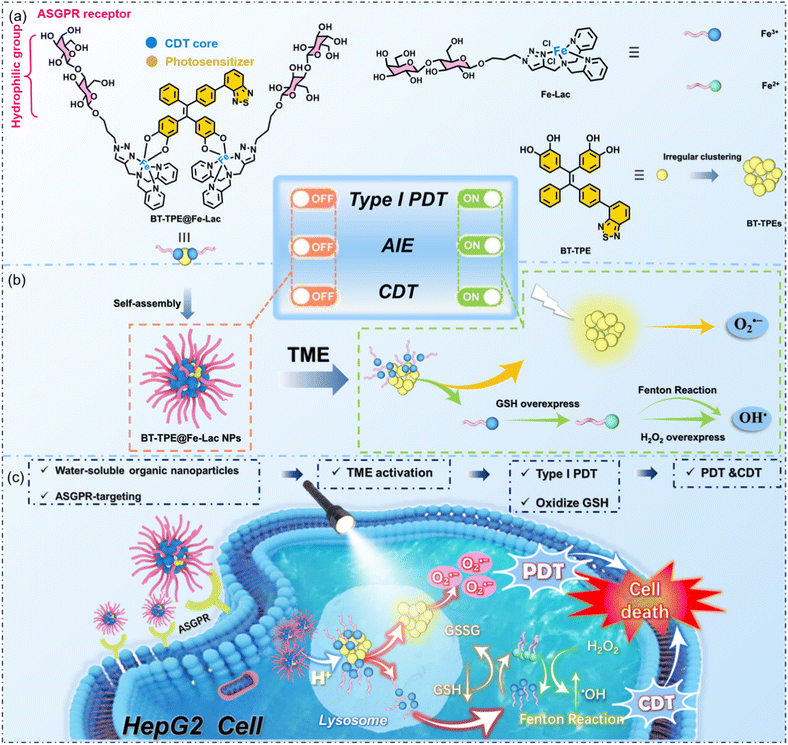
A glycosylated AIE-active Fe(III) photosensitizer activated by the tumor microenvironment for synergistic type I photodynamic and chemodynamic therapy†
Gai-Li
Feng
,
Wei
Zhou
,
Jin-ping
Qiao
,
Guang-Jian
Liu
 and
Guo-Wen
Xing
and
Guo-Wen
Xing
 *
*
College of Chemistry, Beijing Normal University, China. E-mail: gwxing@bnu.edu.cn
First published on 6th November 2024
Abstract
Photodynamic therapy (PDT) and chemodynamic therapy (CDT) are both promising cancer treatments to inhibit tumor cells by generating highly cytotoxic reactive oxygen species (ROS). Herein, we report a novel tumor microenvironment (TME) stimulus-responsive water-soluble glycosylated photosensitizer (BT-TPE@Fe-Lac), which can serve as a high-efficiency antitumor agent by combining PDT and CDT, based on the coordination of Fe3+ with lactosyl bis(2-pyridylmethyl)amine and an AIE luminogen (benzothiazole-hydroxytetraphenylethene, BT-TPE). BT-TPE@Fe-Lac is stable under physiological conditions and selectively targets HepG2 cells via asialoglycoprotein receptor (ASGPR)-mediated endocytosis. It rapidly dissociates into AIE-active BT-TPE molecules and a lactosyl ferric(III) complex in the acidic lysosomes of cancer cells. Upon exposure to light, BT-TPE produces O2˙− radicals for type I PDT. The ferric(III) complex is reduced to an Fe(II) complex upon depletion of glutathione, which primes the breakdown of endogenous H2O2 within the tumor microenvironment, thus generating highly toxic ˙OH for enhanced CDT. Compared with the monotherapy of PDT or CDT, BT-TPE@Fe-Lac can significantly increase the intracellular ROS levels to induce more tumor cell death under low drug doses and hypoxia-dependent conditions. This strategy leverages the unique properties of the TME to optimize therapeutic outcomes, offering a promising approach for the TME-responsive nanoplatform in advanced cancer therapy.
Introduction
The occurrence, progression, and metastasis of tumors are highly correlated with the specific tumor microenvironment (TME), which is characterized by overexpression of hydrogen peroxide and glutathione (GSH), low pH, hypoxia, and vascular abnormalities.1–3 Glutathione is a highly expressed reducing agent in tumor cells and is responsible for eliminating oxidative stress to maintain the intracellular redox balance.4–6 The consumption of intracellular GSH is considered as an effective additional means of improving treatment outcomes through reactive oxygen species (ROS) generation strategies.7,8 The tumor microenvironment has been recognized as a significant factor affecting the effectiveness of the aforementioned treatments, and more and more studies are incorporating the tumor microenvironment into the diagnosis and treatment of tumors.9Photodynamic therapy (PDT) has been considered as a novel alternative to traditional oncology treatments with high selectivity, low toxicity, no drug resistance and lower non-invasiveness.10–14 Although PDT has shown great potential in cancer treatment, many challenges still need to be overcome in practical applications. The hypoxia of the TME seriously restricts the PDT effect of conventional type-II photosensitizers (PSs), which are highly dependent on O2.15,16 Type-I photosensitizers predominantly generate superoxide radicals (O2˙−) through a cascade of electron transfer involving excited PSs, adjacent substrates, and molecular oxygen.17,18 Subsequently, through the superoxide dismutase (SOD)-mediated disproportionation reaction and the subsequent Fenton reaction, O2˙− is converted into highly cytotoxic hydroxyl radicals (˙OH) and causes oxidative stress.19–21 However, due to the low concentration of endogenous ferric ions and the presence of peroxidase in tumor cells, most H2O2 is decomposed into water and oxygen.22 Thus, the generation of ˙OH is often limited, resulting in reduced therapeutic efficacy. Therefore, it is crucial to develop strategies to improve the controllability and efficiency of ˙OH generation, in order to enhance the therapeutic outcomes of PDT. Chemodynamic therapy (CDT) involves the production of highly reactive ˙OH in the tumor microenvironment through Fenton or Fenton-like reactions with H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23–25 which provides a method for directly killing cancer cells using endogenous H2O2, and has become a new strategy for cancer treatment. So far, many research groups have explored the use of different materials as CDT reagents to trigger cancer cell apoptosis and inhibit tumor growth.26–31 However, the relatively weak catalytic efficiency of CDT leads to the limited production of ROS. The combination of PDT and CDT will be an effective strategy to increase the generation of ROS.32,33 From the perspective of synergistic therapy, the combination of PDT and CDT is considered as a breakthrough tactic to achieve superimposed effects while overcoming their respective obstacles. Currently, most studies on CDT/PDT synergistic therapy involve the intricate doping or assembly of photosensitizers and Fenton agents,34–36 and the resulting nanosystems usually have poor bioavailability due to low water solubility.
23–25 which provides a method for directly killing cancer cells using endogenous H2O2, and has become a new strategy for cancer treatment. So far, many research groups have explored the use of different materials as CDT reagents to trigger cancer cell apoptosis and inhibit tumor growth.26–31 However, the relatively weak catalytic efficiency of CDT leads to the limited production of ROS. The combination of PDT and CDT will be an effective strategy to increase the generation of ROS.32,33 From the perspective of synergistic therapy, the combination of PDT and CDT is considered as a breakthrough tactic to achieve superimposed effects while overcoming their respective obstacles. Currently, most studies on CDT/PDT synergistic therapy involve the intricate doping or assembly of photosensitizers and Fenton agents,34–36 and the resulting nanosystems usually have poor bioavailability due to low water solubility.
Furthermore, to achieve excellent tumor theranostic efficiency, tumor targeted delivery is essential. Due to enhanced permeability and retention (EPR) effects, most of the drug-loaded micelles can be passively accumulated in tumors but often suffer from low efficiency.37 It still remains a great challenge to smartly construct an integrated and actively targeted nanoplatform with the simplest and most biocompatible components to promote multimodal tumor treatment diagnosis. The asialoglycoprotein receptor (ASGPR), which is overexpressed on the surface of liver cells,39 provides an ideal target for hepatocyte-specific delivery,38 and specifically recognizes galactose residues. As a disaccharide composed of galactose and glucose, lactose has gained popularity due to easy preparation, nontoxicity, non-immunogenicity, easy carrying and release of various drugs, excellent water solubility, biocompatibility, and delivery of the target drug to hepatocellular carcinoma cells.
Compared with the aggregation caused quenching (ACQ) of traditional fluorescent dyes, aggregation-induced emission (AIE) has excellent photophysical properties. However, the low water solubility of most AIE fluorescent dyes limits their application scopes in the detection and biosensing fields to a certain extent.40 In recent years, our group has developed a series of glycosyl AIE luminogens for bioluminescence imaging, tumor therapy, small molecule detection and other related fields.41 Based on the above analysis, herein, we constructed a multifunctional photosensitizer BT-TPE@Fe-Lac with TME stimuli-responsive capacities, which is facilely prepared via the coordination of Fe3+ with an AIE luminogen (benzothiazole-hydroxytetraphenylethene, BT-TPE) and lactosyl bis(2-pyridylmethyl)amine. The amphiphilic BT-TPE@Fe-Lac could self-assemble into nanoparticles (BT-TPE@Fe-LacNPs) in aqueous solution (Scheme 1a and b). Compared with the typical reported drugs used for PDT/CDT (Table S1†), the designed BT-TPE@Fe-LacNPs are endowed with the following significant characteristics: (i) by convenient and facile synthesis, composites with synergistically enhanced functions can be fabricated utilizing the coordination characteristics of Fe(III), and easy assembly of the glycosylated photosensitizer can be achieved; (ii) lactosyl BT-TPE@Fe-LacNPs improve both aqueous solubility and targeted delivery, and are mainly accumulated in lysosomes through ASGPR-mediated endocytosis, leading to more effective precision therapy; (iii) the fluorescence, photodynamic therapy, and chemodynamic therapy functions are quenched under normal physiological conditions, which can minimize adverse effects on normal tissues; (iv) BT-TPE@Fe-Lac is a TME stimulus-responsive photosensitizer, which rapidly dissociates into AIE-active BT-TPE molecules and a ferric(III) complex in the acidic lysosomes of cancer cells; (v) BT-TPE can produce O2˙− under light irradiation and realize oxidative damage induced cell death by PDT. Notably, even under severe hypoxic conditions (2% O2), it still generates sufficient cytotoxicity via type I photoreactions; (vi) the ferric(III) complex is reduced to an Fe(II) complex upon depletion of glutathione, which primes the breakdown of endogenous H2O2 within the tumor microenvironment, thus disrupting the redox balance and generating highly toxic ˙OH for enhanced CDT; (vii) compared with the monotherapy of PDT or CDT, BT-TPE@Fe-LacNPs have been shown to remarkably suppress the growth of HepG2 cells through a synergistic combination of CDT and PDT (Scheme 1c), achieving an impressive IC50 value of 1.2 μM. Based on the above descriptions, BT-TPE@Fe-Lac is considered to provide a promising platform for synergistic tumor therapy, facilitating the precise diagnosis and targeted treatment of HepG2 cells. To the best of our knowledge, this is the first report on utilizing dual coordination of Fe3+ to construct glycosylated photosensitizer aggregates for tumor microenvironment synergistic therapy.
Results and discussion
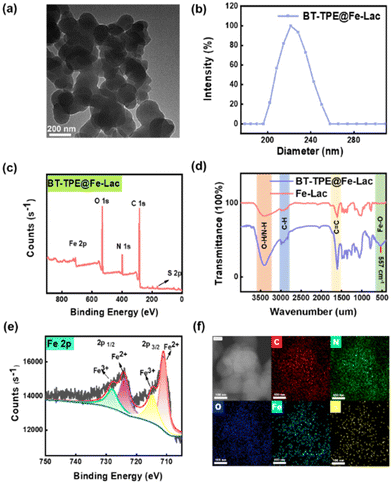
Firstly, BT-TPE and Fe-Lac were synthesized and characterized by electrospray ionization mass spectrometry, nuclear magnetic resonance spectroscopy, etc. The polyphenolic structure of BT-TPE can coordinate with the Fe(III) complexes (Fe-Lac) to afford the desired BT-TPE@Fe-Lac. The detailed preparation process of BT-TPE@Fe-Lac is illustrated in Scheme S1 in the ESI.†The successful assembly of BT-TPE@Fe-Lac into BT-TPE@Fe-LacNPs in aqueous solution was initially characterized by transmission electron microscopy (TEM). The TEM images (Fig. 1a) demonstrated that BT-TPE@Fe-LacNPs are monodisperse with an approximate size of 200 nm. Due to the hydration layer formed on the surface of the NPs, the particle size observed using dynamic light scattering (DLS) is slightly larger, measuring approximately 220 nm (Fig. 1b). An obvious Tyndall effect could be observed in the aqueous solution (10 μM) of BT-TPE@Fe-Lac (Fig. S1†), indicating that abundant nanoparticles exist and form large hydrodynamic particle size micelles. Furthermore, BT-TPE@Fe-LacNPs had an excellent stability in phosphate buffered saline (PBS) for up to 7 days (Fig. S2†). Meanwhile, the TEM elemental mapping showed the uniform distribution of carbon (C), oxygen (O), nitrogen (N), sulfur (S) and iron (Fe) atoms (Fig. 1f). The FTIR spectra (Fig. 1c) showed distinct peaks at around 1650, 2800, and 3500 cm−1, attributed to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–H, and O–H, respectively. Compared with Fe-Lac, the FTIR spectrum of BT-TPE@Fe-Lac exhibited a low-frequency band at approximately 557 cm−1, corresponding to the Fe–O vibration peak. In addition, X-ray photoelectron spectroscopy (XPS) was carried out to investigate the chemical composition (Fig. 1d). The measured spectrum exhibited five characteristic peaks: C 1s (284.9 eV), N 1s (400.0 eV), O 1s (532.8 eV), S 2p (165.72 eV), and Fe 2p (711.12 eV, 714.62 eV, 724.27 eV, 727.82 eV) (Fig. S3†). Furthermore, the high-resolution XPS spectrum of Fe 2p (Fig. 1e) showed two main peaks located at 714.62 eV and 727.82 eV respectively, corresponding to Fe 2p3/2 and Fe 2p1/2, which had been proved to be the typical peaks of Fe3+. All these data demonstrated the successful synthesis and self-assembly of BT-TPE@Fe-Lac and the excellent stability, uniformity and potential for the following drug delivery to targeted tumors.
C, C–H, and O–H, respectively. Compared with Fe-Lac, the FTIR spectrum of BT-TPE@Fe-Lac exhibited a low-frequency band at approximately 557 cm−1, corresponding to the Fe–O vibration peak. In addition, X-ray photoelectron spectroscopy (XPS) was carried out to investigate the chemical composition (Fig. 1d). The measured spectrum exhibited five characteristic peaks: C 1s (284.9 eV), N 1s (400.0 eV), O 1s (532.8 eV), S 2p (165.72 eV), and Fe 2p (711.12 eV, 714.62 eV, 724.27 eV, 727.82 eV) (Fig. S3†). Furthermore, the high-resolution XPS spectrum of Fe 2p (Fig. 1e) showed two main peaks located at 714.62 eV and 727.82 eV respectively, corresponding to Fe 2p3/2 and Fe 2p1/2, which had been proved to be the typical peaks of Fe3+. All these data demonstrated the successful synthesis and self-assembly of BT-TPE@Fe-Lac and the excellent stability, uniformity and potential for the following drug delivery to targeted tumors.
Fe3+ is an excellent electron transfer agent.42 When complexed with Fe3+, the electrons from BT-TPE are more likely to transfer to the metal Fe in its empty orbitals, which implies that the fluorescence imaging and photosensitization functions of BT-TPE in the BT-TPE@Fe-LacNPs are in the quenched state under neutral conditions. As shown in Fig. S4,† the fluorescence of BT-TPE is dramatically quenched when assembled into BT-TPE@Fe-LacNPs. It is generally believed that lower pH can promote the cleavage of Fe–O bonds. The increased electrostatic repulsion between H+ and metal ions in acidic media weakens the coordination between Fe3+ and BT-TPE, leading to the disintegration of the complex. As a result, the fluorescence imaging and photosensitization functions of BT-TPE in the BT-TPE@Fe-LacNPs can be restored under acidic conditions (Scheme 1b).
As a designed specific antitumor theranostic agent, the responses of BT-TPE@Fe-LacNPs were evaluated in solution. Mass spectrometry (Fig. S5 and S6†) revealed peaks corresponding to BT-TPE and Fe-Lac after acid addition (PBS solution with pH = 5.4), indicating the successful molecular complexation and acid-promoted dissociation. The images of TEM and DLS (Fig. S7†) demonstrated that BT-TPE@Fe-LacNPs degraded upon incubation at pH 5.4 for 24 hours. Additionally, the fluorescence intensity of the BT-TPE@Fe-LacNPs reliably increases as the pH value decreases (Fig. S8a†). To gain further insight into the stability of the NPs, we quantified their release over time. It was observed that the BT-TPE@Fe-LacNPs remained stable at pH 7.4, and the nanomaterial decomposed at pH 5.4 (Fig. S8b, c and d†). In addition, no significant absorbance change of BT-TPE or BT-TPE@Fe-LacNPs was observed in the presence of light illumination even after 80 minutes, manifesting the excellent photostability (Fig. S9†). Combining these results, the acid stimuli-responsive disassembly feature of BT-TPE@Fe-LacNPs was carefully verified.
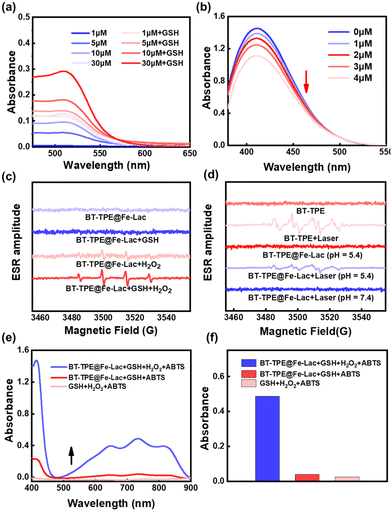
It is known that Fe(III) ions can be reduced to Fe(II) upon depletion of glutathione (GSH).43 To investigate this reduction process, BT-TPE@Fe-LacNPs were incubated with high levels of GSH (2 mM) and the Fe(II) chelator phenanthroline, whose coordination was monitored by UV/VIS absorption spectroscopy (Fig. 2a). The color of the solution (Fig. S10c†) and the characteristic absorption band of the Fe(II)-phenanthroline complex at 512 nm increased significantly with increasing concentration of GSH. Meanwhile, the consumption of GSH by BT-TPE@Fe-Lac was investigated using the GSH-specific probe 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) via UV/VIS absorption spectroscopy. As shown in Fig. 2b, the absorption band at 412 nm decreased markedly, suggesting the substantial depletion of GSH.
Next, to evaluate the BT-TPE@Fe-LacNPs’ ability to generate ROS, we examined the production of superoxide anions (O2˙−) and singlet oxygen (1O2) using specific indicators: dihydroethidium (DHE) for O2˙− and 9,10-anthracenediyl-bis(methylene)-dimalonic acid (ABDA) for 1O2. As shown in Fig. S11a and b,†BT-TPE@Fe-Lac was a type-I PS, which could generate O2˙− upon light irradiation at pH 5.4. We further investigated the generation of free radicals by using electron spin resonance (ESR) spectroscopy. Both BT-TPE and BT-TPE@Fe-LacNPs were incubated with the O2˙− scavenger 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (Fig. 2c). The mixtures were then exposed to radiation or kept in the dark. No characteristic signal of DMPO-OH (peak integral ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was detected in either BT-TPE or BT-TPE@Fe-Lac, but DMPO-O2˙− (peak integral ratio 1
1) was detected in either BT-TPE or BT-TPE@Fe-Lac, but DMPO-O2˙− (peak integral ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was generated upon irradiation. Further studies explored the ability of BT-TPE@Fe-Lac to decompose hydrogen peroxide (H2O2) into ˙OH via the Fenton reaction in the presence of Fe(III). The ESR spectrum revealed a characteristic signal corresponding to the presence of ˙OH (peak integral ratio 1
1) was generated upon irradiation. Further studies explored the ability of BT-TPE@Fe-Lac to decompose hydrogen peroxide (H2O2) into ˙OH via the Fenton reaction in the presence of Fe(III). The ESR spectrum revealed a characteristic signal corresponding to the presence of ˙OH (peak integral ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), with the signal intensity increasing under acidic conditions (Fig. 2d). Importantly, BT-TPE alone could not generate ˙OH under these conditions (Fig. S11c†). The ability of BT-TPE@Fe-Lac to decompose H2O2 into ˙OH was further confirmed using UV/VIS absorption spectroscopy with the specific probe 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate) (ABTS) (Fig. 2e). In the presence of hydroxyl radicals, colorless ABTS is oxidized to green oxABTS. As shown in Fig. 2f, only in the co-existence of GSH, H2O2 and BT-TPE@Fe-Lac, can ABTS be oxidized into the green product (Fig. S10f†). These results indicate that Fe2+, generated after acid addition, can react with H2O2 through the Fenton reaction to produce hydroxyl radicals. Above all, these properties illustrate the potential of BT-TPE@Fe-LacNPs for effectively enhancing cell death through the activation of oxidative stress signaling pathways upon the consumption of GSH and the generation of reactive oxygen species (˙OH, O2˙−).
1), with the signal intensity increasing under acidic conditions (Fig. 2d). Importantly, BT-TPE alone could not generate ˙OH under these conditions (Fig. S11c†). The ability of BT-TPE@Fe-Lac to decompose H2O2 into ˙OH was further confirmed using UV/VIS absorption spectroscopy with the specific probe 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate) (ABTS) (Fig. 2e). In the presence of hydroxyl radicals, colorless ABTS is oxidized to green oxABTS. As shown in Fig. 2f, only in the co-existence of GSH, H2O2 and BT-TPE@Fe-Lac, can ABTS be oxidized into the green product (Fig. S10f†). These results indicate that Fe2+, generated after acid addition, can react with H2O2 through the Fenton reaction to produce hydroxyl radicals. Above all, these properties illustrate the potential of BT-TPE@Fe-LacNPs for effectively enhancing cell death through the activation of oxidative stress signaling pathways upon the consumption of GSH and the generation of reactive oxygen species (˙OH, O2˙−).
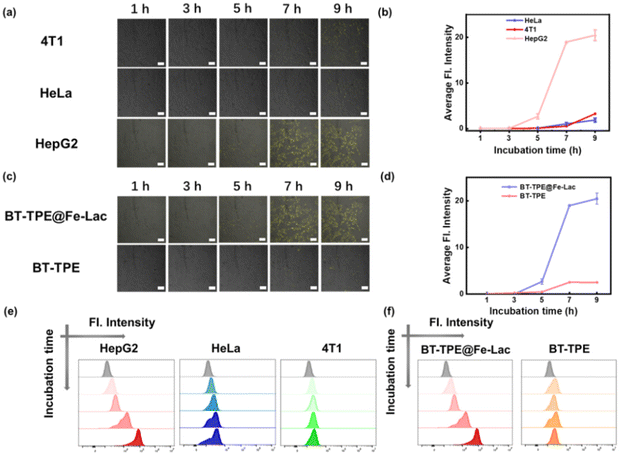
In view of the excellent performance of BT-TPE@Fe-LacNPsin vitro, we further explored the applications of BT-TPE@Fe-LacNPs at the cellular level. ASGPR is an asialoglycoprotein receptor overexpressed significantly on the membrane of HepG2 cells,44–48 which can specifically recognize galactose residues, potentially providing an ideal platform for the uptake of lactose-complexed BT-TPE@Fe-Lac. Thus, we conducted internalization and selective experiments of BT-TPE@Fe-Lac on three different cell types (HepG2, 4T1 and HeLa cells) through confocal laser scanning microscopy (CLSM) and flow cytometry (Fig. 3a and e). As illustrated in Fig. 3a and b, HepG2 cells treated with BT-TPE@Fe-LacNPs emitted obvious fluorescence after 9 h, whereas weak fluorescence was observed in 4T1 or HeLa cells. In addition, the cellular uptake experiment of BT-TPE was also conducted through CLSM and flow cytometry (Fig. 3c and f). Fluorescence imaging revealed weaker fluorescence signals upon incubation with BT-TPE than with BT-TPE@Fe-Lac in HepG2 cells, which were quantified using Image-J (Fig. 3d). Due to the lactose residues in BT-TPE@Fe-Lac as specific ligands for ASGPRs, they facilitate a faster uptake of BT-TPE@Fe-Lac into HepG2 cells compared with BT-TPE. On this basis, the cellular localization ability of BT-TPE@Fe-Lac was further studied by CLSM. As illustrated in Fig. S12,† the green fluorescence from the BT-TPE@Fe-LacNPs inside the cells was well colocalized with the red fluorescence from lysosomes, and the Pearson correlation coefficient was calculated to be 0.85. The above results confirmed that BT-TPE@Fe-LacNPs mainly accumulated in lysosomes after being endocytosed into the cells by ASGPR-mediated recognition.
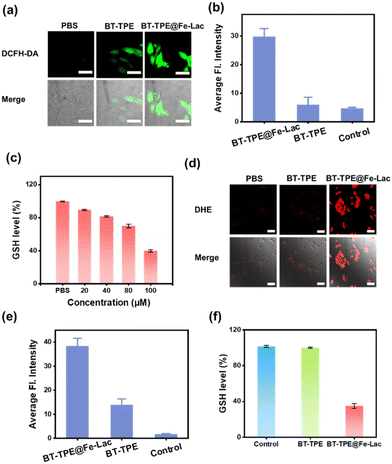
Considering the aforementioned results of ROS generation in aqueous solution, the ability of BT-TPE@Fe-LacNPs to produce ROS in HepG2 cells was evaluated using 2-dichlorodihydrofluorescein-diacetate (DCFH-DA). Upon light exposure treatment, an obvious green fluorescence signal was observed via CLSM imaging, and BT-TPE@Fe-Lac efficiently generated ROS under normoxic and hypoxic conditions (Fig. S13†). Meanwhile, the dihydroethidium (DHE) staining assay was also carried out to detect the generation of intracellular O2˙−. HepG2 cells treated with BT-TPE@Fe-Lac and DHE followed by photoirradiation showed the characteristic red fluorescence, indicating DHE exposure to O2˙− (Fig. S13†). Furthermore, we evaluated the generation of reactive oxygen species (ROS) by BT-TPE in living cells treated with DCFH-DA and DHE respectively (Fig. 4a and d). It showed that weaker fluorescence signals were obtained upon incubation with BT-TPE than with BT-TPE@Fe-Lac in HepG2 cells, which were quantified using Image-J (Fig. 4b and e). The results revealed a stronger ROS and O2˙− production capacity of BT-TPE@Fe-LacNPs in comparison with BT-TPE.
Based on the fact that GSH in aqueous solution can be consumed during the reduction of Fe(III) to Fe(II) in BT-TPE@Fe-LacNPs, we evaluated the intracellular GSH consumption. As shown in Fig. 4c, the concentration of GSH in HepG2 cells was also reduced with increasing concentration of BT-TPE@Fe-LacNPs. While the treatment with BT-TPE did not influence the GSH concentration, the incubation with BT-TPE@Fe-LacNPs caused the depletion of GSH (Fig. 4f). These findings suggest that BT-TPE@Fe-LacNPs should stimulate glutathione oxidative stress after entering into tumor cells and create conditions for further intracellular Fenton reactions. As a result, BT-TPE@Fe-LacNPs have the potential to disrupt the redox homeostasis of tumor cells.
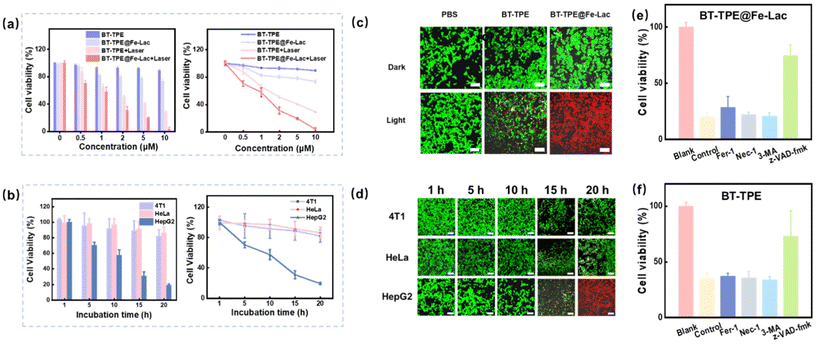
Considering the ability to produce ROS and consume GSH in living cells, the synergistic inhibitory effect of BT-TPE@Fe-Lac on the growth of HepG2 cells was examined. In detail, the cytotoxicity of BT-TPE@Fe-Lac towards HepG2 cells was assessed using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay (Fig. 5a). Compared to BT-TPE, BT-TPE@Fe-Lac displayed a certain degree of dark toxicity, which we speculated was caused by CDT. Specifically, BT-TPE@Fe-LacNPs were dissociated in the acidic tumor microenvironment and reacted with excess endogenous hydrogen peroxide in tumor cells via the Fenton reaction to generate ˙OH, resulting in cell apoptosis. Notably, both BT-TPE and BT-TPE@Fe-Lac exhibited phototoxicity after light exposure (Fig. 5a). BT-TPE@Fe-LacNPs contributed to the most striking inhibition of cell viability under light treatment. Under normoxic conditions, the BT-TPE@Fe-LacNPs exhibited significant cytotoxicity towards HepG2 cells under white LED light irradiation for 40 min. The half-maximal inhibitory concentration (IC50) for BT-TPE@Fe-Lac was 1.2 μM. Excitingly, even under hypoxic conditions, BT-TPE@Fe-Lac retained its original phototoxicity, indicating its function was independent of O2 (Fig. S14†). To visualize the therapeutic effect, HepG2 cells were stained with the cell viability probes calcein AM and propidium iodide (PI) after treatment with BT-TPE@Fe-Lac (Fig. S15†). When incubated with BT-TPE@Fe-Lac and exposed to light, the proportion of red fluorescence increased, suggesting most HepG2 cells were inhibited after 20 hours. Compared to BT-TPE@Fe-Lac, only a minimal amount of cell death was observed on exposure to irradiation after being incubated with BT-TPE (Fig. 5c). In addition, the cancer cell apoptosis was further studied using an Annexin V-FITC/propidium iodide dual staining assay. As shown by flow cytometry, BT-TPE@Fe-LacNPs + light treatment produced the highest apoptosis rate of 94.04% (Fig. S16†), which was in good agreement with the cytotoxicity results from the MTT assay. The results suggest that BT-TPE@Fe-LacNPs induced HepG2 cell death through the synergistic effects of type I PDT and CDT. Moreover, we conducted MTT assays (Fig. 5b) and live/dead cell staining experiments (Fig. 5d) respectively to compare the inhibition effects of BT-TPE@Fe-Lac in three types of tumor cells. It revealed that the inhibition effect of BT-TPE@Fe-Lac in HepG2 cells was superior compared with the other two cell types. Overall, these results show that BT-TPE@Fe-LacNPs can induce cell death of HepG2 cells by a combination of PDT and CDT.
The above results made us further explore the mechanism of cell death induced by BT-TPE@Fe-LacNPs. HepG2 cells in a 96-well plate were incubated with various cell death pathway inhibitors: apoptosis inhibitors (z-VAD-fmk), necrosis inhibitors (Nec-1), autophagy inhibitors (3-methyladenine, 3-MA), and ferroptosis inhibitors (ferrostatin-1). After treatment with these inhibitors, the cells were exposed to BT-TPE@Fe-LacNPs and cell viability was assessed using MTT assay (Fig. 5e). The results showed that BT-TPE@Fe-Lac treatment primarily triggered cell death through a combination of apoptosis and a small part of ferroptosis. In contrast, treatment with BT-TPENPs alone induced cell death exclusively through the apoptotic pathway, without ferroptosis (Fig. 5f). These findings highlight that BT-TPE@Fe-Lac is crucial for activating ferroptosis, suggesting a dual mechanism of action involving both apoptosis and ferroptosis, which could have significant implications for therapeutic applications.
Conclusions
In summary, a novel tumor microenvironment-activated nanoplatform (BT-TPE@Fe-Lac) was successfully developed in this study, which integrates an Fe(III) ion, the AIE-active BT-TPE photosensitizer, and a lactose molecule through dual coordination. The nanoplatform was then utilized for combined photodynamic therapy and chemodynamic therapy. BT-TPE@Fe-Lac was stable under physiological conditions and selectively targeted HepG2 cells which overexpressed ASGPR. To our delight, it rapidly dissociated into BT-TPE molecules and a ferric(III) complex in the acidic lysosomes of cancer cells. Upon irradiation, the BT-TPE molecules only generated O2˙−, inducing cell apoptosis primarily. The released ferric(III) complex played a crucial role in depleting intracellular glutathione, thereby amplifying oxidative stress and enhancing the efficacy of ROS-related therapies. Additionally, the ferric(III) complex reacted with excessive H2O2 in the tumor microenvironment to produce ˙OH, thereby facilitating the CDT function. In vitro experiments demonstrated that BT-TPE@Fe-Lac exhibits a significant photodynamic and chemodynamic synergistic antitumor effect on HepG2 cells. It is believed that the innovative combination therapy leveraging both PDT and CDT should open up a new strategy for the treatment of cancer tumors, offering a promising approach to enhance therapeutic outcomes through targeted, multi-modal interventions.Author contributions
Guo-Wen Xing: conceptualization, formal analysis, funding acquisition, investigation, project administration, resources, supervision, visualization, writing – original draft, and writing – review & editing. Gai-Li Feng: data curation, writing – original draft, and writing – review & editing. Wei Zhou: writing – review & editing. Jin-ping Qiao: data curation and writing – review & editing. Guang-Jian Liu: conceptualization, project administration, supervision, validation, and writing – review & editing.Data availability
All the data associated with the research in this manuscript are available on reasonable request and can be acquired from the corresponding authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21977014).References
- Z. Tang, Y. Liu, M. He and W. Bu, Chemodynamic Therapy: Tumour Microenvironment–Mediated Fenton and Fenton–like Reactions, Angew. Chem., 2019, 131(4), 958–968 CrossRef.
- Y. Dai, C. Xu, X. Sun and X. Chen, Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment, Chem. Soc. Rev., 2017, 46(12), 3830–3852 RSC.
- L. Wang, M. Huo, Y. Chen and J. Shi, Tumor Microenvironment-Enabled Nanotherapy, Adv. Health. Mat., 2018, 7(8), e1701156 CrossRef PubMed.
- E. Ju, K. Dong, Z. Chen, Z. Liu, C. Liu, Y. Huang, Z. Wang, F. Pu, J. Ren and X. Qu, Copper(II)–Graphitic Carbon Nitride Triggered Synergy: Improved ROS Generation and Reduced Glutathione Levels for Enhanced Photodynamic Therapy, Angew. Chem., Int. Ed., 2016, 55(38), 11467–11471 CrossRef CAS PubMed.
- Y. Xiong, C. Xiao, Z. Li and X. Yang, Engineering nanomedicine for glutathione depletion-augmented cancer therapy, Chem. Soc. Rev., 2021, 50(10), 6013–6041 CAS.
- A. Chatterjee and S. Gupta, The role of glutathione in cancer, Cell Biochem. Funct., 2018, 433, 33–42 CAS.
- F. Zeng, L. Tang, Q. Zhang, C. Shi, Z. Huang, S. Nijiati, X. Chen and Z. Zhou, Coordinating the Mechanisms of Action of Ferroptosis and the Photothermal Effect for Cancer Theranostics, Angew. Chem., Int. Ed., 2022, 61, e202112925 CrossRef CAS.
- X. Cheng, H. Xu, H. Ran, G. Liang and F. Wu, Glutathione-Depleting Nanomedicines for Synergistic Cancer Therapy, ACS Nano, 2021, 15(5), 8039–8068 CrossRef CAS.
- C. Cao, X. Wang, N. Yang, X. Song and X. Dong, Recent advances of cancer chemodynamic therapy based on Fenton/Fenton-like chemistry, Chem. Sci., 2022, 13(4), 863–889 RSC.
- W. Fan, P. Huang and X. Chen, Overcoming the Achilles’ heel of photodynamic therapy, Chem. Soc. Rev., 2016, 45(23), 6488–6519 RSC.
- C. A. Robertson, D. H. Evans and H. Abrahamse, Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT, J. Photochem. Photobiol., B, 2009, 96(1), 1–8 CrossRef CAS PubMed.
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Photodynamic therapy of cancer: An update, CA: A Cancer J. Clin., 2011, 61(4), 250–281 Search PubMed.
- Z. Zhou, J. Song, L. Nie and X. Chen, Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy, Chem. Soc. Rev., 2016, 45(23), 6597–6626 RSC.
- X. Zhao, J. Liu, J. Fan, H. Chao and X. Peng, Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application, Chem. Soc. Rev., 2021, 50(6), 4185–4219 RSC.
- H. Chen, Y. Wan, X. Cui, S. Li and C. S. Lee, Recent Advances in Hypoxia–Overcoming Strategy of Aggregation–Induced Emission Photosensitizers for Efficient Photodynamic Therapy, Adv. Healthcare Mater., 2021, 10, 2101607 CrossRef CAS PubMed.
- X. Deng, Z. Shao and Y. Zhao, Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy, Adv. Sci., 2021, 8, 2002504 CrossRef CAS PubMed.
- M. Ochsner, Photophysical and photobiological processes in the photodynamic therapy of tumours, J. Photochem. Photobiol., B, 1997, 39(1), 1–18 CrossRef CAS.
- L. Huang, Y. Xuan, Y. Koide, T. Zhiyentayev, M. Tanaka and M. R. Hamblin, Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram–negative and gram–positive bacteria, Lasers Surg. Med., 2012, 44(6), 490–499 CrossRef PubMed.
- E. L. G. Samuel, D. C. Marcano, V. Berka, B. R. Bitner, G. Wu, A. Potter, R. H. Fabian, R. G. Pautler, T. A. Kent, A. Tsai and J. M. Tour, Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(8), 2343–2348 CrossRef CAS.
- L. Brunet, D. Y. Lyon, E. M. Hotze, P. J. J. Alvarez and M. R. Wiesner, Comparative Photoactivity and Antibacterial Properties of C60 Fullerenes and Titanium Dioxide Nanoparticles, Environ. Sci. Technol., 2009, 43(12), 4355–4360 CrossRef CAS PubMed.
- J. Du and J. M. Gebicki, Proteins are major initial cell targets of hydroxyl free radicals, Int. J. Biochem. Cell Biol., 2004, 36(11), 2334–2343 CrossRef CAS PubMed.
- M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long, X. Song, J. W. Foley and X. Peng, Near-Infrared Light-Initiated Molecular Superoxide Radical Generator: Rejuvenating Photodynamic Therapy against Hypoxic Tumors, J. Am. Chem. Soc., 2018, 140(44), 14851–14859 CrossRef CAS PubMed.
- L. Shi, Y. Wang, C. Zhang, Y. Zhao, C. Lu, B. Yin, Y. Yang, X. Gong, L. Teng, Y. Liu, X. Zhang and G. Song, An Acidity–Unlocked Magnetic Nanoplatform Enables Self–Boosting ROS Generation through Upregulation of Lactate for Imaging–Guided Highly Specific Chemodynamic Therapy, Angew. Chem., Int. Ed., 2021, 60(17), 9562–9572 CrossRef CAS PubMed.
- B. Ma, S. Wang, F. Liu, S. Zhang, J. Duan, Z. Li, Y. Kong, Y. Sang, H. Liu, W. Bu and L. Li, Self-Assembled Copper–Amino Acid Nanoparticles for in Situ Glutathione “AND” H2O2 Sequentially Triggered Chemodynamic Therapy, J. Am. Chem. Soc., 2019, 141(2), 849–857 Search PubMed.
- Z. Tang, P. Zhao, H. Wang, Y. Liu and W. Bu, Biomedicine Meets Fenton Chemistry, Chem. Rev., 2021, 121(4), 1981–2019 Search PubMed.
- W. Ying, Y. Zhang, W. Gao, X. Cai, G. Wang, X. Wu, L. Chen, Z. Meng, Y. Zheng, B. Hu and X. Lin, Hollow Magnetic Nanocatalysts Drive Starvation–Chemodynamic–Hyperthermia Synergistic Therapy for Tumor, ACS Nano, 2020, 14(8), 9662–9674 CrossRef CAS.
- J. Fu, Y. Shao, L. Wang and Y. Zhu, Lysosome-controlled efficient ROS overproduction against cancer cells with a high pH-responsive catalytic nanosystem, Nanoscale, 2015, 7(16), 7275–7283 RSC.
- L. Shi, Y. Wang, C. Zhang, Y. Zhao, C. Lu, B. Yin, Y. Yang, X. Gong, L. Teng, Y. Liu, X. Zhang and G. Song, An Acidity–Unlocked Magnetic Nanoplatform Enables Self–Boosting ROS Generation through Upregulation of Lactate for Imaging–Guided Highly Specific Chemodynamic Therapy, Angew. Chem., Int. Ed., 2021, 60(17), 9562–9572 CrossRef CAS PubMed.
- S. Dong, J. Xu, T. Jia, M. Xu, C. Zhong, G. Yang, J. Li, D. Yang, F. He, S. Gai, P. Yang and J. Lin, Upconversion-mediated ZnFe2O4 nanoplatform for NIR-enhanced chemodynamic and photodynamic therapy, Chem. Sci., 2019, 10(15), 4259–4271 RSC.
- T. Chen, Q. Chu, M. Li, G. Han and X. Li, Fe3O4@Pt nanoparticles to enable combinational electrodynamic/chemodynamic therapy, J. Nanobiotechnol., 2021, 19, 206 CrossRef CAS.
- L. Zhang, Z. Liu, Q. Deng, Y. Sang, K. Dong, J. Ren and X. Qu, Nature–Inspired Construction of MOF@COF Nanozyme with Active Sites in Tailored Microenvironment and Pseudopodia–Like Surface for Enhanced Bacterial Inhibition, Angew. Chem., Int. Ed., 2021, 60(7), 3469–3474 CrossRef CAS PubMed.
- T. Feng, Z. Tang, J. Karges, J. Shen, C. Jin, Y. Chen, Y. Pan, Y. He, L. Ji and H. Chao, Exosome camouflaged coordination-assembled Iridium(III) photosensitizers for apoptosis-autophagy-ferroptosis induced combination therapy against melanoma, Biomaterials, 2023, 301, 122212 CrossRef CAS.
- R. Sun, W. Ma, M. Ling, C. Tang, M. Zhong, J. Dai, M. Zhu, X. Cai, G. Li, Q. Xu, L. Tang, Z. Yu and Z. Peng, pH-activated nanoplatform for visualized photodynamic and ferroptosis synergistic therapy of tumors, J. Controlled Release, 2022, 350, 525–537 CrossRef CAS.
- C. Liu, D. Wang, S. Zhang, Y. Cheng, F. Yang, Y. Xing, T. Xu, H. Dong and X. Zhang, Biodegradable Biomimic Copper/Manganese Silicate Nanospheres for Chemodynamic/Photodynamic Synergistic Therapy with Simultaneous Glutathione Depletion and Hypoxia Relief, ACS Nano, 2019, 13(4), 4267–4277 CrossRef CAS.
- J. Kim, H. R. Cho, H. Jeon, D. Kim, C. Song, N. Lee, S. H. Choi and T. Hyeon, Continuous O2 -Evolving MnFe2O4 Nanoparticle-Anchored Mesoporous Silica Nanoparticles for Efficient Photodynamic Therapy in Hypoxic Cancer, J. Am. Chem. Soc., 2017, 139(32), 10992–10995 CrossRef CAS.
- L. Feng, F. He, Y. Dai, S. Gai, C. Zhong, C. Li and P. Yang, Multifunctional UCNPs@MnSiO3 @g-C3N4 nanoplatform: improved ROS generation and reduced glutathione levels for highly efficient photodynamic therapy, Biomater. Sci., 2017, 5(12), 2456–2467 RSC.
- C. Jin, H. Zhang, J. Zou, Y. Liu, L. Zhang, F. Li, R. Wang, W. Xuan, M. Ye and W. Tan, Floxuridine Homomeric Oligonucleotides “Hitchhike” with Albumin In Situ for Cancer Chemotherapy, Angew. Chem., Int. Ed., 2018, 57(29), 8994–8997 Search PubMed.
- J. Lu, Y. Dai, Y. He, T. Zhang, J. Zhang, X. Chen, C. Jiang and H. Lu, Organ/Cell-Selective Intracellular Delivery of Biologics via N-Acetylated Galactosamine-Functionalized Polydisulfide Conjugates, J. Am. Chem. Soc., 2024, 146(6), 3974–3983 Search PubMed.
- T. A. Su, D. S. Shihadih, W. Cao, T. C. Detomasi, M. C. Heffern, S. Jia, A. Stahl and C. J. Chang, A Modular Ionophore Platform for Liver-Directed Copper Supplementation in Cells and Animals, J. Am. Chem. Soc., 2018, 140(42), 13764–13774 Search PubMed.
- Y. Wang, S. Xu, L. Shi, C. Teh, G. Qi and B. Liu, Cancer-Cell-Activated in situ Synthesis of Mitochondria-Targeting AIE Photosensitizer for Precise Photodynamic Therapy, Angew. Chem., Int. Ed., 2021, 60(27), 14945–14953 Search PubMed.
- G. Liu, J. Zhang, W. Zhou, G. Feng and G. Xing, Recent advance in sugar-based AIE luminogens and their applications in sensing and imaging, Chem. Commun., 2024, 60, 11899–11915 RSC.
- D. D. Zhang, Ironing out the details of ferroptosis, Nat. Cell Biol., 2024, 26, 1386–1393 Search PubMed.
- Y. Huang, Y. Jiang, Z. Xiao, Y. Shen, L. Huang, X. Xu, G. Wei, C. Xu and C. Zhao, Three birds with one stone: A ferric pyrophosphate based nanoagent for synergetic NIR-triggered photo/chemodynamic therapy with glutathione depletion, J. Chem. Eng., 2020, 380, 122369 Search PubMed.
- W. Zhou, Y. Liu, Q. Ma, G. Feng, J. Zhang, G. Liu, Y. Zhang and G. Xing, AIE-active lysosome-targeted fluorescent organic nanoparticles for leucine aminopeptidase-activatable fluorescent imaging and precision photodynamic therapy potential, Dyes Pigm., 2024, 221, 111781 CrossRef CAS.
- A. A. D'Souza and P. V. Devarajan, Asialoglycoprotein receptor mediated hepatocyte targeting - strategies and applications, J. Controlled Release, 2015, 203, 126–139 CrossRef PubMed.
- M. Wang, Z. Li, F. Liu, Q. Yi, C. Pu, Y. Li, T. Luo, J. Liang and J. Wang, Development of Asialoglycoprotein-Mediated Hepatocyte-Targeting Antitumor Prodrugs Triggered by Glutathione, J. Med. Chem., 2021, 64(19), 14793–14808 Search PubMed.
- Y. C. Liu, G. J. Liu, W. Zhou, G. L. Feng, Q. Y. Ma, Y. Zhang and G. W. Xing, In Situ Self–Assembled J–Aggregate Nanofibers of Glycosylated Aza–BODIPY for Synergetic Cell Membrane Disruption and Type I Photodynamic Therapy, Angew. Chem., Int. Ed., 2023, 62, e202309786 CrossRef CAS.
- J. K. Nair, J. L. Willoughby, A. Chan, K. Charisse, M. R. Alam, Q. Wang, M. Hoekstra, P. Kandasamy, A. V. Kel'In, S. Milstein, N. Taneja, J. O'Shea, S. Shaikh, L. Zhang, R. J. van der Sluis, M. E. Jung, A. Akinc, R. Hutabarat, S. Kuchimanchi, K. Fitzgerald, T. Zimmermann, T. J. van Berkel, M. A. Maier, K. G. Rajeev and M. Manoharan, Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing, J. Am. Chem. Soc., 2014, 136(49), 16958–16961 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03871a |
| This journal is © The Royal Society of Chemistry 2025 |