Facile synthesis of in situ carbon-coated CoS2 micro/nano-spheres as high-performance anode materials for sodium-ion batteries†
Lingling
Chen
a,
Pengfei
Wang
a,
Chen
Bao
b,
Yanyan
Li
b,
Bo
Fan
b,
Gaofeng
Li
 *b and
Dianbo
Ruan
b
*b and
Dianbo
Ruan
b
aSchool of Materials Science and Chemical Engineering, Ningbo University, Ningbo 315211, China
bSchool of Mechanical Engineering and Mechanics, Ningbo University, Ningbo, 315211, China. E-mail: ligaofeng@nbu.edu.cn
First published on 22nd November 2024
Abstract
In situ carbon-coated CoS2 micro/nano-spheres were successfully prepared by sulfuric calcination using the solvothermal method with glycerol as the carbon source without introducing extraneous carbon. This method prevents carbon agglomeration and avoids the cumbersome steps of the current technology. The composite demonstrates excellent sodium storage capacity as an anode material for sodium-ion batteries. The initial charge and discharge capacities were 1027 and 1224 mA h g−1 at 50 mA g−1, respectively, with an initial coulombic efficiency of 83.9%. The capacity of CoS2@C at 350 °C was maintained at 937 mA h g−1 after 140 cycles at a current density of 2 A g−1. The outstanding electrochemical performance is mainly attributed to the nanostructure design and the presence of in situ carbon. As revealed by the kinetic analysis, the pseudo-capacitive behaviour also contributed to the excellent electrochemical performance.
1. Introduction
For numerous energy devices, lithium-ion batteries (LIBs) have become the core focus of the new energy market, with their rapid rise in the field of new energy vehicles. Nevertheless, the shortage and high cost of lithium resources impede the continued development of LIBs in the long run.1–3 Therefore, sodium-ion batteries (SIBs) have attracted attention as a consequence of their cost-effectiveness, abundant availability and physicochemical characteristics similar to those of LIBs.4,5 As a commonly used negative electrode material in LIBs, graphite exhibits a notably limited sodium storage capacity of only 12 mA h g−1 for thermodynamic reasons. Moreover, the large ionic radii cause significant volume changes, resulting in slow kinetics and limiting the application of SIBs.6–9 Consequently, developing anode materials with high specific capacity and excellent rate performance is vital for the future advancement of SIBs.On the basis of the storage mechanism of sodium, anode materials can be classified into three types. Among them, intercalation-type materials, such as hard and soft carbon, have low specific capacity and low-voltage platforms.10–12 Although alloy-type materials have a high theoretical capacity, their volume changes substantially during cycling, leading to pulverisation and loss of electrochemical activity.13–15 In many conversion-type materials, transition-metal sulfides (TMSs) have become an ideal choice for anode materials in SIBs because of their suitable redox potentials and high theoretical capacity.16,17 The electrochemical reaction mechanism typically involves the reduction or oxidation of transition metals, accompanied by the formation or decomposition of corresponding sodium-containing compounds. Amongst them, cobalt sulfide (CoS2) has garnered considerable attention because of its remarkable characteristics, particularly its high theoretical capacity (871 mA h g−1) and facile reaction with sodium ions (Na+) during the charge and discharge processes.18–20 However, during the sodiation process, it experiences significant volume expansion, generating large internal stresses that lead to capacity decay, low coulombic efficiency, suboptimal rate capability and poor cycling stability. Additionally, the poor electrical conductivity of TMCs may cause an imbalance between electron and ion transport, which limits the rapid diffusion kinetics of Na+.
Recently, various strategies and methodologies have been employed to address these concerns, including reducing particle size to the nanoscale, designing microstructures and nanostructures21,22 and incorporating buffer matrix materials.23,24 Among them, nanostructure design is an effective method to mitigate volume changes. This approach creates abundant internal spaces and efficient migration channels for electrons and ions, significantly reducing ion migration distance, providing more electrochemical active sites and consequently achieving high specific capacity and superior rate performance. For instance, Zhao et al. synthesised the CoS2@C composite with a hollow nanocubic structure via carbon coating and vulcanisation process. This unique structural material has a high sodium storage rate capacity (302.8 mA h g−1 at 10 A g−1).20 Introducing a carbon material substrate effectively prevents particle agglomeration and growth during preparation. It also avoids direct contact between active particles and the electrolyte, minimising side reactions and improving the first-cycle Coulomb efficiency. The electrode rate performance can be enhanced by introducing an electrically conductive carbon framework or network. Currently, carbon coating is generally achieved through multiple steps, and an obvious interface is formed during the coating process. For example, by polymerising layers of polydopamine onto the surface of NiCoCP, Lin successfully prepared NiS2@CoS2 core–shell nanocubes. Its capacity remained stable at 600 mA h g−1 during the 250th cycle at 1000 mA g−1, demonstrating its superior capacity and remarkable cycling stability.25 Therefore, nanostructures and the introduction of carbon materials have significant applications in SIBs.
In this study, a straightforward solvothermal method was used to synthesise a homogeneous Co-glycerol acid microspheres using glycerol as a carbon source without introducing extraneous carbon. Subsequently, CoS2@C nanospheres with micro nano structures were obtained through calcination and sulfurisation at different temperatures. During the vulcanisation process, a thin layer of amorphous carbon is formed at the periphery of the sphere. This nanostructure provides abundant internal space and more active sites, effectively shortening the diffusion path of Na+ and relieving volume expansion. As the SIB anode, CoS2@C exhibited first charge and discharge capacities of 1027 and 1224 mA h g−1 at 50 mA g−1, a first-cycle Coulomb efficiency of 83.9% and superior cycling performance. At a low current density of 100 mA g−1, the reversible capacity can be maintained at 1282 mA h g−1 after 50 cycles, whereas at a high current density of 2 A g−1, it was maintained at 937 mA h g−1 after 140 cycles.
2. Experimental part
2.1. Synthesis of CoS2@C composites
First, 0.5 mmol of Co(NO3)2·6H2O was dissolved in 40 mL of 2-propyl. Then, 8 mL of glycerol was introduced into the mixture under strong magnetic agitation. Upon homogeneity, the admixture was transferred to a 100 mL polytetrafluoroethylene autoclave and heated in an oven at 180 °C for 16 h. The resulting precipitate was cleaned twice with pure ethanol and oven-dried at 60 °C.26The obtained product and sublimed sulfur powder (mass ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were placed on both sides of the crucible and heated in a nitrogen atmosphere at 350 °C, 450 °C and 550 °C for 4 h at a heating rate of 2 °C min−1.
3) were placed on both sides of the crucible and heated in a nitrogen atmosphere at 350 °C, 450 °C and 550 °C for 4 h at a heating rate of 2 °C min−1.
2.2. Material characterisation
Field emission scanning electron microscopy (SEM; Hitachi US-70, Japan) was used to observe the morphology of the samples, and X-ray diffraction (XRD; D8Advance, Germany) was used to analyse the phase of the material and the degree of crystallinity. The surface chemistry was further characterised by X-ray photoelectron spectroscopy (XPS; Thermo ESCALAB 250XI). Element mapping was performed using energy-dispersive X-ray spectroscopy (EDS). Transmission electron microscopy (TEM) and high-resolution TEM (G2 20, Tecnai, Hillsboro, OR, USA) provided more structural information. The selected area electron diffraction (SAED) mode was recorded digitally by a Gatan CCD camera.2.3. Electrochemical measurements
The CR2032 coin cell was assembled in a glove box fully saturated with argon gas. The working electrode was fabricated by applying a mixture of CoS2 as the active material, carbon black, and polyvinylidene fluoride binder in a weight ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 onto a copper foil substrate. The process employed N-methyl-2-pyrrolidone as the solvent to facilitate the uniform coating of the mixture. The coated copper foil was vacuum-dried at 110 °C for 10 h. A sodium metal sheet and glass fibre were used as the electrode and diaphragm, respectively, and the electrolyte was sodium hexafluorophosphate dissolved in diethylene glycol dimethyl ether solvent. The dried electrode was cut into a 13 mm-diameter disc. The mass loading of active material was about 0.7–1.0 mg cm−2. All electrochemical performance tests were conducted at room temperature. Furthermore, electrochemical impedance spectroscopy (EIS) was performed over the frequency range of 100 kHz–0.01 Hz on a CHI760E electrochemical workstation, and cyclic voltammetry (CV) tests were conducted in the potential window of 0.01–3 V. Constant current charge and discharge and rate and cycle performance were tested at various current densities using a LAND CT2001A test instrument (China).
1 onto a copper foil substrate. The process employed N-methyl-2-pyrrolidone as the solvent to facilitate the uniform coating of the mixture. The coated copper foil was vacuum-dried at 110 °C for 10 h. A sodium metal sheet and glass fibre were used as the electrode and diaphragm, respectively, and the electrolyte was sodium hexafluorophosphate dissolved in diethylene glycol dimethyl ether solvent. The dried electrode was cut into a 13 mm-diameter disc. The mass loading of active material was about 0.7–1.0 mg cm−2. All electrochemical performance tests were conducted at room temperature. Furthermore, electrochemical impedance spectroscopy (EIS) was performed over the frequency range of 100 kHz–0.01 Hz on a CHI760E electrochemical workstation, and cyclic voltammetry (CV) tests were conducted in the potential window of 0.01–3 V. Constant current charge and discharge and rate and cycle performance were tested at various current densities using a LAND CT2001A test instrument (China).
3. Results and discussion
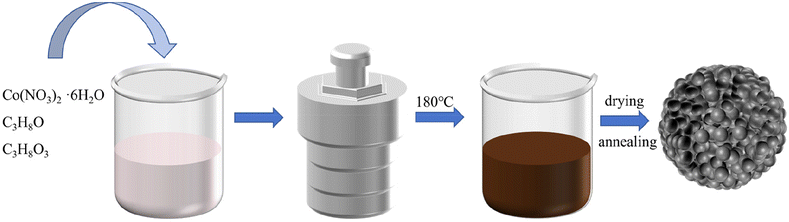
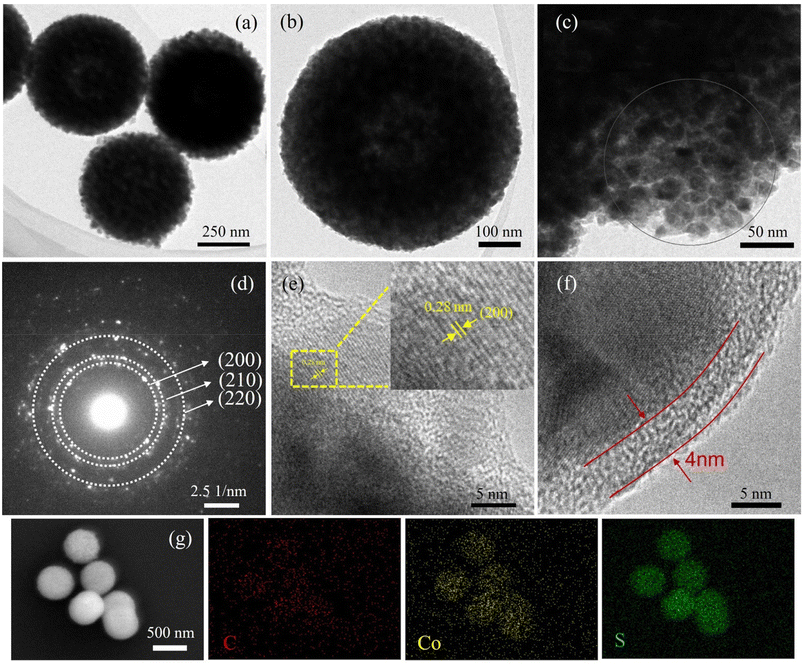
The preparation of CoS2@C nanospheres is illustrated in Fig. 1. Various glycerate nanosphere precursors were synthesised using the solvothermal method. Under a nitrogen atmosphere, the precursors were sulfurised in a tube furnace with sulfur powder, yielding CoS2@C nanospheres.The morphological characteristics of CoS2@C nanospheres at 350 °C are studied via SEM and TEM. Fig. 2a displays an SEM image of the Co-glycerate nanosphere precursor. The precursor comprises uniform solid spheres with an average diameter of approximately 500 nm. The surface of CoS2@C at 350 °C formed after calcination in the tube furnace was surrounded by numerous nanoparticles, resulting in a slight increase in particle size. Measurements were performed at approximately 600 nm (Fig. 2b–d). TEM confirmed the structure (Fig. 3a and b). Fig. 3c and d shows the SAED patterns, which reveal the polycrystalline properties of CoS2@C at 350 °C. Fig. 3e shows the lattice fringes with a d-spacing of 0.28 nm. It matches the (200) crystal faces of CoS2@C nanospheres at 350 °C.27 The thickness of the carbon layer was approximately 4 nanometres in depth (Fig. 3f). The EDS demonstrates that carbon, sulfur and cobalt are uniformly distributed throughout the structure of CoS2@C nanospheres at 350 °C (Fig. 3g). On the basis of elemental analysis, sulfur, cobalt and carbon contents were 53.07%, 24.93% and 7.69%, respectively (Table S1†). The presence of carbon increases the electrical conductivity and alleviates volume expansion during the reaction, thereby improving the performance.
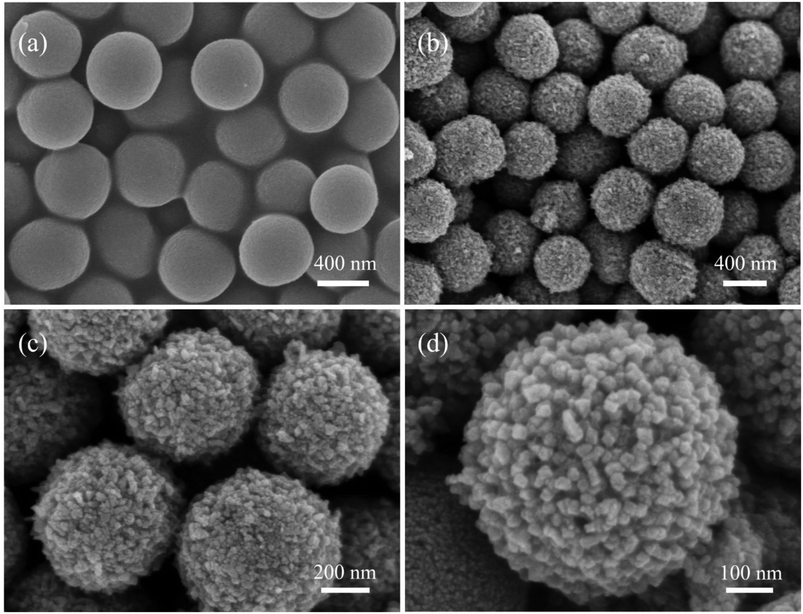 | ||
| Fig. 2 Scanning electron microscopy images of the precursor: (a) Co-glycerate and (b–d) CoS2@C nanospheres at 350 °C. | ||
As a comparison, SEM observations of CoS2@C at 450 °C (Fig. S1†) and 550 °C (Fig. S2†) shows that the particle size is slightly reduced to approximately 500 nm, with minimal morphological difference. However, the spherical structures calcined at 450 °C and 550 °C were less complete and uniform than those calcined at 350 °C. The mapping images of CoS2@C at 450 °C and 550 °C are shown in Fig. S1c–f and S2c–f,† respectively. The results also reveal a uniform distribution of carbon, sulfur and cobalt in the nanospheres. The carbon content is 5.61% and 3.71%, respectively, based on elemental analysis (Table S1†). The carbon content gradually decreases with increasing calcination temperature.
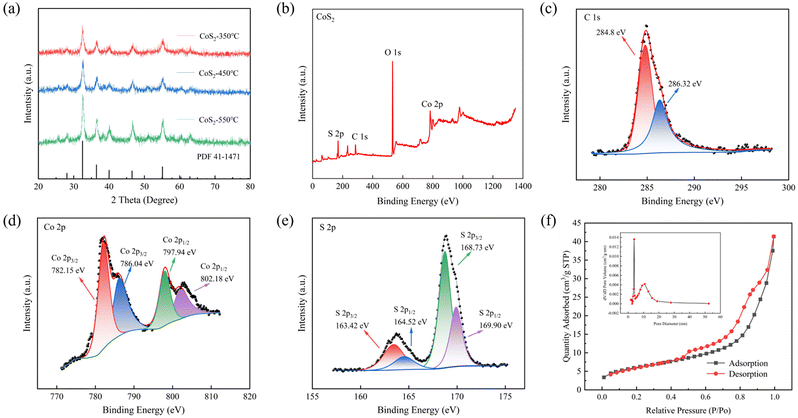
Fig. 4a displays the XRD patterns of the synthesised CoS2@C nanospheres at various temperatures. The outcomes indicate that the locations of all diffraction peaks observed in the prepared samples align with the CoS2 standard card (JCPDS no. 41-1471). Notably, no other significant impurity peaks were detected. The positions of the peaks of the CoS2 nanospheres calcined at 450 °C and 550 °C match well with the CoS2 standard card, and the peak intensity increased with increasing calcination temperature. A graphical representation of the XRD profile of the cobalt precursor is presented in Fig. S3.†28,29
To explore the phase and surface chemical properties of CoS2@C composites at 350 °C, XPS was used to analyse their chemical and electronic states. The spectrum in Fig. 4b displays four elements in the samples: carbon, sulfur, cobalt and oxygen. Fig. 4c displays two peaks in the carbon 1s region at 284.8 and 286.32 eV, belonging to sp2 and graphitic carbon, respectively.30,31Fig. 4d shows the high-resolution XPS spectrum pertaining to cobalt 2p, where two peaks centred at 782.15 and 797.94 eV belong to Co2+, and two satellite peaks associated with cobalt 2p are observed at 786.04 and 802.18 eV, respectively.32–34 In the XPS spectrum of sulfur 2p in Fig. 4e, the two peaks centred at 163.42 and 164.52 eV can be assigned to sulfur 2p3/2 and S 2p1/2 of cobalt–sulfur in the CoS2 compound. However, the binding energies of 168.73 and 169.90 eV indicate the production of sulfur oxides due to exposure to air.9,31,35,36
Fig. 4f shows the BET-specific surface area of CoS2@C at 350 °C, which is approximately 21.88 m2 g−1. The curve confirms the existence of mesoporous pores with an average pore size of 12.84 nm. A large specific surface area helps alleviate volume expansion by shortening the diffusion distance of ions and improving their kinetic properties.
The Raman spectrum of CoS2@C at 350 °C (Fig. S4†) shows peaks at 390 and 470 cm−1, corresponding to CoS2, which lacks representative D and G band features but has two significant peaks in the range of 1000–2000 cm−1. This result reveals the incompleteness of its graphitised structure and the abundance of defects. Interestingly, this partially graphitised structure and numerous defects generally help improve conductivity.37 In order to determine the carbon content of CoS2@C material, a thermogravimetric test was carried out under air atmosphere at a heating rate of 10 °C min−1 as shown in Fig S5.† According to the thermogravimetric results, it can be calculated that the carbon content of CoS2@C at the calcination temperature of 350 °C, 450 °C and 550 °C is 27.3%, 26.3% and 24.4%, respectively. With the increase of temperature, the carbon content of the composite decreases gradually.
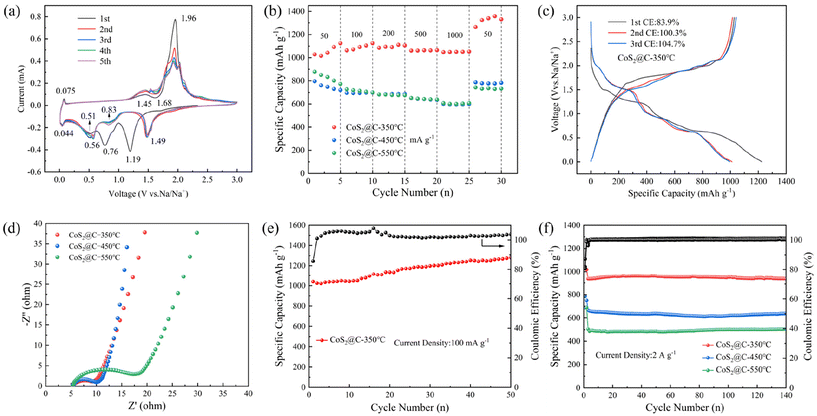
The properties and mechanisms of the electrode reactions of CoS2@C at 350 °C are studied using CV within a potential window spanning 0.01 to 3 V at a scan rate of 0.1 mV s−1 (Fig. 5a). During the initial cathodic sweep, four cathodic peaks appeared at 1.68, 1.19, 0.76 and 0.51 V, which may be related to the formation of NaxCoS2 and cobalt. After the first cycle, a shift in the reduction peaks was observed at 1.49, 0.83 and 0.51 V, exhibiting good reversibility due to the overlapping nature of the peaks. On the anodic scan curve, three discernible oxidation peaks appeared at 1.45, 1.96 and 2.02 V. These peaks are likely attributable to the reaction between CoS2 and Na+. The CV curves of CoS2@C at 450 °C and 550 °C (0.1 mV s−1) are displayed in Fig. S6,† which shows redox characteristics similar to those of CoS2@C at 350 °C. The battery's reaction mechanism is depicted as follows:9
Discharge process:
| CoS2 + xNa+ + xe− → NaxCoS2 | (1) |
| NaxCoS2 + (4 − x)Na+ + (4 − x)xe− → 2Na2S + Co | (2) |
Charge process:
| 2Na2S + Co → NaxCoS2 + (4 − x)Na+ + (4 − x)xe− | (3) |
| NaxCoS2 → CoS2 + xNa+ + xe− | (4) |
Notably, XRD tests were conducted at various discharge and charge phases to study the reaction mechanism of CoS2@C at 350 °C, which can further substantiate the aforementioned conversion reaction, as shown in Fig. S7.† When the discharge reached 1.6 V, the CoS2 peak weakened, and two new peaks corresponding to Na2S (JCPDS no. 47-0178) appeared at 31.5° and 38.9°. As the discharge process progresses, the CoS2 phase gradually disappears, and the diffraction peaks of cobalt (JCPDS no. 02-0727) appear at 42.5° and 44.6°. During the charging phase, the metallic cobalt peak gradually weakens and disappears, whereas the peak of CoS2 begins to reappear, although its intensity is not significant. This may be because the surface of the pole sheet is covered by binders, copper foils and diaphragms, which affect the strength of the CoS2 peak.9
The magnification capacity of CoS2@C at 350 °C under various current densities is studied. The findings are depicted in Fig. 5b. The respective charging capacities at 0.05, 0.1, 0.2, 0.5 and 1 A g−1 exhibit values of 1124, 1091, 1085, 1063 and 1048 mA h g−1. When the current density is reverted to 50 mA g−1, a reversible capacity of 1263 mA h g−1 is still achieved. However, CoS2@C at 450 °C and 550 °C demonstrates poor rate capability at the same current density.
Fig. 5c presents the constant current charge and discharge profiles of the initial three cycles of CoS2@C, which were obtained within a voltage window ranging from 0.01 to 3 V, under conditions of 350 °C. Under 50 mA g−1, the first cycle exhibited a charge and a discharge capacity of 1027 and 1224 mA h g−1, yielding a first-cycle coulombic efficiency of 83.9%. Additionally, CoS2@C at 450 °C displays first-cycle capacities of 796.2 and 969.1 mA h g−1 at 50 mA g−1, with a coulombic efficiency of 82.1%. At 50 mA g−1, the first cycle of CoS2@C at 550 °C exhibits a charging capacity of 878.3 mA h g−1 and a discharging capacity of 1026.1 mA h g−1. This translates to a coulombic efficiency of 85.5% (Fig. S8†). The observed irreversible capacity decline stems from electrolyte decomposition and some side reactions.17Fig. 5e displays the cycling ability of CoS2@C at 350 °C at 100 mA g−1. After 50 cycles, the retained capacity was 1282 mA h g−1, and the capacity slowly rises. Because of the repeated conversion reaction, the nanoparticles are further crushed into smaller particles with a larger surface area, which may contribute to a gradual increase in capacity.17Fig. 5f shows the cyclic performance of CoS2@C at 350 °C, 450 °C and 550 °C at 2 A g−1. After 140 cycles, the capacity reached 937 mA h g−1, with 100% Coulomb efficiency. When calcined at 450 °C and 550 °C in the 140th cycle, the capacities were 637 and 500 mA h g−1, respectively. Fig. S9† shows Long-term cycling performance of CoS2@C at 350 °C at 5 A g−1. After 300 cycles, the reversible capacity of CoS2 nanomaterial at 350 °C is 590 mA h g−1, and the CE reaches 100%, which indicates that it has excellent electrochemical properties. Fig. S10† depicts SEM images of CoS2@C at 350 °C after cycling at 2 A g−1, where the microsphere structure and some smaller nanospheres are visible. This anode material exhibits high sodium storage capacity due to its micro nano structures, which effectively alleviate volume expansion, provide abundant internal space and numerous active sites and shorten the ion migration path. Additionally, in situ carbon increases the electrical conductivity.
The reaction kinetics of the battery were studied using EIS. Fig. 5d shows the outcomes of EIS at frequencies ranging from 100 kHz to 0.01 Hz at the CHI760E electrochemical station. On the basis of equivalent circuit simulation, under the open-circuit voltage state, the charge transfer resistance for CoS2@C at 350 °C is 2.783 Ω, significantly lower than that for CoS2@C at 550 °C (9.794Ω). This indicates that CoS2@C composites at 350 °C have good ion transport and fast sodium-ion diffusion capacity. Fig S11† compares the rate performance of this work and the reported CoS2 based composites, including NC@CoS2@CNTs,38 CoS2/NGC-1:30,39 CoS2/carbon composites,40 H-CoS2,41 N–C/CoS2,42 CoS2/NiS2,43 S/CoS2@C.27 The results show that the material has good electrochemical performance.
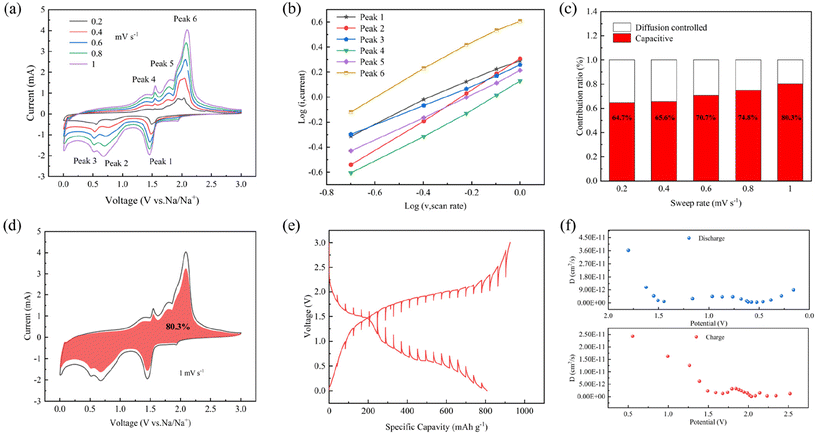
To probe the reaction kinetics of the CoS2@C at 350 °C sample, CV was tested at various scan rates, as depicted in Fig. 6a. The curves show comparable trends across scanning rates, where i represents the peak current density and v represents the scanning rate. Fig. 6b shows the log(i) and log(v) graphs for CoS2@C at 350 °C electrodes using the following equations:
| i = avb | (5) |
In eqn (5), b is the slope of the fitting line, representing the contribution of pseudo-capacitance to capacity, and a is constant. At a value of b equalling 0.5, the electrochemical process is primarily governed by ionic diffusion mechanisms with minimal involvement of pseudo-capacitance effects. When b = 1, the pseudo-capacitance dominates, positively affecting the rate capacity.30,44,45 The results in Fig. 6b show that the b values are 0.86, 1.21, 0.78, 1.05, 0.92 and 1.04, respectively, implying that the electrochemical reaction of the CoS2@C electrode at 350 °C is predominantly governed by the pseudo-capacitance.
| i(v) = k1v + k1v1/2 | (6) |
Eqn (6) is introduced to better understand the region where capacitance contributions occur, where k1v and k1v1/2 are ascribed to capacitance and diffusion control effects.46,47Fig. 6c shows the contribution of pseudo-capacitance behaviour at various scan rates. At scan rates of 0.2, 0.4, 0.6, 0.8 and 1 mV s−1, the contribution rate is 64.7%, 65.6%, 70.7%, 74.8% and 80.6%, respectively. The consequence depict that the pseudo-capacitance-to-capacity ratio is high, and the capacitive contribution ratio increases significantly as the scanning rate increases. Fig. 6d shows the pseudo-capacitance ratio (red) at 1 mV s−1. The diffusion behaviour and thermodynamics of Na+ in the materials were determined by galvanostatic intermittent titration technique (Fig. 6e). It charges or discharges by applying a constant current, then disconnects the current and records the voltage change. The formula is as follows:48–50
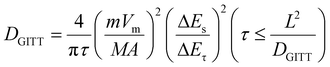 | (7) |
4. Conclusions
The CoS2@C nanospheres with micro nano structures are synthesised using simple solvothermal and annealing methods as anode materials for SIBs. The results show that CoS2@C at 350 °C has excellent rate capabilities and a high sodium storage capacity. The reversible capacities of CoS2@C at 350 °C are 1124, 1091, 1085, 1063 and 1048 mA h g−1 at 0.05, 0.1, 0.2, 0.5 and 1 A g−1, respectively. At 2 A g−1, 937 mA h g−1 can be reached after 140 cycles. At a high current density of 5 A g−1, the reversible capacity can reach 590 mA h g−1 after 300 cycles. This outstanding electrochemical performance of CoS2@C at 350 °C materials is attributed to the micro nano structures and carbon coating, which mitigate volume expansion and improve electrical conductivity during cycling. As revealed by the kinetic analysis, the pseudo-capacitance behaviour also contributed to the remarkable electrochemical performance.Author contributions
Lingling Chen – Writing – original draft; methodology; data curation. Pengfei Wang – validation. Chen Bao – validation. Yanyan Li: supervision. Bo Fan – supervision. Gaofeng Li – writing – review and editing; formal analysis; funding acquisition; project administration. Dianbo Ruan – supervision; resources.Data availability
The data has been included in the paper or ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by the Science and Technology Innovation 2025 Major Project of Ningbo, Ningbo Municipal Science and Technology Bureau, grant number 2022Z022.References
- A. Kumar Prajapati and A. Bhatnagar, J. Energy Chem., 2023, 83, 509–540 CAS.
- T. Perveen, M. Siddiq, N. Shahzad, R. Ihsan, A. Ahmad and M. I. Shahzad, Renewable Sustainable Energy Rev., 2020, 119, 109549 CAS.
- F. Xiao, W. Lai, S. Zeng, L. He, M. Ge, F. Luo, P. Xiong, H. Lin, C. Lin, Y. Luo, J. Zhang, Q. Qian, Q. Chen and L. Zeng, Adv. Sustainable Syst., 2024, 8, 2400098 CAS.
- Q. Zhou, L. Liu, Z. Huang, L. Yi, X. Wang and G. Cao, J. Mater. Chem. A, 2016, 4, 5505–5516 Search PubMed.
- Y. Wang, F. Xiao, X. Chen, P. Xiong, C. Lin, H. E. Wang, M. Wei, Q. Qian, Q. Chen and L. Zeng, InfoMat, 2023, 5(9), e12467 CrossRef CAS.
- L. Fang, N. Bahlawane, W. Sun, H. Pan, B. B. Xu, M. Yan and Y. Jiang, Small, 2021, 17, 2101137 CrossRef CAS.
- B. Guan, S.-Y. Qi, Y. Li, T. Sun, Y.-G. Liu and T.-F. Yi, J. Energy Chem., 2021, 54, 680–698 CrossRef CAS.
- S. Qiao, Q. Zhou, M. Ma, H. K. Liu, S. X. Dou and S. Chong, ACS Nano, 2023, 17, 11220–11252 CrossRef CAS.
- W. Zhang, Z. Yue, Q. Wang, X. Zeng, C. Fu, Q. Li, X. Li, L. Fang and L. Li, Chem. Eng. J., 2020, 380, 122548 CrossRef CAS.
- J. C. Hyun, H. M. Jin, J. H. Kwak, S. Ha, D. H. Kang, H. S. Kim, S. Kim, M. Park, C. Y. Kim, J. Yoon, J. S. Park, J.-Y. Kim, H.-D. Lim, S. Y. Cho, H.-J. Jin and Y. S. Yun, Energy Environ. Sci., 2024, 17, 2856–2863 RSC.
- S. Tan, H. Yang, Z. Zhang, X. Xu, Y. Xu, J. Zhou, X. Zhou, Z. Pan, X. Rao, Y. Gu, Z. Wang, Y. Wu, X. Liu and Y. Zhang, Molecules, 2023, 28, 3134 CrossRef CAS.
- Y. Zhen, Y. Chen, F. Li, Z. Guo, Z. Hong and M.-M. Titirici, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2111119118 CrossRef CAS.
- W. T. Jing, C. C. Yang and Q. Jiang, J. Mater. Chem. A, 2020, 8, 2913–2933 RSC.
- S.-M. Zheng, Y.-R. Tian, Y.-X. Liu, S. Wang, C.-Q. Hu, B. Wang and K.-M. Wang, Rare Met., 2020, 40, 272–289 CrossRef.
- X. Yang, H.-J. Liang, H.-Y. Yu, M.-Y. Wang, X.-X. Zhao, X.-T. Wang and X.-L. Wu, J. Phys. Mater., 2020, 3, 042004 CrossRef CAS.
- T. Hou, B. Liu, X. Sun, A. Fan, Z. Xu, S. Cai, C. Zheng, G. Yu and A. Tricoli, ACS Nano, 2021, 15, 6735–6746 CrossRef CAS.
- M. Yang, X. Chang, L. Wang, X. Wang, M. Gu, H. Huang, L. Tang, Y. Zhong and H. Xia, Adv. Mater., 2023, 35, 2208705 CrossRef CAS PubMed.
- Q. Li, Q. Jiao, Y. Yan, H. Li, W. Zhou, T. Gu, X. Shen, C. Lu, Y. Zhao, Y. Zhang, H. Li and C. Feng, Chem. Eng. J., 2022, 450, 137922 CrossRef CAS.
- F. Xiao, X. Yang, D. Wang, H. Wang, D. Y. W. Yu and A. L. Rogach, ACS Appl. Mater. Interfaces, 2020, 12, 12809–12820 CrossRef CAS.
- Z. Zhao, S. Li, C. Li, Z. Liu and D. Li, Appl. Surf. Sci., 2020, 519, 146268 CrossRef CAS.
- S. Gao, Y. He, H. Li, G. Yue, Z. Cui, Y. Li, J. Bai, N. Wang, Q. Zhang, Y. Yu and Y. Zhao, Energy Storage Mater., 2024, 65, 103170 CrossRef.
- R. Wei, Y. Dong, Y. Zhang, X. Kang, X. Sheng and J. Zhang, Nano Res., 2021, 15, 3273–3282 CrossRef.
- Z. Zhang, Y. Huang, X. Gao, Z. Xu and X. Wang, ACS Appl. Energy Mater., 2020, 3, 6205–6214 CrossRef CAS.
- L. Ren, Y. Huyan, Y. Cao, Z. Luo, Y. Zhang and J.-G. Wang, Electrochim. Acta, 2023, 467, 143077 CrossRef CAS.
- Y. Lin, Z. Qiu, D. Li, S. Ullah, Y. Hai, H. Xin, W. Liao, B. Yang, H. Fan, J. Xu and C. Zhu, Energy Storage Mater., 2018, 11, 67–74 CrossRef.
- I. Saptiama, Y. V. Kaneti, Y. Suzuki, K. Tsuchiya, N. Fukumitsu, T. Sakae, J. Kim, Y. M. Kang, K. Ariga and Y. Yamauchi, Small, 2018, 14, 1800474 CrossRef PubMed.
- Q. He, F. Xiao, R. Chen, T. Yao, Y. Wu and H. Wang, J. Alloys Compd., 2023, 960, 170866 CAS.
- X. He, L. Bi, Y. Li, C. Xu and D. Lin, Electrochim. Acta, 2020, 332, 135453 CAS.
- J. Wang, J. Qin, Y. Jiang, X. Wang and M. Cao, Nanoscale, 2020, 12, 13781–13790 RSC.
- C. Dong, L. Guo, H. Li, B. Zhang, X. Gao, F. Tian, Y. Qian, D. Wang and L. Xu, Energy Storage Mater., 2020, 25, 679–686 Search PubMed.
- X. Lu, A. Liu, Y. Zhang and S. Liu, Electrochim. Acta, 2021, 371, 137773 CAS.
- H. A. Abubshait, T. Alshahrani, H. H. Alhashim, T. H. Flemban, G. Ali, Q. Mahmood, A. Laref and N. A. Kattan, Int. J. Energy Res., 2020, 45, 5283–5292 Search PubMed.
- D. Han, G. Yu, A. Liu, G. Li, W. Wang, B. He, Z. Hou and H. Yin, Carbon Lett., 2023, 33, 1839–1846 CrossRef.
- H. Xia, K. Li, Y. Guo, J. Guo, Q. Xu and J. Zhang, J. Mater. Chem. A, 2018, 6, 7148–7154 RSC.
- C. Yang, Y. Li, W. Peng, F. Zhang and X. Fan, Chem. Eng. J., 2022, 427, 131792 CrossRef CAS.
- M. Yin, K. Wang, C. Gao, R. Yang, Y. Huang and L. Yu, Mater. Res. Bull., 2024, 179, 112943 CrossRef CAS.
- F. Zeng, L. Li, C. Liu and Z. Lin, ChemistrySelect, 2021, 6, 4344–4353 CrossRef CAS.
- Y. Li, R. Guo, Y. Sun, Y. Wang, W. Liu, H. Pei, H. Zhao, J. Zhang, D. Ye, J. Xie and J. Kong, ChemElectroChem, 2020, 7, 2752–2761 CrossRef CAS.
- Y. Zheng, L. He, X. Kong, Y. Song and Y. Zhao, Appl. Surf. Sci., 2022, 603, 154481 CrossRef CAS.
- Y. Zhang, N. Wang, C. Sun, Z. Lu, P. Xue, B. Tang, Z. Bai and S. Dou, Chem. Eng. J., 2018, 332, 370–376 CrossRef CAS.
- X. Liu, K. Zhang, K. Lei, F. Li, Z. Tao and J. Chen, Nano Res., 2016, 9, 198–206 CrossRef CAS.
- Y. Pan, X. Cheng, L. Gong, L. Shi and H. Zhang, Nanoscale, 2018, 10, 20813–20820 RSC.
- J. Wang, X. Yue, X. Cao, Z. Liu, A. M. Patil, J. Wang, X. Hao, A. Abudula and G. Guan, Chem. Eng. J., 2022, 431, 134091 CrossRef CAS.
- Y. Ma, Y. Ma, D. Bresser, Y. Ji, D. Geiger, U. Kaiser, C. Streb, A. Varzi and S. Passerini, ACS Nano, 2018, 12, 7220–7231 CrossRef CAS PubMed.
- D. Su, Y. Pei, L. Liu, Z. Liu, J. Liu, M. Yang, J. Wen, J. Dai, H. Deng and G. Cao, Nano-Micro Lett., 2021, 13, 107 CrossRef CAS PubMed.
- L. Cao, X. Gao, B. Zhang, X. Ou, J. Zhang and W.-B. Luo, ACS Nano, 2020, 14, 3610–3620 CrossRef CAS PubMed.
- Z. Lin, X. Xiong, M. Fan, D. Xie, G. Wang, C. Yang and M. Liu, Nanoscale, 2019, 11, 3773–3779 RSC.
- H. Li, Y. He, Y. Dai, Y. Ren, T. Gao and G. Zhou, Chem. Eng. J., 2022, 427, 131784 CrossRef CAS.
- Y.-F. Liu, T. Zhang, H.-H. Zhang, T.-T. Huang, K. Wang, Y.-X. Song, J.-F. Liang, Y.-G. Zhang, W. Fan and X.-B. Zhong, Dalton Trans., 2024, 53, 3573–3578 RSC.
- S. Luo, J. Cui, S. Liang, Y. Guo, B. Yuan, L. Xu, R. Zheng, J. Li, W. Yang, M. Chen, Y. Lu and Y. Luo, ACS Appl. Nano Mater., 2024, 7, 1655–1663 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03503h |
| This journal is © The Royal Society of Chemistry 2025 |