Open Access Article
Open Access ArticleQuaternary ammonium-functionalized polysilsesquioxanes for antifogging coating†
Takashi
Hamada
 *ab,
Tetsuya
Sugimoto
acd,
Arata
Tanaka
d,
Tetsuya
Maeda
ac,
Daiji
Katsura
ac,
Hiroyuki
Koga
ac and
Joji
Ohshita
*ab,
Tetsuya
Sugimoto
acd,
Arata
Tanaka
d,
Tetsuya
Maeda
ac,
Daiji
Katsura
ac,
Hiroyuki
Koga
ac and
Joji
Ohshita
 *de
*de
aCollaborative Research Laboratory, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-4-1 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8527, Japan. E-mail: hama@hiroshima-u.ac.jp
bQuantum Materials and Applications Research Center, Takasaki Institute for Advanced Quantum Science, National Institutes for Quantum Science and Technology (QST), 1233 Watanuki-machi, Takasaki, Gunma 370-1292, Japan. E-mail: hamada.takashi@qst.go.jp
cTechnical Research Center, Mazda Motor Corporation, Aki-gun, Hiroshima 730-8670, Japan
dSmart Innovation Program, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-4-1 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8527, Japan. E-mail: jo@hiroshima-u.ac.jp
eDivision of Materials Model-Based Research, Digital Monozukuri (Manufacturing) Education and Research Center, Hiroshima University, 3-10-32 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-0046, Japan
First published on 25th November 2024
Abstract
Aiming to develop antifogging materials with enhanced antifogging properties and scratch resistance, polysilsesquioxane films containing quaternary ammonium groups were prepared via a sol–gel reaction and quaternization. Owing to their high water uptake, scratch hardness, and transparency, the prepared quaternary ammonium-functionalized polysilsesquioxane films are promising for application in antifogging coatings.
Fogging has a negative impact on our daily life; for example, the fogging of eyeglasses, camera lenses, and bathroom mirrors makes daily life uncomfortable.1 In particular, the fogging of automobile windshields leads to serious problems, including traffic accidents due to reduced visibility.2 The mechanism of fogging involves light scattering by water droplets on transparent materials, considerably reducing both transparency and visibility.3,4 Therefore, the development of antifogging materials that prevent fogging when applied as a coating on transparent solid surfaces such as lenses, mirrors, and automobile windshields attracts considerable research attention.1–4
Antifogging materials can be broadly classified into three categories, i.e., hydrophilic polymers, inorganic oxides, and organic–inorganic hybrid polymers. Hydrophilic polymers containing hydroxy, carboxyl, amine, and ammonium groups prevent the formation of water droplets by absorbing water via hydrogen bonding.1,5,6 However, on solid surfaces, hydrophilic polymers are prone to poor scratch resistance, which reduces film transparency, hindering their application in eyeglasses, optical lenses, and automobile windshields. Inorganic oxides, especially titanium oxides (TiO2), exhibit superhydrophilic surfaces and excellent scratch resistance.7,8 Water droplets spread on the surface of a TiO2 film as water contact angles are below 5° under UV irradiation, thereby preventing the formation of water droplets. Unfortunately, the superhydrophilicity of TiO2 film surfaces is not long-lasting. Meanwhile, organic–inorganic hybrid polymers such as polysilsesquioxanes (PSQs) have recently attracted attention because the inorganic Si–O–Si framework exhibits high scratch resistance.9–15
So far, we have reported antifogging materials based on PSQs containing amino groups, hydroxy groups, tetraethylene glycol chains, and amine hydrochloride salt, which exhibit good water uptake (WU) and scratch resistance.12–15 As a continuation of our work, we are interested in exploring PSQs bearing quaternary ammonium groups, which are used in polymer electrolyte membranes and show high WU due to the hydration of ions.16 Herein, we report the facile synthesis of PSQ films containing quaternary ammonium groups, which does not require immersion into harsh reagents such as sulfuric acid and hydrochloric acid. We found that the quaternary ammonium group solves the coloring problem of PSQ films while maintaining excellent antifogging properties.
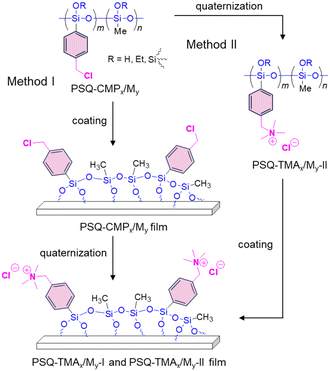
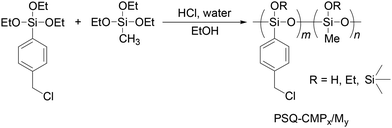
We designed two routes to synthesize PSQ films containing quaternary ammonium groups, as illustrated in Fig. 1. The quaternary ammonium group can be easily formed by reacting benzyl chloride with trimethylamine (TMA).16 Therefore, we selected 4-(chloromethyl)phenyltriethoxysilane (CMPTES) for the preparation of PSQ having quaternary ammonium groups. CMPTES was synthesized via the reaction of 4-(chloromethyl)phenyltrichlorosilane and ethanol and obtained as a colorless liquid in a yield of 75% after vacuum distillation (112 °C at 0.5 mmHg)17 (Scheme S1†). Then, poly[(4-chloromethyl)phenyl-co-methyl]silsesquioxane (PSQ-CMP1/M4) was synthesized at a CMPTES/triethoxymethylsilane (MTES) feed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 via hydrolysis and polycondensation at 90 °C for 3 h. Other PSQs with different ratios of benzyl chloride and methyl groups (PSQ-CMPx/My) were also prepared. In our design, MTES was used to prevent excess water absorption by the quaternary ammonium group. The chemical structure of PSQ-CMP1/M4 was confirmed via1H nuclear magnetic resonance (NMR) spectroscopy. As shown in Fig. S1,† broad signals attributable to the methyl protons of the silylmethyl group and the methylene and phenyl protons of the benzyl group were observed at 0.16, 4.59, 7.39, and 7.66 ppm. Broad signals of residual ethoxy groups were observed at 1.23 and 3.81 ppm, from which the amount of residual ethoxy groups was calculated to be 10%. The weight-average molar mass (Mw) and polydispersity (Mw/Mn) of PSQ-CMP1/M4 were estimated to be 1700 and 2.0 via size exclusion chromatography (SEC). The same procedure was used to synthesize PSQ-CMP1/M1 and PSQ-CMP1/M0, which showed a decrease in Mw from 1700 to 940 and an increase in the amount of residual ethoxy groups from 10 to 23% with an increasing feed ratio of CMPTES (Table 1). This trend is in agreement with a previous report, according to which the steric hindrance of the phenyl group on the silicon atom decreases the Mw and the amount of residual ethoxy groups retained in PSQ-CMPx/My.18
4 via hydrolysis and polycondensation at 90 °C for 3 h. Other PSQs with different ratios of benzyl chloride and methyl groups (PSQ-CMPx/My) were also prepared. In our design, MTES was used to prevent excess water absorption by the quaternary ammonium group. The chemical structure of PSQ-CMP1/M4 was confirmed via1H nuclear magnetic resonance (NMR) spectroscopy. As shown in Fig. S1,† broad signals attributable to the methyl protons of the silylmethyl group and the methylene and phenyl protons of the benzyl group were observed at 0.16, 4.59, 7.39, and 7.66 ppm. Broad signals of residual ethoxy groups were observed at 1.23 and 3.81 ppm, from which the amount of residual ethoxy groups was calculated to be 10%. The weight-average molar mass (Mw) and polydispersity (Mw/Mn) of PSQ-CMP1/M4 were estimated to be 1700 and 2.0 via size exclusion chromatography (SEC). The same procedure was used to synthesize PSQ-CMP1/M1 and PSQ-CMP1/M0, which showed a decrease in Mw from 1700 to 940 and an increase in the amount of residual ethoxy groups from 10 to 23% with an increasing feed ratio of CMPTES (Table 1). This trend is in agreement with a previous report, according to which the steric hindrance of the phenyl group on the silicon atom decreases the Mw and the amount of residual ethoxy groups retained in PSQ-CMPx/My.18
| Entry | PSQ-CMPx/My | M w (g mol−1) | M w/Mn | Residual ethoxy group (%) |
|---|---|---|---|---|
| a Monomer/HCl molar ratio = 0.105. b Estimated by SEC using a polystyrene standard as a reference. c The residual ethoxy group was estimated via1H NMR spectroscopy. | ||||
| 1 | PSQ-CMP1/M0 | 940 | 1.4 | 23 |
| 2 | PSQ-CMP1/M1 | 1200 | 1.6 | 13 |
| 3 | PSQ-CMP1/M4 | 1700 | 2.0 | 10 |
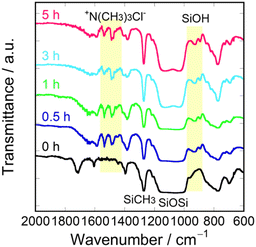
Next, we prepared PSQ films containing quaternary ammonium groups using two approaches. In method I, a 50 wt% PSQ-CMP1/M4 tetrahydrofuran (THF) solution was coated and cured on a silicon wafer and the quaternization was conducted in the film state. To investigate the progress of the polycondensation during annealing, a PSQ-CMP1/M4 film was monitored via Fourier transform infrared (FTIR) spectroscopy during curing at 180 °C. A peak around 960 cm−1 corresponding to the silanol group decreases with increasing curing time (Fig. S2†). Conversely, the peak at 1150–1000 cm−1, which corresponds to the Si–O–Si moiety, increases. This indicates that the polycondensation of PSQ-CMP1/M4 proceeds to form a highly crosslinked structure. Indeed, the PSQ-CMP1/M4 film does not dissolve in THF after curing at 180 °C for 3 h. Overall, the synthesis of the PSQ-CMP1/M4 film involved curing at 80 °C for 1 h and then at 180 °C for 3 h. Next, quaternization of the PSQ-CMP1/M4 film was conducted by immersing it in 13 wt% TMA THF solution. The quaternization was also monitored via FTIR spectroscopy (Fig. 2). Peaks at 1550–1460 cm−1 corresponding to the ammonium group appear after immersion for 1–5 h. Unfortunately, the quaternization degree could not be quantitatively calculated using the FTIR spectra. Therefore, the progress of the quaternization of the PSQ-CMP1/M4 film was monitored via13C solid-state NMR spectroscopy. For this measurement, a free-standing gel film was prepared by curing at 180 °C for 3 h because the 13C solid-state NMR spectra could not be recorded using a film on a glass substrate. The obtained free-standing gel film was immersed in 13 wt% TMA THF solution for 1–5 h. Before immersion, signals corresponding to the methyl carbon of the silylmethyl group and the methylene and phenyl carbons of the benzyl group were observed at −6.3, 43.3, and 120–140 ppm in the 13C solid-state NMR spectrum (Fig. 3). Signals of the residual ethoxy group were also observed at 15.6 and 55.5 ppm. The signal of methylene carbon of the benzyl group at 43.3 ppm decreased after immersion for 1–3 h, and a signal due to the methyl carbon of the trimethylammonium group appeared at 50.2 ppm. After quaternization, the signal for the methylene carbon of the benzyl group might overlap with that of the remaining THF (65.3 ppm). After immersion for 5 h, the signal of the methylene carbon at 43.3 ppm disappeared, whereas the intensity of the signal of the methyl carbon of the trimethylammonium group increased. The quaternization degrees were estimated to be 53%, 91%, and >99% after 1, 3, and 5 h of immersion based on the integral ratio of the remaining methylene carbon at 43.3 ppm to the integral ratio of methyl carbon at −6.3 ppm. Therefore, the quaternization of PSQ-CMP1/M4 coated on a glass substrate proceeds quantitatively with a quaternization degree of >99%. Overall, the optimal conditions for quaternization of a PSQ-CMP1/M4 film on a glass substrate were immersion in 13 wt% TMA THF solution for 5 h. The quaternized film was labeled as the PSQ-TMA1/M4-I film. For the investigation of antifogging properties, PSQ-TMA1/M0-I and PSQ-TMA1/M1-I films were similarly prepared using PSQ-CMP1/M0 and PSQ-CMP1/M1 films.
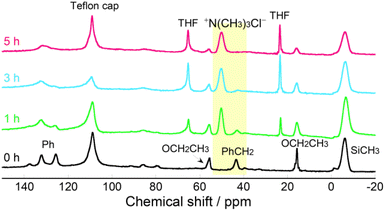 | ||
| Fig. 3 13C solid-state NMR spectra of a PSQ-CMP1/M4 film prepared at 180 °C for 3 h and after immersion of PSQ-CMP1/M4 films in 13 wt% TMA THF solution at room temperature for 1, 3, and 5 h. | ||
In method II, the quaternization of PSQ-CMP1/M4 was conducted and the obtained polymer was coated on a glass substrate. First, PSQ-CMP1/M4 was stirred in 13 wt% TMA THF solution overnight, and the solvent was then removed under reduced pressure. The obtained white solid was named PSQ-TMA1/M4-II. The solubility of PSQ-CMP1/M4 changed dramatically after quaternization; the resulting quaternized PSQ-TMA1/M4-II dissolved easily in water. The chemical structure of PSQ-TMA1/M4-II was investigated via1H NMR spectroscopy in D2O. Broad signals corresponding to the methyl protons of the silylmethyl group and the methylene and phenyl protons of the benzyl group were observed at 0.11, 4.50, and 7.60 ppm, respectively (Fig. S3†). A new signal attributable to the methyl group of the ammonium group appeared at 3.03 ppm. The quaternization degree of PSQ-CMP1/M4-II was estimated to be >99% based on 1H NMR in a similar manner. Then, a 50 wt% PSQ-TMA1/M4-II THF solution was coated onto a glass substrate, and the resulting PSQ-TMA1/M4-II film was cured at 80 °C for 1 h and then at 180 °C for 3 h. In the FTIR spectrum, the peak of the silanol group at around 960 cm−1 decreased and the peaks at 1150–1000 cm−1 corresponding to the Si–O–Si moiety increased (Fig. S4†). This indicates that this annealing resulted in the formation of the siloxane network.
To evaluate the properties of the PSQ films containing trimethylammonium groups, the WU and pencil hardness of PSQ-TMAx/My-I and PSQ-TMAx/My-II films prepared using methods I and II, respectively, were measured, and the results are presented in Table 2. The PSQ-TMA1/M0-I film exhibited a high WU of 41% and a pencil hardness of 6B (Table 2, entry 1) because water is easily absorbed by the ammonium group. The PSQ-TMA1/M0-I film was extremely soft, most likely due to the steric hindrance of the benzyl group decreasing the crosslinking density during film preparation. Indeed, the Mw of PSQ-CMP1/M0 was also lower due to the steric hindrance of the benzyl chloride group. However, the WU of the PSQ-TMA1/M0-I film was considerably high and comparable with that of anion exchange membranes.16 Although the WU decreased with the decreasing amount of trimethylammonium groups, the PSQ-TMA1/M4-I film showed a high WU of 25% (Table 2, entry 3), which was similar to that of poly(vinyl alcohol) films.12 In addition, PSQ-TMA1/M4-I exhibited a high pencil hardness of HB compared with that of PSQ-TMA1/M0-I. The bulky benzyl group hindered the formation of the siloxane network structure, while copolymerization with MTES promoted a highly cross-linked network and improved the scratch resistance. The PSQ-TMA1/M4-I film obtained from PSQ-CMP1/M4, which was prepared by curing at 200 °C, showed a high pencil hardness of H. However, the WU of the PSQ-TMA1/M4-I film decreased to 19% (Table 2, entry 4), indicating that the WU is also affected by the siloxane network. Conversely, the WU of the PSQ-TMA1/M4-II film considerably decreased to 4% (Table 2, entry 5), even though the amount of trimethylammonium groups was similar to that of the PSQ-TMA1/M4-I film. This indicates that the trimethylammonium groups decomposed during film preparation. In the FTIR spectrum of the PSQ-TMA1/M4-II film, the peak around 1550–1460 cm−1, which corresponds to the ammonium group, almost disappeared after curing at 180 °C for 3 h. Along with the decomposition of the trimethylammonium groups, the PSQ-TMA1/M4-II film showed a pencil hardness of 2H. In the case of the PSQ-TMA1/M4-II film prepared by curing at 150 °C for 3 h, the decomposition of the trimethylammonium groups was partly suppressed; however, this film exhibited a low pencil hardness of 6B (Table 2, entry 6).
Antifogging materials require antifogging properties and transparency. The PSQ-TMA1/M4-I film exhibited a high WU of 25%, which should prevent fogging by vapor. For example, the bare glass substrate and the PSQ-TMA1/M4-I film on the glass substrate were exposed to hot vapor at 40 °C for 10 seconds at a distance of 2 cm. The glass substrate quickly fogged due to the formation of water droplets, whereas the PSQ-TMA1/M4-I film on the glass substrate prevented the formation of water droplets and retained the transparency (inset of Fig. 4), demonstrating its excellent antifogging properties. Then, the transparency of the PSQ-TMA1/M4-I film was evaluated via UV–visible spectroscopy. The PSQ-TMA1/M4-I film on the glass substrate exhibited a high transmittance of over 98% in the range of 400–800 nm. We previously reported that a poly[3-(2-aminoethylamino)propyl]silsesquioxane (PAEAPS) film possesses high antifogging properties stemming from its high water absorption properties.12 However, the PAEAPS film has a light yellow color and the transmittance rapidly decreases below 450 nm (Fig. 4).12 This color change may be due to the denaturation of the amino group by oxidation.12 In contrast, in the present films, the nitrogen atom of the trimethylammonium group is protected by the methyl groups against denaturation.
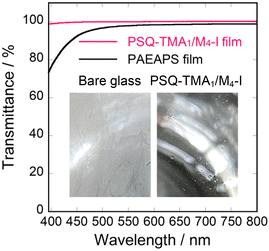 | ||
| Fig. 4 UV–visible spectra of PSQ-TMA1/M4-I and PAEAPS films. Reproduced from ref. 12. Copyright 2021 American Chemical Society. Photograph of the glass substrate and PSQ-TMA1/M4-I film on the glass substrate exposed to hot vapor at 40 °C for 10 seconds at a distance of 2 cm (inset). | ||
In summary, PSQ films containing quaternary ammonium groups were successfully prepared by reacting PSQ-CMPx/My films with TMA. The quaternization of PSQ-CMPx/My films proceeded with a quaternization degree of >99%. The PSQ-TMA1/M0-I film showed a high WU of 41%, which is comparable with that of anion exchange membranes. The PSQ-TMA1/M4-I film exhibited a high WU of 25%, which is similar to that of poly(vinyl alcohol) films. The PSQ-TMA1/M4-I film exhibited a high pencil hardness of HB. The PSQ-TMA1/M4-I film on a glass substrate prevented fogging for 10 seconds under hot vapor at 40 °C. The PSQ-TMA1/M4-I film showed a high transparency of over 98%. A PAEAPS film containing amino groups exhibits a light yellow color. The PSQ-TMA1/M4-I film is colorless and possesses high transparency because denaturation of the nitrogen atom is prevented. Overall, the PSQ-TMA1/M4-I film is a promising material for antifogging coatings.
Author contributions
T. H. designed this study and wrote the paper. T. S., A. T., and T. M. contributed to all of the experimental work. D. K. and H. K. contributed to the investigation. J. O. contributed to supervision and project administration.Data availability
All data are provided in the manuscript and the ESI.†Conflicts of interest
There are no conflicts to declare.References
- X. Gong, H. Yu, L. Wang, X. Liu, S. Ren, Y. Huang and Z. Huang, Adv. Colloid Interface Sci., 2022, 309, 102794 CrossRef CAS PubMed.
- I. R. Durán and G. Laroche, Adv. Colloid Interface Sci., 2019, 263, 68–94 CrossRef PubMed.
- J. Yoon, M. Ryu, H. Kim, G.-N. Ahn, S.-J. Yim, D.-P. Kim and H. Lee, Adv. Mater., 2020, 32, 2002710 CrossRef CAS PubMed.
- X. Gong, H. Yu, L. Wang, J. Hu, L. Wang and C. Li, Prog. Org. Coat., 2023, 179, 107523 CrossRef CAS.
- Z. Zheng, Y. Liu, L. Wang, L. Yu, Y. Cen, T. Zhu, D. Yu and C. Chen, Prog. Org. Coat., 2020, 142, 105578 CrossRef CAS.
- L. Zhang, K. Feng, Y. Liu, F. Wu, Y. Liu, B. Yu, X. Pei, L. Liu, C. Zhang, Y. Wu and F. Zhou, ACS Appl. Polym. Mater., 2024, 6, 6645–6657 CrossRef CAS.
- I. F. Wahab, A. R. Bushroa, S. W. Teck, T. T. Azmi, M. Z. Ibrahim and J. W. Lee, J. Mater. Res. Technol., 2023, 23, 687–714 CrossRef CAS.
- Y. Wei, Q. Wu, H. Meng, Y. Zhang and C. Cao, RSC Adv., 2023, 13, 20584–20597 RSC.
- T. Kozuma, A. Mihata and Y. Kaneko, Materials, 2021, 14, 3178 CrossRef CAS PubMed.
- Q. Liu, J. Cui, T. Kaneko, W. Dong, M. Chen, J. Luo and D. Shi, Prog. Org. Coat., 2024, 196, 108690 CrossRef CAS.
- J. Nakagawa, S. Morinaga and Y. Kaneko, ACS Omega, 2024, 9, 28895–28902 CrossRef CAS PubMed.
- T. Maeda, T. Hamada, S. Tsukada, D. Katsura, K. Okada and J. Ohshita, ACS Appl. Polym. Mater., 2021, 3, 2568–2575 CrossRef CAS.
- T. Hamada, T. Sugimoto, T. Maeda, D. Katsura, S. Mineoi and J. Ohshita, Langmuir, 2022, 38, 5829–5837 CrossRef CAS PubMed.
- T. Maeda, T. Sugimoto, T. Hamada, D. Katsura, S. Mineoi and J. Ohshita, ACS Appl. Polym. Mater., 2022, 4, 7599–7606 CrossRef CAS.
- T. Hamada, T. Maeda, K. Kawashima, T. Sugimoto, A. Tanaka, Y. Tanaka, D. Katsura, S. Mineoi, Y. Kaneko and J. Ohshita, ACS Appl. Polym. Mater., 2023, 5, 1596–1605 CrossRef CAS.
- T. Hamada, Y. Zhao, K. Yoshimura, A. Radulescu, K. Ohwada and Y. Maekawa, ChemistrySelect, 2021, 6, 8879–8888 CrossRef CAS.
- C. Tian, C. Bao, A. Binder, Z. Zhu, B. Hu, Y. Guo, B. Zhao and S. Dai, Chem. Commun., 2013, 49, 8668–8670 RSC.
- T. Hamada, T. Goto, S. Takase, K. Okada, A. Uedono and J. Ohshita, ACS Appl. Polym. Mater., 2022, 4, 2851–2859 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details for the monomer and polymer, 1H nuclear magnetic resonance spectra of the polymer, and Fourier transform infrared spectra of a film. See DOI: https://doi.org/10.1039/d4lp00322e |
| This journal is © The Royal Society of Chemistry 2025 |