Chemical analysis of Chang'e-5 lunar soil using LA-ICP-MS in highly diluted fused glass discs †
Shitou
Wu
 *ab,
Dingshuai
Xue
*ab,
Dingshuai
Xue
 ab,
Yueheng
Yang
ab,
Yueheng
Yang
 ab,
Hao
Wang
ab,
Chunlai
Li
c and
Fuyuan
Wu
ab
ab,
Hao
Wang
ab,
Chunlai
Li
c and
Fuyuan
Wu
ab
aState Key Laboratory of Lithospheric and Environmental Coevolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, 100029, P.R. China. E-mail: shitou.wu@mail.iggcas.ac.cn
bCollege of Earth and Planetary Science, University of Chinese Academy of Sciences, Beijing, 100049, P.R. China
cKey Laboratory of Lunar and Deep Space Exploration, National Astronomical Observatories, Chinese Academy of Sciences, 100101, Beijing, P.R. China
First published on 31st October 2024
Abstract
The bulk chemical compositions of extraterrestrial materials can provide critical information on the evolution and magmatism of planetary bodies. However, accurate measurements are challenging because these samples, particularly returned samples, are extremely limited and valuable. We demonstrate the practicality of chemical analysis using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) on highly diluted fused glass discs (lithium borate flux![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sample = 100
sample = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Only 30 mg of sample is required to produce a lithium borate disc, which is one magnitude lower than previously used (>300 mg). We proposed a modified Jet sample cone (the orifice diameter enlarging from 1.1 mm to 1.2 mm) to improve the sensitivity by a factor of ∼4.5 compared to that of standard sample cone. The interferences from the lithium borate discs were evaluated systemically, and corrections are necessary for some elements (e.g., Si, Ca, Pb). The limits of detection for most elements are in the range of 0.1–1.0 μg g−1. The analytical precision is better than 30% (relative standard deviation) for elements with concentrations of >0.5 μg g−1. The accuracy is better than 15% (relative deviation), as demonstrated by analyses of BCR-2 and BHVO-1 rock powder reference materials. This innovative technique was applied to lunar samples collected by Chang'e-5 (CE-5) mission, and an independent dataset of 38 major and trace elements was obtained. We report the average chemical composition of the CE-5 lunar soil samples, which will be an important reference for future study. This method will be useful for other precious extraterrestrial samples (e.g., CE-6 lunar samples).
1). Only 30 mg of sample is required to produce a lithium borate disc, which is one magnitude lower than previously used (>300 mg). We proposed a modified Jet sample cone (the orifice diameter enlarging from 1.1 mm to 1.2 mm) to improve the sensitivity by a factor of ∼4.5 compared to that of standard sample cone. The interferences from the lithium borate discs were evaluated systemically, and corrections are necessary for some elements (e.g., Si, Ca, Pb). The limits of detection for most elements are in the range of 0.1–1.0 μg g−1. The analytical precision is better than 30% (relative standard deviation) for elements with concentrations of >0.5 μg g−1. The accuracy is better than 15% (relative deviation), as demonstrated by analyses of BCR-2 and BHVO-1 rock powder reference materials. This innovative technique was applied to lunar samples collected by Chang'e-5 (CE-5) mission, and an independent dataset of 38 major and trace elements was obtained. We report the average chemical composition of the CE-5 lunar soil samples, which will be an important reference for future study. This method will be useful for other precious extraterrestrial samples (e.g., CE-6 lunar samples).
1 Introduction
The bulk chemical compositions of extraterrestrial materials can provide critical information about the evolution and magmatism of planetary bodies.1,2 For example, the incompatible trace element ratio (e.g., La/Sm, Nb/Ta, and Zr/Y) can constrain the degree of magmatic differentiation and assimilation of host rocks,3 and Ni contents can be used to quantify exotic addition.4 However, cosmochemical materials, particularly returned samples, are valuable; therefore, non-destructive techniques or techniques using small sample sizes are preferred.5 Instrumental neutron activation analysis (INAA), as a non-destructive technique, has previously been used for analyzing the chemical compositions of geochemical and cosmochemical samples.6,7 However, the analytical efficiency of this technique is very low due to the requirement of the radioactive source, and several important elements could not be analyzed, e.g., Si, Er.The introduction of ICP-MS to geochemistry and cosmochemistry fields in the 1980s led to more elemental data being reported for extraterrestrial materials.8 The advantages of ICP-MS are low detection limits (sub-ng g−1 level), the ability of multiple element analyses, and high analysis efficiency, making it as the most popular technique for multiple element analyses. However, this technique generally requires 50–100 mg of sample and uses acid digestion to dissolve powders, inevitably destroying the sample. Although previous studies attempted to reduce the sample size to <50 mg using low-dilution ratio and highly sensitive ICP-MS, it is still not able to avoid destroying the samples and may suffer from matrix effects due to the high salinity effect.4 Another issue faced by ICP-MS is the instability or adsorption of high field strength elements (e.g., Ta) in acid solutions, leading to inaccurate results for these elements.9
Laser ablation (LA)-ICP-MS is another technique for measuring bulk chemical compositions, which requires converting samples to homogeneous solid state materials (e.g., glass and powder pellets).10–19 Flux-free fusion leads to less contamination, but it may be affected by the loss of volatile elements (e.g., Pb) during the high temperature melting (>1500 °C).15,17 In addition, flux-free fusion is not able to produce homogeneous glasses for ultramafic rocks (e.g. CE-5 basalt, SiO2 < 47%).20 The addition of flux is necessary to avoid mineral crystallization during the rapid cooling of the melt. Lithium borate is a commonly used flux. Traditionally, the flux![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sample ratio is in a range of 3
sample ratio is in a range of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–10
1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This dilution factor requires 300–1250 mg of sample, which is cleatly a large amount for valuable samples.
1. This dilution factor requires 300–1250 mg of sample, which is cleatly a large amount for valuable samples.
In this study, we firstly demonstrate the practicality of chemical analysis using LA-ICP-MS on highly diluted fused glass discs (flux![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sample = 100
sample = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Only 30 mg of sample is required to produce the standard lithium borate discs (for XRF analysis). The sample size could be down to sub-mg levels with the proportionally reduced flux. The challenges of LA-ICP-MS analysis on such highly diluted fused glass discs are: (1) requirement of high sensitivity due to the low sample proportion (∼1%); (2) identification and correction of interferences. The Jet sample cone was modified with the orifice diameter enlarging from 1.1 mm to 1.2 mm, leading to ∼4.5 fold sensitivity improvement compared to that using standard sample cone. We systematically evaluated the interferences from lithium borate discs, and corrections are necessary for some elements (e.g., Si, Ca, Pb). The analytical precision and accuracy were evaluated using the BCR-2 and BHVO-1 rock powder reference materials. We then applied this method to the Chang'e-5 (CE-5) lunar soil sample and obtained an independent chemical concentration dataset for 38 elements.
1). Only 30 mg of sample is required to produce the standard lithium borate discs (for XRF analysis). The sample size could be down to sub-mg levels with the proportionally reduced flux. The challenges of LA-ICP-MS analysis on such highly diluted fused glass discs are: (1) requirement of high sensitivity due to the low sample proportion (∼1%); (2) identification and correction of interferences. The Jet sample cone was modified with the orifice diameter enlarging from 1.1 mm to 1.2 mm, leading to ∼4.5 fold sensitivity improvement compared to that using standard sample cone. We systematically evaluated the interferences from lithium borate discs, and corrections are necessary for some elements (e.g., Si, Ca, Pb). The analytical precision and accuracy were evaluated using the BCR-2 and BHVO-1 rock powder reference materials. We then applied this method to the Chang'e-5 (CE-5) lunar soil sample and obtained an independent chemical concentration dataset for 38 elements.
2 Experiment and methods
2.1 Sample information
The CE-5 lunar soil sample analyzed here is CE5C0800YJFM002 obtained from China's CE-5 mission to the Moon.21–26 The soil samples are composed mostly of basalt, impact glass, agglutinates, and mineral fragments. Detailed information on the CE-5 lunar sample is reported elsewhere21–26 and summarized in the ESI file.† Two certified basaltic reference materials (BCR-2 and BHVO-1) were used for quality control. The recommended values of these two materials were taken from GeoReM database (http://georem.mpch-mainz.gwdg.de/).272.2 Preparation of highly diluted fused glass discs (flux![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) sample = 100
sample = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1)
1)
Pre-mixed anhydrous lithium borate powder (67% lithium tetraborate, 33% lithium metaborate; Claisse, Quebec, Canada) was used as the flux. The lithium borate flux (3000.0 ± 0.2 mg) and sample (30.0 ± 0.1 mg) were weighed into a Pt–Au crucible and melted to the highly diluted fused glass discs (Fig. 1). The preparation method is largely followed from Xue et al.28 and the detailed descriptions are summarized in the ESI file.†
 | ||
| Fig. 1 Photographs of (a) CE-5 lunar soil samples and (b) the highly diluted lithium borate disc used for LA-ICP-MS analysis. | ||
2.3 LA-ICP-MS analysis
Elemental abundances in the fused glass discs were determined by LA-ICP-MS at the Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, China. The approach was similar to that outlined by Wu et al.29 The instrument parameters are listed in ESI Table S1.† A modified Jet sample cone (orifice diameter enlarged from 1.1 mm to 1.2 mm) for element XR SF-ICP-MS was used to improve the instrument sensitivity. ARM-2 reference glass was used as the external calibration29,30 and BCR-2 and BHVO-1 glass discs were analyzed for quality control. The bulk normalization strategy based on Li2O, B2O3, SiO2, TiO2, Al2O3, FeO, MnO, MgO, CaO, K2O, and P2O5 as 100 wt% was used for data processing.31 The data were reduced using an in-house built DRS31 in the iolite software platform.32 This approach could calculate the concentration data without applying a known internal standard obtained from an independent analysis (e.g., XRF). The detailed descriptions of this approach were included in a previous study.313 Results and discussion
3.1 Sensitivity improvement using the modified sample cone
The precise determination of a low proportion of sample (1%) in lithium borate discs requires the improvement of instrument sensitivity. The previous study demonstrated that the Jet sample cone has ∼3-fold sensitivity improvement than the standard sample cone.33 To further improve the sensitivity, we enlarged the orifice diameter of Jet sample cone from 1.1 mm to 1.2 mm (ESI Fig. S1†) as a larger orifice allows more ions to pass through the sample cone, leading to better sensitivity. Although the first stage vacuum pressure (between the sample and skimmer cones) increases by a factor of 1.5, it does not affect the sustainability of system vacuum. It should be mentioned that the orifice diameter could not be further enlarged due to the deterioration of the interface vacuum to a value that the instrument could not open the skimmer value.Fig. 2a shows the intensities of the 139La and 238U signals using the standard cone, Jet cone and modified Jet cone, indicating ∼4.5-fold sensitivity improvement. Such improved sensitivity is helpful for the LA-ICP-MS analysis in highly diluted fused glass discs. The limits of detections (LODs) of 38 elements were calculated following Pettke et al.34 (Fig. 2b; ESI Table S3†). The LODs using the modified Jet cone are clearly better than those using the standard cone (Fig. 2b). In general, the LODs of most trace elements are in a range of 0.1–1.0 μg g−1. The light elements have higher LODs than heavy elements. The detection capability of our analytical protocol is enough for CE-5, BCR-2 and BHVO-1 samples (Fig. 2b).
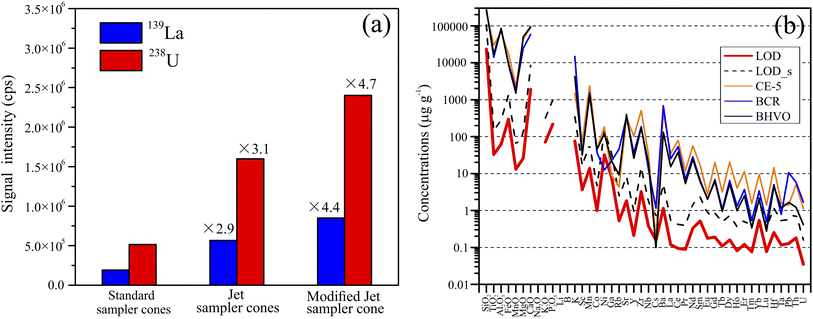 | ||
| Fig. 2 (a) Maximum 137La and 238U intensities using standard, Jet, and modified Jet sample cones in Ar plasma while maintaining a Th+/U+ ratio of 0.9–1.1 and ThO+/Th+ ratio of <0.5%. The sample is NIST SRM 612 with laser parameters of 50 μm spot size, 5 Hz frequence, and 3.0 J cm−2 energy density. (b) Limits of detections (LODs) of the 38 elements calculated using the protocol of Pettke et al. (2012)34 using the modified Jet cones and “LOD_s” represents the values using standard cones. The compositions of BCR-2, BHVO-1, and CE-5 are shown for comparison. | ||
3.2 Interferences from lithium borate discs and their corrections
Interferences from the lithium borate discs could heavily bias the data. Such interferences in highly diluted fused glass discs are much severer than those in standard fused glass discs because of the low-proportion sample (∼1%). ESI Fig. S2† shows the chemical compositions of the lithium borate discs. For some elements (e.g., Si and Ti), multiple isotopes were analyzed to identify the sources of the interferences. Two sources of interferences were identified: (1) impurities in the reagent and contaminations during preparation and (2) Li-, B-, O-, and Ar-based polyatomic ions. ESI Table S2† lists the potential Li-, B-, O-, and Ar-based polyatomic interferences on targeted isotopes (e.g., 11B16O on 27Al, 7Li40Ar on 47Ti, and 11B40Ar on 51V).These interferences must be identified and cottected before obtaining high quality data. Isotopes affected by impurities in the flux reagent include 29Si, 43Ca, 60Ni, 65Cu, 66Zn, and 137Ba. Contamination affects mainly 208Pb (ESI Fig. S2†). In general, the lithium borate discs are clean, and only a few elements (Al, Si, P, Ca, Ti, and V) have contents higher than 10 μg g−1; however, these interferences could greatly affect the accuracy due to the high dilution of the fused glass discs. For example, the P interference from lithium borate discs on the sample is about 1000 μg g−1, which could not be effectively corrected for the CE-5 lunar sample, while Si, Fe, Ti, Mg, Cr, and Mn could be effectively corrected. ESI Fig. S3† compares the interference-corrected and uncorrected SiO2 results, indicating such corrections are necessary to obtain the accurate data. The recommended approach is measuring the minor and trace elements in blank flux discs and subtracting those values from each analysis of the lunar soil sample.
3.3 Analytical precision and accuracy
The analytical accuracy and precision were evaluated using the BCR-2 and BHVO-1 lithium borate discs. The data are summarized in ESI Table S4† and plotted in ESI Fig. S4.† The accuracies of major elements and trace elements are better than 10% and 15% (ESI Fig. S4†) respectively, except for a few elements with low concentrations (<0.5 μg g−1) (e.g., Cs and Lu in BCR-2 and Lu, Yb, Ta in BHVO-1). There is a negative linear correlation between the concentrations and RSD on logarithmic plots (ESI Fig. S5†). For the trace elements with concentrations of >0.50 μg g−1, the analytical precision is better than 30%.3.4 Application to Chang'e-5 lunar soil
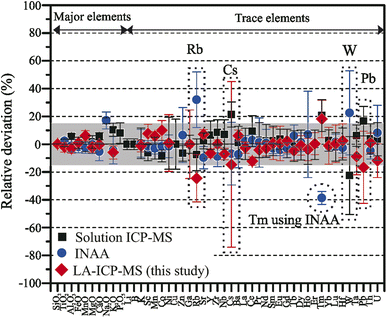
A total of 26 spot analyses were made on CE-5 lunar soil lithium borate discs. The laser spots were randomly distributed across the discs to check their homogeneity. The data are listed in Table 1, showing that the discs are relatively homogeneous. Previous INAA and solution ICP-MS data4,21,35 are also listed for comparison. Fig. 3 plots the deviation of the data acquired using different techniques relative to the mean values. The data match within 15% relative deviation (Fig. 3), except for Rb, Cs, W, and Pb.| Samples | CE5C0400 | CE5C0800 | CE5C0800 | CE5C0800 | Overall mean | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Techniques | ICP-MS | INAA | XRF | LA-ICP-MS | ||||||
| Values | 2SD | Values | 2SD | Values | 2SD | Values | 2SD | Values | 2SD | |
| n = 7 | n = 1 | n = 1 | n = 1 | |||||||
| SiO2 | — | — | — | — | 42.2 | 0.3 | 42.3 | 1.0 | 42.3 | 0.3 |
| TiO2 | 5.12 | 0.14 | 5.19 | 0.03 | 5.00 | 0.06 | 4.97 | 0.36 | 5.07 | 0.21 |
| Al2O3 | 11.6 | 0.2 | 10.8 | 0.5 | 10.8 | 0.2 | 10.6 | 0.7 | 10.9 | 0.8 |
| FeO | 22.7 | 0.9 | 22.0 | 0.9 | 22.5 | 0.3 | 22.7 | 1.8 | 22.5 | 0.7 |
| MnO | 0.280 | 0.010 | 0.278 | 0.011 | 0.280 | 0.030 | 0.303 | 0.022 | 0.285 | 0.024 |
| MgO | 6.52 | 0.29 | 6.40 | 0.41 | 6.48 | 0.35 | 6.26 | 0.46 | 6.42 | 0.23 |
| CaO | 11.6 | 0.3 | 10.4 | 0.7 | 11.0 | 0.1 | 11.0 | 0.7 | 11.0 | 1.0 |
| Na2O | 0.460 | 0.010 | 0.461 | 0.028 | 0.26 | 0.21 | — | — | 0.39 | 0.23 |
| K2O | 0.210 | 0.010 | 0.182 | 0.018 | 0.19 | 0.15 | 0.179 | 0.017 | 0.190 | 0.028 |
| P2O5 | 0.270 | 0.020 | — | — | 0.230 | 0.050 | — | — | 0.250 | 0.057 |
| Li | 15.4 | 0.6 | — | — | — | — | — | — | 15.4 | 0.6 |
| Be | 2.84 | 0.11 | — | — | — | — | — | — | 2.84 | 0.11 |
| K | 1510 | 166 | 1510 | 151 | — | — | 1480 | 125 | 1500 | 32 |
| Sc | 62.9 | 3.6 | 66.0 | 2.6 | — | — | 72.1 | 9.6 | 67.0 | 9.4 |
| Mn | 2150 | 170 | 2150 | 86 | — | — | 2350 | 150 | 2220 | 225 |
| Co | 37.2 | 1.6 | 40.0 | 1.6 | — | — | 44.7 | 6.3 | 40.6 | 7.6 |
| Ni | 139 | 26 | 136 | 11 | — | — | 139 | 57 | 138 | 4 |
| Cu | 12.2 | 2.2 | — | — | — | — | — | — | 12.2 | 2.2 |
| Zn | 14.2 | 0.8 | 16.2 | 3.2 | — | — | — | — | 15.2 | 2.8 |
| Ga | 5.79 | 0.38 | — | — | — | — | 5.8 | 2.9 | 5.79 | 0.01 |
| Rb | 5.23 | 0.60 | 7.5 | 1.5 | — | — | 4.3 | 1.5 | 5.7 | 3.3 |
| Sr | 313 | 18 | 276 | 22 | — | — | 328 | 21 | 306 | 53 |
| Y | 116 | 12 | — | — | — | — | 102 | 6 | 109 | 20 |
| Zr | 545 | 50 | 458 | 34 | — | — | 503 | 29 | 502 | 87 |
| Nb | 35.6 | 3.8 | — | — | — | — | 30.9 | 2.6 | 33.3 | 6.6 |
| Cs | 0.220 | 0.020 | 0.169 | 0.038 | — | — | 0.16 | 0.19 | 0.181 | 0.069 |
| Ba | 395 | 46 | 362 | 35 | — | — | 415 | 34 | 391 | 53 |
| La | 35.4 | 4.0 | 36.1 | 1.4 | — | — | 34.4 | 2.8 | 35.3 | 1.7 |
| Ce | 99 | 11 | 92.8 | 3.7 | — | — | 79.2 | 4.9 | 90 | 20 |
| Pr | 12.7 | 1.6 | 12.5 | 2.2 | — | — | 11.8 | 1.3 | 12.3 | 0.9 |
| Nd | 59.3 | 7.0 | 58.4 | 5.8 | — | — | 56.2 | 5.9 | 58.0 | 3.2 |
| Sm | 17.0 | 1.8 | 16.1 | 0.6 | — | — | 16.7 | 3.8 | 16.6 | 0.9 |
| Eu | 2.77 | 0.20 | 2.56 | 0.10 | — | — | 2.69 | 0.67 | 2.67 | 0.21 |
| Gd | 19.6 | 2.0 | 18.9 | 0.8 | — | — | 19.9 | 3.3 | 19.5 | 1.1 |
| Tb | 3.27 | 0.36 | 3.51 | 0.28 | — | — | 3.16 | 0.43 | 3.31 | 0.36 |
| Dy | 20.5 | 2.2 | 20.9 | 1.4 | — | — | 20.4 | 2.9 | 20.6 | 0.5 |
| Ho | 4.07 | 0.46 | 4.50 | 1.40 | — | — | 4.04 | 0.65 | 4.20 | 0.51 |
| Er | 11.3 | 1.2 | — | — | — | — | 11.4 | 1.8 | 11.3 | 0.1 |
| Tm | 1.57 | 0.18 | 0.800 | 0.040 | — | — | 1.54 | 0.42 | 1.30 | 0.87 |
| Yb | 9.90 | 0.76 | 9.49 | 0.57 | — | — | 9.6 | 2.5 | 9.65 | 0.44 |
| Lu | 1.36 | 0.14 | 1.41 | 0.08 | — | — | 1.39 | 0.37 | 1.39 | 0.05 |
| Hf | 14.0 | 1.6 | 13.6 | 0.5 | — | — | 14.3 | 3.5 | 14.0 | 0.7 |
| W | 0.50 | 0.14 | 0.79 | 0.24 | — | — | — | — | 0.65 | 0.41 |
| Ta | 1.83 | 0.14 | 1.77 | 0.24 | — | — | 1.57 | 0.42 | 1.72 | 0.27 |
| Pb | 1.89 | 0.20 | — | — | — | — | 1.35 | 0.70 | 1.62 | 0.76 |
| Th | 5.14 | 0.56 | 4.72 | 0.28 | — | — | 5.00 | 0.71 | 4.95 | 0.43 |
| U | 1.35 | 0.16 | 1.41 | 0.28 | — | — | 1.15 | 0.28 | 1.30 | 0.27 |
The CE-5 lunar sample has an Rb content of ∼5.0 μg g−1, higher than the LODs of the three techniques; therefore, the large variation may suggest that Rb is heterogeneous in the CE-5 samples. The Cs and W data also show large scatter, which is probably related to the low contents of those elements. The slight scatter in Pb contents is probably due to the contamination issue of ICP-MS and LA-ICP-MS. It should be noted that the Tm content determined by INAA is clearly lower than that determined by ICP-MS and LA-ICP-MS. The reason for this is unclear, but it may be related to analytical issues with the INAA technique. In general, the data presented in this study further support the conclusion that the CE-5 lunar soil samples are homogeneous with only a few volatile elements showing slight heterogeneity.
Although the sample size used in this study is 30 mg (this size is required for the XRF standard glass disc), the amount of sample size could be down to sub-mg levels with proportionally reduced flux. The highly diluted fused glass discs transform samples from their original state to glass, but this glass disc can be long-term and low-cost preserved for future usage. In addition, it is practically to carry out isotopic ratio measurements (e.g. U) in these glass discs using LA-MC-ICP-MS and SIMS. Our method could yield precise major element concentration data that are comparable to XRF analysis (Table 1). The main drawback of this technique is the interferences from the flux and contamination during preparation, particularly Li and B, implying that these elements cannot be analyzed. Future study may be focusing on the purification of Li–B flux or selection of other fluxes.
With the new data reported in this study, the average chemical composition of the CE-5 lunar soil sample could be calculated. The dataset was obtained for the samples from different batches (CE5C0400, CE5C0600, and CE5C0800) using different methods (solution ICP-MS, INAA, XRF, and LA-ICP-MS; Table 1). Further interpretation of these data is outside of the scope of this study. However, the mean data reported here could represent the bulk compositions of the CE-5 lunar soils, and it will be an important reference for the future studies.
4 Conclusions
We demonstrated the practicality of chemical analysis using LA-ICP-MS on highly diluted fused glass discs. 30 mg of sample is required to produce a standard lithium borate disc (for XRF analysis), which could be down to sub-mg levels with proportionally reduced flux. We modified the Jet sample cone by enlarging the orifice diameter from 1.1 mm to 1.2 mm, and this approach leads to ∼4.5-fold sensitivity improvement compared to the standard cone. The interferences from the lithium borate discs were evaluated systemically; corrections are necessary for some elements. The limits of detection, analytical precision, and accuracy of this technique were evaluated using the BCR-2 and BHVO-1 rock powder reference materials. We applied this method to CE-5 lunar samples and provided an independent dataset of 38 elements. We reported the average chemical composition of CE-5 lunar samples, which will be an important reference for future study. This innovative technique will be useful for other precious extraterrestrial samples (e.g., CE-6 returned lunar samples).Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Author contributions
Shitou Wu led the project, conceived the study, conducted the experimental analyses, interpreted the data and substantially edited the manuscript. Dingshuai Xue and Yueheng Yang conceived the study, conducted the experimental analyses, interpreted the data and edited the manuscript. Hao Wang, Chunlai Li, and Fuyuan Wu revised the manuscript and provided supervision; and all authors finalized the manuscript for publication.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Qin Zhou for providing CE-5 lunar soil samples, and Danping Zhang for assistance during the preparation of lithium borate discs. This research was supported by the National Natural Science Foundation of China (42273034), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2022066), and the Key Research Program of the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS-202204).References
- W. F. McDonough and S. s. Sun, Chem. Geol., 1995, 120, 223–253 CrossRef CAS.
- R. Rudnick and S. Gao, Treatise Geochem., 2003, 3, 1–64 Search PubMed.
- Z. Wang, K. Zong, Y. Li, J. Li, Q. He, Z. Zou, H. Becker, F. Moynier, J. M. D. Day, W. Zhang, Y. Qian, L. Xiao, Z. Hu, Z. She, H. Hui, X. Wu and Y. Liu, Geochim. Cosmochim. Acta, 2024, 373, 17–34 CrossRef CAS.
- K. Zong, Z. Wang, J. Li, Q. He, Y. Li, H. Becker, W. Zhang, Z. Hu, T. He, K. Cao, Z. She, X. Wu, L. Xiao and Y. Liu, Geochim. Cosmochim. Acta, 2022, 335, 284–296 CrossRef CAS.
- J.-H. Li, Q.-L. Li, L. Zhao, J.-H. Zhang, X. Tang, L.-X. Gu, Q. Guo, H.-X. Ma, Q. Zhou, Y. Liu, P.-Y. Liu, H. Qiu, G. Li, L. Gu, S. Guo, C.-L. Li, X.-H. Li, F.-Y. Wu and Y.-X. Pan, Geosci. Front., 2022, 13, 101367 CrossRef CAS.
- C. Koeberl, J. Radioanal. Nucl. Chem., 1993, 168, 47–60 CrossRef CAS.
- E. Orvini and M. Speziali, Microchem. J., 1998, 59, 160–172 CrossRef CAS.
- G. A. Jenner, H. P. Longerich, S. E. Jackson and B. J. Fryer, Chem. Geol., 1990, 83, 133–148 CrossRef CAS.
- C. Münker, Chem. Geol., 1998, 144, 23–45 CrossRef.
- C. Zhang, Z. Hu, W. Zhang, Y. Liu, K. Zong, M. Li, H. Chen and S. Hu, Anal. Chem., 2016, 88, 10088–10094 CrossRef CAS PubMed.
- Z. He, F. Huang, H. Yu, Y. Xiao, F. Wang, Q. Li, Y. Xia and X. Zhang, Geostand. Geoanal. Res., 2016, 40, 5–21 CrossRef CAS.
- Z. Yu, M. D. Norman and P. Robinson, Geostand. Geoanal. Res., 2003, 27, 67–89 CrossRef CAS.
- J. Fedorowich, J. Richards, J. Jain, R. Kerrich and J. Fan, Chem. Geol., 1993, 106, 229–249 CrossRef CAS.
- S. M. Eggins, Geostand. Geoanal. Res., 2003, 27, 147–162 CrossRef CAS.
- B. Stoll, K. P. Jochum, K. Herwig, M. Amini, M. Flanz, B. Kreuzburg, D. Kuzmin, M. Willbold and J. Enzweiler, Geostand. Geoanal. Res., 2008, 32, 5–26 CAS.
- L. Y. Zhu, Y. S. Liu, Z. C. Hu, Q. H. Hu, X. R. Tong, K. Q. Zong, H. H. Chen and S. Gao, Geostand. Geoanal. Res., 2013, 37, 207–229 CrossRef CAS.
- S. Wu, V. Karius, B. C. Schmidt, K. Simon and G. Wörner, Geostand. Geoanal. Res., 2018, 42, 575–591 CrossRef CAS.
- J. Zheng, T. Chen, H. C. Ng, L. F. Robinson, X.-Y. Zheng, X. Shi and M. Huang, Anal. Chem., 2022, 94, 7576–7583 CrossRef CAS.
- Z. Hu, W. Zhang, Y. Liu, S. Gao, M. Li, K. Zong, H. Chen and S. Hu, Anal. Chem., 2015, 87, 1152–1157 CrossRef CAS PubMed.
- K. P. Jochum, D. B. Dingwell, A. Rocholl, B. Stoll, A. W. Hofmann, S. Becker, A. Besmehn, D. Bessette, H. J. Dietze and P. Dulski, Geostand. Newsl., 2000, 24, 87–133 CrossRef CAS.
- C. Li, H. Hu, M.-F. Yang, Z.-Y. Pei, Q. Zhou, X. Ren, B. Liu, D. Liu, X. Zeng, G. Zhang, H. Zhang, J. Liu, Q. Wang, X. Deng, C. Xiao, Y. Yao, D. Xue, W. Zuo, Y. Su, W. Wen and Z. Ouyang, Natl. Sci. Rev., 2022, 9(2), 1–13 Search PubMed.
- Q.-L. Li, Q. Zhou, Y. Liu, Z. Xiao, Y. Lin, J.-H. Li, H.-X. Ma, G.-Q. Tang, S. Guo, X. Tang, J.-Y. Yuan, J. Li, F.-Y. Wu, Z. Ouyang, C. Li and X.-H. Li, Nature, 2021, 600(7887), 54–58 CrossRef CAS.
- H.-C. Tian, H. Wang, Y. Chen, W. Yang, Q. Zhou, C. Zhang, H.-L. Lin, C. Huang, S.-T. Wu, L.-H. Jia, L. Xu, D. Zhang, X.-G. Li, R. Chang, Y.-H. Yang, L.-W. Xie, D.-P. Zhang, G.-L. Zhang, S.-H. Yang and F.-Y. Wu, Nature, 2021, 600(7887), 59–63 CrossRef CAS PubMed.
- S. Hu, H. He, J. Ji, Y. Lin, H. Hui, M. Anand, R. Tartèse, Y. Yan, J. Hao, R. Li, L. Gu, Q. Guo, H. He and Z. Ouyang, Nature, 2021, 600(7887), 49–53 CrossRef CAS PubMed.
- F.-Y. Wu, Q.-L. Li, Y. Chen, S. Hu, Z.-Y. Yue, Q. Zhou, H. Wang, W. Yang, H.-C. Tian, C. Zhang, J.-H. Li, L.-X. Li, H.-J. Hui, C.-L. Li, Y.-T. Lin, X.-H. Li and J. W. Delano, Annu. Rev. Earth Planet. Sci., 2024, 52, 159–1943 CrossRef CAS.
- X. Che, A. Nemchin, D. Liu, T. Long, C. Wang, M. D. Norman, K. H. Joy, R. Tartese, J. Head, B. Jolliff, J. F. Snape, C. R. Neal, M. J. Whitehouse, C. Crow, G. Benedix, F. Jourdan, Z. Yang, C. Yang, J. Liu, S. Xie, Z. Bao, R. Fan, D. Li, Z. Li and S. G. Webb, Science, 2021, 374(6569), 887–890 CrossRef CAS PubMed.
- K. P. Jochum, U. Nohl, K. Herwig, E. Lammel, B. Stoll and A. W. Hofmann, Geostand. Geoanal. Res., 2005, 29, 333–338 CrossRef CAS.
- D.-S. Xue, B.-X. Su, D.-P. Zhang, Y.-H. Liu, J.-J. Guo, Q. Guo, J.-F. Sun and S.-Y. Zhang, J. Anal. At. Spectrom., 2020, 35, 2826–2833 RSC.
- S. Wu, G. Wörner, K. P. Jochum, B. Stoll, K. Simon and A. Kronz, Geostand. Geoanal. Res., 2019, 43, 567–584 CrossRef CAS.
- S. Wu, Y. Yang, K. P. Jochum, R. L. Romer, J. Glodny, I. P. Savov, S. Agostini, J. C. M. De Hoog, S. T. M. Peters, A. Kronz, C. Zhang, Z. Bao, X. Wang, Y. Li, G. Tang, L. Feng, H. Yu, Z. Li, Z. Le, J. Lin, Y. Zeng, C. Xu, Y. Wang, Z. Cui, L. Deng, J. Xiao, Y. Liu, D. Xue, Z. Di, L. Jia, H. Wang, L. Xu, C. Huang, L. Xie, A. Pack, G. Wörner, M. He, C. Li, H. Yuan, F. Huang, Q. Li, J. Yang, X. Li and F. Wu, Geostand. Geoanal. Res., 2021, 45, 719–745 CrossRef CAS.
- S. Wu, C. Huang, L. Xie, Y. Yang and J. Yang, Chin. J. Anal. Chem., 2020, 46(10), 1628–1636 Search PubMed.
- C. Paton, J. Hellstrom, B. Paul, J. Woodhead and J. Hergt, J. Anal. At. Spectrom., 2011, 26(12), 2508–2518 RSC.
- S. Wu, M. Yang, Y. Yang, L. Xie, C. Huang, H. Wang and J. Yang, Int. J. Mass Spectrom., 2020, 456, 116394 CrossRef CAS.
- T. Pettke, F. Oberli, A. Audétat, M. Guillong, A. C. Simon, J. J. Hanley and L. M. Klemm, Ore Geol. Rev., 2012, 44, 10–38 CrossRef.
- Y. Yao, C. Xiao, P. Wang, C. Li and Q. Zhou, J. Am. Chem. Soc., 2022, 144(12), 5478–5484 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ja00329b |
| This journal is © The Royal Society of Chemistry 2025 |