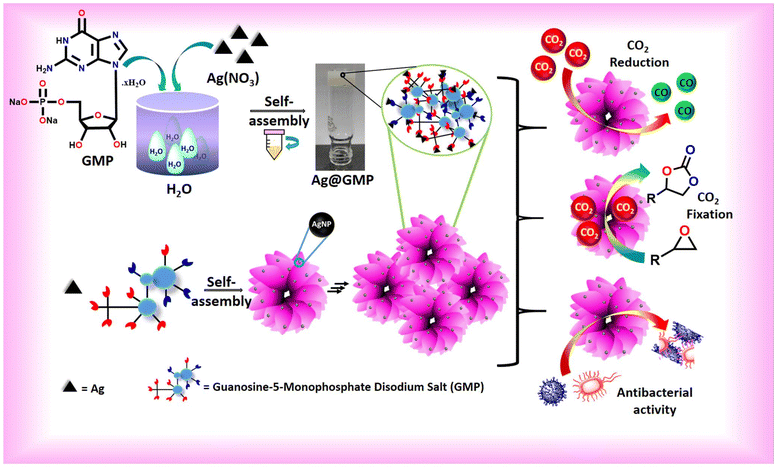
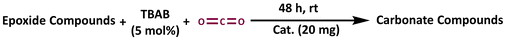Self-template impregnated silver nanoparticles in coordination polymer gel: photocatalytic CO2 reduction, CO2 fixation, and antibacterial activity†
Noohul
Alam
 a,
Sumit
Mondal
a,
Sumit
Mondal
 a,
Niwesh
Ojha
a,
Niwesh
Ojha
 b,
Subham
Sahoo
b,
Subham
Sahoo
 a,
Mohammad Tarique
Zeyad
a,
Mohammad Tarique
Zeyad
 c,
Sushant
Kumar
c,
Sushant
Kumar
 b and
Debajit
Sarma
b and
Debajit
Sarma
 *a
*a
aSolid State and Inorganic Chemistry Group, Department of Chemistry, Indian Institute of Technology Patna, Bihar 801106, India. E-mail: debajit@iitp.ac.in
bGas-solid Interaction Laboratory, Department of Chemical and Biochemical Engineering, Indian Institute of Technology Patna, Bihar 801106, India
cDepartment of Agricultural Microbiology, Faculty of Agricultural Science, Aligarh Muslim University, Aligarh, India
First published on 1st November 2024
Abstract
CO2 fixation and light-assisted conversion of CO2 in the presence of water into fuels and feedstocks are clean and sustainable techniques to alleviate the energy crisis and global climate change. In this regard, herein, a waterborne multifunctional metal–organic coordination polymer gel (Ag@GMP) was prepared from silver nitrate and guanosine 5′-monophosphate. Electron microscopy exhibits that Ag@GMP has a flower-like structure, which is composed of vertically grown sheets, and corresponding high magnification images display the presence of silver nanoparticles on the vertically grown sheets. Ag@GMP demonstrates remarkable photocatalytic performance, achieving a CO2 conversion rate of 18.6 μmol g−1 with approximately 85% selectivity towards CO at ambient temperature without using sacrificial agents. In situ diffuse reflectance infrared Fourier transform spectroscopy was employed to elucidate the proposed mechanism for photocatalytic CO2 reduction. Additionally, Ag@GMP exhibits significant catalytic activity in the fixation of CO2 with epoxides, leading to the formation of valuable chemicals under atmospheric pressure. Ag@GMP demonstrated efficient antibacterial activity against both Gram-negative and Gram-positive bacteria. The highest zone of inhibition was observed against S. aureus MTCC 3160 (15.83 ± 1.1 mm), and for E. coli, P. aeruginosa PAO1, and B. subtilis, it was found to be 12.66 ± 0.9, 14.33 ± 0.8 and 12.8 ± 0.8 mm, respectively.
Introduction
The ever-increasing concentration of carbon dioxide in the earth's atmosphere is a threat for the existence of life on the earth.1,2 CO2 concentration has steeply risen from 280 ppm in the pre-industrial era to 410 ppm at present.3,4 The high level of CO2 in the atmosphere will directly affect humans.5,6 The utilization of renewable energy (nuclear power, wind, hydrogen, and solar energy) is still lower than that of fossil fuels. Therefore, since the last few decades, there is a global demand for carbon dioxide capture and utilization (CCU).7 CCU technique is an exciting strategy for achieving sustainable energy.8–10 Particularly, the photocatalytic conversion of CO2 in the presence of water into fuels and value-added feedstocks is a viable technique to alleviate the greenhouse effect and energy demand.11–13 Similarly, the coupling of CO2 with epoxides to produce cyclic carbonates is an eco-friendly approach for the development of value-added products from CO2 as cyclic carbonates find a wide range of applications as electrolytes in batteries, in polymer synthesis, and as green solvents.14 However, the activation of carbon dioxide is extremely challenging owing to its high endergonic nature; therefore, catalysts play a key role in the activation of CO2.15,16To this end, the design and development of advanced, “soft” processable metal–organic coordination polymer gel (CPG) hybrid materials with high catalytic activity for CO2 fixation and photocatalytic CO2 reduction is a stirring and emerging area of research in heterogeneous catalysis.17–20 CPGs are prepared through the coordination-driven self-assembly of metal ions and organic linkers and have attracted plenty of attention because of their wide range of applications ranging from catalysis to medicine.21–24 The choice of metal ions plays an important role in designing CPGs for desired applications.25–27 Plasmonic nanoparticle (PNP)-incorporated CPGs are attractive materials for catalysis because of their remarkable absorption and nanoscale control of light and heat.28 An increasing number of these so-called hybrid plasmonic nanomaterials have been studied for chemical reactions in recent years, resulting in the development of plasmon-assisted catalysis.29
An increasing number of these so-called hybrid plasmonic nanomaterials have been studied for chemical reactions in recent years, resulting in the development of plasmon-assisted catalysis.29 As silver-based plasmonic materials have been established for photocatalytic CO2 reduction, therefore, the use of silver metal ion and π-conjugated organic linkers could be an ideal combination for the preparation of soft metal–organic CPG-based active catalytic materials.30 However, only a handful of CPG-based heterogeneous catalysts have been reported for photocatalytic carbon dioxide reduction as well as CO2 fixation.11,17–20,31 Therefore, there is wide open space for the design and the development of metal–organic CPG hybrid materials for catalysis.
Antibiotics entered in their golden era with the discovery of Penicillin in 1928; since then, several antibiotics have been either extracted from natural sources or chemically prepared, and countless lives have been saved.32 However, antibiotic resistance in bacteria has been rapidly growing.33,34 Therefore, it is essential to develop the next generation of antibiotics against a variety of life-threatening bacterial infections. Engineered nanoparticles (NPs) have the capability to mitigate the issue by providing a viable alternative for treating a variety of illnesses, most notably those generated by multidrug-resistant (MDR) bacteria.35–37 Amongst various engineered nanoparticles that have been utilized as antibacterial agents, silver nanoparticles (AgNPs) or AgNPs-containing materials have been found to be excellent antibacterial agents because of their robust antimicrobial effectiveness against different fungi, viruses, and bacteria.38–40 The bare AgNPs are susceptible to aggregation when disseminated in aqueous solution, which decreases their stability and drastically reduces their antibacterial activity.36 This problem with AgNPs can be overcome by affixing them to a support material.39 CPGs exhibit remarkable adaptability for incorporating various polymers, nanomaterials, and metal ions by in situ co-gelation without affecting the basic gelation process.41,42 Therefore, CPGs could be utilized to prepare highly dispersed AgNPs-loaded CPG for excellent antibacterial activity, wherein the gel matrix can act as a template (or fixed platform) and offers anchoring sites for in situ reduced or presynthesized AgNPs to avoid aggregation.
In this regard, a waterborne CPG (Ag@GMP) was synthesized by the direct mixing of silver nitrate and the low molecular weight gelator guanosine 5′-monophosphate disodium salt hydrate (Fig. 1). The silver nanoparticle-loaded Ag@GMP has the ability to convert CO2 with over ∼85% selectivity into CO in the presence of moisture and light without any sacrificial agent. Moreover, the as-synthesized Ag@GMP also exhibits excellent catalytic activity toward CO2 conversion into cyclic carbonate at atmospheric pressure. Additionally, Ag@GMP has an inhibitory ability for Gram-negative as well as Gram-positive bacteria. The maximum zone of inhibition against S. aureus MTCC 3160 was found to be 15.83 ± 1.1 mm, and for E. coli, P. aeruginosa PAO1, and B. subtilis, it was 12.66 ± 0.9, 14.33 ± 0.8 and 12.8 ± 0.8 mm, respectively.
Results and discussion
The coordination-driven gelation approach for the fabrication of silver nanoparticles-anchored metal–organic CPG with silver nitrate and GMP is demonstrated in Fig. 1. The structure of guanine in GMP is rich in carbon and nitrogen, providing a rigid skeleton with multiple coordination sites. The gel nature of Ag@GMP was checked by the “inversion vial” method. Upon turning the vial upside down, the gel did not flow due to the gravitational forces, indicating the gel nature of Ag@GMP CPG (Fig. 1). The binding model of different metal ions with nucleic acids is a well-established field of research.43,44 At neutral pH, the binding stability suggests that the best possible interaction may happen with the N7/O6 centre of the guanosine moiety to the metal ions.45 Importantly, Ag+, Pt2+, and Hg2+ have the affinity to bind with nucleobase rather than phosphate groups.46,47 Herein, Ag+ is prone to forming adducts with the electron-rich nitrogen (N7) and oxygen (O6) sites43 in the purine ring of the guanosine moiety, which is elaborately illustrated in Fig. 2. The self-assembly process may encompass both metal–linker coordination and additional supramolecular non-covalent interactions that contribute to robust gelation. The nucleobase and phosphate groups regulate the π⋯π-stacking and hydrogen bonding, respectively, to strengthen the gel synthesis via 3D supramolecular structure formation. As we know, the semi-solid gel materials are viscoelastic in nature. Therefore, the mechanical strength and the viscoelastic nature of the synthesized CPG were investigated by rheological analysis. For gel materials, the storage modulus (G′) is always higher than the loss modulus (G′′) of the materials. Freshly prepared Ag@GMP CPG was used for rheological study. Fig. S1† demonstrates the G′ and G′′ analysis against angular frequency sweep at constant 0.01% strain, wherein the G′ and G′′ values remained almost constant in the entire test region and did not cross each other (i.e., G′ > 105, G′′ < 105). The rheological study confirms the viscoelastic nature of the synthesized Ag@GMP CPG.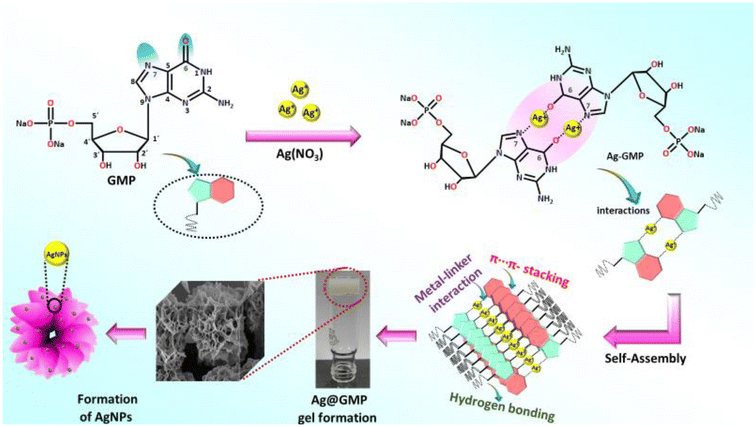 | ||
| Fig. 2 Schematic representation of the potential interactions between silver ions and the GMP moiety. | ||
The interaction with Ag+ and GMP moiety was further explained through FTIR analysis (Fig. S2a, ESI†). The blue shift from 1689 cm−1 to 1606 cm−1 and 3311 cm−1 to 3216 cm−1 is due to the alteration in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and N–H stretching band, respectively. This clearly indicates the interaction between Ag+ with purine ring's carbonyl groups. The hydrogen bonding in the supramolecular structure may be the reason behind the NH2 band shifting. Furthermore, the peak at about 1523 cm−1 remains unshifted, but the intensity of the peak is drastically diminished after complex formation between Ag+ and the GMP moiety, which may be due to the C
O stretching vibration and N–H stretching band, respectively. This clearly indicates the interaction between Ag+ with purine ring's carbonyl groups. The hydrogen bonding in the supramolecular structure may be the reason behind the NH2 band shifting. Furthermore, the peak at about 1523 cm−1 remains unshifted, but the intensity of the peak is drastically diminished after complex formation between Ag+ and the GMP moiety, which may be due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrational stretching band of the imidazole moiety.48–51 It also suggests the interaction between Ag+ and the purine unit. In addition, the effect of π–π stacking interactions on the gelation process was investigated using diffuse-reflectance spectroscopy (DRS) (Fig. S2b, ESI†). The spectrum of Ag@GMP shows that the pristine GMP absorbance band was slightly red shifted (272 nm to 276 nm) due to the π–π stacking in the supramolecular gel structure.52 Along with this, a clear broad absorbance peak at about 440 nm dictates the surface plasmon resonance peak (SPR) of AgNPs, which is absent in pristine GMP.41 The generation of AgNPs during the assembly process is likely associated with the heterocyclic and glycosyl groups. The electron-rich nitrogen-containing moiety and the hydroxyl group of glycosyl may take part in stabilizing the AgNPs with the gel matrix. The AgNPs were then incorporated due to the interaction between the amino or hydroxyl groups and AgNPs. Therefore, the supramolecular gel-phase network encapsulates and stabilizes these nascent metal nanoparticles.53,54
N vibrational stretching band of the imidazole moiety.48–51 It also suggests the interaction between Ag+ and the purine unit. In addition, the effect of π–π stacking interactions on the gelation process was investigated using diffuse-reflectance spectroscopy (DRS) (Fig. S2b, ESI†). The spectrum of Ag@GMP shows that the pristine GMP absorbance band was slightly red shifted (272 nm to 276 nm) due to the π–π stacking in the supramolecular gel structure.52 Along with this, a clear broad absorbance peak at about 440 nm dictates the surface plasmon resonance peak (SPR) of AgNPs, which is absent in pristine GMP.41 The generation of AgNPs during the assembly process is likely associated with the heterocyclic and glycosyl groups. The electron-rich nitrogen-containing moiety and the hydroxyl group of glycosyl may take part in stabilizing the AgNPs with the gel matrix. The AgNPs were then incorporated due to the interaction between the amino or hydroxyl groups and AgNPs. Therefore, the supramolecular gel-phase network encapsulates and stabilizes these nascent metal nanoparticles.53,54
The synthesized Ag@GMP CPG was lyophilized to get the Ag@GMP xerogel, and then PXRD analysis was performed for the phase purity and crystallinity of the synthesized xerogel (Fig. S3, ESI†). The recorded PXRD pattern of the Ag@GMP xerogel suggests the crystalline nature of the material, which contrasts with the usually amorphous nature of the CPG. The obtained diffraction was matched with that of the reported Ag(0) (ICSD No. 22434), indicating the formation of AgNPs within the Ag@GMP xerogel.
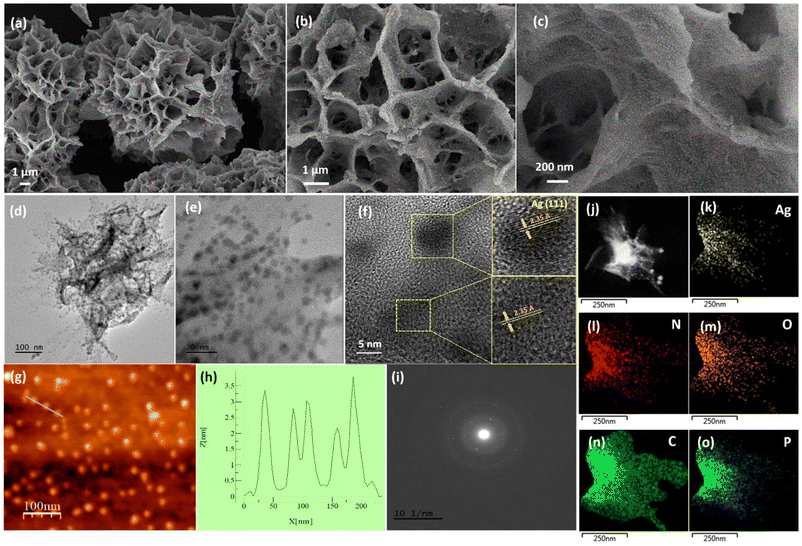
The FESEM micrograph of Ag@GMP exhibits a flower-like structure made up of vertically grown sheets. The high-magnification FESEM images reveal that these sheets contain silver nanoparticles (Fig. 3a–c). The TEM micrograph also confirms the presence of AgNPs in the as-synthesized Ag@GMP (Fig. 3d). The HRTEM images revealed the dispersion of the spherical and quasi-spherical AgNPs within the Ag@GMP xerogel, and the average particle size of AgNPs was found to be approximately 4.46 nm (Fig. 3e and Fig. S4, ESI†).
The lattice fringes of Fig. 3e are presented in Fig. 3f, which exhibits a clear view of the d-spacing for the (111) plane of Ag with a d-spacing of 2.35 Å. Moreover, the topological study of Ag@GMP was performed by AFM analysis in the noncontact mode, which further confirmed the formation of AgNPs within the as-synthesized Ag@GMP material. The AFM image height profile measurement displayed that the Ag@GMP xerogel consists of about 3 nm AgNPs on the sheet-like structure (Fig. 3g and h). The SAED pattern of the material indicates the crystalline nature (Fig. 3i). The STEM study was also performed, which demonstrated the homogenous distribution of all the elements within the synthesized material (Fig. 3j–o).
Furthermore, X-ray photoelectron spectroscopy (XPS) was also performed to investigate the electronic state of the surface and the chemical composition of the synthesized Ag@GMP xerogel. The survey spectrum peaks clearly exhibit that the xerogel is composed of silver (Ag), nitrogen (N), carbon (C), oxygen (O), and phosphorus (P) elements (Fig. S5a, ESI†). The core-level spectrum of Ag 3d was deconvoluted to investigate the valence state of silver in the synthesized xerogel. The XPS spectrum of Ag 3d exhibits two peaks at 368.3 eV and 374.3 eV assigned to 3d5/2 and 3d3/2, respectively (Fig. S5b, ESI†). The deconvoluted spectra display the presence of both Ag(I) and Ag(0) in the material. Next, we performed a temperature-programmed desorption (TPD) experiment to evaluate the active binding sites on the synthesized Ag@GMP material (Fig. S6, ESI†). The CO2-TPD spectrum of the Ag@GMP xerogel shows CO2 desorption peaks at low temperatures (i.e., about 80 °C) and also at high temperatures (i.e., about 600 °C). The CO2 desorption peaks observed at about 80 °C indicate the presence of weak binding sites, and the peaks found at higher temperatures indicate the existence of strong basic sites (i.e., –NH/–OH groups) that are capable of binding with CO2 even at higher temperatures. The obtained CO2-TPD results suggest the presence of binding sites for CO2 gas at low as well as high temperatures in the as-synthesized Ag@GMP material.
The ζ-potential value of the Ag@GMP xerogel was found to be −54.9 mV (Fig. S7, ESI†). The high negative value of ζ-potential indicates that the Ag@GMP xerogel has a negative surface charge. As we know, carbon dioxide is a Lewis acid; therefore, it is expected that the negative surface of the Ag@GMP xerogel can facilitate the binding of the CO2 molecules.
Optoelectronic properties
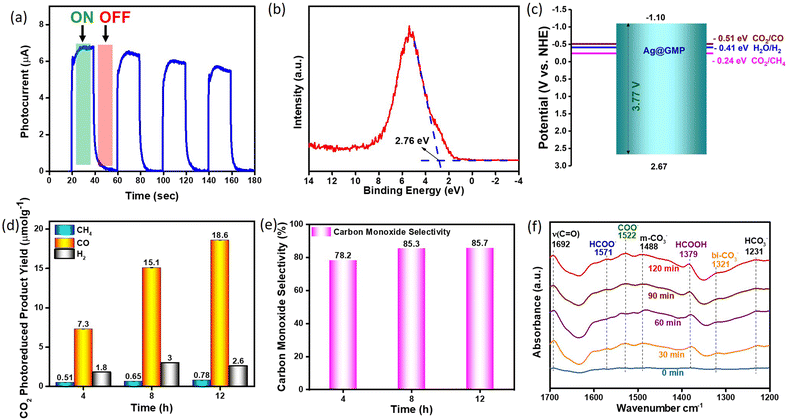
The optical band gap of the Ag@GMP xerogel was calculated to be 3.77 eV using the Tauc plot from the UV-vis absorption spectra (Fig. S8, ESI†). Thereafter, the photocurrent response of Ag@GMP was analysed to explore the efficiency of photoinduced charge carriers. Fig. 4a clearly shows that the photocurrent surged spontaneously under light irradiation and subsequently reduced to zero when the light was turned off, showing that the Ag@GMP catalyst exhibited photoresponse. Additionally, the photocurrent response remained stable after four ON/OFF illumination cycles, suggesting the excellent photoelectrochemical stability of Ag@GMP. However, the slight decrease in the photocurrent intensity is due to the back-electron transfer process. Furthermore, valence band (VB) XPS analysis was performed to get information of the valence band edge, which was found to be 2.76 eV (Fig. 4b). Therefore, the corresponding conduction and valence bands can be calculated from the following eqn (1).| EVB = ECB + Eg | (1) |
Photocatalytic activity
The transformation of carbon dioxide gas into valuable chemical compounds in the presence of moisture and light is a sustainable approach for addressing environmental concerns. The band alignment of the Ag@GMP material suggests that it can reduce CO2 gas. Moreover, the CO2-TPD and zeta potential results also indicate the presence of basic sites within Ag@GMP.Therefore, we checked the applicability of Ag@GMP to reduce CO2 gas in the presence of water and light. Here, water molecules work as a source of H+ ions or reducing agents without adding sacrificial agents. Several catalytic measurements were performed under identical conditions without CO2 gas, photocatalysts, and light irradiation (Fig. S9, ESI†). No products (CO, CH4 and H2) were detected in the GC equipped with TCD, FID, and methaniser, suggesting that the reduced products were derived only from CO2 photoreduction. Fig. 4d demonstrates the formation rate of CO, CH4, and H2 over Ag@GMP catalysts for different illumination times, i.e., four, eight, and twelve hours of experiment. The yield of the photo-reduced products increased with the irradiation time. The Ag@GMP was found to be highly selective (>85%) toward CO formation over CH4 and H2 (Fig. 4e). The CO, CH4, and H2 formation rates were 18.6 μmol g−1, 0.78 μmol g−1, and 2.6 μmol g−1, respectively, under 12 h of irradiation. The PXRD patterns after catalysis (Fig. S10, ESI†) suggest the retention of the framework of Ag@GMP catalysts. The photocatalytic performance of the Ag@GMP catalysts is highly comparable with several reported benchmark hybrid materials (Table S1, ESI†).
In situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy and photocatalytic CO2 reduction mechanism
The in situ DRIFTS technique was employed under UV-vis illumination at 1800–1300 cm−1 to investigate the light-induced generation of active intermediates throughout the catalytic process, thereby offering essential insights into the plausible CO2 reduction mechanism (Fig. 4f). Initially, Ag@GMP was subjected to vacuum at 100 °C for 2 hours to remove any adsorbed species, followed by the passage of water-moist CO2 from the reaction vessels to maintain the adsorption–desorption process until a stable IR signal was obtained. The IR spectrum was initially acquired under dark condition, labeled as 0 min. Subsequently, the xenon arc lamp was activated to facilitate the monitoring of intermediate products formed under UV-vis light exposure. Upon light exposure, new peaks emerged, and the intensity of some peaks changed over time, indicating a reaction intermediate transformation. Initially, upon light illumination, CO2 accepts photoconducting electrons from the catalyst surface to undergo a single-electron reduction process, resulting in the formation of reactive intermediate COO− species, which is indicated by the emergence of a peak at 1522 cm−1.55In addition, two peaks that arise with time at 1379 and 1571 cm−1 could be attributed to formic acid (HCOOH) and formate (HCOO−) species, respectively.56 The peak appearing at 1321 cm−1 is attributed to the bidentate carbonate species, while the emergence of the peak at 1231 cm−1 corresponds to bicarbonates.57,58 These peaks arise due to the interaction between reactive intermediate molecules and the available free OH group on the catalyst's surface. The peak appearing at 1488 cm−1 corresponds to monodentate carbonates. Additionally, the emergence of the peak at 1231 cm−1 corresponds to the bicarbonates. Moreover, a notable peak emerged at 1692 cm−1 over time, corresponding to the CO peak, thus confirming the formation of CO due to light exposure over time.59
We have proposed the plausible reaction pathway for photocatalytic CO2 reduction based on the obtained experimental results and in situ DRIFT analysis (R1–R10, ESI†). The zeta potential and CO2-TPD analyses indicate the basic nature of the catalyst surface, which could facilitate CO2 interaction with the material. Fig. 4f implies that Ag@GMP would have a multielectron reduction process. The photo-generated electron–hole pair reacted with CO2 on the catalyst surface, yielding the COO− species (Fig. 4f, 1522 cm−1, R1 & R2, ESI†). This implies the formation of a reactive intermediate, which subsequently undergoes a series of intricate reactions to yield CO and CH4 (R4–R10, ESI†). The signals correspond to HCO3− (1231 cm−1), CO3− (1321 & 1488 cm−1), and HCOOH (1379 cm−1), which appeared with time in the in situ DRIFT analysis, affirming the formation of intermediates for product formation. Notably, these entities can receive both protons and electrons, leading to carbon monoxide production, as depicted in the reactions (R5–R9, ESI†).
Catalytic CO2 cycloaddition reactions
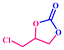
Silver nanoparticles functionalized metal–organic hybrid materials have shown efficient catalytic activity in facilitating the carboxylation of epoxides to yield the corresponding esters. The as-synthesized Ag@GMP material contains catalytically active sites comprising silver nanoparticles, alongside polar functional groups such as –OH and –NH, which endow them with great potential for catalyzing CO2 cycloaddition reactions. Therefore, we have investigated the applicability of Ag@GMP as a catalyst for CO2 cycloaddition reactions under solvent-free conditions. Bifunctional catalysts are crucial for driving both photocatalytic CO2 reduction and CO2 cycloaddition reactions efficiently. However, to the best of our knowledge, there are no reports on specifically tailored CPGs for these dual functions.Ag@GMP was activated prior to catalysis by keeping it under vacuum for 24 h. The catalytic reactions were carried out in the presence of carbon dioxide at atmospheric pressure (balloon) and under solvent-free conditions at room temperature. 20 mmol epoxide and 5 mol% tetrabutylammonium bromide (TBAB) were used as the substrate and cocatalyst, respectively. The 1H NMR spectrum of the filtrate was recorded to calculate the conversion. Controlled reactions were carried out without the catalyst, without the cocatalyst, using ligands (GMP) and AgNO3 as the catalyst (Table 1). In all the cases, the conversion was found to be very low. A time-dependent kinetic study was also carried out to check the efficiency of the catalyst (considering the conversion of epichlorohydrin to the corresponding cyclic carbonate (CC) as a model reaction).
Fig. 5 shows the conversion of the CO2 cycloaddition reaction of epichlorohydrin in the presence of Ag@GMP catalyst at different time intervals. The conversion was found to be 9%, 22%, and 36% after 2 h, 5 h, and 10 h respectively. Finally, the conversion was found to be 99% after 48 h.
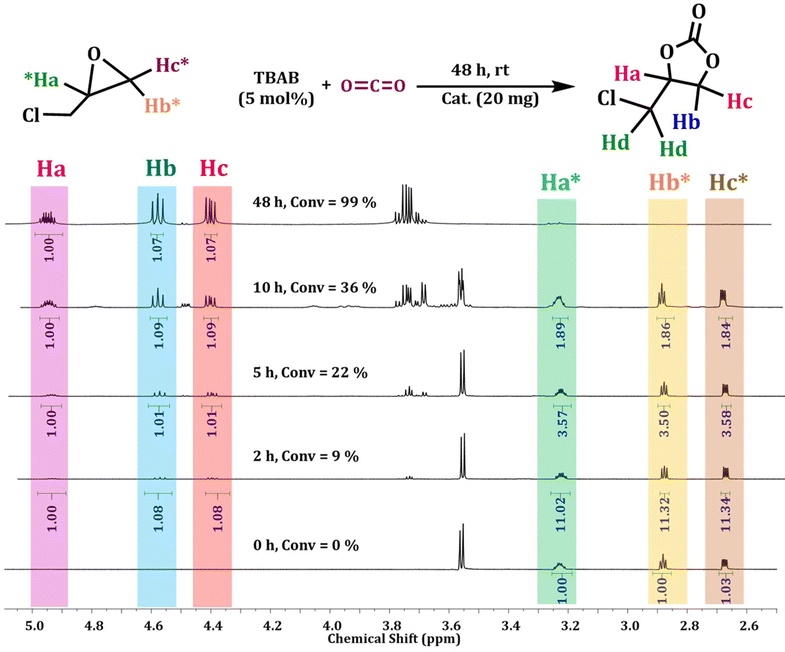 | ||
| Fig. 5 1H NMR (CDCl3, 500 MHz) spectra for the optimization of the reaction with time for the cycloaddition reaction of epichlorohydrin with CO2 using Ag@GMP as a catalyst. | ||
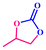
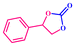
The catalytic activity was also explored using other epoxides (Table 2), viz., a small epoxide, propyleneoxide; relatively larger epoxide, 1,2-epoxybutane; and an aromatic epoxide, styrene oxide. The conversion was found to be 82% for propyleneoxide towards the corresponding CC (Fig. S11 and Table 2, 2.2, ESI†). The relatively lower conversion may be due to the absence of an electron-withdrawing group. However, the catalytic activity was slightly reduced for considerably larger epoxides. 60% conversion was found for 1,2-epoxybutane under the same reaction conditions (Fig. S12 and Table 2, 2.3, ESI†). A low conversion of about 46% was observed in case of styrene oxide (Fig. S13 and Table 2, 2.4, ESI†).
The cycloaddition reaction is directly linked to the ring-opening process. The ring-opening process of the epoxide acts as the rate-determining step of the catalytic conversion reaction.60,61 The electron-withdrawing effect of the chloromethyl group eases the pathway and helps in the ring-opening process. Consequently, the conversion to the respective cyclic carbonates occurs far more rapidly compared to other substrate scopes. Meanwhile, larger substituents on the aromatic ring provide steric hindrance, which impedes the diffusion rate of larger epoxides to the Lewis acidic site. It is reflected in the conversion rate of the corresponding epoxide to cyclic carbonates.62 The CO2 cycloaddition reaction of epichlorohydrin towards the respective carbonate compound (CC) shows 99% conversions in the presence of the catalyst Ag@GMP (Fig. 5). After the first cycle, the catalyst was isolated via filtration and then washed with acetone for the next cycles. The recyclability of Ag@GMP was studied for up to three cycles and 99, 97, and 95% conversions were obtained, respectively (Fig. 6).
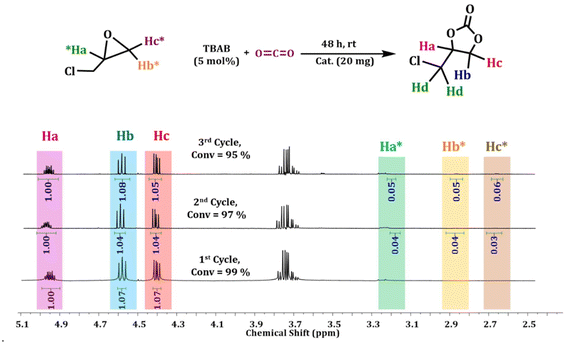 | ||
| Fig. 6 1H-NMR (CDCl3, 500 MHz) spectra of recyclability for the cycloaddition reaction of epichlorohydrin with CO2 using Ag@GMP as a catalyst. | ||
A comparison table (Table S2, ESI†) has been made to compare the activity of the catalysts Ag@GMP within the family of CPGs as well as other well-known reported compounds for the conversion of epichlorohydrin to CC via CO2 cycloaddition reactions. Ag@GMP is well compared within the family of the reported gel-based materials. Cu(II)-MOG shows 80% conversion in 48 h at RT.19 MOG at RT exhibits about 78% conversion in 48 h.31 NiXero shows 48% conversion at 80 °C under 5 bar.20 UMCM-1-NH2 at RT shows about 78% conversion in 24 h,63 whereas MOF-5 shows a slightly higher conversion of 93% in 12 h.64 Almost complete conversion was found using Zn-DAT as a catalyst at 8 bar pressure in 24 h.65 Eu-MOF and Cu–K-MOF show full conversions at higher temperatures of about 80 °C and 60 °C, respectively.66,67 In this context, the utilization of CPG as a catalyst for CO2 cycloaddition remains largely unexplored. Ag@GMP could convert epichlorohydrin completely to its respective CC only within 48 h. The obtained results affirmed that the performance of the catalyst Ag@GMP stands out as highly competitive within the coordination polymer gel family and the best-reported catalysts.19,20,31 Moreover, Ag@GMP proves to be a particularly advantageous catalyst due to its straightforward scalability for bulk production.
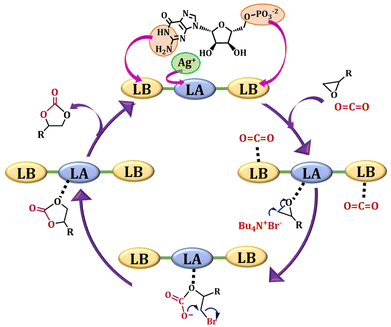
Based on our results and through the literature survey,68–72 the mechanism of the catalytic cycle is shown in Fig. 7. The Lewis acidic silver centre acts as a catalytic active centre. The unsaturated acidic metal centres bind the nucleophilic oxygen atoms of the epoxide. The nucleophilic bromide ion obtained from TBAB attacks the polarized epoxide for ring opening, which subsequently interacts with carbon dioxide to create an intermediate. This anion has a high tendency to close the ring to form a cyclic carbonate product. The XPS spectra of Ag@GMP indicate that the material comprises both Ag(0) and Ag+ states. The zero-oxidation state of silver is present as nanoparticles (NPs), and the +1 oxidation silver ions interact with the GMP moiety to form the structural arrangement.73 The electron-rich NPs takes part as an electron donor source and Ag+ stabilizes the intermediates during the catalytic process. Hence, the surface motif featuring Ag+ oxidation state and the electron-rich NPs containing Ag(0) both play a synergistic role in enhancing the catalytic activity.
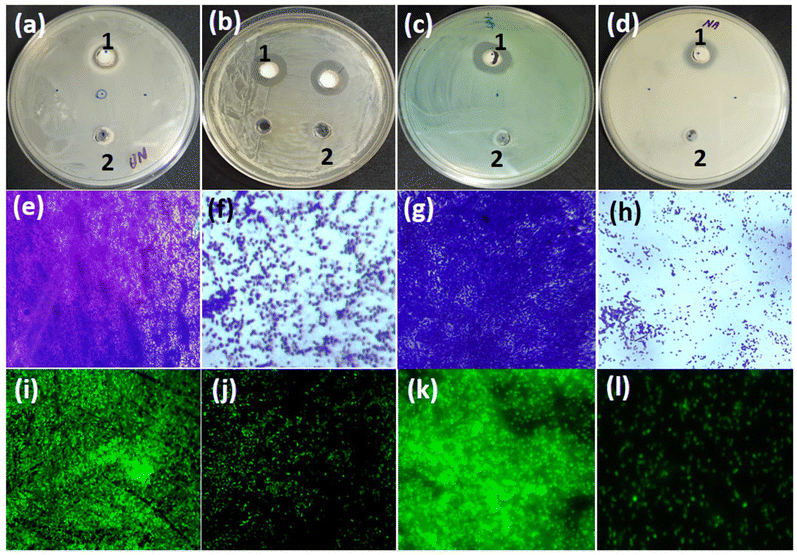
Antibacterial studies
Silver nanoparticle-containing materials are well-known to demonstrate significant antibacterial activity. Therefore, we further explored the applicability of these materials as antibacterial agents.40 The antibacterial activity of the as-synthesized silver nanoparticles containing the Ag@GMP material was tested against Gram-negative (E. coli ATCC 25922 and P. aeruginosa PAO1) and Gram-positive (S. aureus MTCC 3160 and B. subtilis) bacteria. Ag@GMP showed varying degrees of inhibitory impacts against the tested bacteria (Fig. 8a–d). The maximum zone of inhibition (15.83 ± 1.1 mm) was observed against S. aureus MTCC 3160. Similarly, the zone of inhibition against E. coli, P. aeruginosa PAO1, and B. subtilis were found to be 12.66 ± 0.9, 14.33 ± 0.8, and 12.8 ± 0.8 mm, respectively. Furthermore, the inhibitory impact of Ag@GMP materials on biofilm development was also investigated through microscopy. The bacterial cultures were grown in the absence and presence of Ag@GMP on glass coverslips in 24-well polystyrene tissue culture plates.The light microscopic images of P. aeruginosa PAO1 and S. aureus MTCC 3160 exhibited that both the bacterial pathogens had colonised on the glass surface in large numbers, producing a dense cluster of cells in the untreated control (Fig. 8e and h).
However, the biofilm-forming ability of both bacteria was significantly decreased when the cultures were treated with Ag@GMP material (Fig. 8f and h).
Additionally, confocal laser scanning microscopy (CLSM) was also used to examine the antimicrobial activity of the as-synthesized Ag@GMP. For that, biofilms were stained with acridine orange and then the confocal microscopy images of P. aeruginosa PAO1 and S. aureus MTCC 3160 biofilms were recorded with and without treating with Ag@GMP (Fig. 8i–l). The CLSM micrographs also exhibit that both the bacteria densely colonized on the glass surface in the absence of Ag@GMP, although their ability to form biofilm significantly reduced after treatment with Ag@GMP. Additionally, scanning electron microscopy was used to examine the influence of Ag@GMP treatment on biofilm architecture. Moreover, the SEM images of untreated and Ag@GMP-treated biofilms of the test bacteria (P. aeruginosa PAO1 and S. aureus MTCC 3160) are presented in Fig. S14, ESI.† The untreated P. aeruginosa PAO1 developed dense biofilms on a glass surface, with normal and smooth bacterial cell shapes (Fig. S14a, ESI†). Ag@GMP reduced the biofilm formation and decreased bacterial colonization (Fig. S14b, ESI†). Similarly, the biofilm development in untreated S. aureus MTCC 3160 was higher as compared to that in treated S. aureus MTCC 3160 (Fig. S14c and S14d, ESI†).
Conclusions
In summary, a waterborne silver nanoparticle-incorporated multifunctional Ag@GMP material was obtained from guanosine 5′-monophosphate and silver nitrate. Electron microscopy reveals the presence of silver nanoparticles on the vertically grown sheets. The PXRD pattern of the materials matches with that of the reported Ag NPs, which also favoured the SEM and TEM results. The significantly negative ζ-potential values suggest a predominantly negatively charged surface, which could facilitate CO2 binding, also corroborated by the CO2-TPD results, indicating the presence of basic binding sites. Ag@GMP displays excellent photocatalytic activity (18.6 μmol g−1) with high selectivity (over ∼85%) for CO2 conversion into CO at room temperature without any sacrificial agent. Moreover, Ag@GMP also displays excellent catalytic activity for the fixation of CO2 with epoxides to afford cyclic carbonates at atmospheric pressure. The presence of Ag NPs makes Ag@GMP an efficient material for antibacterial agents, and the obtained results demonstrate efficient antibacterial activity for both Gram-negative and Gram-positive bacteria. The maximum zone of inhibition against S. aureus MTCC 3160 was found to be 15.83 ± 1.1 mm, and for E. coli, P. aeruginosa PAO1 and B. subtilis, it was 12.66 ± 0.9, 14.33 ± 0.8 and 12.8 ± 0.8 mm, respectively. The multifunctional nature of silver-based CPGs underscores their importance in addressing environmental and health challenges simultaneously, showcasing the immense potential of CPGs in advancing multifaceted solutions for a range of societal needs.Author contributions
NA, SM, NO, SS, MTZ, SK and DS designed and conducted the research and co-wrote the manuscript. The final version of the manuscript is approved by all the authors.Data availability
All the data supporting this article have been included in the main manuscript as well as ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Authors acknowledge IIT Patna for providing the research facilities. D. S. acknowledges the core research grant under sanction CRG/2023/003002 from the Science and Engineering Research Board, DST, Government of India. The anonymous reviewers are acknowledged for their efforts in reviewing and enhancing the quality of the work.References
- Z. Liu, W. Hou, H. Guo, Z. Wang, L. Wang and M. Wu, ACS Appl. Mater. Interfaces, 2023, 15, 33868–33877 CrossRef CAS
.
- M. Laghaei, M. Ghasemian, W. Lei, L. Kong and Q. Chao, J. Mater. Chem. A, 2023, 11, 11925–11963 RSC
.
- E. Gong, S. Ali, C. B. Hiragond, H. S. Kim, N. S. Powar, D. Kim, H. Kim and S.-I. In, Energy Environ. Sci., 2022, 15, 880–937 RSC
.
- S. Singh, A. Modak, K. K. Pant, A. Sinhamahapatra and P. Biswas, ACS Appl. Nano Mater., 2021, 4, 8644–8667 CrossRef CAS
.
- Z. Zhang, E. Jansen, S. P. Sobolowski, O. H. Otterå, G. Ramstein, C. Guo, A. Nummelin, M. Bentsen, C. Dong and X. Wang, Nat. Geosci., 2023, 16, 321–327 CrossRef CAS
.
- J. Song, G. Tong, J. Chao, J. Chung, M. Zhang, W. Lin, T. Zhang, P. M. Bentler and W. Zhu, Sci. Rep., 2023, 13, 5536 CrossRef CAS PubMed
.
- J.-L. K. Gbe, K. Ravi, E. K. Tillous, A. Arya, M. Grafoute and A. V. Biradar, ACS Appl. Mater. Interfaces, 2023, 15, 17879–17892 CrossRef CAS PubMed
.
- J. Hegarty, B. Shindel, D. Sukhareva, M. L. Barsoum, O. K. Farha and V. Dravid, Environ. Sci. Technol., 2023, 57, 21080–21091 CrossRef CAS
.
- X. Zheng, M. C. Drummer, H. He, T. M. Rayder, J. Niklas, N. P. Weingartz, I. L. Bolotin, V. Singh, B. V. Kramar and L. X. Chen, J. Phys. Chem. Lett., 2023, 14, 4334–4341 CrossRef CAS
.
- E. Nishikawa, S. Islam, S. Sleep, V. Birss and J. Bergerson, Green Chem., 2023, 25, 229–244 RSC
.
- N. Alam, N. Ojha, S. Kumar and D. Sarma, ACS Sustainable Chem. Eng., 2023, 11, 2658–2669 CrossRef CAS
.
- W. Dong, C. Wang, Y. Zou, W. Wang and J. Liu, ACS Appl. Mater. Interfaces, 2024 DOI:10.1021/acsami.4c01101
.
- T. Zhang, F. Meng, M. Gao, J. Wei, K. J. H. Lim, K. H. Lim, P. Chirawatkul, A. S. W. Wong, S. Kawi and G. W. Ho, Small, 2023, 19, 2301121 CrossRef CAS
.
- Z. Zhang, H. Gao, H. Wu, Y. Qian, L. Chen and J. Chen, ACS Appl. Nano Mater., 2018, 1, 6463–6476 CrossRef CAS
.
- B. Ge, Y. Ye, Y. Yan, H. Luo, Y. Chen, X. Meng, X. Song and Z. Liang, Inorg. Chem., 2023, 62, 19288–19297 CrossRef CAS
.
- T. Zhang, T. Li, M. Gao, W. Lu, Z. Chen, W. L. Ong, A. S. W. Wong, L. Yang, S. Kawi and G. W. Ho, Adv. Energy Mater., 2024, 2400388 CrossRef CAS
.
- P. Verma, F. A. Rahimi, D. Samanta, A. Kundu, J. Dasgupta and T. K. Maji, Angew. Chem., Int. Ed., 2022, 61, e202116094 CrossRef CAS PubMed
.
- P. Verma, A. Singh, F. A. Rahimi, P. Sarkar, S. Nath, S. K. Pati and T. K. Maji, Nat. Commun., 2021, 12, 7313 CrossRef CAS PubMed
.
- C. K. Karan, M. C. Sau and M. Bhattacharjee, Chem. Commun., 2017, 53, 1526–1529 RSC
.
- E. Saha, H. Jungi, S. Dabas, A. Mathew, R. Kuniyil, S. Subramanian and J. Mitra, Inorg. Chem., 2023, 62, 14959–14970 CrossRef CAS PubMed
.
- N. Alam, S. Mondal and D. Sarma, Coord. Chem. Rev., 2024, 504, 215673 CrossRef CAS
.
- N. Alam, S. Mondal, S. S. Hossain, S. Sahoo and D. Sarma, ACS Appl. Eng. Mater., 2023, 1, 1201–1212 CrossRef CAS
.
- N. Rahman and I. Ahmad, Chemosphere, 2024, 351, 141272 CrossRef CAS PubMed
.
- N. Rahman and I. Ahmad, J. Hazard. Mater., 2023, 457, 131783 CrossRef CAS
.
- N. Alam, S. Majumder, S. J. Ray and D. Sarma, Langmuir, 2022, 38, 10601–10610 CrossRef CAS
.
- S. Dhibar, B. Pal, K. Karmakar, S. Roy, S. A. Hafiz, A. Roy, S. Bhattacharjee, S. J. Ray, P. P. Ray and B. Saha, Nanoscale Adv., 2023, 5, 6714–6723 RSC
.
- Z. Zhan, T. Huang, J. Zhu, X. Cao, Y. Zhou, Y. Tang and P. Wu, CrystEngComm, 2024, 26, 639–646 RSC
.
- N. Sarfraz and I. Khan, Chem. – Asian J., 2021, 16, 720–742 CrossRef CAS
.
- Y. Liu, C.-H. Liu, T. Debnath, Y. Wang, D. Pohl, L. V. Besteiro, D. M. Meira, S. Huang, F. Yang and B. Rellinghaus, Nat. Commun., 2023, 14, 541 CrossRef CAS PubMed
.
- J.-J. Liu, Z.-W. Jiang and S.-W. Hsu, ACS Appl. Mater. Interfaces, 2023, 15, 6716–6725 CrossRef CAS
.
- C. K. Karan and M. Bhattacharjee, Eur. J. Inorg. Chem., 2019, 2019, 3605–3611 CrossRef CAS
.
- M. M. Golden, S. J. Post, R. Rivera and W. M. Wuest, ACS Infect. Dis., 2023, 9, 2386–2393 CrossRef CAS PubMed
.
- W. Li, S. Hadjigol, A. R. Mazo, J. Holden, J. Lenzo, S. J. Shirbin, A. Barlow, S. Shabani, T. Huang and E. C. Reynolds, ACS Appl. Mater. Interfaces, 2022, 14, 25025–25041 CrossRef CAS
.
- H. Liu, Y. Zuo, S. Lv, X. Liu, J. Zhang, C. Zhao, X. Xu, Y. Xu and X. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 17182–17192 CrossRef CAS
.
- S. Kumar, R. K. Majhi, A. Singh, M. Mishra, A. Tiwari, S. Chawla, P. Guha, B. Satpati, H. Mohapatra and L. Goswami, ACS Appl. Mater. Interfaces, 2019, 11, 42998–43017 CrossRef CAS
.
- R. Zhao, M. Lv, Y. Li, M. Sun, W. Kong, L. Wang, S. Song, C. Fan, L. Jia and S. Qiu, ACS Appl. Mater. Interfaces, 2017, 9, 15328–15341 CrossRef CAS
.
- X. Pang, Q. Xiao, Y. Cheng, E. Ren, L. Lian, Y. Zhang, H. Gao, X. Wang, W. Leung and X. Chen, ACS Nano, 2019, 13, 2427–2438 CAS
.
- X. Li, R. Gui, J. Li, R. Huang, Y. Shang, Q. Zhao, H. Liu, H. Jiang, X. Shang and X. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 30434–30457 CrossRef CAS
.
- W. Zhou, Z. Jia, P. Xiong, J. Yan, Y. Li, M. Li, Y. Cheng and Y. Zheng, ACS Appl. Mater. Interfaces, 2017, 9, 25830–25846 CrossRef CAS PubMed
.
- K. Y. Gudz, L. Y. Antipina, E. S. Permyakova, A. M. Kovalskii, A. S. Konopatsky, S. Y. Filippovich, I. A. Dyatlov, P. V. Slukin, S. G. Ignatov and D. V. Shtansky, ACS Appl. Mater. Interfaces, 2021, 13, 23452–23468 CrossRef CAS
.
- R. Kyarikwal, N. Malviya, A. Chakraborty and S. Mukhopadhyay, ACS Appl. Mater. Interfaces, 2021, 13, 59567–59579 CrossRef CAS
.
- Y. Hu, W. Xu, G. Li, L. Xu, A. Song and J. Hao, Langmuir, 2015, 31, 8599–8605 Search PubMed
.
- K. Loo, N. Degtyareva, J. Park, B. Sengupta, M. Reddish, C. C. Rogers, A. Bryant and J. T. Petty, J. Phys. Chem. B, 2010, 114, 4320–4326 CrossRef CAS
.
- H. Sigel, Chem. Soc. Rev., 1993, 22, 255–267 RSC
.
- Y. Fu, X. Wang, J. Zhang and W. Li, Curr. Opin. Biotechnol., 2014, 28, 33–38 CrossRef CAS PubMed
.
- J. V. Burda, J. Šponer, J. Leszczynski and P. Hobza, J. Phys. Chem. B, 1997, 101, 9670–9677 Search PubMed
.
- L. Berti and G. A. Burley, Nat. Nanotechnol., 2008, 3, 81–87 Search PubMed
.
- Y. Zhang, C. He, J. T. Petty and B. Kohler, J. Phys. Chem. Lett., 2020, 11, 8958–8963 Search PubMed
.
- I. Goncharova, Spectrochim. Acta, Part A, 2014, 118, 221–227 Search PubMed
.
- G. I. Dovbeshko, N. Y. Gridina, E. B. Kruglova and O. P. Pashchuk, Talanta, 2000, 53, 233–246 Search PubMed
.
- H. Fidder, M. Yang, E. T. Nibbering, T. Elsaesser, K. Röttger and F. Temps, J. Phys. Chem. A, 2013, 117, 845–854 Search PubMed
.
- S. Mondal and D. Sarma, Soft Matter, 2023, 19, 4926–4938 CAS
.
- S. Ray, A. K. Das and A. Banerjee, Chem. Commun., 2006, 2816–2818 CAS
.
- J. Fei, L. Gao, J. Zhao, C. Du and J. Li, Small, 2013, 9, 1021–1024 Search PubMed
.
- A. Holmgren, B. Andersson and D. Duprez, Appl. Catal., B, 1999, 22, 215–230 CAS
.
- N. Ojha, A. Bajpai and S. Kumar, J. Colloid Interface Sci., 2021, 585, 764–777 Search PubMed
.
- A. Porta, R. Matarrese, C. G. Visconti and L. Lietti, Energy Fuels, 2023, 37, 7280–7290 CAS
.
- Y. Yang, W. Liu, Q. Zhong, J. Zhang, B. Yao, X. Lian and H. Niu, ACS Appl. Nano Mater., 2021, 4, 4735–4745 CAS
.
- L. Zou, Z. A. Chen, D. H. Si, S. L. Yang, W. Q. Gao, K. Wang, Y. B. Huang and R. Cao, Angew. Chem., Int. Ed., 2023, 62, e202309820 CAS
.
- H. Sun and D. Zhang, J. Phys. Chem. A, 2007, 111, 8036–8043 CrossRef CAS
.
- R. Patra and D. Sarma, Dalton Trans., 2023, 52, 10795–10804 RSC
.
- S. S. Dhankhar and C. M. Nagaraja, New J. Chem., 2019, 43, 2163–2170 RSC
.
- R. Babu, A. C. Kathalikkattil, R. Roshan, J. Tharun, D.-W. Kim and D.-W. Park, Green Chem., 2016, 18, 232–242 RSC
.
- J. Song, Z. Zhang, S. Hu, T. Wu, T. Jiang and B. Han, Green Chem., 2009, 11, 1031–1036 RSC
.
- R. Das, S. S. Dhankhar and C. Nagaraja, Inorg. Chem. Front., 2020, 7, 72–81 RSC
.
- M. Sinchow, N. Semakul, T. Konno and A. Rujiwatra, ACS Sustainable Chem. Eng., 2021, 9, 8581–8591 CrossRef CAS
.
- Y. Yang, C. Tu, J. Shi, X. Yang, J.-J. Liu and F. Cheng, Cryst. Growth Des., 2022, 22, 4813–4820 CrossRef CAS
.
- P. Das and S. K. Mandal, Chem. Mater., 2019, 31, 1584–1596 CrossRef CAS
.
- M. Pardakhti, T. Jafari, Z. Tobin, B. Dutta, E. Moharreri, N. S. Shemshaki, S. Suib and R. Srivastava, ACS Appl. Mater. Interfaces, 2019, 11, 34533–34559 CrossRef CAS
.
- H. Chen, L. Fan, X. Zhang and L. Ma, ACS Appl. Mater. Interfaces, 2020, 12, 27803–27811 CrossRef CAS
.
- L.-G. Ding, B.-J. Yao, W.-X. Wu, Z.-G. Yu, X.-Y. Wang, J.-L. Kan and Y.-B. Dong, Inorg. Chem., 2021, 60, 12591–12601 CAS
.
- R. Patra and D. Sarma, ACS Appl. Mater. Interfaces, 2024, 16, 10196–10210 CrossRef CAS PubMed
.
- R. K. Aparna, S. Mukherjee, S. S. Rose and S. Mandal, Inorg. Chem., 2022, 61, 16441–16447 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03254c |
| This journal is © The Royal Society of Chemistry 2025 |