du, Q. Tang, S. Zheng and Q. Miao, et al., Degradation mechanism of organic solar cells with aluminum cathode, Sol. Energy Mater. Sol. Cells, 2011, 95(12), 3303–3310 CrossRef CAS.
- M. De Jong, L. Van Ijzendoorn and M. De Voigt, Stability of the interface between indium-tin-oxide and poly (3, 4-ethylenedioxythiophene)/poly (styrenesulfonate) in polymer light-emitting diodes, Appl. Phys. Lett., 2000, 77(14), 2255–2257 CrossRef CAS.
- M.-F. Xu, L.-S. Cui, X.-Z. Zhu, C.-H. Gao, X.-B. Shi and Z.-M. Jin, et al., Aqueous solution-processed MoO3 as an effective interfacial layer in polymer/fullerene based organic solar cells, Org. Electron., 2013, 14(2), 657–664 CrossRef CAS.
- H. Zhou, Y. Zhang, C.-K. Mai, J. Seifter, T.-Q. Nguyen and G. C. Bazan, et al., Solution-processed pH-neutral conjugated polyelectrolyte improves interfacial contact in organic solar cells, ACS Nano, 2015, 9(1), 371–377 CrossRef CAS.
- E. J. Lee, S. W. Heo, Y. W. Han and D. K. Moon, An organic–inorganic hybrid interlayer for improved electron extraction in inverted polymer solar cells, J. Mater. Chem. C, 2016, 4(13), 2463–2469 RSC.
- H. Xu, A. Sharma, J. Han, B. P. Kirk, A. R. Alghamdi and F. Xu, et al., The Role of Self-Assembled Monolayers in the Performance-Stability Trade-Off in Organic Solar Cells, Adv. Energy Mater., 2024, 14(44), 2401262 CrossRef CAS.
- S. Lee, J. S. Jin, H. Moon, J.-H. Kim, K. Park and J. Oh, et al., Long-Term Thermal Stability of Nonfullerene Organic Solar Cells via Facile Self-Assembled Interface Passivation, ACS Energy Lett., 2023, 8(10), 3989–3998 CrossRef CAS.
- M. Liu, L. Bi, W. Jiang, Z. Zeng, S. W. Tsang and F. R. Lin, et al., Compact hole-selective self-assembled monolayers enabled by disassembling micelles in solution for efficient perovskite solar cells, Adv. Mater., 2023, 35(46), 2304415 CrossRef CAS.
- H. Dietrich and D. Zahn, Molecular mechanisms of solvent-controlled assembly of phosphonate monolayers on oxide surfaces, J. Phys. Chem. C, 2017, 121(33), 18012–18020 CrossRef CAS.
- N. Cernetic, T. Weidner, J. E. Baio, H. Lu, H. Ma and A. K. Y. Jen, Enhanced Performance of Self-Assembled Monolayer Field-Effect Transistors with Top-Contact Geometry through Molecular Tailoring, Heated Assembly, and Thermal Annealing, Adv. Funct. Mater., 2015, 25(33), 5376–5383 CrossRef CAS.
- C. D. Bain, E. B. Troughton, Y. T. Tao, J. Evall, G. M. Whitesides and R. G. Nuzzo, Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold, J. Am. Chem. Soc., 1989, 111(1), 321–335 CrossRef CAS.
- R. Yamada, H. Sakai and K. Uosaki, Solvent effect on the structure of the self-assembled monolayer of alkanethiol, Chem. Lett., 1999, 28(7), 667–668 CrossRef.
- E. L. Hanson, J. Schwartz, B. Nickel, N. Koch and M. F. Danisman, Bonding self-assembled, compact organophosphonate monolayers to the native oxide surface of silicon, J. Am. Chem. Soc., 2003, 125(51), 16074–16080 CrossRef CAS PubMed.
- F. A. Angel, Y. L. Lyubarskaya, A. A. Shestopalov and C. W. Tang, Degradation of self-assembled monolayers in organic photovoltaic devices, Org. Electron., 2014, 15(12), 3624–3631 CrossRef CAS.
- K. Wang, C. Liu, T. Meng, C. Yi and X. Gong, Inverted organic photovoltaic cells, Chem. Soc. Rev., 2016, 45(10), 2937–2975 Search PubMed.
- S. Park and H. J. Son, Intrinsic photo-degradation and mechanism of polymer solar cells: the crucial role of non-fullerene acceptors, J. Mater. Chem. A, 2019, 7(45), 25830–25837 RSC.
- K. Park, J.-H. Kim, J. S. Jin, H. Moon, J. Oh and S. Lee, et al., Overcoming the Interfacial Photocatalytic Degradation of Nonfullerene Acceptor-Based Organic Photovoltaics by Introducing a UV-A-Insensitive Titanium Suboxide Layer, ACS Appl. Mater. Interfaces, 2024, 16(3), 3778–3785 Search PubMed.
- G. Sherafatipour, J. Benduhn, B. R. Patil, M. Ahmadpour, D. Spoltore and H.-G. Rubahn, et al., Degradation pathways in standard and inverted DBP-C70 based organic solar cells, Sci. Rep., 2019, 9(1), 4024 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |