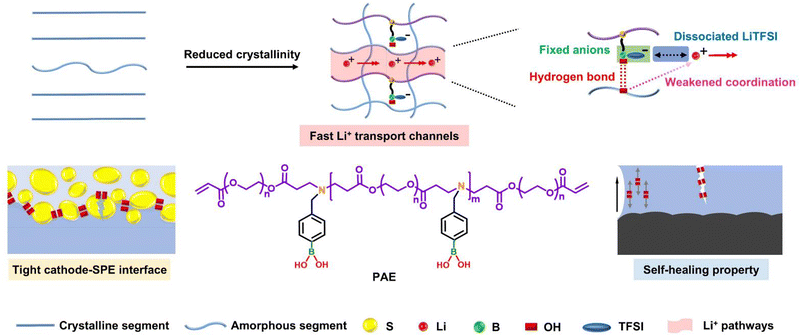
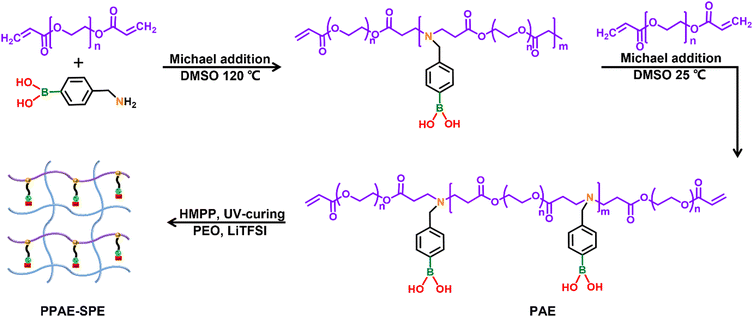
Comb-like poly(β-amino ester)-integrated PEO-based self-healing solid electrolytes for fast ion conduction in lithium–sulfur batteries†
Hui-Min
Wang
ab,
Mengdi
Geng
a,
Jing
Bai
a,
Dezhong
Zhou
*c,
Weibo
Hua
 c,
Sheng
Liu
c,
Sheng
Liu
 *a and
Xueping
Gao
*a and
Xueping
Gao
 *a
*a
aInstitute of New Energy Material Chemistry, School of Materials Science and Engineering, Nankai University, Tianjin 300350, China. E-mail: shengliu@nankai.edu.cn; xpgao@nankai.edu.cn
bSchool of Materials Science and Engineering, Sichuan University of Science & Engineering, Sichuan, Zigong 643000, China
cSchool of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, China. E-mail: dezhong.zhou@xjtu.edu.cn
First published on 15th October 2024
Abstract
All-solid-state lithium–sulfur batteries (ASSLSBs) using poly(ethylene oxide) (PEO) electrolytes offer significant advantages in energy density and safety. However, their development is hampered by the slow Li+ conduction in solid polymer electrolytes and sluggish electrochemical conversion at the cathode–electrolyte interface. Herein, we fabricate a self-healing poly(β-amino ester) with a comb-like topological structure and multiple functional groups, synthesized through a Michael addition strategy. This material modifies the PEO-based solid-state electrolyte, creating fast Li+ transport channels and improving polysulfides conversion kinetics at the electrode surface. Consequently, both modified all-solid-state lithium symmetric cells and lithium–sulfur batteries exhibit improved electrochemical performance. This work demonstrates an expanded interpenetrating macromolecular engineering approach to develop highly ion-conductive solid polymer electrolytes for ASSLSBs.
New conceptsWe pioneer the use of poly(β-amino ester) (PAE), a material with flexible structural design and controllable molecular weight (Mw), to fabricate an integrated PEO-based solid polymer electrolyte (SPE) for all-solid-state lithium–sulfur batteries (ASSLSBs). Unlike previous approaches that often rely on agglomerative inorganic fillers or complex synthesis of macromolecular polymers, we designed and synthesized a novel PAE with a comb-like topological structure and multiple functional groups using a facile Michael addition reaction to modify the PEO-based SPE. The appropriate Mw of PAE ensures good dispersibility, and the integrated PEO–PAE–SPE (PPAE–SPE) exhibits a homogeneous and interpenetrating macromolecular network after the in situ polymerization of PAE within the PEO system. Moreover, the comb-like topological structure and multiple functional groups of PAE (Lewis-acidic boron and nitrogen, hydroxyl groups) synergistically construct rapid ion transport channels by reducing the crystallinity of PEO, fixing TFSI− anions and weakening the coordination between Li+ and PEO chains. Additionally, the electronegative nitrogen and hydroxyl groups in the PPAE–SPE system impart self-healing properties to the cathode–electrolyte interface by forming reversible hydrogen bonds in the composite cathode, resulting in a tightly adherent interface and enhanced LiPSs conversion. This work presents a novel strategy for developing advanced SPEs with superior topological structures and multiple functional groups for ASSLSBs. |
Introduction
Lithium–sulfur (Li–S) batteries with a high theoretical energy density (2600 W h kg−1) meet the increasing demands for energy storage in modern mobile society.1–3 However, the shuttle effect of soluble lithium polysulfides (LiPSs) and safety hazards associated with flammable ether-based liquid electrolytes and uncontrolled lithium dendrite growth severely hinder their practical application.4,5 Solid state electrolytes (SSEs) without the organic liquid component are being extensively researched to combine high energy density and enhanced safety.6 SSEs are mainly categorized into two primary classes: inorganic ceramic electrolytes (ICEs) and solid polymer electrolytes (SPEs).7 ICEs, which can be further divided into oxides and sulfides, typically exhibit high ionic conductivity (σ) up to 10−4–10−2 S cm−1 and can inhibit the shuttle effect since LiPSs are insoluble in the solid phase.8 However, issues such as the high interface impedance of oxide-based ISEs due to the inferior mechanical brittleness and detrimental interfacial chemical reactions in sulfide-based ISEs limit their application.9–11 In contrast, SPEs based on flexible poly(ethylene oxide) (PEO) offer significant advantages, including good fabricating scalability, intimate interfacial contact with electrodes, and high stability with metal lithium. These properties make PEO-based SPEs the most promising electrolytes for all-solid-state Li–S batteries (ASSLSBs).12Despite these advantages, PEO-based ASSLSBs face several significant challenges. Firstly, SPEs based on PEO generally exhibit low ionic conductivity and Li+ transference number (tLi+) due to the high crystallinity of PEO and the coordinated Li+ with polymer chains, resulting in a Li+ concentration gradient and uneven lithium deposition.13,14 Secondly, the C–C–O ether structure of PEO, similar to that in ether-based liquid electrolytes, promotes the generation and dissolution of LiPSs within the PEO, which perpetuates the notorious shuttle effect.15 Moreover, the kinetics of LiPSs conversion at the solid cathode–electrolyte interface are limited due to the poor interfacial contact caused by the pulverization of cathode particles and the formation of vacant cavities during cycling.16,17 The slow migration of Li+ in the SPEs and sluggish LiPSs conversion at the cathode–electrolyte interface significantly aggravate concentration and electrochemical polarization, thus hindering the electrochemical performance of ASSLSBs. Thirdly, SPEs with poor mechanical properties cannot suppress the lithium dendrite growth and withstand cracks caused by changes in the electrode volume, leading to safety and lifespan issues.18,19
Various strategies have been explored to address these challenges, including the addition of organic/inorganic fillers,20,21 polymer grafting,22 cross-linking23 and blending.24 Basically, fillers can suppress the crystallinity of PEO, promote the dissociation of lithium salts, and improve the mechanical strength of SPEs, thereby enhancing ionic conductivity and suppressing lithium dendrite growth.25 However, inorganic fillers tend to agglomerate in the polymer matrix and cannot lead to the construction of fast Li+ transport channels within homogeneous SPEs. It is reported that even after being homogeneously incorporated into PEO, still numerous crystallized polymer regions exist in the SPE matrix.26 In contrast, macromolecular polymers with specific topological structures and multiple functional groups offer good mechanical strength and better compatibility with the PEO matrix, thus introducing them into SPEs is a more effective approach. By rationally designing the structure of the introduced macromolecule, such as a rigid or flexible backbone, functional side chains and interpenetrating networks, integrated SPEs can address the problems in ASSLSBs.27 However, the synthesis of functional polymers is often complex, and phase separation may occur within the macromolecular system of PEO and the involved high molecular weight (Mw) functional polymers, resulting in disadvantageous electrochemical performance. Poly(β-amino ester) (PAE) polymers with flexible structure design and controllable Mw possess abundant interior tertiary amine and ester groups.28 The chemical composition and topological structure (e.g., linear, graft, branched, and cyclized) of PAEs can be easily modulated via a facile Michael addition reaction by selecting appropriate monomers of diacrylates and amines, and Mw can be controlled by adjusting the reaction conditions.29 Therefore, PAEs with specific topological structures and functional groups have significant application potential in ASSLSBs, which has not yet been explored.
Herein, we designed and synthesized a comb-like PAE with multiple functional groups to modify PEO-based SPEs for the first time. The appropriate Mw of PAE ensures good dispersibility, and after in situ polymerization of PAE in the PEO system, the integrated PEO–PAE–SPE (PPAE–SPE) with homogeneous and interpenetrating macromolecular network is obtained. As illustrated in Scheme 1, in PPAE–SPE, the comb-like topological structure reduces crystallinity by disturbing the regular arrangement of PEO segments, thus improving the ionic conductivity of SPE. Besides, multiple functional groups of the PAE, including Lewis-acidic boron sites and electronegative nitrogen as well as oxhydryl groups, synergistically lead to the construction of rapid ion transport channels by fixing TFSI− anions and weakening the coordination between Li+ and PEO chains. In addition, the nitrogen and oxhydryl groups in the PPAE–SPE system provide the cathode–electrolyte interface with self-healing properties by formatting reversible hydrogen bonds with the binder of PEO in the composite cathode, thus filling vacant cavities caused by pulverization of cathode particles. This results in a tightly adherent cathode–electrolyte interface, low interfacial charge transfer resistance and fast LiPSs conversion. Moreover, the abundant hydrogen bonds in the PPAE–SPE also give it bulk self-healing properties, which along with the fast Li+ migration and enhanced mechanical strength due to the rigid phenyl group, can effectively inhibit lithium dendrite growth, thereby improving lifespan and safety performance. This work provides a practical synthesis strategy for advanced SPEs with superior topological structures and multiple functional groups for ASSLSBs.
Results and discussion
As illustrated in Fig. 1, the PAE precursor was synthesized by the copolymerization of poly(ethylene glycol) diacrylate (PEGDA) and [4-(aminomethyl)phenyl] boronic acid via the “A2 + B2” Michael addition strategy.30 The Mw of precursor polymers and the yield increase over time (Fig. S1a, ESI†). Typically, a short reaction time results in a low yield, while an excessively long reaction time leads to a high Mw of the polymers, causing nonuniform dispersion in the PEO system. To balance yield and Mw, the reaction was halted after 3 hours of polymerization when the Mw reached approximately 40 kDa. PEGDA was then added to ensure active vinyl groups on both ends of PAE, converting to interpenetrating macromolecules in the SPE network after in situ polymerization. Notably, the Mw of PAE further increased to 80 kDa due to the increase of concentration during the purification process (Fig. S1b, ESI†), which not only allowed uniform dispersion with PEO and LiTFSI, but also made it suitable for subsequent polymerization. The chemical structure of PAE was confirmed via1H NMR (Fig. S1c, ESI†), indicating the successful synthesis of the PAE functionalized with ethylene oxide, phenyl, boron and oxhydryl groups. Subsequently, the homogeneous solution containing PEO, LiTFSI, active PAE and photoinitiator was poured onto a Teflon membrane and exposed to 365 nm UV light. During UV curing, the PAE underwent free radical polymerization through its vinyl groups, resulting in the formation of PPAE–SPE. The FTIR spectrums of the monomer PEGDA, comb-like polymer PAE, PPAE–SPE (with 6% PAE relative to PEO) and comparative PEO-based SPE (PEO–SPE) were measured (Fig. S1d, ESI†). Compared with PEO–SPE, the presence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in PPAE–SPE confirms the successful introduction of PAE. Meanwhile, the absence of the vinyl characteristic peak indicates the complete polymerization of PAE without any residual monomers. Notably, the abundant electronegative nitrogen and oxhydryl groups in PAE can form quintuple reversible hydrogen bonds with the oxygen and terminal oxhydryl groups of PEO (Fig. S2, ESI†), endowing the PPAE–SPE significant with self-healing properties.31,32 As shown in Fig. S3 (ESI†), the original and artificially stained PPAE–SPE film segments merged into an integral block completely after self-healing for 2 h at room temperature, and the recovered film was strong enough to withstand a 100 g weight along the direction vertical to the cut surface. Furthermore, the SEM images of PEO–SPE and PPAE–SPE membranes were compared. As shown in Fig. S4a and b (ESI†), PEO–SPE exhibits numerous visible holes due to the solvent evaporation during the preparation process. In contrast, the PPAE–SPE membrane shows a dense and smooth surface, attributed to the self-healing properties of PPAE–SPE through abundant reversible hydrogen bonding. This dense and continuous SPE structure facilitates Li+ transport. Besides, because the polymerization of low molecule PAE which has a better dispersibility in the SPE system, the PPAE–SPE membrane has a uniform elemental distribution as shown in the EDS mapping images (Fig. S4c, ESI†). The cross-sectional SEM image of PPAE–SPE (Fig. S4d, ESI†) shows a thickness of about 115 μm, which is the average thickness of SPEs.
O bond in PPAE–SPE confirms the successful introduction of PAE. Meanwhile, the absence of the vinyl characteristic peak indicates the complete polymerization of PAE without any residual monomers. Notably, the abundant electronegative nitrogen and oxhydryl groups in PAE can form quintuple reversible hydrogen bonds with the oxygen and terminal oxhydryl groups of PEO (Fig. S2, ESI†), endowing the PPAE–SPE significant with self-healing properties.31,32 As shown in Fig. S3 (ESI†), the original and artificially stained PPAE–SPE film segments merged into an integral block completely after self-healing for 2 h at room temperature, and the recovered film was strong enough to withstand a 100 g weight along the direction vertical to the cut surface. Furthermore, the SEM images of PEO–SPE and PPAE–SPE membranes were compared. As shown in Fig. S4a and b (ESI†), PEO–SPE exhibits numerous visible holes due to the solvent evaporation during the preparation process. In contrast, the PPAE–SPE membrane shows a dense and smooth surface, attributed to the self-healing properties of PPAE–SPE through abundant reversible hydrogen bonding. This dense and continuous SPE structure facilitates Li+ transport. Besides, because the polymerization of low molecule PAE which has a better dispersibility in the SPE system, the PPAE–SPE membrane has a uniform elemental distribution as shown in the EDS mapping images (Fig. S4c, ESI†). The cross-sectional SEM image of PPAE–SPE (Fig. S4d, ESI†) shows a thickness of about 115 μm, which is the average thickness of SPEs.
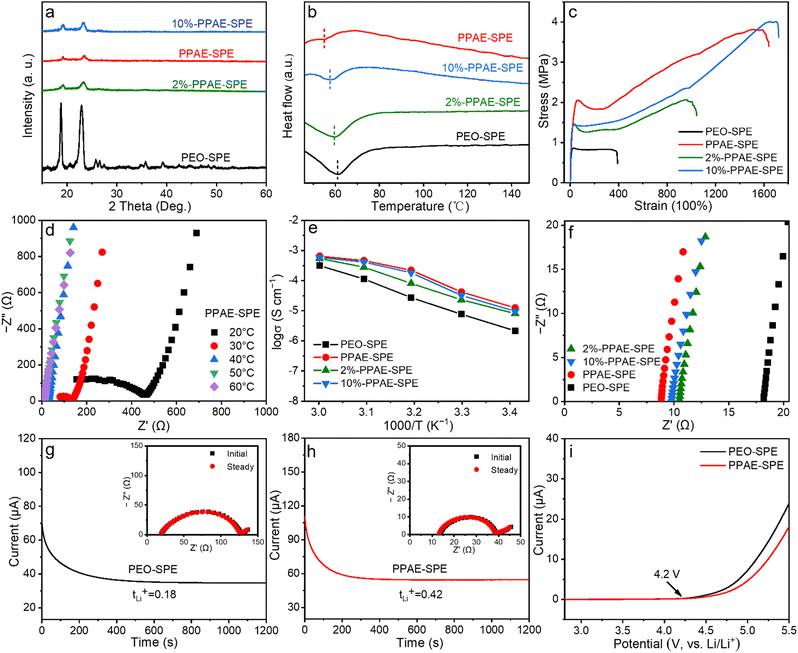
The effect of varying the PAE content in PPAE–SPEs was explored. As shown in Fig. 2a, XRD was used to investigate the crystallinity of different SPEs. Typically, the two characteristic peaks at 19° and 23° are attributed to PEO. Notably, the intensity of these peaks in all PPAE–SPEs is substantially decreased, indicating reduced crystallinity. The DSC tests of different SPEs were further performed. As seen in Fig. 2b, the melting temperature (Tm) of PPAE–SPEs with varying PAE content is lower than PEO–SPE, indicating a reduced crystalline phase in the PEO matrix, consistent with the XRD results. Especially, PPAE–SPE with the PAE content of 6% exhibits the lowest crystallinity, as evidenced by its lowest Tm. Low PAE content cannot sufficiently disrupt the long-chain structure of PEO, whereas excess PAE with rigid phenyl groups impedes segmental motion. Mechanical properties were also tested since they were closely related to the ability to restrain the growth of lithium dendrites. As displayed in Fig. 2c, both the stress and strain of PPAE–SPEs increase with the content of PAE. The 10%-PPAE–SPE possesses the largest tensile strength and impressive elongation strain, the 6%-PPAE–SPE coming second (3.81 MPa, 1522%, respectively), much higher than PEO–SPE (0.8 MPa, 378.1%, respectively). The enhanced mechanical strength is attributed to the introduced rigid aromatic structure of phenyl groups and the interpenetrating macromolecular network, while the improved mechanical elasticity benefits from the strong hydrogen bonding interactions,31,33 which will be sacrificed before the breaking of polymers. The enhanced mechanical properties are beneficial for accommodating the volume change of sulfur and lithium electrodes, alleviating SPE deformation during cycling, inhibiting lithium dendrite growth, and improving the lifespan of batteries.
Ionic conductivity and Li+ transference number are critical factors for SPEs, reflecting their ability to facilitate Li+ movement. To investigate the ionic conductivity of PPAE–SPEs, electrochemical impedance spectroscopy (EIS) was carried out at various temperatures. As shown in the Nyquist plots in Fig. 2d and Fig. S5a–c (ESI†), the impedances of SPEs decrease with increasing test temperature, indicating enhanced polymer chain mobility. Subsequently, the temperature-dependent ionic conductivity of different SPEs was calculated and compared using Arrhenius plots. As shown in Fig. 2e and Table S1 (ESI†), all PPAE–SPEs exhibit improved ionic conductivity compared to PEO–SPE across the measured temperature range. Especially, at 20–50 °C, the ionic conductivity of PPAE–SPE is one order of magnitude higher than that of PEO–SPE. The ionic conductivity at the working temperature of 60 °C was further compared. As seen in Fig. 2f, PEO–SPE displays an ionic conductivity of only 3.14 × 10−4 S cm−1, whereas the PPAE–SPE demonstrates the highest ionic conductivity of 6.48 × 10−4 S cm−1, which is superior to those reported in other published studies (Table S2, ESI†). Since both Li+ and TFSI− participate in the ionic conductivity in the dual-ion SPE system, but only mobile Li+ ions contribute to the electrochemical performance of batteries during cycling, a comparison is made through the Li+ transference number, which is defined as the ratio of transferable Li+ to all mobile ions including anions in SPE (Fig. 2g, h and Fig. S5d, e, ESI†). Unfortunately, the migration of Li+ is significantly slower than its anionic counterpart TFSI− due to its coordination with the polymer chain in the PEO matrix. Therefore, PEO–SPE without any modification displays a very low tLi+ of 0.18 (Fig. 2g). In contrast, all PPAE–SPEs modified with PAE show elevated tLi+, especially PPAE–SPE, which displays the highest value of 0.42. Since TFSI− as an electron donor is a kind of Lewis-base, it can be fixed by Lewis-acid to form a complex through Lewis acid–base interaction.34 Therefore, the enhancement of tLi+ is attributed to the abundant Lewis-acid sites of boron in PPAE–SPE, which effectively immobilize TFSI− anions through strong Lewis acid–base interactions, thereby increasing the mobility of Li+. In addition, the electrochemical stability of PPAE–SPE was assessed using linear sweep voltammetry (LSV), revealing an electrochemical window identical to that of PEO–SPE (4.2 V, Fig. 2i).
Typically, restricted anionic movement leads to decreased ionic conductivity in PEO-based SPE because the cathodic Li+ ions are constantly complexing and decomplexing with the PEO matrix. However, the PPAE–SPE demonstrates both high σ and tLi+, indicating its superior Li+ transfer properties. It is necessary to verify the roles of comb-like topological structure and multiple functional groups in the rapid Li+ conduction. Solid-state 1H nuclear magnetic resonance (NMR) spectroscopy was conducted to research the dynamics of the PEO chains in PEO–SPE and PPAE–SPE. As shown in Fig. 3a, the peak at around 3.5–4.0 ppm belongs to the hydrogen in the PEO segment. Notably, the signal in PPAE–SPE is narrower and the intensity increased, indicating fast segmental motion of PEO chains in the amorphous region, consistent with the XRD and DSC results.35 This is because the abundant branch chains on comb-like PAE can effectively disturb the regular arrangement of the crystalline phase in PEO, leading to increased proportion of soft amorphous regions. Since ion transport in SPEs occurs primarily through segmental motion in the amorphous phase, the introduction of PAE is convenient to improve the ionic conductivity of PPAE–SPE. In addition, 7Li NMR spectra were used to investigate the local Li+ environments and mechanism of Li+ transport in PPAE–SPE. As displayed in Fig. 3b, the overall line-shape between −1.0 and −2.0 ppm is composed of two peaks, corresponding to two different Li+ environments. The right peak (colored pink) is related to the Li+ coordinated by the oxygen in PEO segment, while the left one (colored green) is associated with more mobile Li+ that stems from greater local disorder and weakened interaction with PEO.36 In comparison to PEO–SPE, PPAE–SPE shows a more mobile Li+ region (8.5% and 16.9%, respectively) and up-field shift of the signal, implying weaker PEO–Li+ interaction.37 This is because the hydrogen bonds between ether groups of PEO and nitrogen as well as oxhydryl of PAE effectively reduce the activity of oxygen in PEO, which mitigates the affinity between PEO chains and Li+, thus enhancing the mobility of Li+. This conclusion is further supported by the Raman spectra. As displayed in Fig. 3c, the Raman spectrum of ether groups in PPAE–SPE shows a blue shift compared with PEO–SPE, indicating reduced activity of ether groups in PEO. Moreover, as shown in Fig. 3d, the Raman peak between 720 and 760 cm−1 corresponds to the coupled CF3 bending and S–N stretching of TFSI−, which reflects the cation–anion complexation in the lithium salt of LiTFSI.38 Notably, the signal of PPAE–SPE shifts to a lower wavenumber, indicating a highly dissociated LiTFSI, which is a prerequisite for fast ion conduction. This is attributed to the robust fixation of TFSI− by the Lewis-acid sites of boron in PPAE–SPE. To sum up, the superior Li+ conductivity in PPAE–SPE is achieved through the comb-like topological structure and multiple functional groups of nitrogen, oxhydryl as well as boron Lewis-acid sites, which synergistically form homogeneous and fast Li+ transport channels across the entire electrolyte system. Specifically, the comb-like topological structure of PAE disturbs the regular arrangement of PEO chains, creating a more amorphous region for ion migration. In the dual-ion SPE system, anions of TFSI− are fixed by the Lewis-acid sites of boron, while the coordination between cations of Li+ and PEO is weakened by the nitrogen and oxhydryl in PAE, resulting in the promoted dissociation of lithium salts and accelerated Li+ conduction.
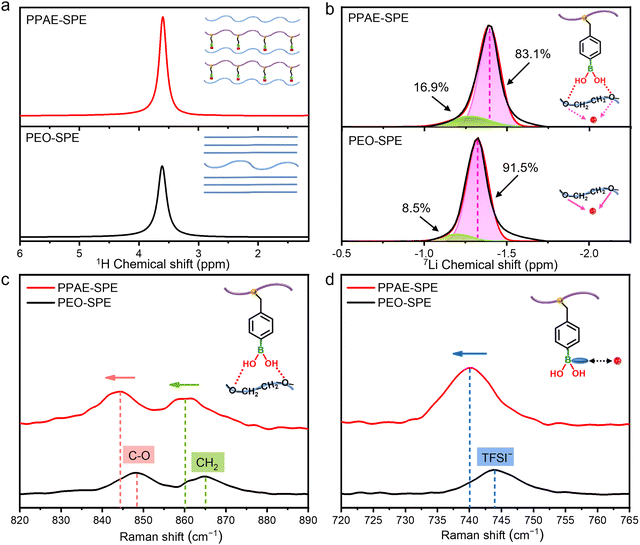 | ||
| Fig. 3 (a) Solid-state 1H, (b) 7Li NMR spectra and Raman spectra in the (c) C–O, CH2, (d) TFSI− bands of PPAE–SPE and PEO–SPE. | ||
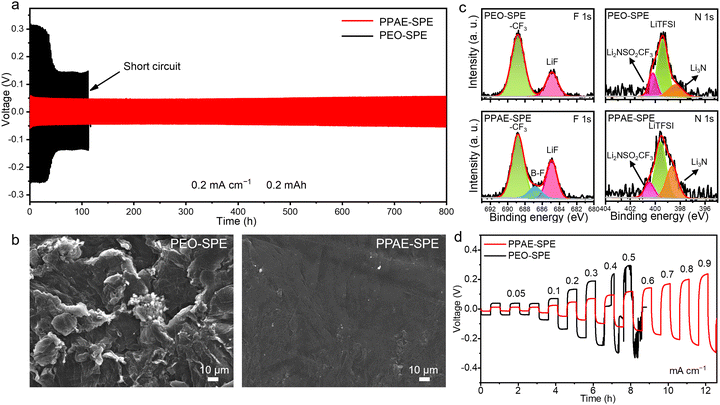
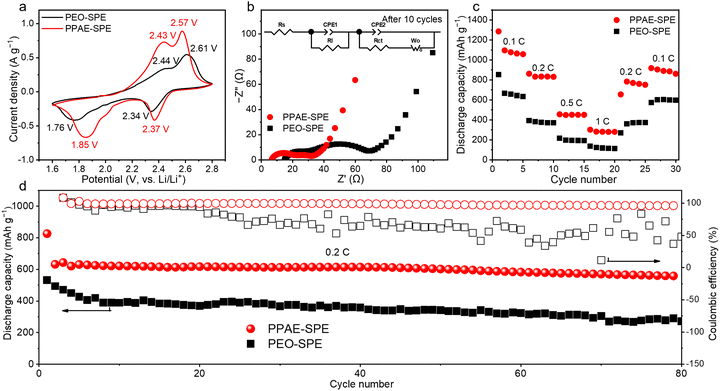
The interface stability of PPAE–SPE toward lithium metal electrodes was evaluated by galvanostatic charge–discharge (GCD) tests in lithium symmetric cells. Typically, the uncontrollable lithium dendrite growth is still challenging in the SPE system with poor mechanical strength, resulting in an unfavorable lifespan. The self-healing ability in PPAE–SPE is expected to address this issue.39 As displayed in Fig. S6 (ESI†), the lithium symmetrical cell with PPAE–SPE shows stable cycling for more than 1700 h at 0.1 mA cm−2, confirming the above conjecture. Besides, as shown in Fig. 4a, during the stripping/plating process under 0.2 mA cm−2 and 0.2 mA h cm−2, the symmetric cells with PEO–SPE show a much higher polarized potential and fail at about 112 hours, which is caused by the inferior electrolyte/electrode interface and uncontrolled growth of lithium dendrites. In contrast, the cell with PPAE–SPE exhibits long-term stability with a small overpotential of about 0.06 V, and no short-circuit occurs over 800 hours, indicating uniform lithium deposition and enhanced interfacial stability. Correspondingly, the surface morphology of lithium anodes retrieved from the cell with PEO–SPE shows a seriously deteriorated surface after cycling for 100 h, whereas the lithium anode based on PPAE–SPE remains dense and smooth, indicating suppressed lithium dendrite growth (Fig. 4b). The improved lithium anode stability in PPAE–SPE is attributed to the following reasons: (1) the fast Li+ transport reduces the Li+ concentration gradient, promoting uniform deposition of Li+ and inhibiting the growth of lithium dendrites. (2) Strong mechanical properties accommodate the volume expansion of lithium anode, and the self-healing property helps it survive occasional lithium dendrites. In addition, to explore the chemical components of SEI, X-ray photoelectron spectra (XPS) analysis was performed on the surface of the anodes after cycling. As previous work has reported,40 the degradation of TFSI− anions occurs in multiple steps, and the sequential reduction of the intermediates contributes to the formation of favorable inorganic SEI components, such as LiF and Li3N. As seen in Fig. 4c, compared with the SEI formed in PEO–SPE, more decomposed species of LiF (684.8 eV), Li3N (398.5 eV) and fewer intermediates of Li2NSO2CF3 (400.2 eV) were detected on the lithium anode equipped with PPAE–SPE, which is attributed to the strong fixation and accelerated degradation of TFSI− anions through the Lewis acid–base interactions. The electrically insulating LiF and fast ionic conductor Li3N prevent electron migration at the interface and facilitate the Li+ transport, thus inhibiting the formation of lithium dendrites and achieving a stable lithium plating/stripping process. It is worth mentioning that SEI formed with PPAE–SPE also contains B–F species (686.8 eV), which likely originate from the boron in PAE. Additionally, to determine the highest current density that different SPEs could withstand, the critical current density (CCD) of lithium symmetric cells was measured at various current densities (from 0.1 to 0.9 mA cm−2). As shown in Fig. 4d, the voltage for both PEO–SPE and PPAE–SPE increases with the current densities. However, PEO–SPE occurs drastic voltage fluctuation at 0.4 mA cm−2. In contrast, the CCD of the PPAE–SPE based symmetrical battery shows much smaller polarization voltage and can reach 0.9 mA cm−2 without any short circuit phenomenon.
All-solid-state batteries with sulfur/carbon (S/C) cathodes were further assembled to evaluate the electrochemical performance of PPAE–SPE in the Li–S system. Both cells with PEO–SPE and PPAE–SPE display two discharge plateaus (Fig. S7, ESI†), corresponding to the continuous conversion from S8 to LiPSs and eventually Li2S2/Li2S, indicating the “solid–liquid–solid” electrochemical transformation mechanism. It is worth mentioning that the cell with PPAE–SPE displays a higher initial discharge capacity than PEO–SPE (1285.8 and 852.8 mA h g−1 at 0.1 C rate, respectively), indicating more complete electrochemical conversion on the cathode–electrolyte interface. To investigate the electrochemical properties of the solid–solid interface in depth, CV and EIS tests were conducted. Consistent with the discharge/charge curves, the CV curves of cells with PEO–SPE and PPAE–SPE show a merged cathodic peak and two anodic peaks, relating to the redox process of active material (Fig. 5a). Notably, the PPAE–SPE based cell exhibits a higher current response, signifying an accelerated conversion of polysulfides. Besides, the higher cathodic peak potential, lower anodic peak potential, and smaller redox peak differences imply less polarization and enhanced electrode reaction reversibility. The EIS results for the cells before and after 10 cycles at 0.2 C are presented in Fig. S8 (ESI†) and Fig. 5b. Before cycling, a semicircle and a low frequency incline were observed, corresponding to the charge transfer resistance (Rct) and the Li+ diffusion process (Wo), respectively. The Rct for the cell with PPAE–SPE is lower, indicating faster carrier transmission, consistent with the CV results. After 10 cycles, a high-frequency semi-cycle corresponding to the surface resistance (RSurf) appeared, which resulted from the additional diffusion in the deposited Li2S2/Li2S layer on Li anode owing to the shuttle effect.41,42 Similarly, the cell based on PPAE–SPE displayed smaller RSurf and Rct, implying ameliorated shuttle of LiPSs and higher electrochemical activity. As the composite cathode using PEO as the binder, PPAE–SPE can also form multiple strong hydrogen bonds through the electronegative nitrogen and oxhydryl with the PEO segment in cathode, resulting in tightly connected electrode–electrolyte interface, which along with the rapid Li+ transmission synergistically enhance the electrochemical reaction kinetics of LiPSs conversion, improve the utilization of active materials and suppress the shuttle effect. In addition, due to the reversibility of hydrogen bonds, the electrode–electrolyte interface also possesses self-healing properties, which can rapidly fill the vacant cavities caused by cathode particle pulverization under large currents, heavy S-loading or long cycling, leading to stable electrochemical performance. As shown in Fig. 5c, due to fast Li+ conduction and the self-healing electrode–electrolyte interface, the cell utilizing PPAE–SPE displays superior rate performance compared to the cell using PEO–SPE, indicating a higher utilization of active materials. On the other hand, this higher utilization also results in a more pronounced sulfur loss since the shuttle effect can be inhibited but not eliminated. As a result, capacity fading is observed when the cell with PPAE–SPE returns to cycling at 0.2 C. To further investigate the stability of PPAE–SPE under high current conditions, Li–S cells using PPAE–SPE were tested at a rate of 0.5 C with the S-loading of 1.0 mg cm−2, which can be stably cycled for more than 30 cycles (Fig. S9, ESI†). Similarly, a cell using PPAE–SPE with a high S-loading of 4.3 mg cm−2 also shows good cycling stability over 20 cycles (Fig. S10, ESI†).
Furthermore, the cycling stability of Li–S cells with PEO–SPE and PPAE–SPE is compared in Fig. 5d. The PPAE–SPE cell delivers an initial discharge capacity of 826.1 and 558.3 mA h g−1 after 80 cycles at 0.2 C, which is superior to that of the PEO–SPE cell (532.1 and 271.8 mA h g−1, respectively). Besides, the cell with PEO–SPE undergoes severe overcharging with very unstable Coulombic efficiency during cycling, which is due to the severe shuttle effect and soft short circuits caused by poor mechanical properties. In contrast, the cell based on PPAE–SPE shows higher and more stable Coulombic efficiency. Besides, in comparison to the reported SPEs, Li–S cells with PPAE–SPE shows substantial electrochemical performance, substantiating the considerable practical application value of PPAE–SPE in the Li–S system (Table S3, ESI†). The lithium surface morphology based on different SPEs after 80 cycles was compared using SEM. As shown in Fig. S11 (ESI†), the lithium anode based on PEO–SPE shows a loose pulverized layer with severe cracks, whereas the lithium surface based on PPAE–SPE is flat and dense. The XPS of the cycled anodes was further conducted to analyse the composition of SEI in full cells. As can be seen in Fig. S12 (ESI†), for the lithium anode equipped with PPAE–SPE, more favorable SEI components of LiF (684.8 eV) and high-valence sulfur-containing species (Li2SO3 and Li2SO4) were detected, meanwhile fewer side reaction products of shuttle effect (Li2S and Li2S2) were observed. This is because the fast Li+ conduction and tightly connected electrode–electrolyte interface enhanced the LiPS conversion, and thus less LiPSs spread to the lithium anode, leading to the inhibited shuttle effect.
Over the past few decades, PEO-based SPEs have been widely researched due to their superior commercialization potential compared to other SSE systems. Innovative SPE structural design is an effective strategy to overcome the Li+ transport and conversion challenges in PEO-based ASSLSBs. Poly(β-amino ester)s, with high structural flexibility and variety achievable through facile Michael addition reaction synthesis methods, hold significant application potential in SPEs. Targeted structural design and the introduction of functional groups make rapid Li+ conduction and conversion possible, thereby effectively improving the performances of ASSLSBs in terms of specific capacity, lithium anode stability and lifespan.
Conclusions
In this study, a poly(β-amino ester) with a comb-like topological structure and multiple functional groups was synthesised and introduced into an PEO-based SPE. Within the homogeneous and interpenetrating macromolecular network, fast Li+ transport channels are realized through the increased amorphous region by the comb-like topological structure of PAE, fixed anions of TFSI− by Lewis-acidic boron and accelerated Li+ mobility due to the electronegative atoms of nitrogen and oxhydryl that weakens the coordination of Li+. Additionally, the hydrogen bonds between PPAE–SPE and the composite cathode facilitate the conversion of LiPSs at the cathode–electrolyte interface, leading to improved cycling stability for ASSLSBs. Moreover, benefitting from the rapid Li+ transport, strong mechanical and self-healing properties of PPAE–SPE, the lithium anode stability is enhanced, allowing the symmetrical lithium battery with PPAE–SPE to cycle stably over 800 h at 0.2 mA cm−2. This work will advance the development of diverse SPE structures and help in achieving high-performance and safe ASSLSBs.Author contributions
Hui-Min Wang: Methodology, investigation, formal analysis, writing – original draft. Mengdi Geng: Formal analysis. Jing Bai: Formal analysis. Dezhong Zhou: Resources, writing – review & editing. Weibo Hua: Writing – review & editing. Sheng Liu: Conceptualization, project administration, writing – review & editing. Xueping Gao: Supervision, funding acquisition.Data availability
All data included in this study are available upon request by contacting the corresponding authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22279064, 21935006 and 22075151).References
- X. Fan, Y. Liu, J. Tan, S. Yang, X. Zhang, B. Liu, H. Cheng, Z. Sun and F. Li, J. Mater. Chem. A, 2022, 10, 7653–7659 RSC.
- Y. Guo, Z. Jin, J. Lu, L. Wei, W. Wang, Y. Huang and A. Wang, Energy Environ. Sci., 2023, 16, 5274–5283 RSC.
- C. Sun, Y. Liu, J. Sheng, Q. Huang, W. Lv, G. Zhou and H.-M. Cheng, Mater. Horiz., 2020, 7, 2487–2518 RSC.
- Y. Guo, Q. Niu, F. Pei, Q. Wang, Y. Zhang, L. Du, Y. Zhang, Y. Zhang, Y. Zhang, L. Fan, Q. Zhang, L. Yuan and Y. Huang, Energy Environ. Sci., 2024, 17, 1330–1367 RSC.
- Z. Pang, H. Zhang, L. Wang, D. Song, X. Shi, Y. Ma, L. Kong and L. Zhang, Front. Mater. Sci., 2023, 17, 230630 CrossRef.
- R. Chen, W. Qu, X. Guo, L. Li and F. Wu, Mater. Horiz., 2016, 3, 487–516 RSC.
- E. Umeshbabu, B. Zheng and Y. Yang, Electrochem. Energy Rev., 2019, 2, 199–230 CrossRef CAS.
- Z. Gao, H. Sun, L. Fu, F. Ye, Y. Zhang, W. Luo and Y. Huang, Adv. Mater., 2018, 30, 1705702 CrossRef PubMed.
- W.-P. Chen, H. Duan, J.-L. Shi, Y. Qian, J. Wan, X.-D. Zhang, H. Sheng, B. Guan, R. Wen, Y.-X. Yin, S. Xin, Y.-G. Guo and L.-J. Wan, J. Am. Chem. Soc., 2021, 143, 5717–5726 CrossRef CAS PubMed.
- J. Wu, S. Liu, F. Han, X. Yao and C. Wang, Adv. Mater., 2021, 33, 2000751 CrossRef CAS PubMed.
- C. Sun, Y. Ruan, W. Zha, W. Li, M. Cai and Z. Wen, Mater. Horiz., 2020, 7, 1667–1696 RSC.
- S. Hong, Y. Wang, N. Kim and S. B. Lee, J. Mater. Sci., 2021, 56, 8358–8382 CrossRef CAS.
- P. Zhai, N. Peng, Z. Sun, W. Wu, W. Kou, G. Cui, K. Zhao and J. Wang, J. Mater. Chem. A, 2020, 8, 23344–23353 RSC.
- A. A. Rojas, S. Inceoglu, N. G. Mackay, J. L. Thelen, D. Devaux, G. M. Stone and N. P. Balsara, Macromolecules, 2015, 48, 6589–6595 CrossRef CAS.
- R. Fang, H. Xu, B. Xu, X. Li, Y. Li and J. B. Goodenough, Adv. Funct. Mater., 2021, 31, 2001812 CrossRef CAS.
- M. Nakayama, S. Wada, S. Kuroki and M. Nogami, Energy Environ. Sci., 2010, 3, 1995–2002 RSC.
- X. Gao, X. Zheng, Y. Tsao, P. Zhang, X. Xiao, Y. Ye, J. Li, Y. Yang, R. Xu, Z. Bao and Y. Cui, J. Am. Chem. Soc., 2021, 143, 18188–18195 CrossRef CAS PubMed.
- H. Wang, Q. Wang, X. Cao, Y. He, K. Wu, J. Yang, H. Zhou, W. Liu and X. Sun, Adv. Mater., 2020, 32, 2001259 CrossRef CAS.
- C.-H. Yu, C.-S. Cho and C.-C. Li, ACS Appl. Mater. Interfaces, 2021, 13, 11995–12005 CrossRef CAS.
- B. Kim and M. J. Park, Mater. Horiz., 2023, 10, 4139–4147 RSC.
- L. Chen, Y. Li, S.-P. Li, L.-Z. Fan, C.-W. Nan and J. B. Goodenough, Nano Energy, 2018, 46, 176–184 CrossRef CAS.
- I. Aldalur, M. Martinez-Ibañez, M. Piszcz, L. M. Rodriguez-Martinez, H. Zhang and M. Armand, J. Power Sources, 2018, 383, 144–149 CrossRef CAS.
- R. Khurana, J. L. Schaefer, L. A. Archer and G. W. Coates, J. Am. Chem. Soc., 2014, 136, 7395–7402 CrossRef CAS.
- J. Wan, J. Xie, X. Kong, Z. Liu, K. Liu, F. Shi, A. Pei, H. Chen, W. Chen, J. Chen, X. Zhang, L. Zong, J. Wang, L.-Q. Chen, J. Qin and Y. Cui, Nat. Nanotechnol., 2019, 14, 705–711 CrossRef CAS.
- T. Yang, C. Wang, W. Zhang, Y. Xia, H. Huang, Y. Gan, X. He, X. Xia, X. Tao and J. Zhang, J. Energy Chem., 2023, 84, 189–209 CrossRef CAS.
- D. Lin, W. Liu, Y. Liu, H. R. Lee, P.-C. Hsu, K. Liu and Y. Cui, Nano Lett., 2015, 16, 459–465 CrossRef.
- Y. Liu, Q. Zeng, Z. Li, A. Chen, J. Guan, H. Wang, S. Wang and L. Zhang, Adv. Sci., 2023, 10, 2206978 CrossRef CAS.
- Y. Zhang, J. Shi, B. Ma, H. Yong, Z. Li, Y.-N. Zhou, J. Li, L. Liang and D. Zhou, ACS Macro Lett., 2023, 12, 626–631 CrossRef CAS PubMed.
- Z. Li, Q. Wo, D. Huang, D. Zhou, L. Guo and Y. Mao, Chin. Chem. Lett., 2024, 35, 109737 CrossRef CAS.
- C. Wang, X. Huang, L. Sun, Q. Li, Z. Li, H. Yong, D. Che, C. Yan, S. Geng, W. Wang and D. Zhou, Chem. Commun., 2022, 58, 2136–2139 RSC.
- J. Lopez, Y. Sun, D. G. Mackanic, M. Lee, A. M. Foudeh, M. S. Song, Y. Cui and Z. Bao, Adv. Mater., 2018, 30, 1804142 CrossRef PubMed.
- L. Chen, J. Xu, M. Zhu, Z. Zeng, Y. Song, Y. Zhang, X. Zhang, Y. Deng, R. Xiong and C. Huang, Mater. Horiz., 2023, 10, 4000–4032 RSC.
- S. Huo, Y. Zhang, Y. He, W. Fan, Z. Hu, W. Bao, X. Jing and H. Cheng, J. Phys. Chem. Lett., 2022, 14, 16–23 CrossRef PubMed.
- Y. Zhao, L. Wang, Y. Zhou, Z. Liang, N. Tavajohi, B. Li and T. Li, Adv. Sci., 2021, 8, 2003675 CrossRef CAS.
- X. Fu, Y. Liu, W. Wang, L. Han, J. Yang, M. Ge, Y. Yao and H. Liu, Macromolecules, 2020, 53, 10078–10085 CrossRef CAS.
- N. Wu, P.-H. Chien, Y. Qian, Y. Li, H. Xu, N. S. Grundish, B. Xu, H. Jin, Y.-Y. Hu, G. Yu and J. B. Goodenough, Angew. Chem., Int. Ed., 2020, 59, 4131–4137 CrossRef CAS PubMed.
- N. Wu, P.-H. Chien, Y. Li, A. Dolocan, H. Xu, B. Xu, N. S. Grundish, H. Jin, Y.-Y. Hu and J. B. Goodenough, J. Am. Chem. Soc., 2020, 142, 2497–2505 CrossRef CAS PubMed.
- H. Wang, J. Song, K. Zhang, Q. Fang, Y. Zuo, T. Yang, Y. Yang, C. Gao, X. Wang, Q. Pang and D. Xia, Energy Environ. Sci., 2022, 15, 5149–5158 RSC.
- Z. Sun, J. Wu, H. Yuan, J. Lan, Y. Yu, Y. Zhu and X. Yang, Mater. Today Energy, 2022, 24, 100939 CrossRef CAS.
- H. Zhang, U. Oteo, X. Judez, G. G. Eshetu, M. Martinez-Ibañez, J. Carrasco, C. Li and M. Armand, Joule, 2019, 3, 1689–1702 CrossRef CAS.
- Z. Ao, Y. Zou, H. Zou, Y. Huang and N. Chen, Chem. – Eur. J., 2022, 28, e202200543 CrossRef CAS PubMed.
- Z. Ao, Y. Zou, H. Li, N. Chen, Y. Huang and Y. Liang, Sustainable Mater. Technol., 2023, 38, e00712 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mh01181c |
| This journal is © The Royal Society of Chemistry 2025 |